Abstract
Aims
The cardiac extracellular matrix is highly involved in regulating inflammation, remodelling, and function of the heart. Whether matrix alterations relate to the degree of inflammation, fibrosis, and overall rejection in the human transplanted heart remained, until now, unknown.
Methods and results
Expression of matricellular proteins, proteoglycans, and metalloproteinases (MMPs) and their inhibitors (TIMPs) were investigated in serial endomyocardial biopsies (n = 102), in a cohort of 39 patients within the first year after cardiac transplantation. Out of 15 matrix-related proteins, intragraft transcript and protein levels of syndecan-1 and MMP-9 showed a strong association with the degree of cardiac allograft rejection (CAR), the expression of pro-inflammatory cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-6 and transforming growth factor (TGF)-β, and with infiltrating CD3+T-cells and CD68+monocytes. In addition, SPARC, CTGF, TSP-2, MMP-14, TIMP-1, Testican-1, TSP-1, Syndecan-1, MMP-2, -9, and -14, as well as IL-6 and TGF-β transcript levels and inflammatory infiltrates all strongly relate to collagen expression in the transplanted heart. More importantly, receiver operating characteristic curve analysis demonstrated that syndecan-1 and MMP-9 transcript levels had the highest area under the curve (0.969 and 0.981, respectively), thereby identifying both as a potential decision-making tool to discriminate rejecting from non-rejecting hearts.
Conclusion
Out of 15 matrix-related proteins, we identified synd-1 and MMP-9 intragraft transcript levels of as strong predictors of human CAR. In addition, a multitude of non-structural matrix-related proteins closely associate with collagen expression in the transplanted heart. Therefore, we are convinced that these findings deserve further investigation and are likely to be of clinical value to prevent human CAR.
Keywords: Heart transplantation, Rejection, Metalloproteinases, Matricellular proteins, Cardiac matrix, Inflammation, Biomarker
Introduction
Cardiac allograft rejection (CAR) is characterized by severe cardiac inflammation, cardiomyocyte damage, fibrosis, and progressive ventricular dysfunction of the transplanted heart.1 Although the 1-year survival rate now approaches 87%, CAR still accounts for most of the early and late deaths after heart transplantation (HTx). The current standard to screen for CAR is the histopathological detection of inflammatory infiltrates and cardiomyocyte necrosis in serial endomyocardial biopsies (EMBs) and grading according to the guidelines of the International Society of Heart and Lung Transplantation (ISHLT).1 Nevertheless, a more in-depth understanding of the biological and molecular changes associated with CAR is mandatory to develop novel diagnostic and therapeutic strategies.
During the last decade, it has become clear that dynamic changes in the cardiac ECM, including altered expression and activity of matricellular proteins, proteoglycans, and the matrix metalloproteinase (MMP) system, do not only determine the mechanical properties of the heart, but also modulate key inflammatory and reparative pathways in animal models of cardiac stress and disease.2–4 As such, reports from several groups advocate a pivotal role for matricellular proteins and proteoglycans, including, thrombospondin (TSP)-1 and -2, connective tissue growth factor (CTGF), secreted protein, acidic and rich in cysteine (SPARC), syndecan (synd)-1 and -4, and testican-1 in the inflammatory process.4–8 Metalloproteinases and their specific tissue inhibitors (TIMPs), on the other hand, are strong regulators of the cardiac ECM and have the capability to directly or indirectly modulate the inflammatory response.2,9 As a result, they determine the structural and functional outcome of diseased hearts. Studies in MMP-2- and -9-deficient mice revealed that both gelatinases influence graft survival by modulating T-cell alloreactivity in a model of acute CAR,10 whereas the use of broad-spectrum MMP-inhibitors significantly enhanced graft survival by suppressing the inflammatory response.11 Besides inflammation, cardiac allograft fibrosis has been identified as an important limiting factor towards long-term graft survival.5 Interestingly, the majority of these ECM-related macromolecules have been reported to influence expression, deposition, assembly, and/or maturation of newly deposited extracellular collagen matrix in various cardiac pathologies.2,4,6
Despite their implication in the inflammatory and reparative pathways of the heart, evaluation of matricellular proteins, proteoglycans, MMPs and TIMPs in relation to inflammation, fibrosis, and the degree of human CAR are scarce and fragmentary. In present study, we focused on the intragraft transcript and protein levels of matricellular proteins (TSP-1 and -2, CTGF and SPARC), proteoglycans (synd-1 and -4, Testican-1), MMPs (MMP-1, -2, -9, MMP-14), and TIMPs (TIMP-1 to -4) in relation to (i) the degree of CAR as measured by ISHLT grading; (ii) the expression of pro-inflammatory cytokines interleukin (IL)-6, tumour necrosis factor (TNF)-α and transforming growth factor (TGF)-β; (iii) the influx of CD3+ T-cells and CD68+ monocytes; and (iv) collagen expression within the first year after HTx. Finally, we also evaluate whether particular EMB-derived transcriptional profiles could potentially provide a valuable diagnostic tool to identify those patients with CAR.
Methods
Study design, patient characteristics, experimental materials and methods, and statistical analysis are described extensively in the Supplementary material online. Briefly, protocol EMBs and plasma samples were simultaneously collected and properly stored during the routine follow-up of 39 patients within the first year after cardiac transplantation (Supplementary material online, Table S1). A first subset of the obtained EMBs were graded for cellular rejection by a pathologist blinded to other clinical or scientific data according to the criteria of the ISHLT, whereas a second subset of EMBs and plasma specimens were processed for further molecular and histological analysis, including, RNA isolation and real-time polymerase chain reaction (RT–PCR), immunohistochemical analysis, in situ gelatin zymography, and enzyme-linked immunosorbent assay. The study was approved by the medical ethical commission of the KULeuven and all patients gave written informed consent, consistent with the Declaration of Helsinki.
Results
Intragraft synd-1, MMP-9, and TIMP-1 transcript levels relate to the degree of cardiac allograft rejection
A total of 102 EMBs from our cohort of 39 HTx patients were examined by real-time quantitative PCR to determine the differential mRNA expression of 15 matrix-related proteins in relation to the different ISHLT rejection grades (Table 1 and Supplementary material online, Table S1). Normalized intragraft transcript levels of synd-1, MMP-9, and TIMP-1 showed the strongest significant and positive correlation with the degree of CAR (Table 1). Whereas no major differences were seen between Grade 0 and Grade 1A EMBs, synd-1, MMP-9, and TIMP-1, respectively, showed a 1.8-, 3.1-, and 1.8-fold increase in Grade 1B (P < 0.001) and a 37.2-, 9.4-, and 7.4-fold increase in Grade 3 (P < 0.001), when compared with Grade 0 EMBs (Table 1). In contrast, only a moderate or no correlations were observed for the other matricellular proteins, proteoglycans, MMPs, and TIMPs included in this study (Table 1).
Table 1.
Intragraft transcript levels in relation to rejection grades on endomyocardial biopsy
| Grade 0 (n = 38) | Grade 1A (n = 31) | Grade 1B (n = 25) | Grade 3 (n = 8) | ANOVA post-test for linearity |
||
|---|---|---|---|---|---|---|
| R2-value | P-value | |||||
| Matrix glycoproteins | ||||||
| Synd-1 | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.4 | 14.9 ± 21.1*,**,*** | 0.286 | <0.0001 |
| Synd-4 | 48 ± 22,4 | 42 ± 21,0 | 49 ± 22,1 | 43 ± 17,7 | 0.001 | 0.803 |
| TSP-1 | 210 ± 159 | 181 ± 117 | 234 ± 107 | 297 ± 170 | 0.034 | 0.073 |
| TSP-2 | 812 ± 574 | 960 ± 850 | 1194 ± 1081 | 1894 ± 1369* | 0.107 | <0.01 |
| CTGF | 0.6 ± 0.6 | 0.4 ± 0.2 | 0.6 ± 0.6 | 1.3 ± 1.1** | 0.069 | <0.05 |
| SPARC | 10.4 ± 16.1 | 11.0 ± 14.3 | 12.4 ± 8.0 | 17.5 ± 12.1 | 0.018 | 0.197 |
| Testican-1 | 19.0 ± 14.0 | 16.4 ± 15.2 | 17.9 ± 12.9 | 22.4 ± 9.6 | 0.006 | 0.516 |
| MMPs and TIMPs | ||||||
| MMP1 | 2.2 ± 1.5 | 3.0 ± 2.2 | 5.4 ± 3.0* | 6.2 ± 3.3*,** | 0.118 | <0.01 |
| MMP2 | 19.8 ± 11.5 | 22.4 ± 13.2 | 22.2 ± 9.6 | 24.4 ± 7.6 | 0.001 | 0.748 |
| MMP9 | 2.7 ± 3.6 | 3.0 ± 3.7 | 8.4 ± 9.2*,** | 25.5 ± 8.2*,**,*** | 0.520 | <0.0001 |
| MMP-14 | 1.3 ± 1.0 | 1.4 ± 1.1 | 1.9 ± 3.4 | 2.3 ± 2.0 | 0.022 | 0.147 |
| TIMP1 | 58 ± 41.8 | 58 ± 32.4 | 104 ± 72*,** | 429 ± 317*,**,*** | 0.479 | <0.0001 |
| TIMP2 | 112 ± 30.07 | 105 ± 22.5 | 116 ± 30.17 | 136 ± 31.6 | 0.046 | <0.05 |
| TIMP3 | 0.7 ± 0.3 | 0.5 ± 0.3 | 0.9 ± 0.6 | 1.8 ± 0.4*,** | 0.267 | <0.01 |
| TIMP4 | 0.10 ± 0.04 | 0.06 ± 0.03 | 0.11 ± 0.20 | 0.31 ± 0.09*,** | 0.167 | <0.05 |
ISHLT grade indicates the histopathological grade of cardiac allograft rejection as set by the International Society of Heart and Lung Transplantation. Transcript levels are given in arbitrary units relative to GAPDH expression and presented as mean ± SD. Total n = 102, including n = 38 for Grade 0 collected from 27 patients, n = 31 for Grade 1A from 22 patients, n = 25 for Grade 1B form 15 patients and n = 8 for Grade 3 from 5 patients.
EMB, endomyocardial biopsy; Synd-1, syndecan-1; TSP, thrombospondin; CTGF, connective tissue growth factor; SPARC, secreted protein acidic and rich in cysteine; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of matrix metalloproteinases.
*P < 0.05 vs. Grade 0.
**P < 0.05 vs. Grade 1A.
***P < 0.05 vs. Grade 1B.
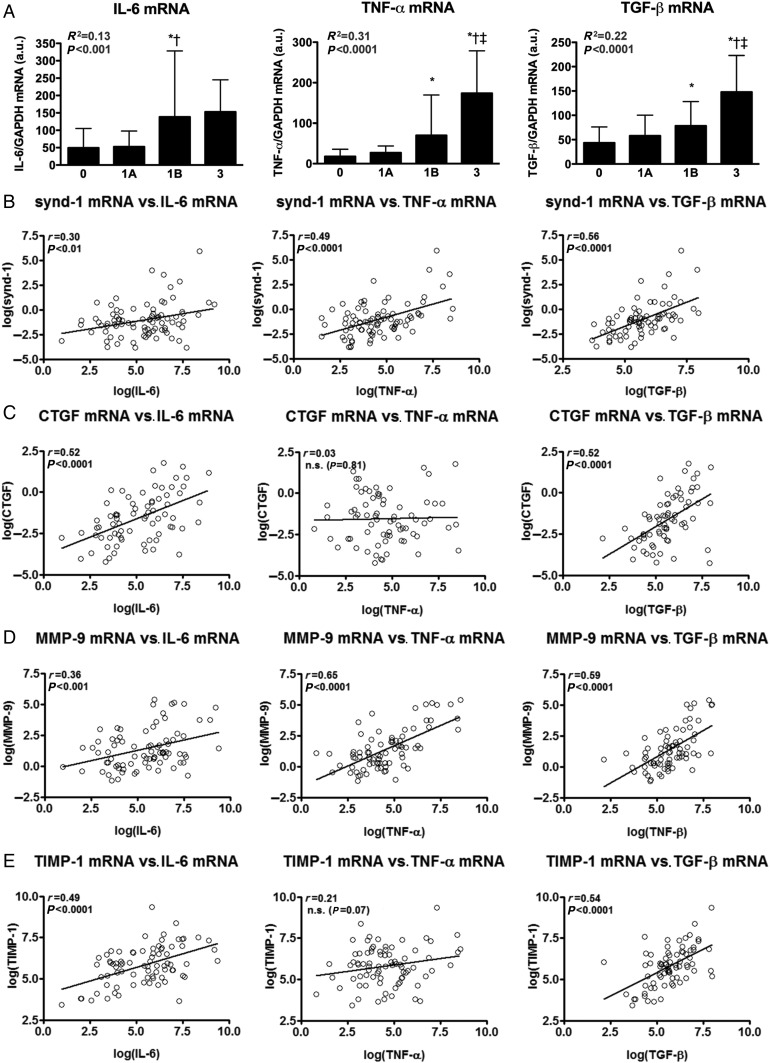
Transcript levels of synd-1, MMP-9, CTGF, and TIMP-1 relate to IL-6, TNF-α, and/or TGF-β
Within our cohort of EMBs, intragraft transcript levels of pro-inflammatory cytokines IL-6, TNF-α, and TGF-β significantly increased in Grade 1B and 3 rejecting EMBs and significantly relate to the degree of CAR (Figure 1A). Interleukin-6, TNF-α, and TGF-β function as potent chemoattractants and provide essential signals for the dynamic trafficking of T-cells and monocytes into allografts.5,12 In addition, both IL-6 and TGF-β negatively regulate cardiac allograft survival through their pro-fibrotic effects.5 Interestingly, normalized synd-1 and MMP-9 transcript levels revealed the strongest association with the expression levels of IL-6, TNF-α, and TGF-β (Figure 1B and D). Whereas TIMP-1 and CTGF, both implicated in stimulating cardiac fibrosis,4,9 showed a strong correlation with IL-6 and TGF-β, but not with TNF-α (Figure 1C and E). Markedly, synd-1, CTGF, SPARC, MMP-1, -9, and TIMP-1 correlated stronger with the expression of one or more pro-inflammatory cytokines when compared with their relation with the degree of CAR (Figure 1, Supplementary material online, Figure S1 and Table S1). Only moderate or no correlations were noted for the other proteins included in this study (Supplementary material online, Figure S1).
Figure 1.
Intragraft transcript levels of in relation to the expression of pro-inflammatory cytokines. (A) Intragraft expression levels (mean ± SD) of IL-6, TNF-α, and TGF-β progressively increase with and significantly relate to the International Society of Heart and Lung Transplantation grade of cardiac allograft rejection. (B–E) Intragraft transcript levels of (B) syndecan-1, (C) CTGF, (D) MMP-9, and (E) TIMP-1 in relation to the mRNA expression levels of IL-6, TNF-α, and TGF-β. *P < 0.05 vs. Grade 0; †P < 0.05 vs. Grade 1A; ‡P < 0.05 vs. Grade 1B; n = 102, including n = 38 for Grade 0 collected from 27 patients, n = 31 for Grade 1A from 22 patients, n = 25 for Grade 1B form 15 patients and n = 8 for Grade 3 from 5 patients.
Together, these data suggest that intragraft expression synd-1, CTGF, MMP-9, and TIMP-1 strongly mirror the expression of IL-6, TNF-α, and/or TGF-β during human CAR.
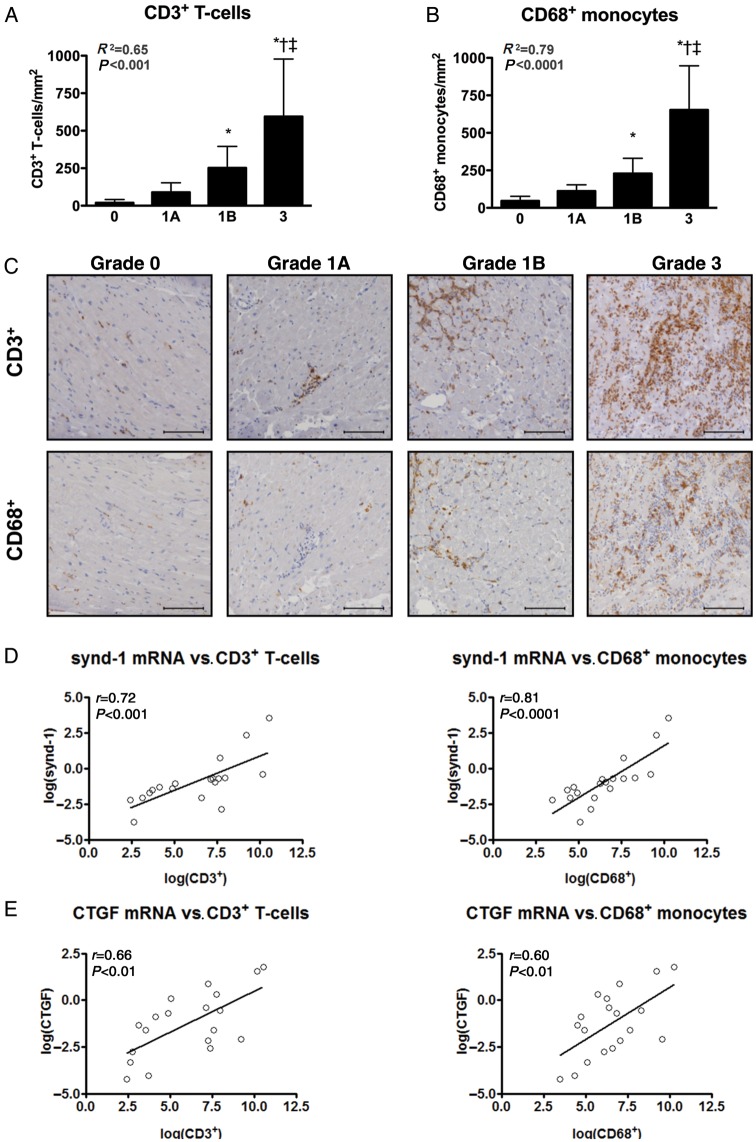
Transcription of synd-1, MMP-9, and CTGF, but not TIMP-1 coincide with the influx of T-cells and monocytes in the transplanted heart
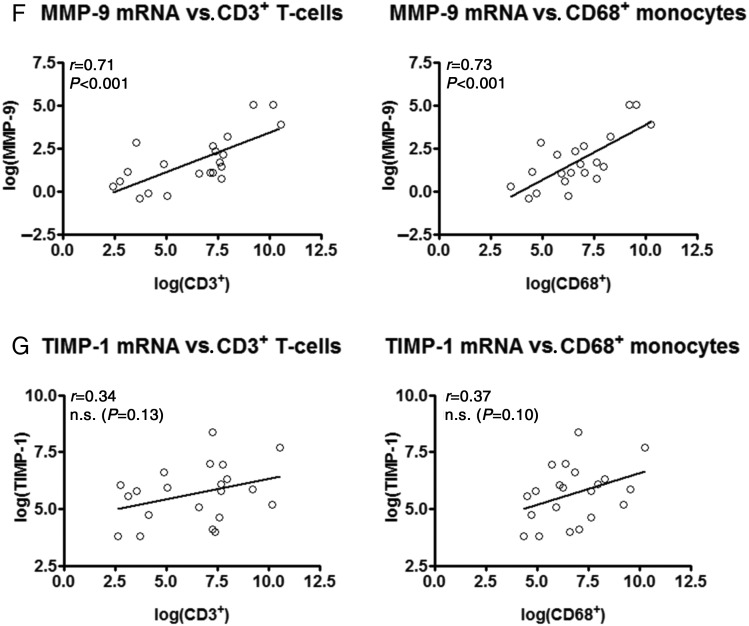
Inflammatory infiltrates associated with CAR, largely consist out of T-cells and monocytes.1 Therefore, a total of 71—randomly chosen—EMBs were examined by immunohistochemistry to evaluate the influx of CD3+ T-cells and CD68+ monocytes (Figure 2A–C). The number of infiltrating CD3+ T-cells and CD68+ monocytes strongly increased with increasing Grade of CAR and these were especially present in EMBs displaying a severe rejection episode accompanied by myocyte necrosis (ISHLT Grade 3; Figure 2A–C). Furthermore, transcript levels of the pro-inflammatory cytokines IL-6, TNF-α, and TGF-β were highly associated with the influx of CD3+ and CD68+ cells (Supplementary material online, Figure S1A–C), indicating a pivotal role for these cytokines in the inflammatory pathways underlying human CAR. More importantly, the influx of CD3+ T-cells and CD68+ monocytes strongly related to the transcription of synd-1, MMP-9, CTGF (from highest to lowest; Figure 2D–F), but not to that of TIMP-1 (Figure 2G). Weak or absence of significant correlations was noted for the remaining proteins included in this study (Supplementary material online, Figure S1D–N).
Figure 2.
Transcription of synd-1, CTGF, MMP-9, but not TIMP-1 coincide with the influx of T-cells and monocytes in the transplanted heart. Immunohistochemical analysis revealed a significantly increased influx of (A) CD3+ T-cells and (B) CD68+ monocytes with increasing grade of human cardiac allograft rejection. Data presented as mean ± SD (C) Representative microphotographs of CD3 and CD68 immunoreactivity in endomyocardial biopsies displaying cardiac allograft rejection Grade 0, 1A, 1B, and 3. Scale-bar: 100 μm. Transcript levels of (D) synd-1, (E) CTGF, (F) MMP-9, and (G) TIMP-1 in relation to the influx of CD3+ T-cells and CD68+ monocytes. *P < 0.05 vs. Grade 0; †P < 0.05 vs. Grade 1A; ‡P < 0.05 vs. Grade 1B; n = 21 for International Society for Heart and Lung Transplantation Grade 0 collected from 21 patients, n = 19 for Grade 1A from 19 patients, n = 16 for Grade 1B from 15 patients and n = 8 for Grade 3 from 5 patients. For histological analysis, seven Grade 3 endomyocardial biopsies were added from patients that were transplanted prior to the start of our study.
Together, these data reveal a close relationship between inflammation and the induction of synd-1, MMP-9, CTGF in the transplanted heart.
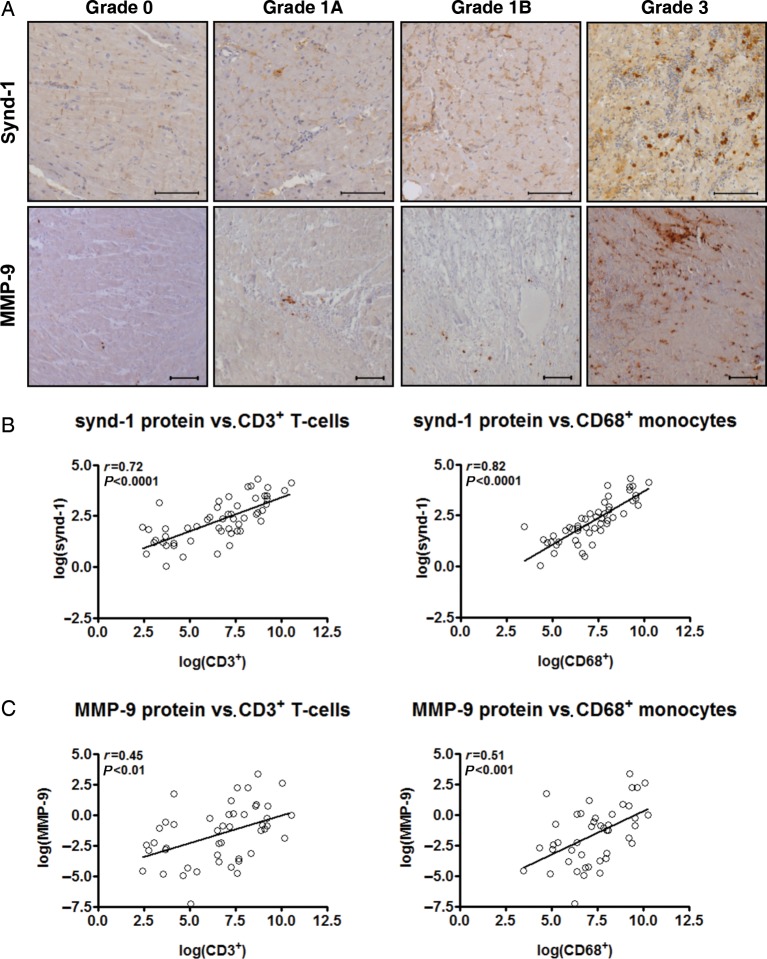
Synd-1 and MMP-9 protein expression mark the influx of T-cells and monocytes during cardiac allograft rejection
Encouraged by our consistent findings on synd-1 and MMP-9, we next investigated whether synd-1- and MMP-9-protein expression marked the degree of inflammation in the transplanted hearts.
In agreement with the mRNA expression profiles, synd-1 and MMP-9 protein levels significantly increased with increasing Grade of CAR (Table 2, Figure 3A). Strikingly, synd-1 and MMP-9 immunoreactivity were minimally detectable in Grade 0 and 1A, but progressively increased in EMBs displaying Grade 1B or Grade 3 rejection episodes. In Grade 3 EMBs, a 6.3-fold increase for synd-1 and a 13.9-fold increase for MMP-9 protein expression were observed, when compared with ISHLT Grade 0 (P < 0.0001 for both; Table 2). Synd-1 and MMP-9 highly co-localized with infiltrating mononuclear cells and strongly correlated to the degree of infiltrating CD3+ T-cells and CD68+ monocytes (Figure 3A–C). In addition, a diffuse immunostaining for MMP-9 and Synd-1 was found in the interstitial matrix and in areas with extensive fibrosis (Figure 3A).
Table 2.
Synd-1 and MMP-9 protein levels in relation to rejection grades on endomyocardial biopsy
| Grade 0 (n = 21) | Grade 1A (n = 19) | Grade 1B (n = 16) | Grade 3 (n = 15) | ANOVA post-test for linearity |
||
|---|---|---|---|---|---|---|
| R2-value | P-value | |||||
| Synd-1, % | 2.04 ± 0.5 | 4.07 ± 0.75 | 5.24 ± 1.34* | 12.89 ± 3.36*,**,*** | 0.692 | <0.0001 |
| MMP-9, % | 0.12 ± 0.07 | 0.35 ± 0.57 | 0.79 ± 0.61* | 1.67 ± 2.03*,**,*** | 0.240 | <0.0001 |
Protein levels are given as percentage of the positive staining area relative to the total tissue area and presented as mean ± SD. N = 71, including n = 21 for ISHLT Grade 0 collected from 21 patients, n = 19 for Grade 1A from 19 patients, n = 16 for Grade 1B from 15 patients and n = 15 for Grade 3 from 12 patients.
EMB, endomyocardial biopsy; Synd-1, syndecan-1; MMP, matrix metalloproteinase.
*P < 0.05 vs. Grade 0.
**P < 0.05 vs. Grade 1A.
***P < 0.05 vs. Grade 1B.
Figure 3.
Intragraft synd-1 and MMP-9 protein expression correlates with the influx of T-cells and monocytes in human cardiac allograft rejection. (A) Representative microphotographs synd-1 and MMP-9 immunoreactivity in endomyocardial biopsies displaying rejection Grade 0, 1A, 1B, and 3. Scale-bar: 100 μm (B and C). Immunohistochemical analysis revealed a strong correlation between (B) synd-1 and (C) MMP-9 and the number of infiltrating CD3+ T-cells and CD68+ monocytes. N = 71, n = 21 for International Society for Heart and Lung Transplantation Grade 0 collected from 21 patients, n = 19 for Grade 1A from 19 patients, n = 16 for Grade 1B from 15 patients and n = 15 for Grade 3 from 12 patients.
Taken together, our data clearly indicate intragraft Synd-1 and MMP-9 protein expression as potent markers for infiltrating T-cells and monocytes in the transplanted heart.
Matricellular proteins, proteoglycans, MMPs, TIMPs, and cardiac allograft fibrosis
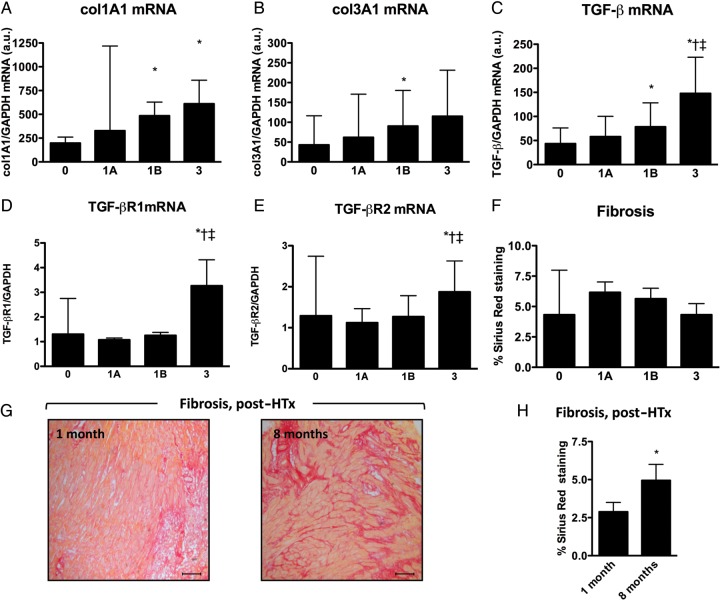
Intragraft transcript levels of collagen type 1α1 (COLIa1) and type IIIα1 (COLIIIa1) significantly increased with the production of pro-inflammatory/pro-fibrotic cytokines TGF-β and IL-6, the influx of CD3+ T-cells and CD68+ monocytes and increasing grade of rejection (Figure 4A and B, Table 3). Together, indicating a close association between inflammation and collagen production during CAR. A closer look at the components of the TGF-β pathway revealed that TGF-β-receptor-1 and -2 expressions are significantly elevated in Grade 3 rejecting EMBs, whereas those of latent TGF-β-binding proteins (LTBP)-1 to -4 did not show any significant changes (Figure 4C–E and Supplementary material online, Table S4). More interesting, however, further analyses revealed that not only the intragraft transcript levels of both TGF-β-receptors, LTBP-1, -2, and -3, but also SPARC, CTGF, TSP-2, MMP-14, TIMP-1, Testican-1, TSP-1, Synd-1, MMP-9, and MMP-2 (from highest to lowest) all positively correlated with the expression levels of COLIa1 and COLIIIa1 (Table 3).
Figure 4.
Cardiac allograft fibrosis. Intragraft transcript levels of (A) collagen type Iα1 (Col1a1) and (B) type IIIα1 (Col3a1) progressively increased with increasing grade of cardiac allograft rejection. (C–E) Intragraft transcript levels of (C) TGF-β, (D) TGF-β receptor-1 (TGF-βR1), and (E) TGF-β receptor-2 in respect to the grade of cardiac allograft rejection. (F) Sirius red stained collagen deposition with respect to the different grades of cardiac allograft rejection. (G) Representative Sirius red stained Grade 3 rejecting endomyocardial biopsies at 1 and 8 months after HTx. Scale-bar: 100 μm. (H) Cardiac allograft fibrosis is significantly increased at 8 months, when compared with 1 month after heart transplantation (*P < 0.01). *P < 0.05 vs. Grade 0 or vs. 1 month post-heart transplantation; †P < 0.05 vs. Grade 1A; ‡P < 0.05 vs. Grade 1B.
Table 3.
Intragraft transcript levels in relation to collagen synthesis
| Col Ia1 mRNA |
Col IIIa1 mRNA |
|||
|---|---|---|---|---|
| r-value | P-value | r-value | P-value | |
| Proteoglycans and matricellular protein transcript levels | ||||
| Synd-1 | 0.45 | <0.0001 | 0.43 | <0.0001 |
| Synd-4 | 0.15 | 0.298 | 0.16 | 0.188 |
| TSP-1 | 0.57 | <0.0001 | 0.54 | <0.0001 |
| TSP-2 | 0.76 | <0.0001 | 0.72 | <0.0001 |
| CTGF | 0.80 | <0.0001 | 0.77 | <0.0001 |
| SPARC | 0.91 | <0.0001 | 0.84 | <0.0001 |
| Testican-1 | 0.58 | <0.0001 | 0.66 | <0.0001 |
| MMP and TIMP transcript levels | ||||
| MMP-1 | 0.09 | 0.649 | 0.07 | 0.566 |
| MMP-2 | 0.40 | <0.001 | 0.40 | <0.001 |
| MMP-9 | 0.42 | <0.0001 | 0.40 | <0.0001 |
| TIMP-1 | 0.63 | <0.0001 | 0.61 | <0.0001 |
| MMP-14 | 0.64 | <0.0001 | 0.65 | <0.0001 |
| TIMP-2 | 0.27 | <0.05 | 0.26 | <0.05 |
| TIMP-3 | 0.08 | 0.934 | 0.16 | 0.206 |
| TIMP-4 | −0.10 | 0.506 | −0.04 | 0.678 |
| Pro-inflammatory cytokine and TGF-β-related transcript levels | ||||
| IL-6 | 0.41 | <0.0001 | 0.38 | <0.0001 |
| TNF-α | 0.12 | 0.452 | 0.20 | <0.05 |
| TGF-β | 0.67 | <0.0001 | 0.72 | <0.0001 |
| TGF-β-R1 | 0.41 | <0.0001 | 0.40 | <0.0001 |
| TGF-β-R2 | 0.21 | <0.05 | 0.22 | <0.05 |
| LTBP-1 | 0.33 | <0.01 | 0.30 | <0.01 |
| LTBP-2 | 0.38 | <0.0001 | 0.38 | <0.0001 |
| LTBP-3 | 0.42 | <0.0001 | 0.40 | <0.0001 |
| LTBP-4 | −0.06 | 0.532 | 0.07 | 0.949 |
| CD3+ leucocytes and CD68+ monocytes | ||||
| CD3+ leucocytes | 0.65 | <0.001 | 0.59 | <0.01 |
| CD68+ monocytes | 0.59 | <0.005 | 0.52 | <0.01 |
Synd-1, syndecan-1; TSP, thrombospondin; CTGF, connective tissue growth factor; SPARC, secreted protein acidic and rich in cysteine; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of matrix metalloproteinases; IL, interleukin; TNF, tumour necrosis factor; TGF-β, transforming growth factor-β; TGF-β-R, TGF-β-receptor; LTBP, latent TGF-β-binding protein.
Next, transmural interstitial fibrosis was visualized and analysed by Sirius red staining (Figure 4F–H). Surprisingly, total collagen deposition did not reflect the mRNA expression profiles of COLIa1 and COLIIIa1 (Figure 4F). Whereas the total amount of deposited collagen showed a trend towards increase in Grade 1A–B compared with Grade 0, no differences were seen in Grade 3 compared with Grade 0 EMBs, suggesting that the increased collagen expression might be counterbalanced by extensive proteolytic degradation in Grade 3 rejecting EMBs (Supplementary material online, Figure S5). In addition, a significant increase in collagen deposition was noted 8 months after transplantation, when compared with 1 month (Figure 4Gand H).
Synd-1 and MMP-9 intragraft transcript levels discriminate rejecting from non-rejecting endomyocardial biopsies
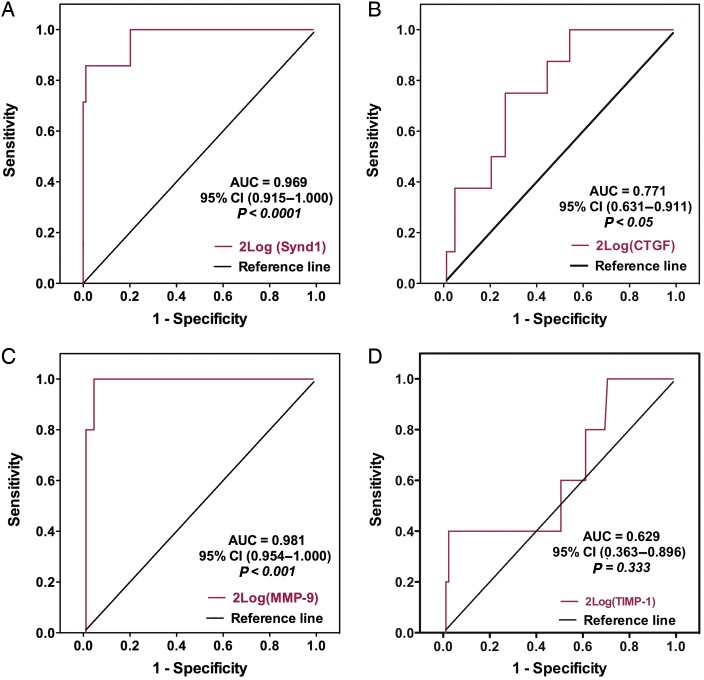
Finally, we set out to determine whether measuring intragraft transcript levels of one or more ECM-related macromolecules could potentially discriminate rejecting from non-rejecting EMBs. For this purpose, EMBs displaying ISHLT Grade 3 were considered as ‘rejecting’, whereas EMBs that exhibit ISHLT Grade 0, 1A, and 1B as ‘non-rejecting’. Next, receiver operating characteristic (ROC) curve analysis was performed for the four parameters that on univariate analysis had a P-value <0.01 (Figure 5). These included the 2log transformed intragraft transcript levels of synd-1 (P < 0 < 0001), CTGF (P < 0.01), MMP-9 (P < 0.0001), and TIMP-1 (P < 0.01). Strikingly, ROC curve analyses showed the most significant discrimination with an area under the curve of 0.969 for 2log(synd-1) [95% confidence interval (CI), 0.915–1.000; P < 0.0001] and 0.981 for 2log(MMP-9) (95% CI: 0.954–1.000; P < 0.001; Figure 5). An optimal cut-off was calculated at −0.44 for 2log(synd-1), representing a specificity of 80.0% and sensitivity of 100% and at 3.81 for 2log(MMP-9), representing a specificity of 95.4% and sensitivity of 100%.
Figure 5.
Intragraft synd-1 and MMP-9 mRNA profiling as potential biomarkers for the grade of cardiac allograft rejection. Receiver operating characteristic curves for (A) 2log(synd-1), (B) 2log(MMP-9), (C) 2log(TIMP-1) and (D) 2log(CTGF). For this model, Grade 3 was considered as ‘rejection’, whereas Grade 0, 1A, and 1B as ‘non-rejection’ and base-2 logarithmic (2log) transformed intragraft transcript levels were used. AUC, area under the curve; 95% CI, 95% confidence intervals.
Taken together, these analyses indicate that the intragraft transcript levels of synd-1 and MMP-9 show potential as a decision-making tool in clinical practice to diagnose human CAR based on EMBs. Nevertheless, future analysis using larger cohorts of EMBs should be performed to confirm our results.
Discussion
Although discoveries of the last decade have made it clear that changes in the cardiac ECM modulate key inflammatory and reparative pathways and thereby co-determine the structural and functional integrity of the heart,3,4 to date, no attempts have been made to investigate the intragraft expression of matricellular proteins, proteoglycans, MMPs and TIMPs in relation to inflammation, fibrosis, and the degree of CAR in the human transplanted heart. Our study demonstrates that—out of 15 ECM-related macromolecules—synd-1 and MMP-9 are the only two that consistently correlate with the degree of human CAR, the expression of pro-inflammatory cytokines TNF-α, IL-6, and TGF-β, infiltrating CD3+ T-cells and CD68+ monocytes, and collagen transcription in the transplanted heart. Furthermore, their protein expression robustly co-localized with areas of inflammation and sites of active remodelling, indicating synd-1 and MMP-9 as potent intragraft markers for the inflammatory response in human cardiac allografts. On the other hand, we also reveal that a multitude of additional non-structural ECM proteins, including SPARC, CTGF, TSP-1 and -2, Testican-1, MMP-2, -14 and, TIMP-1 closely associate with intragraft collagen transcription. More importantly, ROC curve analysis demonstrated that intragraft synd-1 and MMP-9 transcript levels might have potential as a decision-making tool in clinical practice to diagnose Grade 3 rejecting EMBs.
An accumulating body of evidence suggests that CAR surveillance based on intragraft gene-expression profiling my help to improve clinical management of transplant patients.12–14 More specifically, these studies advocate that the use of gene-expression profiling might require fewer EMBs and could potentially provide a more uniform readout with greater sensitivity and limited sampling error when compared with the current histolopathological standard to identify and classify CAR.13 Strikingly, our results indicate that synd-1 and MMP-9 intragraft transcriptional profiling may represent a novel quantitative measure to discriminate rejecting (ISHLT Grade 3) from non-rejecting hearts using a single EMB. Hence, if confirmed in a larger cohort of patients this approach could reduce the inter-reader, subjective variability, as well as grading-inconsistencies among the various transplant centers.15 Furthermore, future development an intragraft transcriptional RT–PCR ‘array’, that not only includes synd-1 and MMP-9, but also other markers of acute CAR (such as FOXP3, Granzyme B, Perforin, and Fas-L),16,17 as well as markers of cardiac allograft vasculopathy (i.e. TSP-1, IFN-γ-inducible CXCR3 chemokines IP-10, and I-TAC, etc.)14,18; markers of reduced long-term graft survival (i.e. VEGF, IL-6, TNF-α, and TGF-β)5,19,20; and others may allow us to detect certain molecular qualities of the transplanted heart that do not meet the eye under the microscope. Taken together, this approach could potentially provide an extended readout that not only determines the degree of CAR, but also reflects the efficacy of the immunosuppression, that identify patients at risk for the development of CAR and vasculopathy, and thereby co-determine the long-term clinical management of HTx patients.
Interestingly, our study is—to our knowledge—the first to support the concept that pro-inflammatory cytokines, T-cells and monocytes are closely associated with or even in part responsible for the induction of several ECM-related macromolecules in the human transplanted heart. It is well established that CAR is the result of a highly complex, multiplayer immunologic reaction in which pro-inflammatory cytokines, T-cells, and monocytes play a prominent role.1,5,14 Our results extend these findings with the observation that the expression of TNF-α, IL-6, and TGF-β, but also the influx of CD3+ T-cells and CD68+ monocytes strongly relate to each other and to the grade of human CAR. Moreover, the presence of these pro-inflammatory cytokines T-cells and/or monocytes is strongly paralleled by an increased expression of primarily synd-1, MMP-9, CTGF, and TIMP-1. These observations are in agreement with previous reports, ranging from the expression and functional implications of synd-1 and MMP-9 in a wide variety of inflammatory disorders (reviewed in by Teng et al.7 and Ram et al.21); the association of synd-1-positive monocytes and MMP-9 expression with rejection of liver, paediatric kidney, and/or pulmonary allografts22–26; increased CTGF and MMP-9 transcript levels in a mouse model of CAR5,10; to the immunomodulatory properties of synd-1,7,23 MMP-9,10 CTGF,5 and TIMP-19 towards T-cells and macrophages. In addition, two-independent studies revealed that in a mouse model of acute CAR, MMP-9 mediates the influx of mononuclear cells into the allografts and regulates the activation and expansion of alloreactive T-cells, whereas the systemic use of a broad-spectrum MMP-inhibitor (GM6001) enhanced allograft survival by directly suppressing the infiltration of inflammatory cells in a mouse model of CAR.10,11 Similarly, the use of CTGF neutralizing antibodies limited graft infiltration by T-cells in a mouse model of chronic CAR.27 On the other hand, our group recently revealed that gene-overexpression of synd-1 or TIMP-1 protects the heart against exaggerated inflammation and adverse remodelling in a mouse model of myocardial infarction.6,28 However, whether up-regulation of synd-1 and TIMP-1 aims to limit the inflammatory influx, and thereby protects the transplanted heart should be the subject of further animal studies. Taken together, our findings are likely to be indicative of a functional role for synd-1, MMP-9, CTGF, and TIMP-1 in the complex inflammatory pathways underlying human CAR and might, therefore, represent potential therapeutic targets to prevent human CAR.
Besides inflammation, cardiac allograft fibrosis has recently been recognized as an important limiting factor towards graft function and long-term survival.5 We reveal that the production of pro-inflammatory cytokines IL-6 and TGF-β; components of the TGF-β pathway TGF-β-R1 and 2, LTBP-1, -2, and -3; the influx of T-cells and monocytes; as well as the transcription of synd-1, TSP-1 and -2, CTGF, SPARC, Testican-1, MMP-2, -9, -14, TIMP-1, and -2 all strongly relate to the expression of Collagen Ia1 and IIIa1 in the transplanted hearts. Moreover, collagen transcript levels progressively increased with the degree of CAR. Interestingly, IL-6 and TGF-β were previously reported to have a negative impact on graft survival through their chemotactic and pro-fibrotic effects.5 Interestingly, not only IL-6 and TGF-β, but also synd-1, TSP-1 and -2 CTGF, TIMP-1 and -2 either promote fibroblast proliferation or regulate ECM production by regulating collagen synthesis, assembly and/or maturation in the diseased heart.2,4–6,9 In addition, both animal and clinical studies indicated that TSP-1, CTGF, MMP-2, -14, TIMP-2, and TGF-β might directly affect or are associated with the development of cardiac allograft vasculopathy.5,18,29,30 The present study translates these findings to the human clinical situation and indicates that the TGF-β pathway, synd-1, TSP-1 and -2, CTGF, SPARC, testican-1, MMP-2, -9, -14, and TIMP-1 and -2 might withhold important targets and mechanistic insights to limit the deleterious fibrotic response, cardiac allograft vasculopathy, and progressive loss of function of the human transplanted heart.
An intriguing observation is that total collagen deposition within our cohort of EMBs did not reflect the increasing transcript levels of COLIa1 and COLIIIa1 with increasing grade of CAR. This discrepancy between mRNA and protein could be the result of increased MMP-9-mediated collagen degradation as suggested by the strongly increased MMP-9 transcript, protein, and MMP2/9 in situ proteolytic activity levels observed in Grade 3 rejecting EMBs (Supplementary material online, Figure S5).31 In this regard, it might be of future interest to evaluate intragraft as well as circulating markers of collagen metabolism, including N-terminal pro-peptides of pro-collagen I and III (PINP and PIIINP) and carboxyterminal telopeptides, in relation to the degree of human CAR.31–33 Nevertheless, more in-depth investigations will be required to unveil the significance of this phenomenon.
Several limitations should be noted when interpreting our results. As mentioned before, it is important to note that due to our limited number of ISHLT Grade 3 rejecting EMBs, further studies are be mandatory to confirm our results. Furthermore, the amount of endomyocardial tissue obtained did not permit protein analysis by multiple techniques. For this reason, we chose to perform semi-quantitative analysis based on immunohistochemistry, because this permitted the analysis of several protein expressions as well as their localization in the same tissue. The immunohistochemical techniques used in this study have been validated by previous studies from our laboratory.31
Despite the limitations of our study, we identified synd-1 and MMP-9 as novel surrogate markers for the degree of inflammation in the transplanted heart. More importantly, we reveal that synd-1 and MMP-9 intragraft transcriptional profiling represents as a novel quantitative tool to discriminate rejecting (ISHLT Grade 3) from non-rejecting hearts. Furthermore, our data point towards important molecular interactions between the immunologic response and the induction of non-structural matrix proteins and collagen expression in the human transplanted heart. Therefore, future efforts to elucidate the interplay between these factors may direct the development of improved diagnostic tools and targeted therapies that limit CAR, vasculopathy, and progressive loss of function of the transplanted heart.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
The authors would like to thank S. Martin from the Leuven Heart Transplant Program and the staff of the Cardiac Catheterization Laboratory at the University Hospital Leuven (KULeuven, Leuven, Belgium) for their assistance in obtaining endomyocardial biopsy specimens and blood samples for this study. We also thank Dr M. Swinnen, S. Jochems, and K. Smeekes for their technical assistance.
Funding
This work was supported by a research grant from the Research fund K.U.Leuven, Leuven, Belgium (PDMK/08/175) and a postdoctoral fellowship from the Research Foundation Flanders, Belgium (FWO-Vlaanderen 1.2.089.10N) to D.V., a pre-doctoral fellowship from the FWO-Vlaanderen (FWO-Vlaanderen 1.1.676.12N) to L.V.A., a Long Term structural funding—Methusalem funding by the Flemish Government to P.C., the Michael Ondetti Chair in Cardiology to J.V., the European Union (FP7-HEALTH-2011, EU-MASCARA) to Y.J, a VIDI grant from the Netherlands Organization of Scientific Research (NWO, 91796338) to S.H., and research grants from the Netherlands Heart Foundation (2007B036, 2008B011), the Research Foundation Flanders (FWO-Vlaanderen 1.1.676.10N and G074009N) and the European Union (FP7-HEALTH-2010, MEDIA, and FP7-HEALTH-2011, EU-MASCARA) to S.H. No relationship with the industry exists.
Conflict of interest: none declared.
References
- 1.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 4.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth AJ, Bishop DK. TGF-beta, IL-6, IL-17 and CTGF direct multiple pathologies of chronic cardiac allograft rejection. Immunotherapy. 2010;2:511–520. doi: 10.2217/imt.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanhoutte D, Schellings MW, Gotte M, Swinnen M, Herias V, Wild MK, Vestweber D, Chorianopoulos E, Cortes V, Rigotti A, Stepp MA, Van de Werf F, Carmeliet P, Pinto YM, Heymans S. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115:475–482. doi: 10.1161/CIRCULATIONAHA.106.644609. [DOI] [PubMed] [Google Scholar]
- 7.Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3–16. doi: 10.1016/j.matbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Brown LF, Laham RJ, Volk R, Simons M. Macrophage-dependent regulation of syndecan gene expression. Circ Res. 1997;81:785–796. doi: 10.1161/01.RES.81.5.785. [DOI] [PubMed] [Google Scholar]
- 9.Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ‘embracing the MMP-independent-side of the family. J Mol Cell Cardiol. 2010;48:445–453. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Campbell LG, Ramachandran S, Liu W, Shipley JM, Itohara S, Rogers JG, Moazami N, Senior RM, Jaramillo A. Different roles for matrix metalloproteinase-2 and matrix metalloproteinase-9 in the pathogenesis of cardiac allograft rejection. Am J Transplant. 2005;5:517–528. doi: 10.1111/j.1600-6143.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 11.Eaton VL, Lerret NM, Velasquez-Lopera MM, John R, Caicedo M, DeCresce RP, Jaramillo A. Enhanced allograft survival and modulation of T-cell alloreactivity induced by inhibition of MMP/ADAM enzymatic activity. Am J Transplant. 2008;8:507–516. doi: 10.1111/j.1600-6143.2007.02097.x. [DOI] [PubMed] [Google Scholar]
- 12.de Groot-Kruseman HA, Mol WM, Niesters HG, Maat AP, van Gelder T, Balk AH, Weimar W, Baan CC. Differential intragraft cytokine messenger RNA profiles during rejection and repair of clinical heart transplants. A longitudinal study. Transpl Int. 2003;16:9–14. doi: 10.1111/j.1432-2277.2003.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 13.Holweg CT, Potena L, Luikart H, Yu T, Berry GJ, Cooke JP, Valantine HA, Mocarski ES. Identification and classification of acute cardiac rejection by intragraft transcriptional profiling. Circulation. 2011;123:2236–2243. doi: 10.1161/CIRCULATIONAHA.109.913921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, McCarthy PM, Feng J, Novick AC, Fairchild RL. Chemokine and chemokine receptor gene expression indicates acute rejection of human cardiac transplants. Transplantation. 2003;75:72–78. doi: 10.1097/00007890-200301150-00013. [DOI] [PubMed] [Google Scholar]
- 15.Marboe CC, Billingham M, Eisen H, Deng MC, Baron H, Mehra M, Hunt S, Wohlgemuth J, Mahmood I, Prentice J, Berry G. Nodular endocardial infiltrates (Quilty lesions) cause significant variability in diagnosis of ISHLT Grade 2 and 3A rejection in cardiac allograft recipients. J Heart Lung Transplant. 2005;24:S219–S226. doi: 10.1016/j.healun.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Shulzhenko N, Morgun A, Zheng XX, Diniz RV, Almeida DR, Ma N, Strom TB, Gerbase-DeLima M. Intragraft activation of genes encoding cytotoxic T lymphocyte effector molecules precedes the histological evidence of rejection in human cardiac transplantation. Transplantation. 2001;72:1705–1708. doi: 10.1097/00007890-200111270-00025. [DOI] [PubMed] [Google Scholar]
- 17.Ramsperger-Gleixner M, Spriewald BM, Tandler R, Kondruweit M, Amann K, Weyand M, Ensminger SM. Increased transcript levels of TNF-alpha, TGF-beta, and granzyme B in endomyocardial biopsies correlate with allograft rejection. Exp Clin Transplant. 2011;9:387–392. [PubMed] [Google Scholar]
- 18.Zhao XM, Hu Y, Miller GG, Mitchell RN, Libby P. Association of thrombospondin-1 and cardiac allograft vasculopathy in human cardiac allografts. Circulation. 2001;103:525–531. doi: 10.1161/01.CIR.103.4.525. [DOI] [PubMed] [Google Scholar]
- 19.Bayliss J, Maguire JA, Bailey M, Leet A, Kaye D, Richardson M, Bergin PJ, Dowling J, Thomson NM, Stein AN. Increased vascular endothelial growth factor mRNA in endomyocardial biopsies from allografts demonstrating severe acute rejection: a longitudinal study. Transpl Immunol. 2008;18:264–274. doi: 10.1016/j.trim.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Deng MC, Erren M, Kammerling L, Gunther F, Kerber S, Fahrenkamp A, Assmann G, Breithardt G, Scheld HH. The relation of interleukin-6, tumor necrosis factor-alpha, IL-2, and IL-2 receptor levels to cellular rejection, allograft dysfunction, and clinical events early after cardiac transplantation. Transplantation. 1995;60:1118–1124. doi: 10.1097/00007890-199511270-00011. [DOI] [PubMed] [Google Scholar]
- 21.Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 22.Krukemeyer MG, Moeller J, Morawietz L, Rudolph B, Neumann U, Theruvath T, Neuhaus P, Krenn V. Description of B lymphocytes and plasma cells, complement, and chemokines/receptors in acute liver allograft rejection. Transplantation. 2004;78:65–70. doi: 10.1097/01.TP.0000132324.14207.8B. [DOI] [PubMed] [Google Scholar]
- 23.Tsai EW, Wallace WD, Gjertson DW, Reed EF, Ettenger RB. Significance of intragraft CD138+ lymphocytes and p-S6RP in pediatric kidney transplant biopsies. Transplantation. 2010;90:875–881. doi: 10.1097/TP.0b013e3181f24e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigo E, Lopez Hoyos M, Escallada R, Ruiz JC, Fresnedo GF, Heras M, Pinera C, Ramos MA, Cotorruelo JG, Arias M. Changes in serum concentrations of matrix metalloproteinases in kidney transplantation. Transplant Proc. 2000;32:517–518. doi: 10.1016/S0041-1345(00)00869-1. [DOI] [PubMed] [Google Scholar]
- 25.Beeh KM, Beier J, Kornmann O, Micke P, Buhl R. Sputum levels of metalloproteinase-9 and tissue inhibitor of metalloproteinase-1, and their ratio correlate with airway obstruction in lung transplant recipients: relation to tumor necrosis factor-alpha and interleukin-10. J Heart Lung Transplant. 2001;20:1144–1151. doi: 10.1016/S1053-2498(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 26.Ermolli M, Schumacher M, Lods N, Hammoud M, Marti HP. Differential expression of MMP-2/MMP-9 and potential benefit of an MMP inhibitor in experimental acute kidney allograft rejection. Transpl Immunol. 2003;11:137–145. doi: 10.1016/S0966-3274(02)00150-8. [DOI] [PubMed] [Google Scholar]
- 27.Booth AJ, Csencsits-Smith K, Wood SC, Lu G, Lipson KE, Bishop DK. Connective tissue growth factor promotes fibrosis downstream of TGFbeta and IL-6 in chronic cardiac allograft rejection. Am J Transplant. 2010;10:220–230. doi: 10.1111/j.1600-6143.2009.02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 29.Tsukioka K, Suzuki J, Fujimori M, Wada Y, Yamaura K, Ito K, Morishita R, Kaneda Y, Isobe M, Amano J. Expression of matrix metalloproteinases in cardiac allograft vasculopathy and its attenuation by anti MMP-2 ribozyme gene transfection. Cardiovasc Res. 2002;56:472–478. doi: 10.1016/S0008-6363(02)00592-8. [DOI] [PubMed] [Google Scholar]
- 30.Koenig GC, Rowe RG, Day SM, Sabeh F, Atkinson JJ, Cooke KR, Weiss SJ. MT1-MMP-dependent remodeling of cardiac extracellular matrix structure and function following myocardial infarction. Am J Pathol. 2012;180:1863–1878. doi: 10.1016/j.ajpath.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, Van de Werf F, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005;112:1136–1144. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 32.Lopez B, Gonzalez A, Diez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–1654. doi: 10.1161/CIRCULATIONAHA.109.912774. [DOI] [PubMed] [Google Scholar]
- 33.Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem. 2008;307:159–167. doi: 10.1007/s11010-007-9595-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.