Abstract
Immunosuppressive regimens that effectively prevent graft-versus-host disease (GVHD) after allogeneic blood or marrow transplantation (alloBMT) have been associated with an increased incidence of posttransplantation lymphoproliferative disorder (PTLD) in the first year after transplantation. We evaluated the incidence of PTLD associated with the use of high-dose, posttransplantation cyclophosphamide (PTCy) as GVHD prophylaxis. From 2000-2011, 785 adult alloBMT patients received PTCy as GVHD prophylaxis at the Johns Hopkins Hospital, including 313 who received PTCy as sole GVHD prophylaxis. HLA-haploidentical or unrelated donor grafts were used for 526 (67%) patients. There were no cases of PTLD in the first year after alloBMT. PTLD is rare after alloBMT using PTCy, even in high-risk alternative donor transplants.
Introduction
The approach to graft-versus-host disease (GVHD) prophylaxis, including methods of graft manipulation and pharmacologic immunosuppression, impacts immune reconstitution after allogeneic blood or marrow transplantation (alloBMT) and modulates the risk for posttransplantation lymphoproliferative disorder (PTLD). PTLD risk peaks in the early months after alloBMT and the vast majority of cases occur within the first year after transplantation.[1] PTLD lesions that arise after alloBMT typically harbor Epstein-Barr virus (EBV).
In a multi-institutional analysis of PTLD incidence among 26,901 alloBMTs performed at 271 institutions worldwide, selective T-cell depletion of grafts and to a lesser extent other approaches to lymphocyte depletion were associated with increased risk for PTLD.[1] Patients who receive anti-thymocyte globulin (ATG) as GVHD prophylaxis are also at higher risk for PTLD.[1, 2] The use of alternative donors, including unrelated (URD), HLA-haploidentical (haplo), and umbilical cord blood (UCB), has been associated with higher rates of PTLD. This heightened risk for PTLD seems mostly related to the intensified GVHD prophylaxis strategies often employed with alternative donor grafts. Patients receiving URD or haplo grafts accompanied by ATG and/or selective T-cell depletion have an incidence of PTLD of 4-8%.[1] The incidence of PTLD after UCB alloBMT is 2-7%, but rates upwards of 17% have been reported with UCB alloBMTs using ATG.[2-5]
The use of posttransplantation cyclophosphamide (PTCy) as GVHD prophylaxis after alloBMT is associated with comparatively low rates of severe acute and chronic GVHD, even with URD or haplo grafts.[6] However, given that approaches that are highly effective in preventing GVHD can be associated with higher rates of PTLD, we have evaluated the incidence of PTLD associated with PTCy at the Johns Hopkins Hospital (JHH).
Materials and Methods
After JHH Institutional Review Board approval, the JHH Transplant Research databases were queried for patients age 18 or older who received PTCy as GVHD prophylaxis at JHH between January 1, 2000 to December 31, 2011. Clinical notes, pathology reports, and radiology reports up through the first year after alloBMT were reviewed.
For myeloablative conditioning, patients received a regimen of busulfan (Bu) and cyclophosphamide (Cy) as previously described.[7] Reduced-intensity conditioning (RIC) regimens were fludarabine (Flu)-based, with the majority (n=354) consisting of Flu/Cy/total body irradiation (TBI).[8] Other RIC regimens were Flu/TBI (n=72), Bu/Flu (n=14), ATG/Flu/Cy/TBI (n=14),[9] or alemtuzumab/Flu/TBI (n=2). All grafts were T-cell replete.
When used as sole GVHD prophylaxis, PTCy 50 mg/kg/day was given intravenously on days 3 and 4 after alloBMT,[7] with the exception of 11 patients who received PTCy on day 3 only. Most recipients of haplo grafts and/or RIC received additional immunosuppression following PTCy on days 3 and 4 after alloBMT, with the majority receiving mycophenolate mofetil and tacrolimus. GVHD prophylaxis regimens are shown in Table 1.
Table 1.
Patient and transplantation characteristics.
| AlloBMTs n=785 | |
|---|---|
| Age, median (range) | 52 (18-75) |
| Male sex, n (%) | 448 (57%) |
| Diagnosis, n (%) | |
| Acute myeloid leukemia or myelodysplastic syndrome | 308 (39%) |
| Mature B-cell neoplasm | 236 (30%) |
| Acute lymphoblastic leukemia | 69 (9%) |
| Myeloproliferative neoplasm, including CML | 52 (7%) |
| Hodgkin lymphoma | 48 (6%) |
| Mature T-cell or NK-cell neoplasm | 36 (5%) |
| Hemoglobinopathy or thalassemia | 17 (2%) |
| Aplastic anemia or paroxysmal nocturnal hemoglobinuria | 9 (1%) |
| Bilineage leukemia | 7 (1%) |
| Histiocytic or dendritic cell neoplasm | 2 |
| Lymphomatoid granulomatosis | 1 |
| Conditioning regimen, n (%) | |
| Reduced-intensity | 456 (58%) |
| Myeloablative | 329 (42%) |
| ATG-containing conditioning regimen, n (%) | 14 (2%) |
| Donor, n (%) | |
| HLA-haploidenticala | 396 (50%) |
| HLA-matched, related | 259 (33%) |
| HLA-matched or 1 antigen mismatched, unrelated | 130 (17%) |
| Allograft source, n (%) | |
| Marrow, T-cell replete | 748 (95%) |
| Peripheral blood, T-cell replete | 37 (5%) |
| GVHD prophylaxis, n (%) | |
| PTCy alone | 313 (40%) |
| PTCy on days +3 and +4 | 302 |
| PTCy on day +3 | 11 |
| PTCy on days +3 and +4/MMF/tacrolimusb | 382 (49%) |
| PTCy on days +3 and +4/MMFc | 55 (7%) |
| PTCy on day +3/MMF/tacrolimusd | 20 (2%) |
| PTCy on days +3 and +4/MMF/sirolimuse | 15 (2%) |
| Rituximab ≤ 12 months after alloBMT, n (%) | 63 (8%) |
| HLA-haploidentical | 55/396 (14%) |
| HLA-matched, related | 8/259 (3%) |
| HLA-matched or 1 antigen mismatched, unrelated | 0/130 |
| DLI ≤ 12 months after alloBMT, n (%) | 57 (7%) |
| HLA-haploidentical | 13/396 (3%) |
| HLA-matched, related | 41/259 (16%) |
| HLA-matched or 1 antigen mismatched, unrelated | 3/130 (2%) |
Abbreviations: alloBMT, allogeneic blood or marrow transplantation; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; NK, natural killer; ATG, anti-thymocyte globulin; HLA, human leukocyte antigen; GVHD, graft-versus-host disease; PTCy, posttransplantation cyclophosphamide; MMF, mycophenolate mofetil; DLI, donor lymphocyte infusion
395 related donors, 1 unrelated donor
MMF 15 mg/kg, 3 times daily up to 1000 mg/dose on days 5-35 and tacrolimus (goal trough 5-15 ng/mL) on days 5-180. Twenty patients received MMF 15 mg/kg twice daily on days 4-33.
MMF 15 mg/kg, twice daily on days 4-33
MMF 15 mg/kg twice daily on days 4-35, tacrolimus (goal trough 5-15 ng/mL) on days 4-50 (n=10) or days 4-180 (n=10)
MMF 15 mg/kg, 3 times daily up to 1000 mg/dose on days 5-35 and sirolimus daily (goal trough 5-15 ng/mL) on days 5-365
PTLD was defined using the 2008 World Health Organization classification.[10] Data on the use of post-alloBMT therapies that could modulate PTLD risk, including donor lymphocyte infusion (DLI) and rituximab therapy, were compiled. Death was considered a competing risk for PTLD. The R program, version 2.15.2 (R Core Development Team, Vienna, Austria) was used to estimate the cumulative incidence of death.
Results
From 2000-2011, 785 alloBMTs were performed using PTCy as GVHD prophylaxis (Table 1). PTCy was used as sole GVHD prophylaxis in 313 (40%) alloBMTs, 295 of which utilized myeloablative conditioning and HLA-matched grafts. Haplo or URD grafts were used in 67% (n=526) of the alloBMTs.
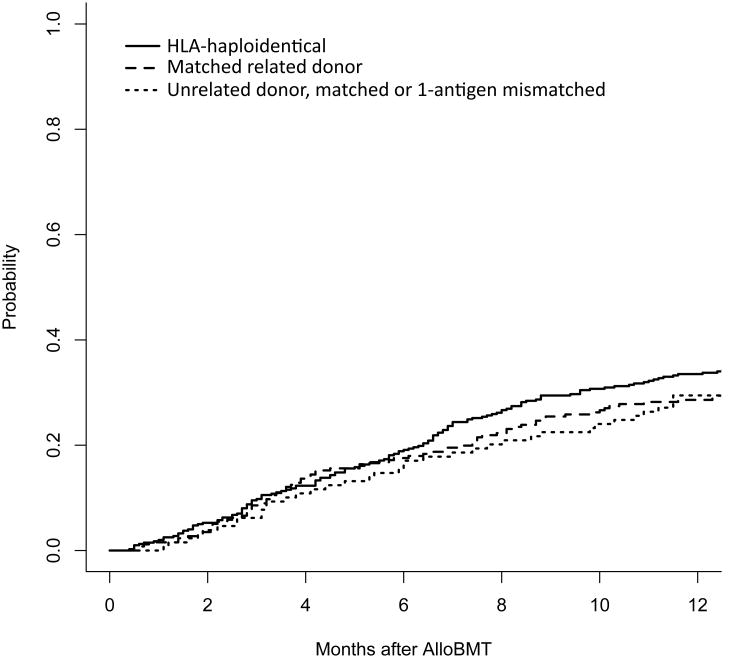
Almost all patients (n=762, 97%) had sufficient follow-up to assess for PTLD in the year following alloBMT, with 505 followed for at least one year by JHH physicians, 203 surviving less than one year but followed until death or hospice enrollment, and 54 resuming care with an outside oncologist before one year post-alloBMT, but corresponding with JHH when clinical issues arose. The cumulative incidence of death at one year was 34% (95% confidence interval (CI) 29-38%) for haplo alloBMTs, 30% (95% CI 22-38%) for URD alloBMTs, and 29% (95% CI 23-34%) for matched, related donor alloBMTs (Figure 1). There were no cases of PTLD identified among the 762 patients with adequate follow-up. Twenty-three patients had insufficient follow-up to assess for PTLD through one year post-alloBMT, but no cases of PTLD were identified during the available follow-up (median 3.7 months, range 1.6-11.9 months).
Figure 1. Cumulative incidence of death by allograft type.
At one year, the cumulative incidence of death was similar for recipients of HLA-haploidentical, unrelated, and HLA-matched related donor allografts at 34% (95% CI 29-38%), 30% (22-38%), and 29% (23-34%), respectively. The cumulative incidence of PTLD is not shown given the absence of events.
Therapies such as DLI, adoptive EBV-specific cellular immunotherapy, and rituximab are effective in treating PTLD. In our cohort, DLI was used in 57 patients within the year following alloBMT, but in no case was DLI given for suspected or confirmed PTLD. Adoptive immunotherapy with EBV-specific cytotoxic T-lymphocytes was not used in this cohort. Sixty-three patients received rituximab during the year following alloBMT. These included 43 patients with CD20(+) lymphomas who received 8 weekly doses of rituximab upon engraftment after RIC alloBMT (41 haplo and 2 HLA-matched related donor grafts) as part of a clinical protocol, 19 patients who received rituximab as part of treatment for relapsed B-lineage malignancy, and one patient who received one dose of pre-emptive rituximab for an asymptomatic, modest elevation of EBV-DNA copy number in the blood 4 months after alloBMT at an outside institution in keeping with that institution's protocol for post-alloBMT EBV monitoring. The patient's elevation in EBV-DNA copy number was not associated with fever, lymphadenopathy, organ dysfunction or other clinical findings concerning for PTLD. EBV DNA monitoring has continued and viral DNA is intermittently detected but the patient remains without signs or symptoms of EBV-associated disease. The patient remains healthy nearly 3 years after alloBMT.
Discussion
The absence of PTLD after alloBMT using PTCy is noteworthy, particularly given that no routine EBV monitoring was performed. Many transplant centers employ EBV monitoring and administer rituximab preemptively in attempts to decrease the risk for PTLD after alloBMT, particularly in high-risk patients.[3, 5, 11-13] Because graft elutriation was used at JHH prior to the implementation of PTCy-based approaches and the associated rate of PTLD was very low,[14, 15] the monitoring of EBV DNA in the blood after alloBMT has not been an institutional standard.[16] As EBV DNA is commonly detected in the blood after alloBMT with wide variation in copy number among patients and in individual patients over time, elevations of EBV DNA in the blood after low-risk alloBMTs should be interpreted with caution.[17, 18] In asymptomatic patients, even sustained elevations of EBV DNA in the blood after alloBMT lack specificity for predicting the development of PTLD.[18]
In reports from other centers that use PTCy, collectively comprising 162 alloBMTs, no cases of PTLD have been reported.[19-24] Of these, 63 alloBMTs were performed at centers that monitor EBV.[20, 24] One center reported that 1 of 13 patients transplanted with PTCy developed elevated EBV-DNA in the blood and no patient developed PTLD, contrasting this finding with a 12% incidence of PTLD at their center with other approaches.[24] The second center reported that 4 of 50 patients developed elevated EBV-DNA in the blood, one of whom was found to have EBV gastritis.[20, 25] While the data are limited, interpretation of EBV-DNA copy number in the blood after alloBMT with PTCy remains uncharted territory.
The absence of PTLD in the year following alloBMT in this series suggests that effective GVHD prophylaxis need not be associated with an increase in PTLD. Our institution and others have reported the effectiveness of PTCy as GVHD prophylaxis in patients receiving HLA-matched related, HLA-matched URD, or haplo grafts, with the cumulative incidence of grade III-IV acute GVHD ranging from 6-12%.[6-8, 20, 21, 23] Thus, it appears that the prevention of PTLD with PTCy does not come at the cost of higher GVHD rates.
In considering death as a competing risk for PTLD, a large majority of patients in this series survived at least one year post-alloBMT, including patients at higher risk for PTLD (recipients of haplo or URD grafts). Furthermore, the vast majority was alive in the first 6 months after alloBMT, when the risk for PTLD is highest. Given that this series includes hundreds of patients who survived more than one year and thus remained at risk for PTLD, it seems unlikely that early deaths would completely account for the absence of PTLD in this series.
Several hypotheses might be considered to explain the lack of PTLD with PTCy, including destruction of donor and host EBV-infected B-cells, relative sparing of EBV-specific memory T-cells, or rapid immune reconstitution, and investigations into these mechanisms including EBV-specific T-cell recovery after alloBMT using PTCy are ongoing. Whatever the explanation(s), PTCy results in very low to absent rates of PTLD, even with unrelated or HLA-mismatched donors.
Acknowledgments
This work was supported by P01CA15396, P50CA96888, P30CA006973, and T32HL7525 from the National Cancer Institute.
Footnotes
Financial Disclosure Statement: The authors have no competing financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Landgren O, Gilbert ES, Rizzo JD, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunstein CG, Weisdorf DJ, DeFor T, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peric Z, Cahu X, Chevallier P, et al. Features of EBV reactivation after reduced intensity conditioning unrelated umbilical cord blood transplantation. Bone Marrow Transplant. 2012;47:251–257. doi: 10.1038/bmt.2011.64. [DOI] [PubMed] [Google Scholar]
- 4.Dumas PY, Ruggeri A, Robin M, et al. Incidence and risk factors of EBV reactivation after unrelated cord blood transplantation: a Eurocord and Societe Francaise de Greffe de Moelle-Therapie Cellulaire collaborative study. Bone Marrow Transplant. 2012 doi: 10.1038/bmt.2012.117. [DOI] [PubMed] [Google Scholar]
- 5.Blaes AH, Cao Q, Wagner JE, Young JA, Weisdorf DJ, Brunstein CG. Monitoring and preemptive rituximab therapy for Epstein-Barr virus reactivation after antithymocyte globulin containing nonmyeloablative conditioning for umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:287–291. doi: 10.1016/j.bbmt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luznik L, Jones RJ, Fuchs EJ. High-dose cyclophosphamide for graft-versus-host disease prevention. Curr Opin Hematol. 2010;17:493–499. doi: 10.1097/MOH.0b013e32833eaf1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of Blood and Marrow Transplantation. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolanos-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. 2008 [Google Scholar]
- 11.van Esser JW, Niesters HG, van der Holt B, et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 2002;99:4364–4369. doi: 10.1182/blood.v99.12.4364. [DOI] [PubMed] [Google Scholar]
- 12.Greenfield HM, Gharib MI, Turner AJ, et al. The impact of monitoring Epstein-Barr virus PCR in paediatric bone marrow transplant patients: can it successfully predict outcome and guide intervention? Pediatr Blood Cancer. 2006;47:200–205. doi: 10.1002/pbc.20604. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad I, Cau NV, Kwan J, et al. Preemptive management of Epstein-Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation. 2009;87:1240–1245. doi: 10.1097/TP.0b013e31819f1c49. [DOI] [PubMed] [Google Scholar]
- 14.Flinn I, Orentas R, Noga SJ, et al. Low risk of Epstein-Barr virus (EBV)-associated posttransplant lymphoproliferative disease (PTLD) in patients receiving elutriated allogeneic marrow transplants may reflect depletion of EBV infected lymphocytes from the graft. Blood. 1995;86:2490–2490. [Google Scholar]
- 15.Gross TG, Hinrichs SH, Davis JR, Mitchell D, Bishop MR, Wagner JE. Depletion of EBV-infected cells in donor marrow by counterflow elutriation. Exp Hematol. 1998;26:395–399. [PubMed] [Google Scholar]
- 16.Hsieh WS, Ambinder RF. Thomas' Hematopoietic Cell Transplantation. Wiley-Blackwell; 2009. Epstein–Barr Virus Infection; pp. 1410–1418. [Google Scholar]
- 17.van Esser JW, van der Holt B, Meijer E, et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell--depleted SCT. Blood. 2001;98:972–978. doi: 10.1182/blood.v98.4.972. [DOI] [PubMed] [Google Scholar]
- 18.Wagner HJ, Cheng YC, Huls MH, et al. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood. 2004;103:3979–3981. doi: 10.1182/blood-2003-12-4287. [DOI] [PubMed] [Google Scholar]
- 19.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117–122. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical Transplantation Using T Cell Replete Peripheral Blood Stem Cells and Myeloablative Conditioning in Patients with High-Risk Hematologic Malignancies Who Lack Conventional Donors is Well Tolerated and Produces Excellent Relapse-Free Survival: Results of a Prospective Phase II Trial. Biology of Blood and Marrow Transplantation. 2012;18:1859–1866. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Bilmon IA, Kwan J, Gottlieb D, et al. Haploidentical bone marrow transplants for hematological malignancies using non-myeloablative conditioning therapy and post-transplant immunosuppression with cyclophosphamide: results from a single Australian centre. Intern Med J. 2012 doi: 10.1111/j.1445-5994.2012.02843.x. [DOI] [PubMed] [Google Scholar]
- 23.Bashey A, Zhang X, Sizemore CA, et al. T-Cell-Replete HLA-Haploidentical Hematopoietic Transplantation for Hematologic Malignancies Using Post-Transplantation Cyclophosphamide Results in Outcomes Equivalent to Those of Contemporaneous HLA-Matched Related and Unrelated Donor Transplantation. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 24.Tischer J, Fritsch S, Teepe C, et al. Incidence of infectious complications after HLA-haplo-identical haematopoetic stem cell transplantation using high-dose cyclophosphamide posttransplantation. Bone Marrow Transplant. 2011;46:S222–S222. [Google Scholar]
- 25.Hisamatsu A, Nagai T, Okawara H, et al. Gastritis associated with Epstein-Barr virus infection. Intern Med. 2010;49:2101–2105. doi: 10.2169/internalmedicine.49.3789. [DOI] [PubMed] [Google Scholar]