Review of ROS in the pathogenesis of acute myeloid leukemia (AML), and the rationale for redox therapies.
Keywords: ROS, cell signaling, leukemogenesis
Abstract
It has become apparent that regulation of ROS is important in cell signaling and homeostasis. Accumulation of ROS triggers oxidative stress in various cell types and contributes to the development, progression, and persistence of cancer. Recent research has demonstrated that redox dysregulation caused by ROS promotes proliferation, differentiation, genomic, and epigenetic alterations; immune evasion; and survival in leukemic cells. ROS act as signaling molecules to regulate redox-sensitive transcriptional factors, enzymes, oncogenes, and other downstream effectors. Thus, a thorough understanding the role of ROS as key mediators in leukemogenesis is likely to provide opportunities for improved pharmacological intervention. In this review, we summarize the recent findings that support a role for ROS in the pathogenesis of AML and outline innovative approaches in the implementation of redox therapies for myeloid malignancies.
Introduction
ROS are a family of chemically active molecules that contain free radicals and are involved in modulating biological cell functions, cell signaling, and homeostasis [1–3]. Cells' fate depends on the levels of ROS in the redox microenvironment. Low levels provide a beneficial effect, supporting cell proliferation and survival pathways. Sublethal levels of ROS are generated during environmental stress, resulting in DNA damage in normal or precancerous cells that promote malignant transformation. However, excessive ROS levels can cause detrimental oxidative stress that results in cell death [4, 5]. Frequently, an adequate balance between producing and eliminating ROS is maintained in cells via antioxidants or specific enzymatic pathways.
ROS have been termed mediators in intracellular signaling. Key to the renewal of this field was the finding that endogenous ROS levels are elevated in tumor cells [6]. Increased levels of steady-state ROS have been linked to numerous cellular processes that are associated with tumor development, such as malignant transformation, prosurvival pathway up-regulation, and DNA damage-induced mutations. ROS also modulate the expression of critical kinases, phosphatases, redox-sensitive transcription factors, and associated genes, suggesting that they promote malignant transformation. The role of ROS in leukemia was supported by the results of several studies [7–9].
AML is an aggressive hematopoietic malignancy that is characterized by highly proliferative blast cells and increasing incidence with age. Clinically, the disease is associated with hyperleukocytosis, extramedullary disease, and abnormal coagulation. Moreover, AML patients inevitably experience relapse, even after standard chemotherapy and stem cell transplantation. Most relapses occur in patients with a poor prognosis index [10, 11]. Current therapeutic strategies appear to have reached the limit of their effectiveness; thus, novel strategies are urgently needed to target leukemic cells exclusively. Several recent studies have demonstrated that altered antioxidant status is a distinct feature of AML, and in blasts isolated from patients with various types of leukemia, oxygen radical levels were significantly higher in malignant cells than in normal leukocytes [12–14]. We hypothesized that altered intracellular and extracellular ROS trigger acute myeloid leukemogenesis.
In this paper, we review the recent medical literature and address the role of intracellular and extracellular ROS in acute myeloid leukemogenesis. Opportunities for pharmacological intervention are identified to demonstrate how recent findings may facilitate the implementation of redox therapies for AML.
ROS REGULATION OF HSCs
ROS are produced as normal products during cellular metabolism and include superoxide anion, H2O2, hydroxyl radical, and NO. They are signal messengers that are generated from multiple sources, including the mitochondrial electron transport system, the NOX complex, and other cellular oxidant-generating systems [15].
ROS have been implicated as the chemical mediators in normal hematopoiesis. HSCs have a self-replicating potential that allows them to constantly replenish blood cells. Fluctuations of relatively low ROS levels control their self-renewal and proliferative capacity [16]. HSCs are found primarily in a quiescent state in the bone marrow stem cell niche [17]. However, HSCs with increased ROS levels undergo proliferation and differentiation after mobilizing to the more oxygen-rich bloodstream. Elevated ROS levels lead to HSCs changing their phenotype [16]. Numerous genes involved in DNA-damage responses or longevity-related signaling contribute to maintaining the self-renewal capacity of HSCs. Repression of intracellular ROS levels in HSCs, after treating with antioxidant ROS scavengers, rescues HSC functions, suggesting that excessive ROS contributes to the phenotypic change in HSCs [18].
Recent studies demonstrated that FoxOs are required for the long-term regenerative potential of the HSC compartment, as FoxOs regulate the response of HSCs to physiologic oxidative stress, quiescence, and survival [19]. Moreover, FoxOs affect HSC integrity by regulating ROS. In particular, a recent finding indicates that the FoxO-deficient HSC phenotype causes elevated ROS concentrations that are correlated with aberrant expression of ROS-regulating genes. Furthermore, daily treatment with the antioxidant N-acetyl-l-cysteine for 5 weeks reverses the FoxO-deficient HSC phenotype [20]. Similarly, germline loss of FoxO3 results in increased ROS levels and increased activation of p38 MAPK in the HSC compartment [21]. In brief, the forkhead transcription factors of the FoxO family participate in diverse physiologic processes by regulating ROS, including cell-cycle arrest, stress resistance, differentiation, apoptosis, and metabolism.
HSCs undergo self-renewing cell divisions and maintain blood production for life. Appropriate control of the self-renewal capacity of HSCs is critical for maintaining hematopoietic homeostasis. Hypoxia helps preserve the progenitor characteristics of HSCs and hematopoietic progenitor cells in the bone marrow. It effectively preserves the biological characteristics of CD34+ cells by regulating NOX, thereby maintaining lower intracellular ROS levels [22]. Clearly, a low-oxygenic niche in the bone marrow limits ROS production to provide long-term protection for HSCs from ROS stress. For example, Mdm2 is an E3 ubiquitin ligase that targets p53 for degradation and is required to control ROS-induced p53 levels for sustainable hematopoiesis [23]. However, excessive oxygen is toxic to HSCs because of a state of unstable, dynamic ROS balance in the surrounding niche. In fact, elevated ROS levels also limit the lifespan of HSCs [24].
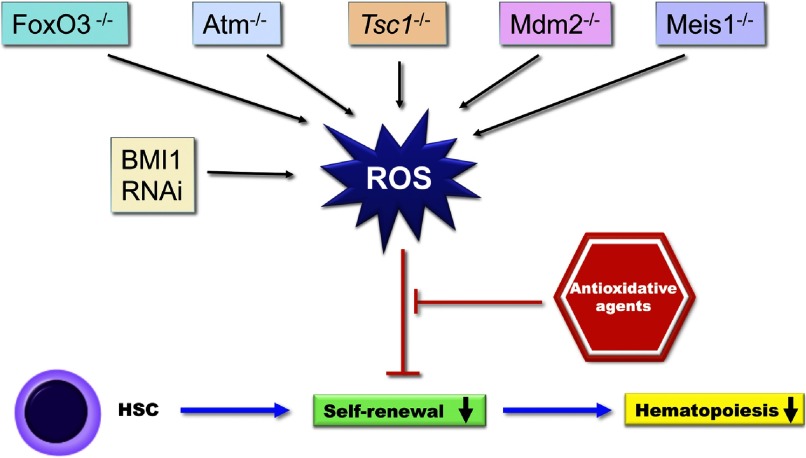
BMI1 has been reported as an oncogene in human carcinogenesis and is necessary for efficient self-renewing cell divisions in adult HSCs. Rizo et al. [25] demonstrated that BMI1 down-regulation impairs the long-term expansion and progenitor-forming capacity of cord blood CD34+ cells in cytokine-driven liquid cultures and in bone marrow stromal cocultures. The Tsc1 acts as a tumor suppressor and is a key regulator of cellular metabolism. The studies by Chen et al. [26] also found that Tsc1 deletion in HSCs drives them from quiescence to rapid cycling, with increased mitochondrial biogenesis and elevated ROS levels, whereas in vivo treatment with a ROS antagonist restores HSC numbers and functions. The ATM gene modulates the cell cycle and is involved in oxidative defense. In ATM-deficient mice, increased levels of ROS induced HSC-specific phosphorylation of p38 MAPK, followed by a defect in maintaining HSC quiescence. p38 MAPK inhibition restored the ROS-defective HSC population capacity and HSC quiescence retention, suggesting that the ROS-p38 MAPK pathway contributes to HSC depletion [24]. Recent findings by Kocabas et al. [27] demonstrated that inducible Meis1 deletion in adult mice with increased ROS levels resulted in apoptosis, loss of HSC quiescence, and failure of bone marrow repopulation after transplantation. ROS act as signal molecules that can control HSC functions via gene regulation. Indeed, this deletion of genes reduced hematopoiesis and HSC self-renewal dramatically (Fig. 1).
Figure 1. ROS regulation of hematopoiesis via stem cell self-renewal.
HSCs have self-replicating potential that allows them to constantly replenish blood cells. Elevated ROS levels, present in normal hematopoiesis, lead to reduced self-renewal and hematopoiesis. Elevated ROS levels reduce the self-renewal of HSCs and inhibit hematopoiesis. Deletion of specific genes affects HSC integrity by modulating ROS levels. −/−, Deficient; RNAi, RNA interference.
ROS-RELATED LEUKEMOGENESIS
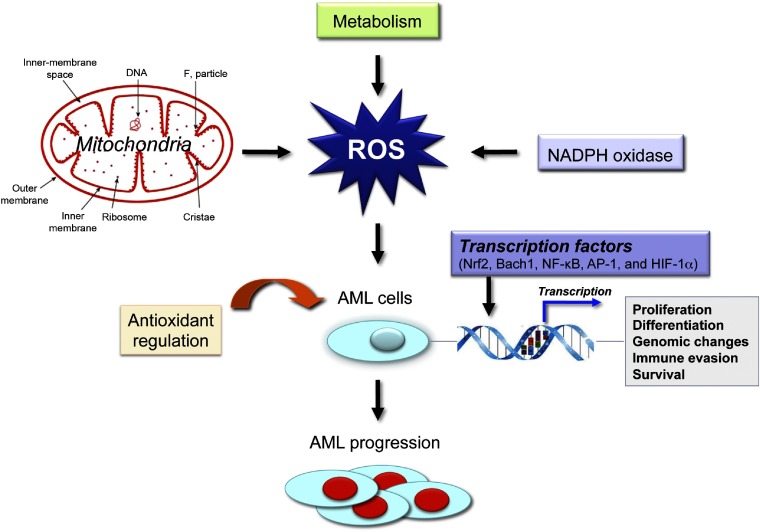
Acute leukemias are malignant clonal disorders of hematopoietic precursor cells, in which leukemic stem cells acquire mutations that confer self-renewal capabilities, altered hematopoietic differentiation, and increased proliferative capacity. ROS modulate the expression of redox-sensitive transcription factors, enzymes, oncogenes, and other downstream effectors [28]. Recent advances in our understanding of AML biology have contributed to better identify genetic and epigenetic abnormalities in leukemic cells and modulate ROS in leukemic hematopoiesis (Fig. 2).
Figure 2. Altered ROS levels stimulate leukemogenesis.
Oxidative stress has been implicated in the pathogenesis of AML. Aberrant ROS signaling triggers damage to nuclear and mitochondrial DNA and compromises DNA repair via increasing a cell's mutation rate and/or promoting and maintaining the oncogenic phenotype as a secondary messenger in intracellular signaling cascades.
Elevated ROS levels in AML cells
In normal mammalian hematopoietic systems, ROS remain at low levels in HSCs. However, common myeloid progenitors produce significantly higher levels of ROS than do normal leukocytes. Leukemic cells are intrinsically under oxidative stress and are therefore more vulnerable to further stress. Scavenging the ROS from hematopoietic progenitors using in vivo genetic tools impairs their differentiation into mature blood cells [29]. In contrast, increasing the hematopoietic progenitor ROS to above basal level triggers precocious differentiation of mature blood cell types in Drosophila, through the JNK signal pathway, FoxO activation, and polycomb down-regulation [29]. In the identification of genes associated with leukemic cell differentiation, Yang et al. [30] reported that ectopic overexpression of mda-7/IL-24 and the splice variant of mda-7/IL-24 lacking exon 5 (IL-24 delE5) significantly promotes ROS production beyond the threshold of tolerability in leukemic cells, resulting in differentiation of leukemic cells [30]. Through a ROS-dependent mechanism, oncoprotein mucin 1-C inhibition also induces a terminally differentiated myeloid phenotype in AML cell lines and primary blasts [31]. These new studies have significantly shifted our understanding of how cell differentiation is controlled via ROS signaling.
It is worth mentioning that the leukemic phenotype is correlated with elevated ROS levels. The serine-threonine kinase Akt is constitutively activated in most primary AML cells, followed by FoxO function inhibition and increased ROS levels [19, 32]. Juntilla et al. [33] found that intracellular ROS levels depend on Akt. Moreover, Akt1/2 double-deficient HSCs competed poorly against WT cells in the development of myeloid and lymphoid cells in in vivo reconstitution assays. Furthermore, constitutively activated Akt and FLT3 regulate leukemic cell survival and resistance to chemotherapy [34]. These results suggest that ROS produced in tumor cells alters the balance of genes expression, which may affect AML progression.
ROS-related genetic instability in AML
Major activated oncogenes, such as Bcr/Abl, Ras, and c-myc, have been found to correlate with enhanced ROS production [35, 36]. For example, Ras-induced ROS promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells [37]. ROS stress causes DSBs and altered repair, leading to genomic changes and carcinogenesis [38, 39]. Defects in nonhomologous end-joining, a main repair pathway for DSBs, lead to abnormal repair, resulting in chromosome deletions and translocations [40]. Genetic instability as a result of increased DNA damage and altered DNA repair contributes to the initiation and progression of inherited and sporadic human leukemias [41]. Furthermore, increased ROS generation enhances the regulatory effect from oncogenic proteins. Woolley et al. [42] found that FLT3 drives H2O2 production in AML cells via the NOX p22phox. Moreover, knocking down p22phox dramatically reduced H2O2 after 24 h in the endoplasmic reticulum. Activating mutations of the FLT3 receptor, seen in ∼30% of AML patients, are associated with the poor prognosis and aggressive clinical behavior of AML. FLT3 mutations, through ITD, cause increased ROS production in FLT3/ITD patients, leading to increased DNA DSBs and repair errors [7]. Alternatively, ROS molecules act as secondary messengers in intracellular signaling cascades, leading to direct DNA damage by increasing the mutation rate of cells. Rassool et al. [43] found that DNA damage and error-prone repair occur after increased ROS in the myeloid leukemic disease progression model. Furthermore, recent studies showed that endogenous levels of 8-hydroxy-2′-deoxyguanosine, an oxidized nucleoside of DNA, were higher in tumor tissues than in controls, suggesting that oxidative DNA damage contributes to AML development [12].
ROS and mitochondrial damage
Mitochondrial dysfunction and oxidative stress have been implicated in the pathophysiology of many diseases [44]. Variations in the mitochondrial basal capacity constitute an important and highly efficient channel for regulating ROS levels in a cell. Disturbances in and weakening of respiratory function in mitochondria further enhance the initial pro-oxidative state of cells. Mutations in mtDNA occur at a high frequency in human tumors and are associated with ROS overproduction [45]. Enhanced ROS not only cause damage to nuclear and mtDNA but also compromise DNA repair. Mitochondria have a limited DNA repair system, and their genome instability was demonstrated recently in AML [46].
Mitochondrial signaling cascades have been implicated in the regulation of cell proliferation and death via multiple pathways [47]. Sirt3 is a mitochondrial tumor-suppressor gene that connects aberrant cellular ROS and carcinogenesis. Murine tumors that lack Sirt3 exhibit abnormally high levels of ROS that directly induce genomic instability and increase HIF-1α protein levels. The subsequent transcription of HIF-1α-targeted genes mediates cellular metabolic reprogramming and increases cellular glucose consumption [48]. In short, it promotes crucial biologic processes in terms of mitochondrial ROS signaling.
Cytokines contribute to myeloid cell growth via ROS
Various tumor-derived factors alter myeloid cell differentiation and maturation via surface receptors and signaling pathways. Recently, several cytokines and growth factors were found to induce ROS production, which may alter the balance of expression of different genes. G-CSF stimulation increases ROS production in a time- and dose-dependent manner and contributes to myeloid cell growth, correlating with activation of Lyn and Akt. ROS production was enhanced further in bone marrow-derived neutrophils that express G-CSFRδ715, a truncated receptor [49]. ROS production induced by IL-3 can protect leukemic cells from apoptosis, but the effect can be counteracted by antioxidants, such as the combined SOD/catalase mimetic, ebselen (a seleno-organic compound and radical scavenger), and hydroxylamine probe. The function of IL-3 and exogenous H2O2 on cell viability seems to be mediated by inhibition of apoptosis [50].
REDOX-DEPENDENT REGULATION OF CELL SIGNALING
Cells maintain a network of sensory mechanisms, signaling pathways, and response systems to cope with environmental challenges, such as hypoxia and inflammation. ROS may regulate signaling pathways specifically, by altering the activity of direct-target molecules. Recent studies have begun to shed light on ROS-dependent regulation of cell signaling and ROS homeostasis in AML.
ROS generated by NOX as a redox messenger
ROS appear to be essential for several downstream signaling events. Piccoli et al. [22] described that ROS generated by NOX are redox messengers that control HSC proliferation and differentiation. ROS production appears to contribute to the proliferation and migration of hematopoietic cells that express a variety of oncogenic tyrosine kinases. NOX is a multiprotein complex that generates ROS in response to a wide range of stimuli. Recent studies by Reddy et al. [9] reported that NOX regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. With the use of targeted approaches against components of the superoxide-producing NOX, myeloid cells showed slow growth and spontaneous migration. Activated Ras strongly up-regulated ROS production by stimulating NOX activity, and excessive ROS production in the context of Ras activation promoted proliferative responses in normal hematopoietic cells [37]. Thus, the combination of a NOX inhibitor and the T cell-derived cytokine IL-2 is an effective relapse-prevention strategy in AML [51, 52].
ROS scavenger as a redox regulator
Trx is a redox-active protein with a molecular mass of 13 Kda, and it is widely distributed in tissues and organs. Reduced Trx has oxygen radical-scavenging and protein-refolding activities in vitro. Inhibition of the Trx system may induce antileukemic effects [53, 54]. Trx enhances the DNA-binding activity of the AP-1 transcription factor via an activation cascade—Trx-redox factor-1-AP-1—and by direct cysteine-mediated associations and reduction processes [55, 56]. To maintain homeostasis, TXNIP Trx-binding protein 2, the endogenous inhibitor of Trx, binds directly to the protein, resulting in decreased antioxidant activity [57]. Deregulated TXNIP expression contributes to experimental murine leukemia and human AML [58]. Furthermore, 3-deazaneplanocin A, a histone methyltransferase inhibitor, targets leukemic cells in AML by up-regulating TXNIP and increases ROS production [59].
Prx, a family of small nonseleno peroxidases, are capable of protecting cells from ROS insult and regulating AML signal transduction pathways. Godfrey et al. [60] reported recently that cell transformation by FLT3 in AML involves oxidative inactivation of the tumor suppressor PTP, DEP-1/PTPRJ. Decreased ROS result in DEP-1 reactivation in FLT3 ITD–expressing cell lines by inhibiting NOX or overexpressing catalase or Prx-1. Prx2 is an epigenetically silenced tumor-suppressor gene, and low expression is clinically associated with poor prognosis in AML patients. Agrawal-Singh et al. [61] found recently that Prx-2 acted as an inhibitor of myeloid cell growth by reducing levels of ROS generated in response to cytokines. Furthermore, forced Prx-2 expression inhibited c-Myc-induced leukemogenes in vivo in bone marrow-transplanted mice. These studies support the concept that ROS scavenger regulates cell signaling to affect pathological responses in redox microenvironment.
Transcription factors controlling gene expression in AML
Several transcription factors, such as Nrf2, Bach1, NF-κB, AP-1, and HIF-1α, regulate the expression of genes that are critically involved in AML pathogenesis in response to redox alterations. In general, transcription factors activate the transcription of genes involved in diverse aspects of cellular and integrative physiology, including energy metabolism, cell growth, survival, invasion, migration, and angiogenesis.
Nrf2 is a positive regulator of myeloid differentiation in AML cells [62]. Indeed, Nrf2 activity is up-regulated in several leukemia types and contributes to leukemogenesis [63]. An essential role of Nrf2 is transactivating antioxidants, such as HO-1, Trx, and enzymes in response to oxidative stress [64]. Rushworth and MacEwan [65] found that Nrf2 plays a key role in up-regulating HO-1 expression and ultimately protects AML cells from TNF-mediated cell death, resulting in clinically devastating levels of apoptosis resistance. Interplay between Bach1 and Nrf2 is apparent in AML cell lines. ROS cause the oxidation of cysteine residues in the Bach1 transcriptional repressor, thereby allowing the dissociation of Bach1 from the antioxidant response element, its translocation to the cytosol, and its degradation [66]. NF-κB (p50/p65 subunits), a ubiquitous transcription factor, is sequestered in an inactive form in the cytoplasm, as a heterotrimeric complex bound to its inhibitory IκBα protein. Upon phosphorylation of the IκBα, NF-κB dissociated from the complex, and the activated NF-κB is then translocated into the nucleus to activate specific target gens. Interestingly, the transcription factor NF-κB (p50/p65) suppressed HO-1 levels in human AML, and chemical inhibition of NF-κB resulted in elevated HO-1 protein levels in THP-1 and HL-60 cells [67]. Miyazaki et al. [68] also found that Bach1 regulated HO-1 gene expression in AML cells, and its low expression enhanced cell survival by up-regulating HO-1 expression. Mullican et al. [69] reported that neuron-derived orphan receptor 1 and orphan nuclear receptor TR3, critical tumor suppressors of myeloid leukemogenesis, promote the expression of the AP-1 transcription factors JunB and c-Jun and inhibit the development of abnormal HSCs and myeloid progenitors.
HIF-1α is also a redox-responsive transcription factor that regulates the expression of numerous genes under physiological and pathological conditions [70]. Interestingly, HIF-1α-induced differentiation of myeloid leukemic cells is independent of the transcriptional activity of HIF-1α. Moreover, HIF-1α increased the DNA-binding ability and hematopoietic transcriptional activity of Runt-related transcription factor 1 (also known as AML-1) [71]. The results of these studies strongly suggest that specific transcription factors play a dominant role in AML pathogenesis, making them redox-sensitive targets.
IMMUNE EVASION VIA ROS
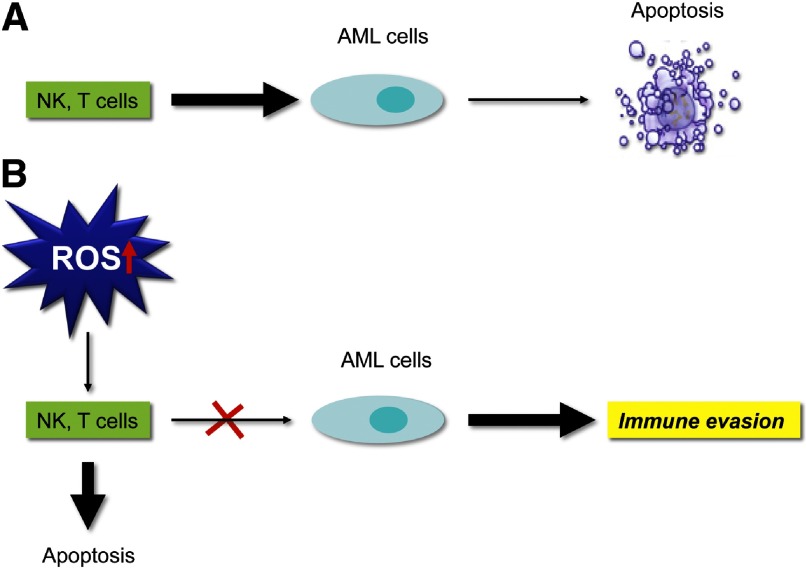
NK cells and T cells play a major role in AML immune surveillance [72]. They both possess efficient immune-modulatory and cytotoxic properties that are essential for recognizing and attacking AML cells. However, multiple deficiencies of T and NK cell functions that confer relapse risk and poor prognosis have been observed in patients with AML. Shifts in the redox balance between ROS and antioxidants regulate immunity at various levels. Tumor-associated myeloid cells are the major type of inflammatory cells involved in regulating antitumor immune responses. Although similar ROS-mediated immunosuppression has been reported in nonmalignant myeloid cells, malignant AML cells use ROS to evade antileukemic effector lymphocytes. Aurelius et al. [73] proposed a novel mechanism, whereby mature, malignant cells, in monocytic forms of leukemia, can produce ROS via the NOX and thus, trigger poly(ADP-ribose) polymerase 1-dependent apoptosis in adjacent NK cells, CD4+ T cells, and CD8+ T cells. In vitro evidence suggests that the generation and release of ROS produced by monocytes and macrophages abrogate cytokine activation of NK and T cells and induce apoptosis of these cells in vitro. Thus, it is probable that inhibition of ROS by histamine will allow cytokines to activate NK and T cells and restore their antineoplastic capabilities [74]. ROS are essential components of the innate immune response [75]. Malignant myeloid cells produce ROS as an immune-evasion strategy to avoid destruction by antileukemic lymphocytes, such as NK cells and CTLs. The proposed model of how the ROS pathway contributes to immune evasion is illustrated in Fig. 3.
Figure 3. Immune evasion via ROS.
(A) NK cells are potential effectors of innate immunity that are regulated by efficient immunomodulatory and cytotoxic mechanisms that are essential for eliminating AML cells. (B) Elevated ROS levels trigger apoptosis in NK cells, CD4+ T cells, and CD8+ T cells, whereas AML cells use ROS signaling as a strategy to evade cell-mediated immunity.
CONCLUDING REMARKS
Accumulating data suggest that ROS are involved in myeloid leukemogenesis. The action of ROS signaling, including intracellular redox state alterations and oxidative protein modifications, is critical in the AML process. Abnormal ROS levels are characteristic of AML and provide a platform for the development of antileukemic drugs. Key to the development of any successful therapy is an understanding of disease etiology and progression [76]. Thus, targeting ROS as an antileukemic strategy is expected to provide a favorable clinical benefit for AML patients.
ACKNOWLEDGMENTS
This project was supported by the Fundamental Research Funds for the Central Universities (No. 0602-08143041; to F.Z.); the National Natural Science Foundation of China (No. 81270597; to F.Z.) for leukemia research; Cancer Prevention and Research Institute of Texas and the National Cancer Institute (R01-CA90853; to F.X.C.).
We thank Markeda L. Wade and Ann Sutton for editing this manuscript.
Footnotes
- ATM
- ataxia telangiectasia-mutated
- Bach1
- BRCA1-associated carboxy-terminal helicase
- BMI1
- B lymphoma MMLV insertion region 1 homolog
- DEP-1
- density-enhanced protein tyrosine phosphatase-1
- DSB
- DNA double-strand break
- FLT3
- FMS-like tyrosine kinase 3
- FoxO
- forkhead box O
- H2O2
- hydrogen peroxide
- HIF-1α
- hypoxia-inducible factor-1α
- HSC
- hematopoietic stem cell
- ITD
- internal tandem duplication
- mda-7
- melanoma differentiation-associated gene-7
- Mdm2
- mouse double-minute 2 homolog
- Meis1
- myeloid ecotrophic insertion site 1
- mtDNA
- mitochondrial DNA
- NOX
- NADPH oxidase
- Nrf2
- nuclear factor erythroid 2 related factor 2
- Prx
- peroxiredoxin
- PTP
- protein tyrosine phosphatase
- Sirt3
- sirtuin-3
- Trx
- thioredoxin
- Tsc1
- tuberous sclerosis complex 1
- TXNIP
- thioredoxin-interacting protein
AUTHORSHIP
All authors wrote the paper.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Finkel T. (2003) Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15, 247–254 [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y., Du Y., Le W., Wang K., Kieffer N., Zhang J. (2011) Redox control of the survival of healthy and diseased cells. Antioxid. Redox Signal. 15, 2867–2908 [DOI] [PubMed] [Google Scholar]
- 3. D'Autreaux B., Toledano M. B. (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 [DOI] [PubMed] [Google Scholar]
- 4. Cairns R. A., Harris I. S., Mak T. W. (2011) Regulation of cancer cell metabolism. Nat. Rev. Cancer 11, 85–95 [DOI] [PubMed] [Google Scholar]
- 5. Toyokuni S. (2006) Novel aspects of oxidative stress-associated carcinogenesis. Antioxid. Redox Signal. 8, 1373–1377 [DOI] [PubMed] [Google Scholar]
- 6. Dickinson B. C., Chang C. J. (2011) Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 7, 504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sallmyr A., Fan J., Datta K., Kim K. T., Grosu D., Shapiro P., Small D., Rassool F. (2008) Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood 111, 3173–3182 [DOI] [PubMed] [Google Scholar]
- 8. Naughton R., Quiney C., Turner S. D., Cotter T. G. (2009) Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia 23, 1432–1440 [DOI] [PubMed] [Google Scholar]
- 9. Reddy M. M., Fernandes M. S., Salgia R., Levine R. L., Griffin J. D., Sattler M. (2011) NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia 25, 281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurosawa S., Yamaguchi T., Miyawaki S., Uchida N., Sakura T., Kanamori H., Usuki K., Yamashita T., Okoshi Y., Shibayama H., Nakamae H., Mawatari M., Hatanaka K., Sunami K., Shimoyama M., Fujishima N., Maeda Y., Miura I., Takaue Y., Fukuda T. (2010) Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica 95, 1857–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rombouts E. J., Pavic B., Lowenberg B., Ploemacher R. E. (2004) Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood 104, 550–557 [DOI] [PubMed] [Google Scholar]
- 12. Zhou F. L., Zhang W. G., Wei Y. C., Meng S., Bai G. G., Wang B. Y., Yang H. Y., Tian W., Meng X., Zhang H., Chen S. P. (2010) Involvement of oxidative stress in the relapse of acute myeloid leukemia. J. Biol. Chem. 285, 15010–15015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Er T. K., Tsai S. M., Wu S. H., Chiang W., Lin H. C., Lin S. F., Tsai L. Y., Liu T. Z. (2007) Antioxidant status and superoxide anion radical generation in acute myeloid leukemia. Clin. Biochem. 40, 1015–1019 [DOI] [PubMed] [Google Scholar]
- 14. Li L., Li M., Sun C., Francisco L., Chakraborty S., Sabado M., McDonald T., Gyorffy J., Chang K., Wang S., Fan W., Li J., Zhao L. P., Radich J., Forman S., Bhatia S., Bhatia R. (2011) Altered hematopoietic cell gene expression precedes development of therapy-related myelodysplasia/acute myeloid leukemia and identifies patients at risk. Cancer Cell 20, 591–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamata H., Hirata H. (1999) Redox regulation of cellular signalling. Cell. Signal. 11, 1–14 [DOI] [PubMed] [Google Scholar]
- 16. Yahata T., Takanashi T., Muguruma Y., Ibrahim A. A., Matsuzawa H., Uno T., Sheng Y., Onizuka M., Ito M., Kato S., Ando K. (2011) Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood 118, 2941–2950 [DOI] [PubMed] [Google Scholar]
- 17. Yahata T., Muguruma Y., Yumino S., Sheng Y., Uno T., Matsuzawa H., Ito M., Kato S., Hotta T., Ando K. (2008) Quiescent human hematopoietic stem cells in the bone marrow niches organize the hierarchical structure of hematopoiesis. Stem Cells 26, 3228–3236 [DOI] [PubMed] [Google Scholar]
- 18. Naka K., Muraguchi T., Hoshii T., Hirao A. (2008) Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid. Redox Signal. 10, 1883–1894 [DOI] [PubMed] [Google Scholar]
- 19. Tothova Z., Gilliland D. G. (2007) FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell 1, 140–152 [DOI] [PubMed] [Google Scholar]
- 20. Tothova Z., Kollipara R., Huntly B. J., Lee B. H., Castrillon D. H., Cullen D. E., McDowell E. P., Lazo-Kallanian S., Williams I. R., Sears C., Armstrong S. A., Passegue E., DePinho R. A., Gilliland D. G. (2007) FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 [DOI] [PubMed] [Google Scholar]
- 21. Miyamoto K., Araki K. Y., Naka K., Arai F., Takubo K., Yamazaki S., Matsuoka S., Miyamoto T., Ito K., Ohmura M., Chen C., Hosokawa K., Nakauchi H., Nakayama K., Nakayama K. I., Harada M., Motoyama N., Suda T., Hirao A. (2007) Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1, 101–112 [DOI] [PubMed] [Google Scholar]
- 22. Piccoli C., D'Aprile A., Ripoli M., Scrima R., Lecce L., Boffoli D., Tabilio A., Capitanio N. (2007) Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem. Biophys. Res. Commun. 353, 965–972 [DOI] [PubMed] [Google Scholar]
- 23. Abbas H. A., Maccio D. R., Coskun S., Jackson J. G., Hazen A. L., Sills T. M., You M. J., Hirschi K. K., Lozano G. (2010) Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell 7, 606–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K., Ohmura M., Naka K., Hosokawa K., Ikeda Y., Suda T. (2006) Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12, 446–451 [DOI] [PubMed] [Google Scholar]
- 25. Rizo A., Olthof S., Han L., Vellenga E., de Haan G., Schuringa J. J. (2009) Repression of BMI1 in normal and leukemic human CD34(+) cells impairs self-renewal and induces apoptosis. Blood 114, 1498–1505 [DOI] [PubMed] [Google Scholar]
- 26. Chen C., Liu Y., Liu R., Ikenoue T., Guan K. L., Zheng P. (2008) TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 205, 2397–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kocabas F., Zheng J., Thet S., Copeland N. G., Jenkins N. A., DeBerardinis R. J., Zhang C., Sadek H. A. (2012) Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood 120, 4963–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyu B. N., Ismailov S. B., Ismailov B., Lyu M. B. (2008) Mitochondrial concept of leukemogenesis: key role of oxygen-peroxide effects. Theor. Biol. Med. Model 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Owusu-Ansah E., Banerjee U. (2009) Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang B. X., Duan Y. J., Dong C. Y., Zhang F., Gao W. F., Cui X. Y., Lin Y. M., Ma X. T. (2011) Novel functions for mda-7/IL-24 and IL-24 delE5: regulation of differentiation of acute myeloid leukemic cells. Mol. Cancer Ther. 10, 615–625 [DOI] [PubMed] [Google Scholar]
- 31. Yin L., Wu Z., Avigan D., Rosenblatt J., Stone R., Kharbanda S., Kufe D. (2011) MUC1-C oncoprotein suppresses reactive oxygen species-induced terminal differentiation of acute myelogenous leukemia cells. Blood 117, 4863–4870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Q., Simpson S. E., Scialla T. J., Bagg A., Carroll M. (2003) Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood 102, 972–980 [DOI] [PubMed] [Google Scholar]
- 33. Juntilla M. M., Patil V. D., Calamito M., Joshi R. P., Birnbaum M. J., Koretzky G. A. (2010) AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115, 4030–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng Z., Samudio I. J., Zhang W., Estrov Z., Pelicano H., Harris D., Frolova O., Hail N., Jr., Chen W., Kornblau S. M., Huang P., Lu Y., Mills G. B., Andreeff M., Konopleva M. (2006) Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 66, 3737–3746 [DOI] [PubMed] [Google Scholar]
- 35. Vafa O., Wade M., Kern S., Beeche M., Pandita T. K., Hampton G. M., Wahl G. M. (2002) c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell 9, 1031–1044 [DOI] [PubMed] [Google Scholar]
- 36. Behrend L., Henderson G., Zwacka R. M. (2003) Reactive oxygen species in oncogenic transformation. Biochem. Soc. Trans. 31, 1441–1444 [DOI] [PubMed] [Google Scholar]
- 37. Hole P. S., Pearn L., Tonks A. J., James P. E., Burnett A. K., Darley R. L., Tonks A. (2010) Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells. Blood 115, 1238–1246 [DOI] [PubMed] [Google Scholar]
- 38. Panayiotidis M. (2008) Reactive oxygen species (ROS) in multistage carcinogenesis. Cancer Lett. 266, 3–5 [DOI] [PubMed] [Google Scholar]
- 39. Clerkin J. S., Naughton R., Quiney C., Cotter T. G. (2008) Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Lett. 266, 30–36 [DOI] [PubMed] [Google Scholar]
- 40. Sallmyr A., Fan J., Rassool F. V. (2008) Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 270, 1–9 [DOI] [PubMed] [Google Scholar]
- 41. Popp H. D., Bohlander S. K. (2010) Genetic instability in inherited and sporadic leukemias. Genes Chromosomes Cancer 49, 1071–1081 [DOI] [PubMed] [Google Scholar]
- 42. Woolley J. F., Naughton R., Stanicka J., Gough D. R., Bhatt L., Dickinson B. C., Chang C. J., Cotter T. G. (2012) H(2)O(2) production downstream of FLT3 is mediated by p22phox in the endoplasmic reticulum and is required for STAT5 signalling. PloS One 7, e34050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rassool F. V., Gaymes T. J., Omidvar N., Brady N., Beurlet S., Pla M., Reboul M., Lea N., Chomienne C., Thomas N. S., Mufti G. J., Padua R. A. (2007) Reactive oxygen species, DNA damage, and error-prone repair: a model for genomic instability with progression in myeloid leukemia? Cancer Res. 67, 8762–8771 [DOI] [PubMed] [Google Scholar]
- 44. Pallardo F. V., Lloret A., Lebel M., d'Ischia M., Cogger V. C., Le Couteur D. G., Gadaleta M. N., Castello G., Pagano G. (2010) Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia-Telangiectasia, Down Syndrome, Fanconi Anaemia and Werner Syndrome. Biogerontology 11, 401–419 [DOI] [PubMed] [Google Scholar]
- 45. Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J. (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 [DOI] [PubMed] [Google Scholar]
- 46. He L., Luo L., Proctor S. J., Middleton P. G., Blakely E. L., Taylor R. W., Turnbull D. M. (2003) Somatic mitochondrial DNA mutations in adult-onset leukaemia. Leukemia 17, 2487–2491 [DOI] [PubMed] [Google Scholar]
- 47. Antico Arciuch V. G., Elguero M. E., Poderoso J. J., Carreras M. C. (2012) Mitochondrial regulation of cell cycle and proliferation. Antioxid. Redox Signal. 16, 1150–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haigis M. C., Deng C. X., Finley L. W., Kim H. S., Gius D. (2012) SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 72, 2468–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu Q. S., Xia L., Mills G. B., Lowell C. A., Touw I. P., Corey S. J. (2006) G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood 107, 1847–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maraldi T., Prata C., Fiorentini D., Zambonin L., Landi L., Hakim G. (2009) Induction of apoptosis in a human leukemic cell line via reactive oxygen species modulation by antioxidants. Free Radic. Biol. Med. 46, 244–252 [DOI] [PubMed] [Google Scholar]
- 51. Brune M., Castaigne S., Catalano J., Gehlsen K., Ho A. D., Hofmann W. K., Hogge D. E., Nilsson B., Or R., Romero A. I., Rowe J. M., Simonsson B., Spearing R., Stadtmauer E. A., Szer J., Wallhult E., Hellstrand K. (2006) Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood 108, 88–96 [DOI] [PubMed] [Google Scholar]
- 52. Martner A., Thoren F. B., Aurelius J., Soderholm J., Brune M., Hellstrand K. (2010) Immunotherapy with histamine dihydrochloride for the prevention of relapse in acute myeloid leukemia. Expert Rev. Hematol. 3, 381–391 [DOI] [PubMed] [Google Scholar]
- 53. Lu J., Chew E. H., Holmgren A. (2007) Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 104, 12288–12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nigro P., Dal Piaz F., Gallotta D., De Tommasi N., Belisario M. A. (2008) Inhibition of the thioredoxin system is a basis for the antileukemic potential of 13-hydroxy-15-oxo-zoapatlin. Free Radic. Biol. Med. 45, 875–884 [DOI] [PubMed] [Google Scholar]
- 55. Das K. C., Muniyappa H. (2010) c-Jun-NH2 terminal kinase (JNK)-mediates AP-1 activation by thioredoxin: phosphorylation of cJun, JunB, and Fra-1. Mol. Cell. Biochem. 337, 53–63 [DOI] [PubMed] [Google Scholar]
- 56. Li Y., Liu W., Xing G., Tian C., Zhu Y., He F. (2005) Direct association of hepatopoietin with thioredoxin constitutes a redox signal transduction in activation of AP-1/NF-κB. Cell. Signal. 17, 985–996 [DOI] [PubMed] [Google Scholar]
- 57. Nakamura H., Masutani H., Yodoi J. (2006) Extracellular thioredoxin and thioredoxin-binding protein 2 in control of cancer. Semin. Cancer Biol. 16, 444–451 [DOI] [PubMed] [Google Scholar]
- 58. Erkeland S. J., Palande K. K., Valkhof M., Gits J., Danen-van Oorschot A., Touw I. P. (2009) The gene encoding thioredoxin-interacting protein (TXNIP) is a frequent virus integration site in virus-induced mouse leukemia and is overexpressed in a subset of AML patients. Leuk. Res. 33, 1367–1371 [DOI] [PubMed] [Google Scholar]
- 59. Zhou J., Bi C., Cheong L. L., Mahara S., Liu S. C., Tay K. G., Koh T. L., Yu Q., Chng W. J. (2011) The histone methyltransferase inhibitor, DZNep, up-regulates TXNIP, increases ROS production, and targets leukemia cells in AML. Blood 118, 2830–2839 [DOI] [PubMed] [Google Scholar]
- 60. Godfrey R., Arora D., Bauer R., Stopp S., Muller J. P., Heinrich T., Bohmer S. A., Dagnell M., Schnetzke U., Scholl S., Ostman A., Bohmer F. D. (2012) Cell transformation by FLT3 ITD in acute myeloid leukemia involves oxidative inactivation of the tumor suppressor protein-tyrosine phosphatase DEP-1/PTPRJ. Blood 119, 4499–4511 [DOI] [PubMed] [Google Scholar]
- 61. Agrawal-Singh S., Isken F., Agelopoulos K., Klein H. U., Thoennissen N. H., Koehler G., Hascher A., Baumer N., Berdel W. E., Thiede C., Ehninger G., Becker A., Schlenke P., Wang Y., McClelland M., Krug U., Koschmieder S., Buchner T., Yu D. Y., Singh S. V., Hansen K., Serve H., Dugas M., Muller-Tidow C. (2012) Genome-wide analysis of histone H3 acetylation patterns in AML identifies PRDX2 as an epigenetically silenced tumor suppressor gene. Blood 119, 2346–2357 [DOI] [PubMed] [Google Scholar]
- 62. Bobilev I., Novik V., Levi I., Shpilberg O., Levy J., Sharoni Y., Studzinski G. P., Danilenko M. (2011) The Nrf2 transcription factor is a positive regulator of myeloid differentiation of acute myeloid leukemia cells. Cancer Biol. Ther. 11, 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rushworth S. A., MacEwan D. J., O'Connell M. A. (2008) Lipopolysaccharide-induced expression of NAD(P)H: quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. 181, 6730–6737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hayes J. D., McMahon M., Chowdhry S., Dinkova-Kostova A. T. (2010) Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid. Redox Signal. 13, 1713–1748 [DOI] [PubMed] [Google Scholar]
- 65. Rushworth S. A., MacEwan D. J. (2008) HO-1 underlies resistance of AML cells to TNF-induced apoptosis. Blood 111, 3793–3801 [DOI] [PubMed] [Google Scholar]
- 66. Ishikawa M., Numazawa S., Yoshida T. (2005) Redox regulation of the transcriptional repressor Bach1. Free Radic. Biol. Med. 38, 1344–1352 [DOI] [PubMed] [Google Scholar]
- 67. Rushworth S. A., Bowles K. M., Raninga P., MacEwan D. J. (2010) NF-κB-inhibited acute myeloid leukemia cells are rescued from apoptosis by heme oxygenase-1 induction. Cancer Res. 70, 2973–2983 [DOI] [PubMed] [Google Scholar]
- 68. Miyazaki T., Kirino Y., Takeno M., Samukawa S., Hama M., Tanaka M., Yamaji S., Ueda A., Tomita N., Fujita H., Ishigatsubo Y. (2010) Expression of heme oxygenase-1 in human leukemic cells and its regulation by transcriptional repressor Bach1. Cancer Sci. 101, 1409–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mullican S. E., Zhang S., Konopleva M., Ruvolo V., Andreeff M., Milbrandt J., Conneely O. M. (2007) Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat. Med. 13, 730–735 [DOI] [PubMed] [Google Scholar]
- 70. Deeb G., Vaughan M. M., McInnis I., Ford L. A., Sait S. N., Starostik P., Wetzler M., Mashtare T., Wang E. S. (2011) Hypoxia-inducible factor-1α protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk. Res. 35, 579–584 [DOI] [PubMed] [Google Scholar]
- 71. Peng Z. G., Zhou M. Y., Huang Y., Qiu J. H., Wang L. S., Liao S. H., Dong S., Chen G. Q. (2008) Physical and functional interaction of Runt-related protein 1 with hypoxia-inducible factor-1α. Oncogene 27, 839–847 [DOI] [PubMed] [Google Scholar]
- 72. Barrett A. J., Le Blanc K. (2010) Immunotherapy prospects for acute myeloid leukaemia. Clin. Exp. Immunol. 161, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aurelius J., Thoren F. B., Akhiani A. A., Brune M., Palmqvist L., Hansson M., Hellstrand K., Martner A. (2012) Monocytic AML cells inactivate antileukemic lymphocytes: role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood 119, 5832–5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Agarwala S. S., Sabbagh M. H. (2001) Histamine dihydrochloride: inhibiting oxidants and synergising IL-2-mediated immune activation in the tumour microenvironment. Exp. Opin. Biol. Ther. 1, 869–879 [DOI] [PubMed] [Google Scholar]
- 75. West A. P., Brodsky I. E., Rahner C., Woo D. K., Erdjument-Bromage H., Tempst P., Walsh M. C., Choi Y., Shadel G. S., Ghosh S. (2011) TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grek C. L., Townsend D. M., Tew K. D. (2011) The impact of redox and thiol status on the bone marrow: pharmacological intervention strategies. Pharmacol. Ther. 129, 172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]