Review of how macrophages switch from an inflammatory to regulatory activation state during disease, and a newly identified mechanism governing this transition.
Keywords: pathology, inflammation, regulation
Abstract
Macrophages make major contributions to inflammatory immunopathology. In this work, we examine three disease scenarios, in which M1s play a major role early in the disease but eventually transitions into a population of cells with immunoregulatory activity. We propose that the transition from an inflammatory to a regulatory phenotype is a natural progression that regularly occurs in stimulated macrophages and that the timing of this transition is critical to maintaining homeostasis. In the first section of this review, we discuss the exogenous microenvironmental cues that may induce macrophages to enter a regulatory state. In the second half of this review, we discuss a novel mechanism, whereby TLR-stimulated macrophages can intrinsically induce their own regulatory activation state. They do so by secreting and synthesizing endogenous “reprogramming” signals that work in an autocrine fashion to promote a regulatory phenotype. We propose that these endogenous regulatory mechanisms exist to prevent macrophage-mediated immunopathology. Thus, macrophages can respond to endogenous and exogenous cues to regulate their activation state, and without these controlled regulatory responses, M1 would persist to the detriment of the host.
Introduction
Macrophages are major cellular contributors to the homeostasis of the host. They can respond to infection to enhance immune responses and conversely, contribute to the repair of tissue in the aftermath of an immune response. It has been appreciated for many decades that exposure of macrophages to bacterial products can induce a profound phenotypic change, resulting in the secretion of myriad inflammatory cytokines and mediators. Under different circumstances, however, these same macrophages can produce anti-inflammatory cytokines, lipid resolvins, and growth factors with the opposite effect. These dramatic changes in their physiology are vital to maintaining homeostasis and protecting the host from severe immunopathology. In this work, we select three disease scenarios, in which macrophages initially assume an inflammatory (M1) phenotype and then transition into a tissue-regenerating, regulatory phenotype. These three scenarios are: muscle development, wound healing, and sepsis. In all three cases, an initial inflammatory response is associated with an M1 phenotype that is replaced over time by a tissue-regenerating response, predominated by macrophages exhibiting a regulatory phenotype (Fig. 1). In vivo observations, in mice and humans, suggest that macrophages exhibit the potential to alter their responses to changing tissue environments, an ability referred to as “functional plasticity”. However, there remains considerable controversy about how this transition from pro- to anti-inflammatory activation occurs in macrophages. One school of thought suggests that the transition is a result of the appearance of new monocyte-derived macrophages that assume a distinct phenotype. The other holds that macrophages themselves can become “reprogrammed” to transition from an inflammatory to a regulatory cell. Definitive in vivo data in support of either of these theories are lacking, and in fact, both may be correct. In the second portion of this review, we describe a recent discovery of a novel mechanism important for the programmed transition from inflammatory to regulatory macrophages. This study reveals the inherent ability of macrophages to initiate this reprogramming to mitigate the activation response. These studies predict that in tissue, macrophages have an intrinsic ability to transition from an inflammatory to a regulatory state and that macrophage inflammatory responses are normally transient, giving way to regulatory responses. Here, we will discuss the dynamic nature of muscle repair, wound healing, and sepsis and focus on the temporal changes that occur in macrophages during these processes. In this brief review, we will focus on the ability of macrophages to modulate immune responses and contribute to homeostasis by down-modulating inflammatory responses.
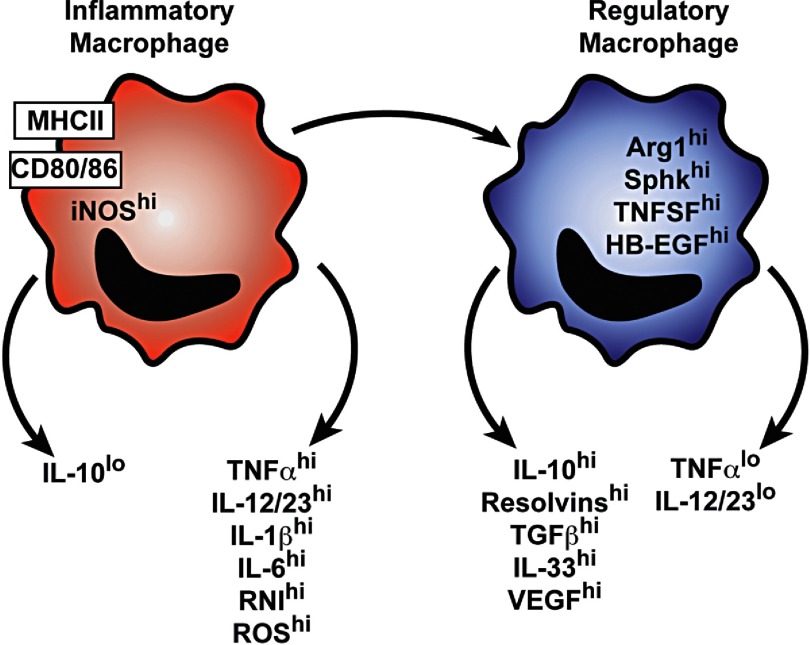
Figure 1. Schematic representation of phenotypic markers of macrophages at different stages of disease.
During muscle development, tissue damage, and sepsis, the initial macrophage activation state is characterized by the expression of biomarkers associated with inflammation. At later stages of these diseases, macrophages switch to a regulatory activation state by expressing phenotypic markers that suppress inflammation and promote tissue repair. RNI, Reactive nitrogen intermediates; TNFSF, TNF superfamily.
EXTRINSIC CONTROL OF MACROPHAGE INFLAMMATORY RESPONSES
Muscle development
The role of macrophages in muscle development has been studied thoroughly, and the reader is referred to many excellent reviews on this subject [1–3]. Muscle has the remarkable ability to regenerate itself after injury, and this regeneration forms the basis for exercise-induced hypertrophy [1–3]. The regenerative potential of muscle lies in satellite cells, muscle stem cells that are perhaps most affected by the changing activation status of neighboring macrophages. In response to muscle exertion and stress, satellite cells become activated to proliferate and eventually differentiate into myogenic precursors that give rise to new myofibers. Several reports have indicated that macrophages associate with satellite cells and play key roles in their differentiation [4, 5]. There appears to be a compartmentalization of macrophage roles, with M1 contributing primarily to the initial activation of satellite cells leading to their proliferation [4]. Regulatory macrophages, in turn, promote the differentiation of these cells into myotubes [6, 7]. While this may be an oversimplification, it certainly suggests that proper muscle development depends on the switch from inflammatory to regulatory macrophage responses, resulting in the differentiation of satellite cells into mature muscle.
Muscle contractions, especially eccentric contractions, result in the tearing of muscle fibers, necessitating the repair of these fibers and the connective tissue surrounding them, ultimately resulting in the formation of new, stronger muscle fibers to replace previous ones. A normal physiological response to muscle exertion is the migration of macrophages into the damaged muscles to participate in the repair process [8, 9]. The analysis of macrophages in muscle shortly after exercise (1–3 days) reveals the presence of macrophages with an inflammatory phenotype, producing inflammatory mediators and oxygen radicals [8, 10]. As resting muscle has only low numbers of resident macrophages [11], it is generally believed that the initial inflammatory response to eccentric exercise comes from the influx of inflammatory monocytes. In the mouse, inflammatory monocytes express high levels of the chemokine receptor CCR2 and the Ly6C marker. In humans, this monocyte subset is typically identified by their expression CD14 and a lack of CD16. Upon CCL-2-dependent migration into the damaged tissue, these monocytes first develop into M1, which serves an important role in the clearance of muscle debris and repair to damaged matrix [12]. Monocyte-derived macrophages play a critical part in muscle regeneration, as in mice lacking CCR2, the inability to recruit M1 into the muscle results in a substantial delay and impairment of muscle regeneration [13, 14].
Although the appearance of M1 has been observed in response to tissue damage, the signals that stimulate macrophages to assume an inflammatory phenotype in muscle are not fully understood. One of the most extensively studied stimuli predicted to be integral in the development of M1 in muscle tissue is cellular debris released from damaged cells within the muscle. Neighboring monocytes/macrophages interpret this debris as “danger”, leading to their production of inflammatory cytokines. High levels of ATP and high-mobility group box 1 that usually reside within cells are released by dying cells into the extracellular tissue milieu to enhance the recruitment of migrating monocytes and activate macrophages to release the proinflammatory cytokine IL-1β [15–17]. Lactic acid and perhaps other products of muscle metabolism have also been associated with the production of inflammatory cytokines by muscle-associated macrophages [18]. Regardless of the stimuli, the consensus is that the early response of macrophages in developing muscle is one predominated by inflammatory mediators, and this response is an integral part of muscle regeneration.
Importantly, the inflammatory response in muscle gradually gives way to an anti-inflammatory-regenerating response. At later times after injury or exercise, muscle-associated macrophages remain plentiful, yet paradoxically, the tissue environment is characterized by a lack of inflammatory cytokines. Moreover, over time, in response to damage, there is an enhancement of anti-inflammatory cytokines and immunoregulatory mediators that dominate the late stages of muscle development [10]. There has been considerable debate about the nature (and the name) of the macrophages that predominate at these later times, and questions remain about the stimuli that promote this phenotypic switch. The well-described response of macrophages to apoptotic cells [19] may certainly contribute to the promotion of the anti-inflammatory environment in the muscle. However, it is likely that multiple, additional exogenous stimuli contribute to the alteration in macrophage physiology. In vitro studies on macrophages have shown that prostaglandin (PG) derivatives, lipid resolvins, cAMP, and adenosine can all contribute to macrophage reprogramming and induce the development of macrophages with a regulatory phenotype [20–24]. All of these mediators have been described in various models of muscle injury. The types of macrophages that arise in muscle, several days to a week after muscle injury, have not been studied systematically. Although a comprehensive transcriptional profiling of muscle over time postinjury has been reported [25], as far as we are aware, the transcriptional profiling of purified, muscle-derived macrophages over time has not yet been performed. Therefore, the products produced by these macrophages can only be inferred. One of the cytokines produced in muscle at later times postinjury is IL-10, and this cytokine is critical for proper muscle development, as in its absence, muscle development is impaired [26]. Although IL-10 makes important contributions to the later (regulatory) stages of muscle development, it is actually detrimental to the early (inflammatory) stages of muscle development [7, 27]. Thus, the blocking of late IL-10 production can interfere with muscle regeneration, just as the addition of exogenous IL-10, early in the muscle repair process, can disrupt regeneration. This differential response to IL-10 (early vs. late) illustrates how carefully the macrophage transition from inflammatory to regulatory must be controlled and how important this transition is to proper muscle development.
In addition to altering their own physiology during the process of muscle repair, macrophages exert a dramatic influence on adjacent cell types that contribute to muscle development. This influence on adjacent cells contributes to the alteration of the microenvironment in the developing muscle. For example, in CCR2-deficient mice, there is not only a decrease in macrophage recruitment to the muscle but also a subsequent decrease in the diameter of myofibres, a reduction in neovascularization, and an increase in fat deposits [13]. These changes in the muscle involve many different cells that respond to products secreted by macrophages and then secrete their own “paracrine trophic factors” [28] to influence muscle development. Macrophages may also induce the activation of the so-called fibro/adipogenic progenitors that have been described recently as playing important roles in muscle development [29]. As the inflammatory environment of a damaged muscle is quite similar to wounded tissue, the repair processes share many characteristics with wound repair (see below).
Not only is the timing of the transition from inflammation to regeneration important in muscle development, but also, the magnitude of these responses must also be controlled carefully. Too much early inflammation, for example, by increasing neutrophil chemotaxis, can lead to necrotic myositis [30], whereas too much late immunoregulation, for example, by ablating arginase (Arg1), can lead to fibrosis [31]. Therefore, we propose that macrophages must be able to regulate their activation responses to prevent detriment to the muscle, suggesting that the functional plasticity of macrophages is vital to proper muscle development.
Wound healing
There are many similarities between muscle development and wound repair, even if the kinetics of these processes varies, and the relative contribution of specific mediators may be specific to each tissue type. In general, all wound healing includes the removal of dead cells and tissue debris, the repair of damaged tissue, the remodeling of the ECM, and the induction of neovascularization. Macrophages participate in all of these processes, and their transition from an inflammatory to a regulatory cell appears to be instrumental in the accomplishment of these diverse tasks.
The destruction of tissue can lead to the release of DAMPs from dying cells, an alteration in the ECM, and the initiation of the coagulation and complement cascades. These events trigger (directly or indirectly) an influx of monocytes, which even in sterile wound environments, assume an inflammatory phenotype [26]. These phagocytic cells clear the injury site of dead cells and debris and participate in host defense against microbes that may occupy the wound site. Early wound-associated macrophages typically release chemokines, lipid mediators, and a number of inflammatory cytokines, including (but not limited to) TNF-α, IL-1β, IL-12, IL-18, and IL-23 [26, 32]. However, the ensuing, regenerative phase of wound healing requires the transition of macrophages from an inflammatory to a wound-healing phenotype [32]. These cells not only produce mediators that contribute directly to wound healing but also exert a dramatic influence on adjacent mesenchymal cells. For example, this phase depends heavily on the participation of fibroblasts, which are major producers of TGF-β and contribute to the restoration of the ECM. In skin wounds, tissue regeneration depends on the ability of local resident stem cells to be activated to proliferate and differentiate. The activation and differentiation of stem cells in the wound are more efficient in the presence of macrophages and similar to muscle development (above), depend on the transition of macrophages from an inflammatory to a regulatory phenotype. In genetically manipulated mice that lack macrophages, wound healing and re-epithelialization are delayed substantially [33]. The activation of stem cells by macrophages does not appear to be restricted to muscle or skin stem cells. Macrophages can also stimulate the growth of mesenchymal stem cells [34] and orchestrate the development of the mammary gland [35]. Thus, macrophages play a role in instructing the wound-healing process and promoting the proliferation and differentiation of stem cells in tissue.
In muscle development and wound healing, both the inflammatory early phase and the regenerative later phase must be controlled carefully. An initial proinflammatory phase is required to recruit innate immune cells and develop M1 macrophages at the site of tissue damage/injury to help fight potential microbial infection. Once the inflammatory environment has subsided, macrophages present in tissue can participate successfully in the regeneration of tissue by producing tissue growth factors and promote collagen synthesis. Importantly, the activation status of macrophages at all stages of wound healing, as well as muscle regeneration, must be tightly regulated. If the early wound-healing response does not transition to a regulatory response, then inflammatory immunopathology will result in necrotic wounds that fail to resolve. If the later regenerative phase is not regulated, then the wound-healing response will progress to an obstructive fibrotic response. Whereas it is perhaps counterproductive to assign fixed names to the macrophage subtypes that promote the regenerative phase of wound healing, the promotion of the late fibrotic response appears to be mediated primarily by the well-characterized “alternatively activated” macrophages that were described originally by Stein, Gordon, and coworkers [36]. In response to IL-4 or IL-13, these macrophages produce collagen and promote collagen production from fibroblasts to cause the fibrosis associated with dysregulated wound responses [37]. The exaggerated wound-healing response is similar in character to some of the fibrotic diseases that occur in helminthic infections that have been described elegantly by Wynn and Barron [38, 39].
Sepsis
The initial phase of sepsis manifests as a SIRS that is triggered by inflammatory responses to TLR agonists, such as bacterial LPS [40]. The severe pathology associated with SIRS occurs in response to the hyperproduction of macrophage-derived inflammatory cytokines, including TNF-α, IL-12, and IL-1β, which coalesce in a “cytokine storm” that can lead to tissue destruction, vascular damage, septic shock, multiple organ failure, and death [40]. It is believed that the inability to control M1 activation responses effectively is what drives this severe immunopathology. Intriguingly, however, in individuals who survive the initial proinflammatory response, a compensatory anti-inflammatory response develops that is accompanied by the appearance of monocytes/macrophages exhibiting a regulatory phenotype [41, 42]. In fact, sepsis represents one of clearest examples of macrophages transitioning from a highly inflammatory population of cells to a population with immunoregulatory properties. This transition has been referred to as “endotoxin tolerance”, a term that implies incorrectly that macrophages become nonresponsive to restimulation by bacterial products. This, of course, is not the case, as these macrophages actually undergo a transcriptional reprogramming, resulting in dramatic shifts, both up and down, in transcript levels. There are many in vitro and in vivo models of endotoxin tolerance, and many of these models produce macrophages with subtle differences in their physiology, leading to some confusion about the specifics of tolerance. Perhaps the most straightforward illustration of this phenomenon comes from an analysis of human monocytes taken from postseptic patients and stimulated ex vivo with LPS. These monocytes produce substantially lower amounts of inflammatory cytokines, such as TNF-α and IL-6, and they increase their production of immunoregulatory molecules, including IL-1R antagonist and IL-10 [43]. It is thought that this modulation in cytokine production is necessary to protect the host from immunopathology associated with prolonged macrophage activation. In fact, in an in vivo mouse model of endotoxin tolerance, pre-exposure of mice to low levels of LPS protected mice from lethal doses of endotoxin [44]. There have been many reports identifying different potential molecular mechanisms behind tolerance [43], and it is likely that multiple molecular mechanisms may be responsible for this transition to a regulatory phenotype. Despite differences of opinion regarding the mechanisms behind tolerance, there is general consensus that this phenomenon is real and that it may actually predispose postseptic patients to subsequent infections. Thus, a mechanism to spare the host of inflammatory tissue damage may present an opportunity that infectious microbes can exploit, thereby highlighting the essential role for tightly regulated macrophage responses to fully protect the host.
Summary
In muscle development, wound healing, and sepsis, the early inflammatory response to injury eventually transitions into a “regulatory” response to promote the repair of damaged tissue. In all three cases, the initial response is the recruitment of inflammatory monocytes and macrophages in response to DAMPs, generated from damaged tissue and/or microbial agonists. In each setting, exogenous factors, such as apoptotic cells, PG derivatives, adenosine, or growth factors, accumulate over time and promote the development of regulatory macrophages. The switch from inflammatory to regulatory macrophages is believed to play an integral role in returning damaged tissue to homeostatic conditions, thereby protecting the host from further detriment. It is important to improve our understanding of the stimuli in tissue that promote regulatory macrophage development and to expand our knowledge of how regulatory macrophage function is controlled to improve treatment options for inflammatory diseases, such as muscular dystrophy, fibrosis, and sepsis. Studying the intrinsic ability of macrophages to transition to a regulatory state may provide some clues about this transition.
INTRINSIC CONTROL OF MACROPHAGE INFLAMMATORY RESPONSES
Although the appearance of disparate macrophage phenotypes has been observed in vivo, the underlying mechanisms supporting the transition from inflammatory to regulatory macrophages remain poorly understood. We have proposed that the ability of macrophages to switch from an inflammatory to a regulatory activation state is dependent on a temporal reprogramming of the macrophage-activation response [45]. We recently identified a mechanism, whereby macrophages intrinsically control their own activation state. We characterized the molecular mechanisms involved in the initiation of the reprogramming, and in this section, we speculate about the physiological significance of this control.
Unlike most mammalian cells, tissue macrophages are primarily dependent on glycolysis for cellular metabolism [46]. Glycolysis also plays a critical role during macrophage inflammatory responses, leading to the generation of intracellular ATP [47–49]. In our most recent study, we demonstrate that following TLR ligation, macrophages release a fraction of glycolysis-generated ATP into the extracellular milieu via pannexin-1 channels [50]. eATP has been characterized previously as a potent danger signal (so-called alarmin), capable of eliciting proinflammatory responses in macrophages via P2X purinoceptor 7 receptors, leading to NLRP3, inflammasome activation [16, 51, 52]. However, these effects are dependent on the presence of high (millimolar) concentrations of eATP that likely derive from dying cells, releasing cellular stores of ATP as a consequence of decreased membrane integrity. We demonstrate that macrophages actively release low levels of ATP upon stimulation, and this previously unrecognized source of eATP can modulate the macrophage activation response.
We monitored the fate of macrophage-derived eATP and found that macrophages hydrolyze eATP rapidly by using ENTPDase1/CD39. Within hours of stimulation, macrophages completely convert released eATP into adenosine. We further show that low (submillimolar) levels of eATP drive a switch in macrophage polarization from an inflammatory to a regulatory activation status. In the time it takes to generate, release, and hydrolyze ATP, TLR-activated macrophages are simultaneously undergoing a dramatic transcriptional reprogramming, culminating in the development of a regulatory activation status (Fig. 2). CD39-mediated generation of adenosine from eATP was integral to this transition, as it is required to initiate the hydrolysis of eATP into eAMP, which then is dephosphorylated selectively by the ecto-adenosine monophosphatase, CD73, to yield adenosine. We observed that adenosine, derived from eATP, potently suppressed TNF-α and IL-12 synthesis, while simultaneously enhancing the expression IL-10, the hallmark of regulatory macrophage activation. Importantly, ATP-derived adenosine also up-regulated an array of immunomodulatory molecules, including HB-EGF, Sphk1, and IL-33 [45, 53, 54]. Interestingly, no up-regulation of Fizz1, Ym1, or mannose receptor was observed, indicating that ATP/adenosine selectively promotes the development of regulatory macrophages and is not representative of IL-4-induced AAMs. Arg1 was also up-regulated in these macrophages. Although, Arg1 has historically been thought of as a distinct AAM marker, it has been demonstrated recently that Arg1 can be induced via IL-4/STAT6-independent mechanisms, including via STAT3 [55] and GPCR/cAMP pathways [56, 57], thus indicating that Arg1 may not be a stable, reliable marker of AAMs and thus, may be expressed more broadly by macrophages. The biomarker signature of TLR-stimulated macrophages in the presence of low levels of ATP/adenosine is therefore consistent with the phenotype associated with regulatory macrophages. CD39, expressed on macrophages, was integral in this reprogramming process, and macrophages from mice lacking CD39 were unable to transition to a regulatory phenotype. Therefore, we believe that in contrast to the inflammatory effects elicited by high levels of ATP, macrophage-derived ATP may be critical in maintaining tissue homeostasis by autoregulating the activation status of macrophages.
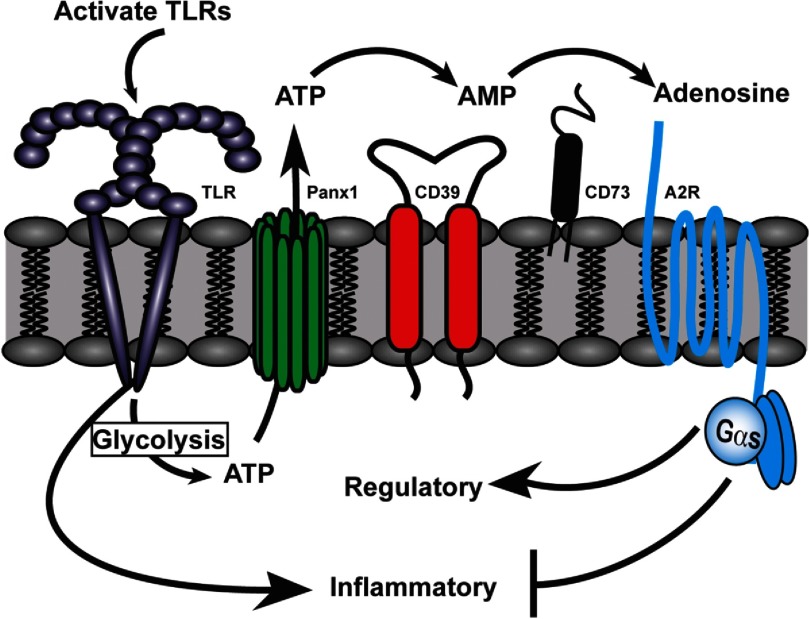
Figure 2. A model depicting intrinsic regulation of the macrophage activation response.
In response to TLR stimulation, macrophages initially induce the expression of proinflammatory mediators and also glycolysis-mediated ATP generation, some of which is released via pannexin-1 (Panx1) channels into the extracellular milieu. Over the next 4 h, macrophages use the ENTPDase1/CD39 to mediate the conversion of eATP to adenosine, which signals via A2bRs (A2R) on the macrophage to dampen inflammatory responses and promote regulatory macrophage development. Blockade of any step of this macrophage-intrinsic regulatory cascade renders macrophages hyperinflammatory.
To address the mechanism by which ATP-derived adenosine may promote regulatory macrophage activation, we investigated the expression of adenosine receptors on activated macrophages over time. Interestingly, we observed that TLR-activated macrophages selectively increase their expression of the A2bR. Thus, these data indicate that upon activation, macrophages not only release and hydrolyze self-derived ATP but also up-regulate A2bR to enhance their sensitivity to extracellular adenosine accumulating in their local extracellular environment. The inhibition of inflammatory cytokines also depended on signaling through the A2bR, and macrophages lacking A2bR exhibited substantial delays in this transition into a regulatory phenotype. Therefore, we reveal a novel, negative-feedback mechanism controlling macrophage responses that is dependent on the macrophage's intrinsic ability to convert eATP into immunosuppressive adenosine. Interestingly, the A2bR is a GPCR that associates with Gαs; therefore, we hypothesize that the generation of cAMP plays an important role in mediating this effect. Previous work demonstrated that regulatory-inducing stimuli can induce ERK activation in macrophages, which leads to phosphorylation of histone H3 at regulatory macrophage-associated loci. ERK-mediated chromatin remodeling was important for increased promoter access by transcription factors, resulting in enhanced transcription of macrophage-derived immunomodulatory mediators, including IL-10 and HB-EGF [58]. Indeed, we demonstrated previously that treating macrophages with cAMP could promote the development of regulatory macrophages via ERK activation [20] and therefore, hypothesize that adenosine signaling through the A2bR may similarly influence macrophage physiology.
Our data suggest that M1 activation is normally transient and strongly dependent on the continued presence of activating stimuli. In the presence of CD39 blockade, macrophages release higher levels TNF-α and IL-12, and this production is sustained over time, even after removal of the stimulus, suggesting that CD39-mediated regulation is critical in maintaining functional plasticity in macrophages. To demonstrate the biological relevance of these observations, we examined a mouse model of septic shock and demonstrated that the transfer of only 1 million CD39-deficient macrophages into WT mice, challenged with a sublethal dose of endotoxin, conferred lethality (<25% survival). This was in stark contrast to mice receiving WT (CD39+/+) macrophages, which largely survived (>75%) the challenge. Furthermore, macrophages isolated from mice receiving WT macrophages exhibited elevated levels of IL-10, Arg1, HB-EGF, and IL-33 and low levels of TNF-α and IL-12, whereas macrophages from mice that received CD39-deficient macrophages continued to exhibit an inflammatory phenotype. This observation supports the theory that the development of regulatory macrophages is associated with increased survival of the host.
Therefore, in contrast to prior reports characterizing eATP as a proinflammatory stimulus, we propose that eATP, derived from TLR-stimulated macrophages, promotes the development of regulatory macrophages. Based on our recent observations, we propose a new model of macrophage activation that places CD39 as the key molecular switch used by macrophages to toggle between inflammatory and regulatory states (Fig. 2). This mechanism suggests that macrophages themselves are capable of intrinsically controlling their own activation state during inflammation. We propose further that the development of macrophage-specific therapeutics, targeting the synthesis, release, and hydrolysis of ATP by macrophages, may greatly broaden the spectrum of treatment options for a variety of diseases, in which the switch from inflammatory to regulatory macrophages is important.
CONCLUDING REMARKS
Macrophages are closely associated with the progression and resolution of inflammation. However, the signals that direct macrophage functional plasticity are only being explored recently. We propose that early in response to exogenous stimuli, macrophages assume an inflammatory phenotype, and during the resolution phase, macrophages switch to an anti-inflammatory, regulatory activation state. In the clinic and in animal models of muscle injury, wound healing, and sepsis, the transition of macrophage activation status has been well-documented. These observations suggest that a switch in macrophage activation states may be critical to maintaining homeostasis. Whereas there may be examples where a new population of regulatory cells replaces M1, we provide direct evidence that macrophages themselves can transition from an inflammatory to a regulatory state. They do so by releasing and converting ATP to adenosine, while selectively up-regulating the A2bR to control their transition into a regulatory macrophage. Thus, we propose that macrophages can change their phenotype in response not only to exogenous signals generated by other cells in tissue, including apoptotic cells, PG derivatives, cAMP activators, or deactivating cytokines, but also, can regulate their own activation status by responding to endogenous signals that they generate themselves. All of these reprogramming signals cooperate with TLR stimulation to induce the production of the anti-inflammatory cytokine IL-10 and down-regulate many inflammatory mediators, while also inducing many other transcripts that are common to regulatory macrophages.
Footnotes
- A2bR
- adenosine 2b receptor
- AAM
- alternatively activated macrophage
- DAMP
- danger-associated molecular pattern
- eATP
- extracellular ATP
- ENTPDase1/CD39
- ecto-nucleoside triphosphate diphosphohydrolase
- ECM
- extracellular matrix
- Gαs
- adelylate cyclase stimulating G-protein alpha subunit
- HB-EGF
- heparin-binding-EGF
- M1
- inflammatory macrophage
- NLRP3
- nucleotide binding oligomerization-like receptor family, pyrin domain-containing 3
- SIRS
- systemic inflammatory response syndrome
- Sphk1
- sphingosine kinase 1
REFERENCES
- 1. Tidball J. G., Villalta S. A. (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. 298, R1173–R1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saclier M., Cuvellier S., Magnan M., Mounier R., Chazaud B. (2013) Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 10.1111/febs.12166 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3. Bosurgi L., Manfredi A. A., Rovere-Querini P. (2011) Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Front. Immunol. 2, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chazaud B., Sonnet C., Lafuste P., Bassez G., Rimaniol A. C., Poron F., Authier F. J., Dreyfus P. A., Gherardi R. K. (2003) Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 163, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cantini M., Massimino M. L., Bruson A., Catani C., Dalla Libera L., Carraro U. (1994) Macrophages regulate proliferation and differentiation of satellite cells. Biochem. Biophys. Res. Commun. 202, 1688–1696 [DOI] [PubMed] [Google Scholar]
- 6. Sonnet C., Lafuste P., Arnold L., Brigitte M., Poron F., Authier F. J., Chrétien F., Gherardi R. K., Chazaud B. (2006) Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J. Cell Sci. 119, 2497–2507 [DOI] [PubMed] [Google Scholar]
- 7. Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLennan I. S. (1996) Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J. Anat. 188, 17–28 [PMC free article] [PubMed] [Google Scholar]
- 9. Pimorady-Esfahani A., Grounds M. D., McMenamin P. G. (1997) Macrophages and dendritic cells in normal and regenerating murine skeletal muscle. Muscle Nerve 20, 158–166 [DOI] [PubMed] [Google Scholar]
- 10. Villalta S. A., Nguyen H. X., Deng B., Gotoh T., Tidball J. G. (2009) Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 18, 482–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abood E. A., Jones M. M. (1991) Macrophages in developing mammalian skeletal muscle: evidence for muscle fibre death as a normal developmental event. Acta Anat. (Basel) 140, 201–212 [DOI] [PubMed] [Google Scholar]
- 12. Summan M., Warren G. L., Mercer R. R., Chapman R., Hulderman T., Van Rooijen N., Simeonova P. P. (2006) Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am. J. Physiol. 290, R1488–R1495 [DOI] [PubMed] [Google Scholar]
- 13. Martinez C. O., McHale M. J., Wells J. T., Ochoa O., Michalek J. E., McManus L. M., Shireman P. K. (2010) Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R832–R842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu H., Huang D., Saederup N., Charo I. F., Ransohoff R. M., Zhou L. (2011) Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 25, 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buvinic S., Almarza G., Bustamante M., Casas M., López J., Riquelme M., Sáez J. C., Huidobro-Toro J. P., Jaimovich E. (2009) ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 284, 34490–34505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrari D., Chiozzi P., Falzoni S., Dal Susino M., Melchiorri L., Baricordi O. R., Di Virgilio F. (1997) Extracellular ATP triggers IL-1 β release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 159, 1451–1458 [PubMed] [Google Scholar]
- 17. Schiraldi M., Raucci A., Muñoz L. M., Livoti E., Celona B., Venereau E., Apuzzo T., De Marchis F., Pedotti M., Bachi A., Thelen M., Varani L., Mellado M., Proudfoot A., Bianchi M. E., Uguccioni M. (2012) HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 209, 551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samuvel D. J., Sundararaj K. P., Nareika A., Lopes-Virella M. F., Huang Y. (2009) Lactate boosts TLR4 signaling and NF-κB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J. Immunol. 182, 2476–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edwards J. P., Zhang X., Mosser D. M. (2009) The expression of heparin-binding epidermal growth factor-like growth factor by regulatory macrophages. J. Immunol. 182, 1929–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strassmann G., Patil-Koota V., Finkelman F., Fong M., Kambayashi T. (1994) Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 180, 2365–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasko G., Szabo C., Nemeth Z. H., Kvetan V., Pastores S. M., Vizi E. S. (1996) Adenosine receptor agonists differentially regulate IL-10, TNF-α, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 157, 4634–4640 [PubMed] [Google Scholar]
- 23. Hong S., Gronert K., Devchand P. R., Moussignac R. L., Serhan C. N. (2003) Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 278, 14677–14687 [DOI] [PubMed] [Google Scholar]
- 24. Titos E., Rius B., Gonzalez-Periz A., Lopez-Vicario C., Moran-Salvador E., Martínez-Clemente M., Arroyo V., Clària J. (2011) Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 187, 5408–5418 [DOI] [PubMed] [Google Scholar]
- 25. Liu D., Sartor M. A., Nader G. A., Gutmann L., Treutelaar M. K., Pistilli E. E., Iglayreger H. B., Burant C. F., Hoffman E. P., Gordon P. M. (2010) Skeletal muscle gene expression in response to resistance exercise: sex specific regulation. BMC Genomics 11, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng B., Wehling-Henricks M., Villalta S. A., Wang Y., Tidball J. G. (2012) IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 189, 3669–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perdiguero E., Sousa-Victor P., Ruiz-Bonilla V., Jardi M., Caelles C., Serrano A. L., Muñoz-Cánoves P. (2011) p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J. Cell Biol. 195, 307–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paylor B., Natarajan A., Zhang R. H., Rossi F. (2011) Nonmyogenic cells in skeletal muscle regeneration. Curr. Top. Dev. Biol. 96, 139–165 [DOI] [PubMed] [Google Scholar]
- 29. Joe A. W., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M. A., Rossi F. M. (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang L., Ran L., Garcia G. E., Wang X. H., Han S., Du J., Mitch W. E. (2009) Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am. J. Pathol. 175, 2518–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wehling-Henricks M., Jordan M. C., Gotoh T., Grody W. W., Roos K. P., Tidball J. G. (2010) Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One 5, e10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Troidl C., Möllmann H., Nef H., Masseli F., Voss S., Szardien S., Willmer M., Rolf A., Rixe J., Troidl K., Kostin S., Hamm C., Elsässer A. (2009) Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J. Cell. Mol. Med. 13, 3485–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goren I., Allmann N., Yogev N., Schurmann C., Linke A., Holdener M., Waisman A., Pfeilschifter J., Frank S. (2009) A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am. J. Pathol. 175, 132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Freytes D. O., Kang J. W., Marcos-Campos I., Vunjak-Novakovic G. (2013) Macrophages modulate the viability and growth of human mesenchymal stem cells. J. Cell. Biochem. 114, 220–229 [DOI] [PubMed] [Google Scholar]
- 35. Gouon-Evans V., Rothenberg M. E., Pollard J. W. (2000) Postnatal mammary gland development requires macrophages and eosinophils. Development 127, 2269–2282 [DOI] [PubMed] [Google Scholar]
- 36. Stein M., Keshav S., Harris N., Gordon S. (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gordon S., Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 38. Barron L., Wynn T. A. (2011) Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur. J. Immunol. 41, 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wynn T. A., Barron L. (2010) Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30, 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stearns-Kurosawa D. J., Osuchowski M. F., Valentine C., Kurosawa S., Remick D. G. (2011) The pathogenesis of sepsis. Annu. Rev. Pathol. 6, 19–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Biswas S. K., Chittezhath M., Shalova I. N., Lim J. Y. (2012) Macrophage polarization and plasticity in health and disease. Immunol. Res. 53, 11–24 [DOI] [PubMed] [Google Scholar]
- 42. Adib-Conquy M., Adrie C., Moine P., Asehnoune K., Fitting C., Pinsky M. R., Dhainaut J. F., Cavaillon J. M. (2000) NF-κB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am. J. Respir. Crit. Care Med. 162, 1877–1883 [DOI] [PubMed] [Google Scholar]
- 43. Biswas S. K., Lopez-Collazo E. (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 [DOI] [PubMed] [Google Scholar]
- 44. Freudenberg M. A., Galanos C. (1988) Induction of tolerance to lipopolysaccharide (LPS)-d-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect. Immun. 56, 1352–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kempner W. (1939) The nature of leukemic blood cells as determined by their metabolism. J. Clin. Invest. 18, 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kellett D. N. (1966) 2-Deoxyglucose and inflammation. J. Pharm. Pharmacol. 18, 199–200 [DOI] [PubMed] [Google Scholar]
- 48. Cramer T., Yamanishi Y., Clausen B. E., Förster I., Pawlinski R., Mackman N., Haase V. H., Jaenisch R., Corr M., Nizet V., Firestein G. S., Gerber H. P., Ferrara N., Johnson R. S. (2003) HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rodríguez-Prados J. C., Través P. G., Cuenca J., Rico D., Aragonés J., Martín-Sanz P., Cascante M., Boscá L. (2010) Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 185, 605–614 [DOI] [PubMed] [Google Scholar]
- 50. Cohen H. B., Briggs K. T., Marino J. P., Ravid K., Robson S. C., Mosser D. M. (2013) TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 10.1182/blood-2013-04-496216 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pelegrin P., Surprenant A. (2006) Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 53. Edwards J. P., Zhang X., Frauwirth K. A., Mosser D. M. (2006) Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 80, 1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang H. R., Milovanović M., Allan D., Niedbala W., Besnard A. G., Fukada S. Y., Alves-Filho J. C., Togbe D., Goodyear C. S., Linington C., Xu D., Lukic M. L., Liew F. Y. (2012) IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur. J. Immunol. 42, 1804–1814 [DOI] [PubMed] [Google Scholar]
- 55. Qualls J. E., Neale G., Smith A. M., Koo M. S., DeFreitas A. A., Zhang H., Kaplan G., Watowich S. S., Murray P. J. (2010) Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci. Signal. 3, ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chang C. I., Zoghi B., Liao J. C., Kuo L. (2000) The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. J. Immunol. 165, 2134–2141 [DOI] [PubMed] [Google Scholar]
- 57. Ben Addi A., Lefort A., Hua X., Libert F., Communi D., Ledent C., Macours P., Tilley S. L., Boeynaems J. M., Robaye B. (2008) Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A(2B) receptor. Eur. J. Immunol. 38, 1610–1620 [DOI] [PubMed] [Google Scholar]
- 58. Lucas M., Zhang X., Prasanna V., Mosser D. M. (2005) ERK activation following macrophage FcγR ligation leads to chromatin modifications at the IL-10 locus. J. Immunol. 175, 469–477 [DOI] [PubMed] [Google Scholar]