Abstract
Chiral vicinal diamines, including 2-aminomethyl indolines and pyrrolidines, are useful as ligands for catalytic asymmetric reactions and are also found as important components of bioactive compounds. Herein is reported the first copper-catalyzed alkene diamination that occurs with high enantioselectivity. The substrate range is the broadest yet reported for this kind of intra-/intermolecular reaction sequence both with respect to γ-alkenyl sulfonamide substrate and external amine nucleophile. The resulting products expand the availability of substituted 2-aminomethyl indolines and pyrrolidines, privileged compounds in asymmetric catalysis and medicinal chemistry. A unique solution to a challenging oxidation problem related to copper catalyst turnover is also presented.
Introduction
Chiral vicinal diamines, including 2-aminomethyl indolines and pyrrolidines, are used as ligands for catalytic asymmetric reactions1-4 and are also found as important components of bioactive compounds.5-7 Development of reactions that allow direct access to chiral vicinal diamines from readily available alkenes has been a longstanding interest and challenge in organic synthesis. Recent advances in alkene diamination technology8-14 have yielded a number of promising strategies, yet catalytic enantioselective alkene diaminations remain rare. Since 2007, Shi and co-workers have developed elegant intermolecular Pd- and Cu-catalyzed enantioselective diene diamination reactions using diaziridinone reagents that serve as both the diamine source and oxidant (Scheme 1).15-18 Additionally, Muniz and co-workers reported the use of a hypervalant chiral iodine(III) reagent for intermolecular diamination of styrenes in 2011.19,20 In 2010, we reported four examples of copper-catalyzed intra/intermolecular alkene diaminations and one promising, albeit unoptimized example of a catalytic enantioselective diamination reaction that resulted in the synthesis of a chiral 2-aminomethyl indoline in 71% ee using the readily available (R,R)-Ph-box ligand (Scheme 1).21 Michael and co-workers reported a Pd-catalyzed enantioselective diamination that provides substituted chiral 2-aminomethylpyrrolidines in very good to excellent enantioselectivities and in moderate to good yields in 2013.22 Several γ-unsaturated amides and carbamates undergo this [Pd(R)-Ph-quinox](TFA)2-catalyzed reaction where (SO2Ph)2NF (NFBS) is used as both the oxidant and amine source (Scheme 1). These enantioselective alkene diamination reactions are largely complementary, where each fits best with specific substrate and product classes.
Scheme 1. Enantioselective Alkene Diamination.
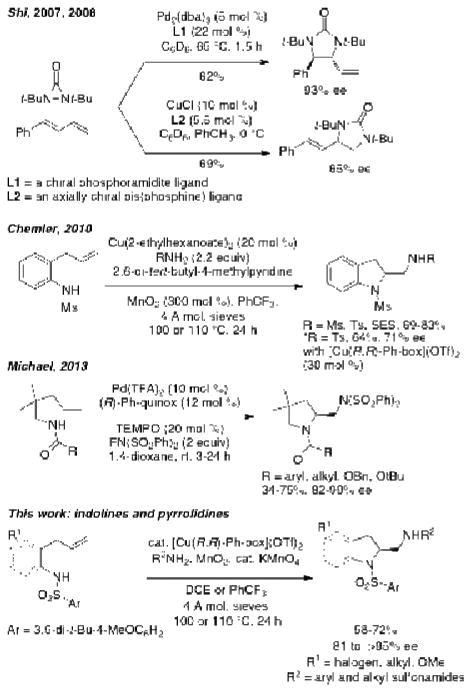
Herein we report a significant expansion of the scope of our copper-catalyzed inter/intramolecular alkene diamination protocol for the synthesis of substituted indolines and pyrrolidines. Importantly, we have optimized and expanded the enantioselective reaction to where we average between 81-91% ee for 2-aminomethyl indolines and 89 to >95% ee for 2-amino pyrrolidines (vide infra). A major new enabling advance in our enantioselective diamination reaction protocol was realized by employing KMnO4 as an oxidation catalyst (vide infra). This enantioselective diamination reaction is distinct from the other protocols in that the amine component is not pre-oxidized, allowing for a generally broader scope of readily available amine sources to be employed.
Results and Discussion
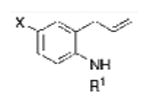
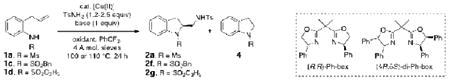
The scope in the racemic, copper-catalyzed alkene diamination21 for the formation of 2-aminomethyl indolines was expanded as summarized in Table 1. Most of these reactions were run using 20 mol % of copper(2-ethylhexanoate)2, 300 mol % of MnO2, 2.2 equiv of external amine nucleophile and 1 equiv of 2,6-di-tert-butyl-4-methyl pyridine as base in α,α,α-trifluorotoluene at 110 °C for 24 h. Other oxidants [e.g. O2 (1 atm), (t-BuO)2] were also examined for the purpose of copper catalyst turnover [believed to be Cu(I) to Cu(II), vide infra]. While O2 was a poor oxidant for this reaction (not shown), (t-BuO)2 proved to be an effective oxidant, though still not as effective as MnO2 (Table 1, entry 1, compare conditions a and b).
Table 1. Scope of the catalytic diamination of 2-allylanilinesa.
| Entry | Substrate | RNH2 | Product | % Yield |
|---|---|---|---|---|
| 1 |
 1a, X = H, R1 = Ms, |
TsNH2 |
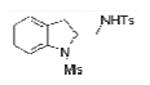 2a |
84 (75)b |
| 2 | 1a | MsNH2 |
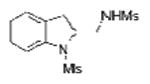 2b |
69 |
| 3 | 1a | SESNH2 |
 2c |
80 |
| 4 | 1a | BzNH2 |
 2d |
50 |
| 5 | 1b, X = H, R1 = Ts | TsNH2 |
 2e |
64c |
| 6 | 1c, X = H, R1 = SO2Bn | TsNH2 |
 2f |
65 |
| 7 | 1d, X = H, R1 = SO2C3H5 | TsNH2 |
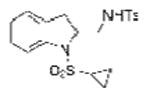 2g |
75 |
| 8 | 1e, X = H, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
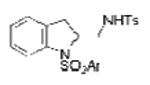 2b, Ar = 3,5-di-t-Bu-4-MeOC6H2 |
84 |
| 9 | 1e | SESNH2 |
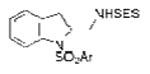 2i |
77 |
| 10 | 1f, X = OMe, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
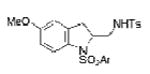 2j |
75 |
| 11 | 1g, X = Br, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
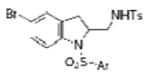 2k |
68 |
| 12 | 1h, X = Cl, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
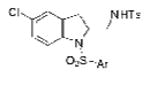 2l |
70 |
| 13 | 1i, X = F, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
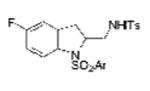 2m |
70 |
| 14 | 1j, X = Me, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
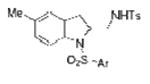 2n |
80 |
| 15d |
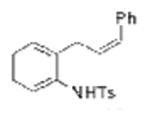 1k |
TsNH2 |
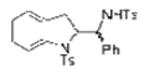 2o, 2p (dr = 58:42) |
76 |
| 16d |
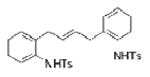 1l |
-- |
 2q |
73 |
Conditions:
20 mol % Cu(2-ethylhexanoate)2, MnO2 (3 equiv), 2,6-di-t-Bu-4-Me-pyridine (1 equiv), PhCF3, RNH2 (2.2 equiv), 110 °C, 24 h.
Using 2 equiv (t-BuO)2 at 100 °C instead of MnO2.
31% of sultam 3 also isolated.
In entry 15, [Cu(bisoxazoline)](OTf)2 was used. For entry 16 [Cu(R,R)-Ph-box](OTf)2 was used. 8% KMnO4 and 1:1 PhCF3:1,2-dichloroethane solvent were used in both reactions. Entries 1-3 (except 1b) were taken from ref. 21.
2-Allylanilines with both alkyl and aryl sulfonamide groups undergo efficient diamination under the optimized reaction conditions (a). External amine nucleophiles including TsNH2, MsNH2, 2-trimethylsilylethylsulfonylamide (SESNH2), and benzamide were effective although benzamide provided a comparatively lower yield (Table 1, entries 1-4).21 N-Tosyl-2-allyl aniline 1b provided 64% of diamine 2e under these catalytic conditions, where an additional 31% of the material was sultam 3 (vide infra, Scheme 2). Use of the hindered 3,5-di-tert-butyl-4-methoxyaryl sulfonamide 1e provided increased diamination yields compared to 1b (68-80% yield, Table 1, entries 8-14), and no sultam products 3, likely due to greater steric blocking of the ortho sites on the sulfonamide's arene (vide infra).
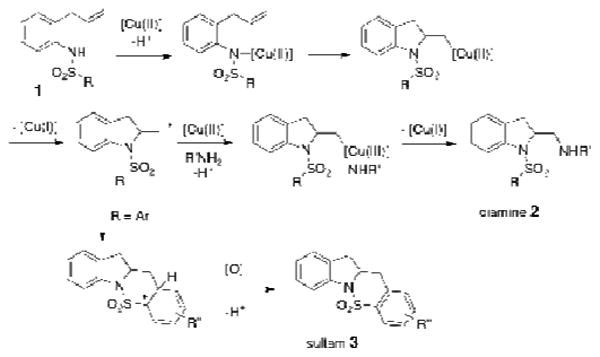
Scheme 2. Proposed copper(II)-catalyzed diamination mechanism.
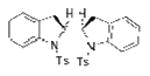
Two substrates with internal alkenes, 1k and 1l, also underwent the copper(II)-catalyzed diamination reaction (Table 1, entries 15 and 16). For these substrates, the N-tosyl sulfonamides were used as no sultam products were observed in their diamination reactions. These substrates did require the use of more reactive Cu(OTf)2•bis(oxazoline) catalyst and highest yields were observed under reaction conditions (solvent, oxidant) optimized for the enantioselective reaction (vide infra). A 58:42 ratio of diamine diastereomers 2o and 2p was formed in 76% combined yield from the (Z)-styrenyl substrate 1k. Use of the chiral (R,R)-Ph-box ligand in this reaction led to only racemic products, however. Meso diamine 2q (73%) was formed from the doubly intramolecular diamination of dimeric substrate 1l. In most of these reactions the remainder of the mass balance was unreacted alkene substrate.
A crystal structure of diamine 2j (Figure 1) confirmed it to be the exo regioisomer.
Figure 1.
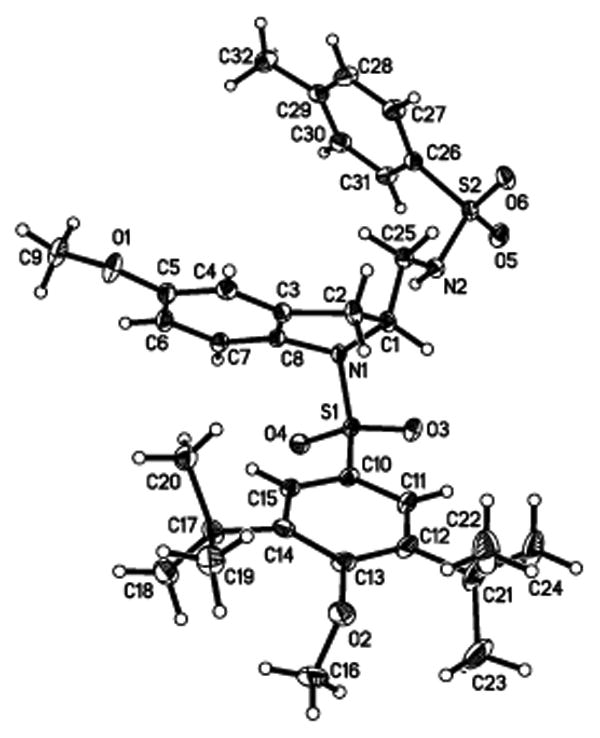
X-ray structure of diamine 2j.
We propose the copper(II)-catalyzed alkene diamination reaction occurs via the mechanism shown in Scheme 2. Coordination of the γ-unsaturated sulfonamide to [Cu(II)] followed by cis-aminocupration23 leads to an unstable organocopper(II) intermediate. Homolysis of the [C-Cu(II)] bond leads to a primary carbon radical. Isotopic labeling studies have supported formation of an sp2-hybridized carbon at this stage since the subsequent C-N bond formation occurs with equal amounts of retention and inversion at this carbon.24 Addition of the carbon radical to a [copper(II)-NHR] species generates a [copper(III)-NHR] species, similar to that implicated in the Kharasch-Sosnovsky reaction.25 Subsequent reductive elimination secures the second C-N bond, giving vicinal diamine 2. Although both reductive elimination and SN2 displacement could secure this second C-N bond, we tentatively favor the reductive elimination mechanism as external amines with electron withdrawing groups usually perform as well if not better than their electron-rich counterparts in this reaction.21,26 For diamination to occur, the copper(II) catalyst is needed in both C-N bond-forming steps. Copper-catalyst turnover in this reaction is thought to involve oxidation of [Cu(I)] to [Cu(II)] with the stoichimetric oxidant, MnO2 (300 mol % of activated ca. 85% MnO2, <5 μm).
When an arylsulfonamide is used (e.g. N-tosyl-2-allylaniline, 1b), an alternative pathway for the carbon radical intermediate is intramolecular addition to the sulfonylarene.27,28 This addition, a net carboamination reaction, results in formation of the corresponding sultam 3 (Scheme 2).27 The competition between diamination and carboamination pathways proved a challenging problem in the optimization of our copper-catalyzed enantioselective diamination (vide infra).
Asymmetric Catalysis
The two most successful ligands for asymmetric catalysis with this class of copper(II)-catalyzed alkene amination/difunctionalization reactions are bis(oxazolines) (R,R)-Ph-box and (4R,5S)-di-Ph-box.28-32 The former is commercially available (in both enantiomers) while the latter is available in one step from a commercially available ligand.24 While arylsulfonamides can give sultam byproducts 3 in this diamination reaction, aliphatic sulfonamides cannot. They do, however, form hydroamination side products 4.18 The affect of substrate, catalyst, base and oxidant were first examined in the copper(II)-catalyzed enantioselective diamination reactions of N-alkylsulfonyl 2-allylanilines 1 (Table 2). While the diamination catalyzed by Cu(2-ethylhexanoate)2 in the presence of (R,R)-Ph-box and MnO2 (3 equiv) provided an excellent yield of diamine 2a, no enantioselectivity was observed (Table 2, entry 1). This is in contrast to the reaction catalyzed with the [Cu(R,R)-Ph-box](OTf)2, which provides diamine 2a in 71% ee (Table 2, entry 2).21 The use of flame-dried 4 Å molecular sieves was important to maintaining reproducible yields and selectivities. We were able to further optimize the diamination of 1a by increasing the amount of TsNH2 nucleophile. This resulted in both increased yield and enantioselectivity at lower catalyst loading (Table 2, entries 2-4, up to 81% ee). Use of the (4R,5S)-di-Ph-box ligand provided slightly higher yield but comparable selectivity (Table 2, entries 4 and 5). Use of K2CO3 as base proved both less efficient (Table 2, entry 6, more hydroamination occurred) and less enantioselective (33% ee), while the reaction with Cs2CO3 provided racemic diamine 2a (Table 2, entry 7). Use of (t-BuO)2 as oxidant provided 59% of diamine 2a but the enantioselectivity was low (21% ee, Table 2, entry 8). It is possible in these latter two cases that a new ligand (e.g. t-BuO- or CO32-) is introduced to the copper center, thereby changing the coordination sphere of the chiral catalyst. By contrast, 2,6-di-tert-butyl-4-methylpyridine is too hindered to coordinate to the copper center. The hydroamination product 4, formed to a larger extent in the reaction using K2CO3 (Table 2, entry 6) is racemic, likely indicating it is formed via H+ promoted cyclization of 1a. This may explain why use of the more soluble hindered pyridine base can minimize this side reaction.
Table 2. Optimization of the enantioselective diamination of 2-allylaniline-derived alkyl sulfonamides.
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Entry | Substrate | [Cu] (mol %) | Ligand (mol %) | Equiv TsNH2 | Oxidant (equiv) | Base | Ratio (2:4) | Yield (%) 2 | ee (%) 2 |
| 1a | 1a | Cu(2-ethylhexanoate)2 (20) | (R,R)-Ph-box (25) | 2.5 | MnO2 (3) | 2,6-di-t-Bu-4-Me-pyridine | Only 2 | 84 | <5 |
| 2a | 1a | Cu(OTf)2 (30) | (R,R)-Ph-box (37) | 1.5 | MnO2 (3) | 2,6-di-t-Bu-4-Me-pyridine | 80:20 | 64 | 71 |
| 3a | 1a | Cu(OTf)2 (20) | (R,R)-Ph-box (25) | 1.2 | MnO2 (3) | 2,6-di-t-Bu-4-Me-pyridine | 55:45 | 45 | 73 |
| 4a | 1a | Cu(OTf)2 (20) | (R,R)-Ph-box (25) | 2.5 | MnO2 (3) | 2,6-di-t-Bu-4-Me-pyridine | 79:21 | 63 | 81 |
| 5a | 1a | Cu(OTf)2 (20) | (4R,5S)-di-Ph-box (25) | 2.5 | MnO2 (3) | 2,6-di-t-Bu-4-Me-pyridine | 80:20 | 68 | 81 |
| 6b | 1a | Cu(OTf)2 (20) | (R,R)-Ph-box (25) | 2.5 | MnO2 (3) | K2CO3 | 53:47 | 35 | 33 |
| 7b | 1a | Cu(OTf)2 (20) | (R,R)-Ph-box (25) | 2.5 | MnO2 (3) | Cs2CO3 | 76:24 | 60 | <5 |
| 8b | 1a | Cu(OTf)2 (20) | (R,R)-Ph-box (25) | 2.5 | (t-BuO)2 (2) | 2,6-di-t-Bu-4-Me-pyridine | 73:27 | 59 | 21 |
| 9a | 1c | Cu(OTf)2 (20) | (4R,5S)-di-Ph-box (25) | 2.5 | MnO2 (3) | 2,6-di-t-Bu-4-Me-pyridine | 55:45 | 49 | 79 |
| 10a | 1d | Cu(OTf)2 (20) | (4R,5S)-di-Ph-box (25) | 2.5 | MnO2 (3) | 2,6-di-t-Bu-4-Me-pyridine | 64:36 | 58 | 76 |
Additional conditions:
110 °C;
100 °C. Diamination to hydroamination (2 to 4) ratios were determined by analysis of the crude 1H NMR. Yields are reported as isolated via flash chromatography. Enantiomeric excess was determined by chiral HPLC.
Under the optimal conditions (Table 2, entry 5), the benzylsulfonamide and cyclopropylsulfonamides 1c and 1d also provided diamine products 2f and 2g in respectable enantiomeric excess, however, these results were not superior to the reaction of 1a, and the ratio of diamination to hydroamination was decreased (Table 2, entries 9 and 10).
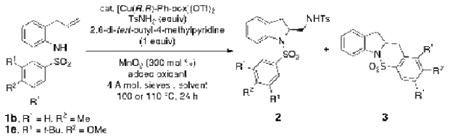
A number of other chiral ligands and sulfonamide nucleophiles were screened in the enantioselective diamination of 1a, but none gave higher selectivity than (R,R)-Ph-box (81% ee). Thus, the copper-catalyzed enantioselective diaminations of N-arylsulfonyl-2-allylanilines 1b and 1e were subsequently re-examined (Table 3). N-tosyl-2-allylaniline 1b gave only sultam 3 under the [Cu(R,R)-Ph-box](OTf)2-catalyzed conditions (Table 3, entry 1). This is in contrast to the reaction with Cu(2-ethylhexanoate)2 as catalyst, where 64% of the diamine adduct 2e was obtained (Table 1, entry 5). This difference indicates relative participation of the copper catalysts in the second C-N bond forming step (Scheme 2, vide supra), which may be due to differential oxidation rates of the corresponding [Cu(I)] complexes (vide infra).33 The more sterically hindered 3,5-di-tert-butyl-4-methoxy phenylsulfonyl group of substrate 1e reduced the rate of sultam formation and allowed formation of diamine adduct 2h in 18% yield (diamine : sultam = 20:80) and in 92% ee under these conditions (Table 3, entry 2). An increase in external amine nucleophile from 2.5 equiv to 5 equiv led to an increase in diamine yield (33% yield, 91% ee, diamine : sultam = 1 : 1.5, Table 3, entry 3). An increase in catalyst loading from 20 to 40 mol % led to a further increase in isolated diamine yield to 56% (diamine : sultam = 63:37, Table 3, entry 4). We interpreted this increase in yield to be a response to the increased amount of available copper(II) needed for the second C-N bond-forming step.
Table 3. Optimization of enantioselective diamination of N-arylsulfonyl-2-allylanilinesa.
| ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Entry | Substrate | [Cu] (mol %) | Additional oxidant | TsNH2 (equiv) | solvent | Ratio 2:3 | Yield (%) 2 | ee (%) 2 |
| 1b | 1b | 20 | -- | 2.5 | PhCF3 | only 3 | -- | -- |
| 2b | 1e | 20 | -- | 2.5 | PhCF3 | 20:80 | 18 | 92 |
| 3b | 1e | 20 | -- | 5.0 | PhCF3 | 40:60 | 33 | 91 |
| 4b | 1e | 40 | -- | 5.0 | PhCF3 | 63:37 | 56 | 90 |
| 5c | 1e | 25 | -- | 5.0 | PhCF3 | 40:60 | 35 | 86 |
| 6c | 1e | 25 | -- | 5.0 | DCE | 46:54 | 40 | 79 |
| 7c | 1e | 25 | -- | 5.0 | 1:1 PhCF3/DCE | 45:55 | nd | nd |
| 8c | 1e | 25 | 8% KMnO4 | 5.0 | 1:1 PhCF3/DCE | 65:35 | 60 | 86 |
| 9b | 1e | 25 | 8% KMnO4 | 5.0 | 1:1 PhCF3/DCE | 69:31 | 64 | 90 |
Conditions:
Reactions in PhCF3 were run at 110 °C. Reactions in DCE were run at 100 °C. Ratio of 2:3 determined by analysis of the crude 1H NMR spectra. Yields of products are given as isolated by flash chromatography. Enantiomeric excess was measured by chiral HPLC analysis.
Run with 24% excess (R,R)-Ph-box ligand compared to Cu(OTf)2;
Run with 8% excess (R,R)-Ph-box ligand compared to Cu(OTf)2.
In order to reduce the catalyst loading from 40 mol % to a more synthetically useful level (25 mol %), we investigated the affect of solvent and oxidant on the reaction. We found a higher ratio of diamine : sultam (46:54) was obtained in 1,2-dichloroethane (DCE) but the diamine product, isolated in 40% yield, had a diminished enantiomeric excess (79% ee). A 1:1 ratio of DCE to CF3Ph provided the same ratio of diamine : sultam (45:55) as in DCE (Table 3, entry 7). In order to maintain a lower catalyst loading (25 mol %) while further maximizing diamine yield, we investigated increasing the oxidizing power of the oxidant. Merely increasing the MnO2 loading from 300 mol % to 400-600 mol % had little effect on the diamination : carboamination ratio (not shown), possibly due to the poor solubility of this reagent in the reaction medium. Inspired by a report by Shaabani and co-workers where a catalytic amount of KMnO4 had been used in an MnO2 oxidation,34 we subjected the reaction to a mixture (mixed with mortar and pestle) of oxidant containing 3 equiv of MnO2 and 0.08 equiv of KMnO4 in the mixed solvent system. To our delight, the diamination : sultam ratio increased to 69 : 31 and diamine 2h was isolated in 64% yield and 90% ee. Use of higher amounts of KMnO4 in these reactions led to formation of side products. We also noted that reactions run with 24% excess ligand, compared to [Cu] [e.g. 25 mol% Cu(OTf)2 and 31 mol % (R,R)-Ph-box] gave higher enantioselectivities than those run with 8% excess ligand [e.g. 25 mol % Cu(OTf)2 and 27 mol % (R,R)-Ph-box] (compare Table 3, entries 8 and 9).
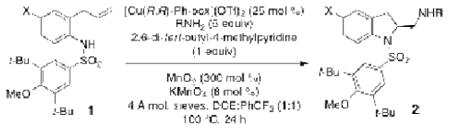
These optimized reaction conditions (Table 3, entry 9) were applied to variously substituted 2-allylanilines, undergoing coupling with TsNH2 and SESNH2 (Table 4). While the yields were all around 60% irrespective of substrate, substituents on the para position of the aniline (Me, OMe, Br, Cl, F) did somewhat diminish the enantioselectivity (enantioselectivity ranged from 81-91% ee).
Table 4. Scope of N-arylsulfonyl-2-allylanilinesa.
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Substrate | RNH2 | Yield (%) | ee (%) |
| 1 | 1e, X = H | SESNH2 | 59 | 91 |
| 2 | 1f, X = OMe | TsNH2 | 61 | 85 |
| 3 | 1g, X = Br | TsNH2 | 59 | 84 |
| 4 | 1h, X = Cl | TsNH2 | 60 | 83 |
| 5 | 1i, X = F | TsNH2 | 60 | 85 |
| 6 | 1j, X = Me | TsNH2 | 63 | 81 |
Same conditions as Table 3, entry 9.
The different sulfonyl groups on the diamine products can undergo orthogonal deprotection, e.g. the arylsulfonyl is susceptible to reduction (Mg, MeOH, Eq 2)35 while the 2-trimethylsilylethyl sulfonamides can be de-sulfonylated with fluoride (e.g. TBAF).
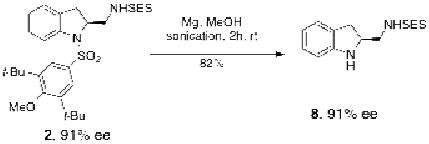 |
1 |
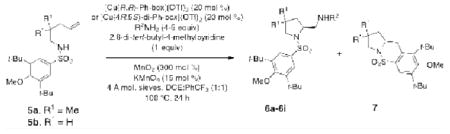
γ-Alkenylsulfonamides 5 also underwent efficient copper-catalyzed diamination (Table 5). Optimal conditions for this reaction involved 20 mol % of [Cu(R,R)-Ph-box ](OTf)2 or [Cu(4R,5S)-di-Ph-box](OTf)2, 3 equiv of MnO2 and 15 mol % KMnO4. The 2,2-dimethyl substrate 5a undergoes the reaction with equal efficiency with both catalysts (although most examples are shown with the (4R,5S)-di-Ph-box ligand, the two ligands are essentially interchangeable: Table 5, entries 1-9) while sulfonamide 5b with the unsubstituted backbone gave the optimal selectivity with the (4R,5S)-di-Ph-box ligand (Table 5, entry 10). High enantioselectivities were obtained when the ligand was only 10% in excess of [Cu] (e.g. 20 mol % Cu(OTf)2, 22 mol % (R,R)-Ph-box).
Table 5. Chiral 2-aminopyrrolidine formation via enantioselective alkene diamination.
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Entry | Substrate | Amine Nucleophile | Product | Ratio 6:7 | Yield (%) 6 | ee (%) 6 |
| 1a | 5a | TsNH2 | 6a, R1 = Me, R2 = Ts | 67:33 | 64 | >95 |
| 2b | 5a | TsNH2 | 6a, R1 = Me, R2 = Ts | 76:24 | 72 | >95 |
| 3c | 5a | SESNH2 | 6b, R1 = Me, R2 = SES | 63:37 | 58 | >95 |
| 4c | 5a | BsNH2 | 6c, R1 = Me, R2 = Bs | 69:31 | 63 | >95 |
| 5c | 5a | 2-MeC6H4SO2NH2 | 6d, R1 = Me, R2 = SO2(2-MeC6H4) | 68:32 | 62 | >95 |
| 6c | 5a | 4-MeOC6H4SO2NH2 | 6e, R1 = Me, R2 = SO2(4-MeOC6H4) | 66:34 | 60 | >95 |
| 7c | 5a | 4-ClC6H4SO2NH2 | 6f, R1 = Me, R2 = SO2(4-ClC6H4) | 64:36 | 60 | >95 |
| 8c | 5a | 4-NO2C6H4SO2NH2 | 6g, R1 = Me, R2 = SO2(4-NO2C6H4) | 64:36 | 58 | >95 |
| 9c | 5a | C3H5SO2NH2 | 6h, R1 = Me, R2 = SO2C3H5 | 67:33 | 61 | >95 |
| 10c | 5b | TsNH2 | 6i, R1 = H, R2 = Ts | 66:34 | 61 | 89 |
Conditions:
Using the (R,R)-Ph-box ligand (22 mol %) with 20 mol % Cu(OTf)2 and 4 equiv R2NH2.
Conditions (a) except using the (R,R)-Ph-box ligand (33 mol %) with 30 mol % Cu(OTf)2.
Conditions (a) except using the (4R,5S)-di-Ph-box ligand (22 mol %) with 20 mol % Cu(OTf)2.
Conditions (c) except using 5 equiv R2NH2. SES = 2-trimethylsilylethylsulfonyl, Bs = benzenesulfonyl.
Enantioselective diaminations of these substrates occurred most efficiently in DCE where ratios of diamines 6 to sultams 7 were 60-70 : 30-40 and isolated diamine yields ranged from 58-64% with enantioselectivities ranging from 89 to >95%. Higher catalyst loading (30 mol % [Cu]) can provide higher isolated yield of diamine 6 (72%, Table 5, entry 2) by diminishing competitive sultam formation.36
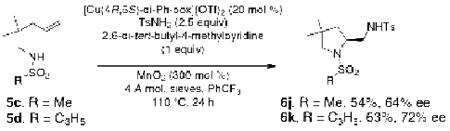
Sulfonamides 5c and 5d with alkyl sulfonyl groups (Ms, cyclopropylsulfonyl) produced diamine products with lower levels of enantioselectivity (64 and 72% ee, respectively, Eq 2).
 |
2 |
The absolute stereochemistry of the indoline and pyrrolidine diamine products is assigned by analogy to similar [Cu(R,R)-Ph-box](OTf)2-catalyzed alkene aminofunctionalizations28-32 and by comparison of the optical rotation of diamine 6i with the same compound, synthesized from a known chiral diamine (see Supporting Information). A transition state model that depicts formation of the major enantiomer via a cis-aminocupration across the substrate's alkene has been calculated for the analogous copper-catalyzed alkene aminooxygenation and should apply to the diamination reaction as well.37
Conclusions
In conclusion we have developed a copper-catalyzed enantioselective intra/intermolecular alkene diamination reaction that forms chiral 2-aminomethyl indolines and pyrrolidines in good yields and very good to excellent enantioselectivities. This is the only reported method for the synthesis of enantiomerically enriched 2-aminomethyl indolines via catalytic enantioselective alkene diamination and thus provides new efficient access to valuable compounds that are otherwise difficult to obtain. A number of external sulfonamide nucleophiles underwent coupling with various γ-alkenylsulfonamide in this reaction. The method does not require use of pre-oxidized amines, instead, inexpensive and earth abundant MnO2 facilitates oxidation for this reaction (oxidation of [Cu(I)] back to the catalytically active [Cu(II)] state). An important finding in this new protocol involves the use of catalytic KMnO4 to aid in the oxidation phase of the catalytic cycle. While the role of KMnO4 (8-15 mol %) is not known for certain, we speculate it serves to regenerate MnO2. Since MnO2 is sparingly soluble in the reaction medium, regenerating the existing MnO2 may be more effective than simply adding more MnO2 to the reaction.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (RO1 GM078383) for generous financial support of this research. We thank William Brennessel and the University of Rochester X-ray facility for the X-ray structure of 2j (CCDC 982216).
References
- 1.Sato S, Watanabe H, Asami M. Tetrahedron Asym. 2000;11:4329–4340. [Google Scholar]
- 2.Zhou Y, Zhu Y, Yan S, Gong Y. Angew Chem Int Ed. 2013;52:10265–10269. doi: 10.1002/anie.201305148. [DOI] [PubMed] [Google Scholar]
- 3.Pansare SV, Pandya K. J Am Chem Soc. 2006;128:9624. doi: 10.1021/ja062701n. [DOI] [PubMed] [Google Scholar]
- 4.Betancort JM, Barbas CF., III Org Lett. 2001;3:3737–3740. doi: 10.1021/ol0167006. [DOI] [PubMed] [Google Scholar]
- 5.Clow A, Jenner P, Marsden CD, Reavill C, Theodorou A. Proc Brit Psych Soc. 1979:433P. [PMC free article] [PubMed] [Google Scholar]
- 6.Lucet D, Le Gall T, Mioskowski C. Angew Chem Int Ed. 1998;37:2580–2627. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Bogatcheva E, Hanrahan C, Nikonenko B, Samala R, Chen P, Gearhart J, Barbosa F, Einck L, Nacy CA, Protopova M. J Med Chem. 2006;49:3045–3048. doi: 10.1021/jm050948+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardona F, Goti A. Nature Chemistry. 2009;1:269–275. doi: 10.1038/nchem.256. [DOI] [PubMed] [Google Scholar]
- 9.de Jong S, Nosal DG, Wardrop DJ. Tetrahedron. 2012;68:4067–4105. doi: 10.1016/j.tet.2012.03.036. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Figueiredo RM. Angew Chem Int Ed. 2009;48:1190–1193. doi: 10.1002/anie.200804362. [DOI] [PubMed] [Google Scholar]
- 11.Wang YF, Zhu X, Chiba S. J Am Chem Soc. 2012;134:3679–3682. doi: 10.1021/ja2120629. [DOI] [PubMed] [Google Scholar]
- 12.Chavez P, Kirsch J, Hovelmann CH, Streuff J, Martinez-Belmonte M, Escudero-Adan EC, Martin E, Muniz K. Chem Sci. 2012;3:2375–2382. [Google Scholar]
- 13.Zhang H, Pu W, Xiong T, Li Y, Zhou X, Sun K, Liu Q, Zhang Q. Angew Chem Int Ed. 2013;52:2529–2533. doi: 10.1002/anie.201209142. [DOI] [PubMed] [Google Scholar]
- 14.Muniz K, Martinez C. J Org Chem. 2013;78:2168. doi: 10.1021/jo302472w. [DOI] [PubMed] [Google Scholar]
- 15.Du H, Yuan W, Zhao B, Shi Y. J Am Chem Soc. 2007;129:11688–11689. doi: 10.1021/ja074698t. [DOI] [PubMed] [Google Scholar]
- 16.Du H, Zhao B, Shi Y. 2008;130:8590–8591. doi: 10.1021/ja8027394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du H, Zhao B, Yuan W, Shi Y. Org Lett. 2008;10:4231–4234. doi: 10.1021/ol801605w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornwall RG, Zhao B, Shi Y. Org Lett. 2013;15:796–799. doi: 10.1021/ol303469a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roben C, Souto JA, Gonzalez Y, Lishchynskyi A, Muniz K. Angew Chem Int Ed. 2011;50:9478–9482. doi: 10.1002/anie.201103077. [DOI] [PubMed] [Google Scholar]
- 20.Muniz K, Nieger M. Chem Comm. 2005:2729–2731. doi: 10.1039/b502150b. [DOI] [PubMed] [Google Scholar]
- 21.Sequeira FC, Turnpenny BW, Chemler SR. Angew Chem Int Ed. 2010;49:6365–6368. doi: 10.1002/anie.201003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingalis EL, Sibbald PA, Kaminsky W, Michael FE. J Am Chem Soc. 2013;135:8854–8856. doi: 10.1021/ja4043406. [DOI] [PubMed] [Google Scholar]
- 23.Paderes MC, Belding L, Fanovic B, Dudding T, Keister JB, Chemler SR. Chem Eur J. 2012;18:1711–1726. doi: 10.1002/chem.201101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sequeira FC, Bovino MT, Chipre AJ, Chemler SR. Synthesis. 2012;44:1481–1484. doi: 10.1055/s-0031-1289762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eames J, Watkinson M. Angew Chem Int Ed. 2001;40:3567–3571. doi: 10.1002/1521-3773(20011001)40:19<3567::AID-ANIE3567>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.This observation, however, could also be due to over-coordination of electron-rich amines to the copper(II), which may cause a general decrease in its ability to catalyze the diamination reaction
- 27.Sherman ES, Chemler SR, Tan TB, Gerlits O. Org Lett. 2004;6:1573–1575. doi: 10.1021/ol049702+. [DOI] [PubMed] [Google Scholar]
- 28.Zeng W, Chemler SR. J Am Chem Soc. 2007;129:12948–12949. doi: 10.1021/ja0762240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuller PH, Kim JW, Chemler SR. J Am Chem Soc. 2008;130:17638–17639. doi: 10.1021/ja806585m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bovino MT, Chemler SR. Angew Chem Int Ed. 2012;51:3923–3927. doi: 10.1002/anie.201109044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liwosz TW, Chemler SR. J Am Chem Soc. 2012;134:2020–2023. doi: 10.1021/ja211272v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paderes MC, Keister JB, Chemler SR. J Org Chem. 2013;78:506–515. doi: 10.1021/jo3023632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller Y, Miao L, Hosseini AS, Chemler SR. J Am Chem Soc. 2012;134:12149–12156. doi: 10.1021/ja3034075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaabani A, Mirzaei P, Naderi S, Lee DG. Tetrahedron. 2004;60:11415–11420. [Google Scholar]
- 35.Lee GH, Youn IK, Choi EB, Lee HK, Yon GH, Yang HC, Pak CS. Current Organic Chemistry. 2004;8:1263–1287. [Google Scholar]
- 36.Benzamide and CbzNH2 failed as nucleophiles in this reaction
- 37.Belding L, Chemler SR, Dudding T. J Org Chem. 2013;78:10288–10297. doi: 10.1021/jo401665n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


