TSP-1-positive DCs promote the Th-17 immune response in the eye, after exposure to desiccating surface stress.
Keywords: TGF-β, IL-17A, IFN-γ, dry eye, conjunctiva, corneal permeability, goblet cells
Abstract
TSP-1 is a physiologic activator of TGF-β, a critical induction factor for Th17-mediated immunity. The purpose of this study was to investigate the role of TSP-1 in the induction of the Th17 ocular surface response to DS. TSP-1KO and WT mice were subjected to DS5 and DS10), and parameters of ocular surface disease, including corneal barrier function, conjunctival CD4+ T cell infiltration, and GC density, were evaluated. TSP-1KO mice subjected to DS had less corneal barrier disruption, reduced loss of PAS+ GC, and decreased CD4+ T cell infiltration in the conjunctiva. In contrast to WT, TSP-1KO mice failed to up-regulate MMP-3 and MMP-9 mRNA transcripts in the cornea and IL-17A mRNA transcripts in the conjunctiva. RAG-1KO recipients of adoptively transferred CD4+ T cells isolated from TSP-1KO mice subjected to DS5 showed milder dry-eye phenotype and less conjunctival inflammation than recipients of CD4+ T cells from DS5 WT control. Reconstitution of TSP-1KO mice with WT DCs prior to DS reversed the resistance of the TSP-1KO to DS-induced immunopathology. In conclusion, DC-derived TSP-1 is critical for generating the Th17 ocular surface response to DS.
Introduction
Dry eye is one of the most common ophthalmic diseases, afflicting tens of millions of patients. Dry eye causes irritation symptoms, blurred vision, and corneal barrier disruption that can lead to sight-threatening corneal ulceration [1]. Dry eye, regardless of cause, is often associated with an autoimmune reaction on the ocular surface, particularly in Sjögren's syndrome, the most severe dry-eye condition [2]. IL-17A, produced by Th17 cells, has been found to contribute to the disruption of the epithelial barrier function that results in an irregular corneal surface with reduced optical quality.
The pleiotropic cytokine TGF-β can stimulate the immune system and induce immune responses. Specifically, TGF-β1, in conjunction with IL-6 (released by activated DCs), encourages the development of Th17 cells from Th0 cells [3].
IL-17A, a product of Th17 cells, has been associated with a variety of autoimmune conditions, including autoimmune encephalomyelitis, collagen-induced arthritis, acute and chronic colitis, systemic lupus erythematosus [4], and autoimmune uveitis [5]. IL-17A has been found to stimulate production and release of various chemokines (IL-8, CXCL1, CXCL6, GM-CSF) by a variety of cells, including epithelial cells, endothelial cells, fibroblasts, synoviocytes, and myeloid cells [6]. Additionally, IL-17A has been noted to increase expression of metalloproteinases (e.g., MMP-3 and MMP-9), IL-6, TNF-α, and IL-1-β in macrophages [5, 7].
We have previously found a significant increase in IL-17A levels in the corneal epithelium and conjunctiva, in addition to the up-regulation of Th17 promoters (IL-6, IL-23, IL-23R, and TGF-β) on the ocular surface, in response to experimental DS [8]. They collectively lead to corneal epithelial barrier dysfunction that is detected by increased corneal permeability to 70 kDa OGD 488. Antibody neutralization of IL-17 prevented corneal barrier disruption in the presence of DS, indicating that IL-17 has a prominent role in desiccation-induced corneal epithelial barrier disruption [8, 9].
TSP-1 is an ECM protein that activates TGF-β1. When covalently bound to TGF-β1, the LAP blocks its active site and renders the molecule inactive. This immature form of TGF-β1 naturally equilibrates with its active state via detachment from LAP. TSP-1 stabilizes the covalent binding sites on the disassociated LAP, thereby preventing its interaction and subsequent inhibition of TGF-β1 [10]. As a result, increased levels of TSP-1 in the presence of TGF-β1 are linked to greater levels of active TGF-β1 [10, 11].
The purpose of this study was to investigate whether the loss of TSP-1 interferes with the induction of the Th17 ocular surface response to DS in the TSP-1KO mouse.
MATERIALS AND METHODS
Mice
C57BL6/J, TSP-1KO (B6.129S2-Thbs1tm1Hym/J), and RAG-1KO (B6.129S7-Rag-1tm1Mom/J) mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All mice had C57BL6/J background. Breeding colonies were set up in our vivarium. C57BL6/J mice were used as WT control. Mice of both genders were used at 12 weeks.
Murine DS model
DS was induced by s.c. injection of scopolamine hydrobromide (0.5 mg/0.2 ml; Sigma-Aldrich, St. Louis, MO, USA), quarter in die (four times/day; 08:00, 12:00, 14:00, and 17:00 h), for DS5 or DS10 in 12-week-old TSP-1KO and WT mice, as published previously [2, 8, 12]. Mice were placed in a cage with a perforated plastic screen on one side to permit air flow from a fan placed 6 inches away for 16 h/day. Room humidity was maintained at 30–35%. Control mice were maintained in a NS environment containing 50–75% relative humidity without exposure to an air draft.
Local neutralization of TGF-β
To evaluate the role of TGF-β during DS, a separate group of WT mice received subconjunctival injections of anti-TGF-β antibody (25 μg/eye/injection, clone 1D11; R&D Systems, Minneapolis, MN, USA) or IC (25 μg/eye/injection; R&D Systems) at Days 0 and 2 during DS5. Mice were used for histology (n=4) and gene expression analysis (n=6).
Quantification of TGF-β activity
Tear fluid washings were collected from NS C57BL/6 and TSP-1KO mice using a method reported previously [13]. One sample consisted of tear washings from both eyes of one mouse pooled (2 μl) in PBS + 0.1% BSA (8 μl). TGF-β activity in tears (n=5/strain) was measured in three independent sets of experiments using a TGF-β reporter assay, as reported previously [14, 15]. Briefly, mink lung epithelial cells transfected with a plasminogen activator inhibitor-luciferase construct (obtained from Dr. Brendan Lee, Baylor College of Medicine, Houston, TX, USA) were maintained in media (DMEM, supplemented with 10% FBS, 0.1% BSA, 50 μg/ml gentamicin, and 1.25 μg/ml amphotericin B). Cells were serum-starved for 24 h in serum-free media (DMEM, supplemented with 0.1% BSA, 50 μg/ml gentamicin, and 1.25 μg/ml amphotericin B) and then incubated at 37°C with samples for 16 h. Cells were then processed as instructed by the Luciferase Assay System Kit (Promega, Madison, WI, USA), and samples were analyzed in an Infinite 200 PRO (Tecan Systems, San Jose, CA, USA).
Histology and PAS staining
GC density was evaluated in NS WT and TSP-1KO, and both strains after DS5 and DS10 (n=5/mouse strain/time-point). Eyes and ocular adnexa were excised surgically, fixed in 10% formalin, paraffin embedded, and cut into 8-μm sections. Ocular sections were cut at the center of the eye, where the lens has its maximum diameter. GCs in sections were stained with PAS reagent and were examined and photographed with a microscope equipped with a digital camera (Eclipse E400 with a DS-Fi1; Nikon, Melville, NY, USA). The number of GCs in the superior and inferior conjunctiva was measured in three distinct sections, 300 μm apart from each other from each eye, using image-analysis software (NIS Elements software, version 3.0, BR; Nikon) and expressed as number of GCs/mm.
For immunohistochemistry, the eyes and adnexa of mice at each time-point (n=5) were excised, embedded in optimal cutting temperature compound (VWR, Suwanee, GA, USA), and flash-frozen in liquid nitrogen. Sagittal, 8-μm sections were cut with a cryostat (HM 500; Micron, Waldorf, Germany), placed on glass slides, and stored at −80°C.
Immunohistochemistry
The following primary antibodies were used to detect immune cells in conjunctival frozen sections: CD4 (clone H129.9, 10 μg/mL; BD Biosciences, San Jose, CA, USA) and TSP-1 (ab85762, 10 μg/mL; Abcam, San Francisco, CA, USA). Cryosections were stained with this primary antibody and appropriate biotinylated secondary antibodies (BD Biosciences) and Vectastain Elite ABC using NovaRed reagents (Vector Laboratories, Burlingame, CA, USA), as described previously [16]. Secondary antibody alone and appropriate isotype (BD Biosciences) controls were also performed. Coverslips were applied after alcohol dehydration without counterstaining. The number of cells staining positively for each antigen was counted in tissue sections. Two sections (cut at least 200 μm apart), from five different animals of each strain and at each time-point, were evaluated. Positively stained cells were counted in the GC-rich areas of the conjunctival epithelium (across a length of at least 700 μm) and stroma (across a length of at least 500 μm and to a depth of 75 μm below the epithelial basement membrane), using image-analysis software (NIS Elements software, version 3.0, BR; Nikon). Values are expressed as number of positive cells/100 μm.
Confocal LSM
Whole, freshly harvested murine corneas and conjunctivas from WT control C57BL/6 mice (n=3) were used for confocal LSM. Whole conjunctiva and corneas were excised surgically, processed, and dual-stained with hamster anti-CD11c (clone HL-3, 12.5 μg/ml; BD Biosciences) or rat anti-CD11b (clone M1/70, 6.25 μg/ml; BD Biosciences) and rabbit anti-TSP-1 (ab85762, 10 μg/mL; Abcam, San Francisco, CA, USA) primary antibodies and secondary Alexa Fluor 488-conjugated goat anti-hamster (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) or Alexa Fluor 488-conjugated goat anti-rat and Alexa Fluor 594-conjugated goat anti-rabbit antibodies without counterstaining. Whole conjunctivas were flattened on a microscope slide, covered with anti-fade Gel/Mount (Fisher, Atlanta, GA, USA), and overlaid with coverslips. Whole-mount digital images (512×512 pixels) were captured with a confocal LSM (LSM 510, with krypton-argon and helium-neon lasers; Zeiss, Thornwood, NY, USA) with 488-nm excitation and 543-nm emission filters, LP505 and LP560, respectively. They were acquired with a 40/1.3× oil-immersion objective, as described previously [16]. Images were processed using Zeiss LSM-PC software and Adobe Photoshop 7.0 (Adobe, San Jose, CA, USA).
Corneal permeability
Corneal epithelial permeability to OGD 488 (70,000 MW; Invitrogen, Eugene, OR, USA) was assessed, as described previously [8], with minor modification. Briefly, 0.5 μl 50 mg/mL OGD was instilled onto the ocular surface, 1 min before euthanasia. Corneas (n=30/mouse strain/time-point/experiment) were rinsed with PBS and photographed with a high-resolution digital camera (CoolSNAP HQ2; Photometrics, Tucson, AZ, USA) attached to a stereoscopic zoom microscope (SMZ 1500; Nikon) under fluorescence excitation at 470 nm. The severity of corneal OGD staining was graded in digital images using NIS-Elements (version 3.0; Nikon) within a 2-mm diameter circle placed on the central cornea by two masked observers. The mean fluorescence intensity, measured by the software inside this central zone, was transferred to a database, and the results were averaged within each group. Results are presented as mean ± sd of gray levels.
Isolation of murine CD4+ T cells and adoptive transfer
Superior CLNs and spleens from WT and TSP-1KO mice donor mice were surgically removed and meshed gently between the frosted ends of two glass slides, as described previously [2]. Untouched CD4+ cells were isolated using magnetic beads, according to the manufacturer's instructions (MACS system; Miltenyi Biotec, Auburn, CA, USA). One donor equivalent of cells was transferred i.p. to T cell-deficient mice (RAG-1KO). One donor equivalent is defined as the number of cells remaining after the respective in vitro manipulation (e.g., CD4+ T cells) of a single set of LNs or spleen (∼5×106 CD4+ cells). The adoptive transfer recipients were killed 72 h after the initial adoptive transfer. In some experiments, eyes were collected for histology, whereas in others, cornea and conjunctiva were processed for RNA analysis.
RNA isolation and real-time PCR
Total RNA collected was extracted from the conjunctiva using an Arcturus PicoPure RNA Isolation Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions, quantified by a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and stored at −80°C. Eight samples/group/strain/experiment were used, and one sample consisted of pooled eyes of the same animal. Samples were treated with DNAse to prevent genomic DNA contamination, according to the manufacturer's instructions (Qiagen, Valencia, CA, USA). The RNA concentration was measured by its absorption at 260 nm, and samples were stored at −80°C until use.
First-strand cDNA was synthesized with random hexamers by MMLV reverse transcription (Ready-To-Go You-Prime First-Strand Beads; GE Healthcare, Arlington Heights, NJ, USA), as described previously [8]. Real-time PCR was performed with specific MGB probes (Taqman; Applied Biosystems) and PCR master mix (Taqman Gene Expression Master Mix) in a commercial thermocycling system (Mx3005P qPCR system; Stratagene, La Jolla, CA, USA), according to the manufacturer's recommendations. Murine MGB probes were: HPRT1 (Mm00446968), IL-17A (Mm00439618), IL-6 (Mm00446490), IL-13 (Mm99999190), IFN-γ (Mm00801778), MMP-3 (Mm00440295), and MMP-9 (Mm00442991) (Taqman; Applied Biosystems). The results of qPCR were analyzed by the CT method, where target change = 2−ΔΔCT. The results were normalized by the CT value of HPRT1. In the DS model, the mean CT of relative mRNA level in the NS control group of each strain was used as the calibrator. The IL-13:IFN-γ ratio was calculated using the mean fold expression of both genes.
Generation of murine bone marrow-derived DCs
Bone marrow-derived DCs were generated as described previously [17, 18] with minor modifications. Briefly, WT and TSP-1KO mice (n=2/strain) were killed, femurs and tibiae were removed, and the marrow was flushed with RPMI 1640 using a syringe with a 0.3-mm needle. Clusters within the marrow suspension were disassociated by vigorous pipetting. Bone marrow cells were suspended in CM, RPMI 1640, supplemented with 10% FBS, 50 μg/ml gentamicin, and 1.25 μg/ml amphotericin B. Cells were adjusted to 1.0 × 106/ml and plated on a dish at 10 ml/well. They were cultured for up to 9 days in the presence of 15 ng/ml rmGM-CSF and 5 ng/ml rmIL-4 (both from PeproTech, Rocky Hill, NJ, USA) at 37°C and 5% CO2. On Day 3 of culture, 10 ml CM with the same amount of rmGM-CSF and rmIL-4 was added to the cells. On Days 6 and 8 of culture, 10 ml supernatant was collected and centrifuged at 300 g for 10 min at room temperature; the cells were suspended in 10 ml CM with the same amount of rmGM-CSF and rmIL-4 and then plated. On Day 9, the nonadherent and loosely adherent DCs were used for experiments. An aliquot of cultured cells prior to adoptive transfer was analyzed by flow cytometry using CD11c-FITC- and CD11b-APC-conjugated antibodies, as described previously [19].
Adoptive transfer of DCs
One million (1×106) DCs were injected i.p. into mice, and they were allowed to rest for 72 h. There were three different groups of NS and DS5 mice: (1) WT mice that received WT DCs, (2) TSP-1KO mice that received WT DCs, and (3) TSP-1KO mice that received TSP-1KO-DCs. There was a total of 20 mice/group/time-point. Mice were used for corneal permeability evaluation (n=15) or histology (n=5).
Statistical analysis
The normality of data was checked with the Kolmogorov-Smirnov test using the Dallal and Wilkinson approximation. Two-tailed t-test was used to evaluate individual differences before and after DS and to compare WT with TSP-1KO, using P ≤ 0.05 as statistically significant. These tests were performed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA).
RESULTS
TSP-1 expression in the ocular surface
We investigated the presence and localization of TSP-1+ cells on the ocular surface by immunohistochemistry and flow cytometry. With the use of conjunctival sections obtained from WT mice, we observed strong immunoreactivity to cells with a dendritic appearance localized in the basal conjunctival epithelium at the GC-rich area and some round cells in the adjacent stroma (Fig. 1A). We also observed that the conjunctival epithelium had weak immunoreactivity to TSP-1. As we observed TSP-1+ cells with a dendritic appearance, we performed dual staining for CD11c or CD11b with TSP-1 in whole-mount conjunctiva. Dual CD11c+TSP-1+ cells with a dendritic morphology (Fig. 1B and C) were visualized easily in the conjunctival epithelium and were often in close proximity to the GC openings (asterisk in Fig. 1B and C). In contrast, CD11b+TSP-1+ cells were rounder, with very few dendrites and were located immediately below the basal epithelium in the conjunctival stroma (Fig. 1B and C).
Figure 1. TSP-1KO mice are resistant to experimental dry eye.
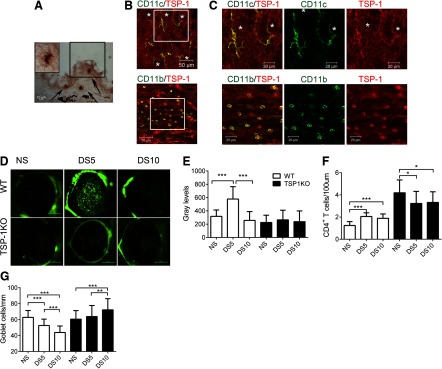
(A) Representative Immunohistochemistry of conjunctival frozen section obtained from a WT mouse showing positive staining for TSP-1 (in red, black arrows) with no counterstaining. Note strong immunoreactivity in cells with dendritic shape and weak immunoreactivity of the conjunctival epithelium. (Inset) High magnification of dendritic-like TSP-1+ cell in black square. (B) Representative merged images of immunofluorescent confocal LSM of whole-mount conjunctiva from NS WT mice dual-labeled for CD11c (green) and TSP-1 (red). Colocalization of markers yields a yellow color. CD11c/TSP-1 dual-positive cells are visualized easily and are concentrated around the GC openings (asterisks). CD11b/TSP-1 dual-positive cells were more frequently found below the basal membrane of the conjunctival epithelium. Original magnification, 40×; original scale bars, 50 μm. (C) High magnification of merged white boxes in B showing dual-positive cells for CD11c and CD11b. Asterisks indicate GC openings. Colocalization of markers yields yellow color. TSP-1+ cells are red, whereas CD11b or CD11c are green. Original magnification, 80×; original scale bars, 20 μm. (D) Representative images of corneas stained with 70 kDa OGD that was used to generate the OGD intensity scores in F in WT and TSP-1KO mice under NS conditions and after DS5 or DS10. (E) Bar graphs showing mean ± sd of OGD intensity. The results represent a mean of three independent experiments that used five mice for each group/time-point/experiment (final n=15/group/time-point). (F) CD4+ T cell infiltration in the conjunctival epithelium in the GC-rich area. Bar graphs show mean ± sd of two independent experiments (final n=5 group/time-point). (G) PAS+ conjunctival GC counts. Bar graphs show mean ± sd of two independent experiments (final n=5 for each group). *P < 0.05; **P < 0.01; ***P < 0.001.
Taken together, these findings show that resident CD11c- and CD11b-positive cells in the normal conjunctiva are TSP-1+ and probably involved in local TGF-β activation.
Reduced TGF-β activity on the ocular surface in TSP-1KO mice
The lacrimal gland produces and secretes TGF-β, and high levels of TGF-β (both total and activated) have been reported in tears of desiccated mice and dry-eye human subjects [20–22]. We investigated the levels of active TGF-β in tears of NS WT and TSP-1KO mice using a reporter assay, as described previously [14]. We measured TGF-β activity in tear samples obtained from both strains. We observed a marked reduction in active TGF-β levels in tears from TSP-1KO (2.12±1.53 ng/mL) compared with WT (3.97±2.58 ng/mL; P<0.05). Similarly, other studies have shown a parallel reduction in TGF-β activity in the retinal pigment epithelial cells [23] and iris pigment epithelial cells [24] of TSP-1-deficient mice. Furthermore, earlier investigations have illustrated the systemic dependence on TSP-1 for TGF-β activity [10]. These studies confirm that TSP-1 has a physiological role in TGF-β activation on the ocular surface.
TSP-1KO mice are resistant to desiccation-induced ocular surface inflammation
To investigate further the contribution of TSP-1 in Th17-mediated ocular surface immune response, we subjected 12-week-old WT and TSP-1KO mice to experimental DS5 and DS10. NS mice served as controls.
We used fluorescent, 70 kDa OGD 488 dye to evaluate corneal epithelial barrier function, as increased corneal permeability to fluorescent dyes is a hallmark of dry-eye disease. Unlike the WT, TSP-1KO mice had no change in OGD uptake from baseline after DS5, a time-point where corneal barrier disruption is maximally observed in this model (Fig. 1D and E).
To examine the extent of ocular surface inflammation secondary to desiccation, we counted CD4+ T cells in conjunctival frozen sections obtained from WT and TSP-1KO mice at NS, DS5, and DS10. The number of CD4+ T cells increased significantly in the conjunctival epithelium of WT mice with desiccation, whereas TSP-1KO showed a paradoxical decrease in the number of CD4+ T cells in response to DS (Fig. 1F). Loss of mucin-filled conjunctival GCs is observed in this murine model of DS. Therefore, we measured PAS+ cells in paraffin-embedded conjunctival sections. WT mice exhibited a significant, progressive decrease in PAS+-filled GCs during DS. In contrast, a significant, progressive increase in the number of PAS+-filled GCs was noted in TSP-1KO (Fig. 1G).
TSP-1KO fails to up-regulate inflammatory cytokines and MMPs in response to DS
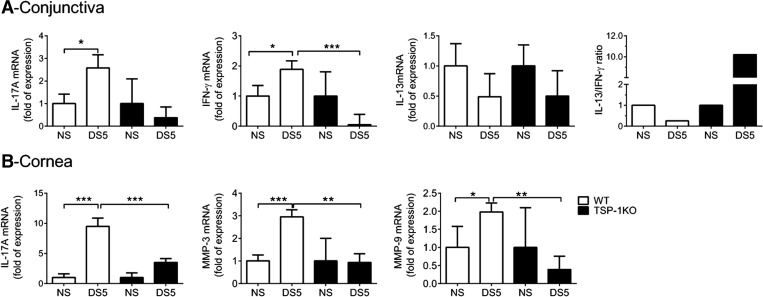
CD4+ T cells from TSP-1KO mice have been reported to proliferate but not produce IL-17A in an EAE model [25]. Consequently, we evaluated expression of IL-17A and MMP-3 and MMP-9, which are stimulated by IL-17A and are involved in proteolysis of corneal epithelial tight-junction proteins [8, 26]. Unlike WT mice that had a threefold increase in IL-17A expression in the conjunctiva after DS5 (Fig. 2A), levels of IL-17A transcripts in the conjunctiva did not change with DS in TSP-1KO. A similar pattern was observed for IFN-γ transcripts. The IL-13/IFN-γ ratio decreased in WT mice, whereas it increased in TSP-1KO mice. Furthermore, levels of MMP-3, MMP-9, and IL-17A mRNA transcripts in the cornea epithelia failed to increase in TSP-1KO mice after DS (Fig. 2B).
Figure 2. TSP-1KO mice failed to up-regulate IL-17A and MMPs after DS.
Relative fold of expression of IL-17A, IFN-γ, IL-13, and IL-13:IFN-γ ratio in the conjunctiva (A) and IL-17A, MMP-3, and MMP-9 in the corneal epithelium, using the NS of each strain as the strain calibrator. Bar graphs show mean ± sd of three independent experiments (final n=9/group/time-point). *P < 0.05; **P < 0.01; ***P < 0.001.
Local neutralization of TGF-β during DS mimics TSP-1KO phenotype
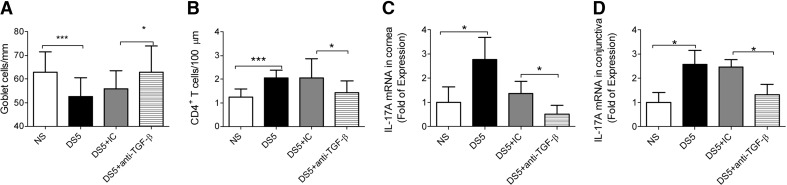
It has been shown that in an experimental model of arthritis in rats and EAE, local antibody neutralization of TGF-β blocked acute inflammation [27, 28] and prevented generation of Th17 cells [29]. To investigate if local neutralization of TGF-β mimicked the response to DS seen in TSP-1KO, we administered subconjunctival injections of TGF-β antibody or IC to mice subjected to DS5 [30]. Neutralization of TGF-β during DS increased significantly the number of filled GCs (Fig. 3A), decreased the number of infiltrating CD4+ T cells to the conjunctiva (Fig. 3B), and decreased expression of IL-17A in cornea and conjunctiva (Fig. 3C and D). Taken together, these results imply that active TGF-β is critical for the initiation of the immunopathologic response to DS, including the production of IL-17A. This phenomenon suggests that the inverse response to DS observed in the TSP-1KO stems from reduced TGF-β activity.
Figure 3. Results of TGF-β neutralization in the WT mice during DS.
A separate group of mice received subconjunctival injections of TGF-β (DS5+anti-TGF-β) or IC antibody during DS5. (A) PAS+ conjunctival GC counts. Bar graphs show mean ± sd of two independent experiments (final n=4 for each group). (B) CD4+ T cell infiltration in the conjunctival epithelium in the GC-rich area. Bar graphs show mean ± sd of two independent experiments (final n=4 group/time-point). (C and D) Relative fold of expression of IL-17A (C) in the corneal epithelium and IL-17A (D) in the conjunctival using the NS groups as the calibrator. Bar graphs show mean ± sd of three independent experiments (final n=6/group/time-point). *P < 0.05; ***P < 0.001.
TSP-1 is essential for the generation of pathogenic CD4+ T cells
CD4+ T cells from TSP-1KO mice have been reported to be less pathogenic in an adoptive-transfer model of EAE [25]. To determine if the generation of pathogenic CD4+ T cells in response to DS was abrogated in the TSP-1KO, we isolated CD4+ T cells from the superior CLNs and spleen of NS and DS5 WT and TSP-1KO mice and adoptively transferred them into immunodeficient RAG-1KO mice.
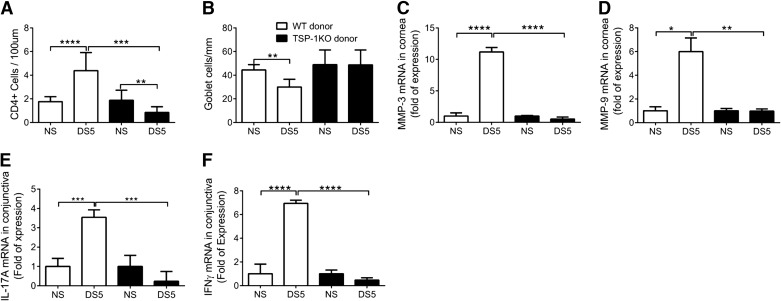
The results of these experiments are summarized in Fig. 4. RAG-1KO-recipient mice receiving WT-DS5 CD4+ T cells showed significantly greater CD4+ T cell infiltration in the conjunctiva and loss of PAS+-filled GCs in the same area than recipients of CD4+ T cells from NS donors. In contrast, there were significantly fewer infiltrating cells in the DS TSP-1KO group with no change in GC density (Fig. 4A and B).
Figure 4. Results of adoptive transfer of CD4+ T cells into RAG-1KO.
RAG-1KO mice received CD4+ T cells isolated from WT or TSP-1KO prior to (NS) or after DS5. (A) CD4+ T cell infiltration of the conjunctival epithelium in the GC-rich area. Bar graphs show mean ± sd of two independent experiments (final n=5 for each group). (B) PAS+ conjunctival GC density. Bar graphs show mean ± sd of two independent experiments (final n=5 for each group). (C–F) Relative fold of expression of MMP-3 (C) and MMP-9 (D) in the corneal epithelium and IL-17A (E) and IFN-γ (F) in the conjunctival epithelium of RAG-1KO recipients using the NS recipient of each strain as the strain calibrator. Bar graphs show mean ± sd of three independent experiments (final n=9/group/time-point). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
PCR analyses of mRNA isolated from the cornea and conjunctiva showed that unlike the RAG-1KO recipients of WT-DS5 donor cells, RAG-1KO recipients of CD4+ T cells from TSP-1KO-DS5 failed to increase levels of MMP-3 and MMP-9 mRNA transcripts in the cornea or IL-17A and IFN-γ transcripts in the conjunctiva (Fig. 4C–F).
DC-associated TSP-1 is critical to the inflammatory response on the ocular surface
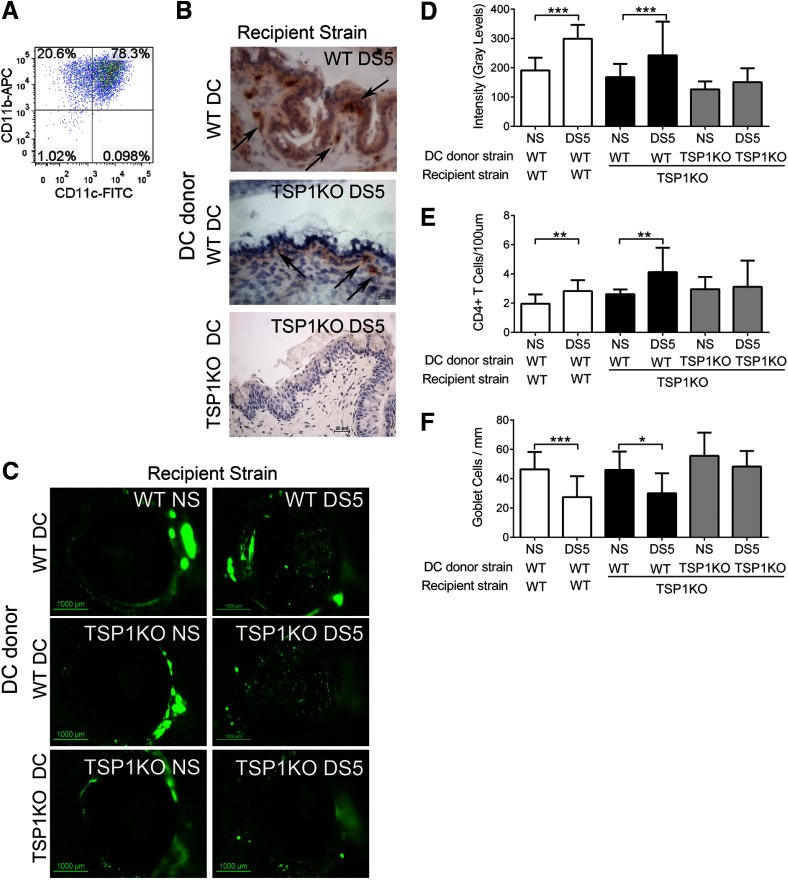
The experimental dry-eye model that we use is very unique in that CD4+ T cells are primed in vivo, without the use of adjuvants. The putative antigen(s) remains to be elucidated. As we observed the expression of TSP-1 in DCs and resistance to dry eye-induced phenotype, in the TSP-1KO donor mice and in the RAG-1KO recipients of TSP-1KO CD4+ T cells, we hypothesize that the Th17 response is dependent on TGF-β1 activation through DC-derived TSP-1. To test this hypothesis, we isolated and expanded bone marrow-derived DCs from WT and TSP-1KO mice in vitro using an established protocol [17, 18] and performed adoptive transfer of DCs from either strain into TSP-1KO mice. At the day of transfer, cells were phenotypically analyzed by flow cytometry and noted to be ∼80% CD11c+ CD11b+ (Fig. 5A). WT mice that received WT DCs served as controls.
Figure 5. Results of DC-adoptive transfer.
Adoptive transfer of WT or TSP-1KO DCs into WT or TSP-1KO mice. Bone marrow-derived DCs from WT or TSP-1KO mice were adoptively transferred into WT and TSP-1KO mice that were allowed to rest for 72 h. Dry -eye phenotype was evaluated before (NS) and after DS5. (A) Representative dot plot of unattached, cultured bone marrow-derived cells at Day 9, double-stained for CD11c and CD11b. Numbers in the quadrants indicate the percentage of positive cells. (B) Representative immunohistochemistry of conjunctival frozen sections obtained from DC recipient mice showing staining of TSP-1-positive cells (in red, black arrows). (C) Representative images of OGD corneal staining that were used to an generate OGD intensity score in WT and TSP-1KO after DC adoptive transfer. (D) Bar graphs show mean ± sd of three independent experiments using 15 mice for each group/time-point/experiment. Note that reconstitution of TSP-1KO with WT DC impairs TSP-1KO resistance to desiccation, whereas transfer of TSP-1KO-DC does not. (E) CD4+ T cell infiltration of the conjunctival epithelium in the CG-rich area. Bar graphs show mean ± sd of two independent experiments (final n=5 for each group). (F) PAS+ conjunctival GC density. Bar graphs show mean ± sd of two independent experiments (final n=5 for each group). *P < 0.05; **P < 0.01; ***P < 0.001.
TSP-1+ cells were noted by immunohistochemical staining in the conjunctival epithelium and subjacent stroma of tissue sections from TSP-1KO mice reconstituted with WT DCs, showing that adoptively transferred cells migrated back to the ocular surface (Fig. 5B). No TSP-1 staining was noted in the TSP-1KO recipients of TSP-1KO DCs.
Reconstitution of TSP-1KO mice with WT DCs prior to DS abrogated the resistance of TSP-1KO mice to corneal-barrier disruption (Fig. 5C and D), whereas transfer of TSP-1KO DCs did not. Adoptive transfer of DCs did not further worsen corneal barrier function in WT mice.
In addition to restoring the normal response of the cornea to DS, adoptive transfer of WT DC to TSP-1KO was accompanied by (1) a significant increase in CD4+ T cells in the conjunctival epithelium and (2) a significant decrease in PAS+-filled GCs in the conjunctiva (Fig. 5E and F).
Taken together, these results show that DC-derived TSP-1 is critical to the ocular surface immunoinflammatory response, presumably through the activation of TGF-β1.
DISCUSSION
Our results suggest that DC-associated TSP-1 promotes the generation of a proinflammatory response to DS on the ocular surface. TSP-1KO mice exhibited an inverse immune phenotype when subjected to DS compared with WT mice: their conjunctival GC density increased, the number of CD4+ T cells in the conjunctiva decreased, corneal barrier function was preserved, and they failed to up-regulate MMPs and T cell-related cytokines. Local neutralization of TGF-β in WT during DS recapitulated the phenotype observed with TSP-1KO under the same conditions. Transfer of TSP-1KO DS5 CD4+ T cells into naive RAG-1KO mice failed to cause dry-eye disease, indicating that TSP-1 is essential to the activation of CD4+ T cells in the ocular surface response to desiccation.
TSP-1 has been shown to be a potent, systemic activator of TGF-β1 by binding to the LAP, preventing its inactivation of TGF-β1. As a result, increased levels of the TSP-1 glycoprotein generate biologically functional TGF-β1 molecules [10]. Previous investigations have implicated the role of TSP-1 in TGF-β1 activation in the eye, specifically in the iris pigment epithelial cells and the retinal pigment epithelial cells [23, 24]. The lacrimal gland produces and secretes TGF-β1, and high levels of TGF-β1 (total and activated) have been reported in tears of desiccated mice and dry-eye human subjects [20–22]. We found a marked reduction in TGF-β activity in the tears of TSP-1KO mice, suggesting that TSP-1 is a physiological activator of TGF-β1 on the ocular surface.
There have been numerous studies describing the pro- and anti-inflammatory consequences of TSP-1 deficiency in murine models. The pro- or anti-inflammatory effects of TSP-1 appear to be tissue/model-specific. We have surveyed the Th1 response in the conjunctiva of WT and TSP-1KO mice. Our results showed that there is a significant increase in IFN-γ in the WT conjunctiva after DS; however, no such change was observed in the TSP-1KO. Other studies present analogous data, in which IFN-γ production is decreased in TSP-1KO mice in an EAE model [25]. Similar results were found in studies where TSP-1/CD47 restriction reduced IFN-γ levels significantly [31]. Moreover, previous studies examining the inflammatory cascade surrounding encephalomyelitis and ureteric obstruction have shown that TSP-1 is critical for generation of a Th17 response [25, 32]. These studies have identified a proinflammatory effect of TSP-1 via a Th17-mediated pathway. Additionally, they have shown that TSP-1KO has a diminished Th17 response. In a model of EAE, TSP-1 deficiency was shown to correspond to diminished Th1 and Th17 responses [25]. Our results showed a Th2 skewing (increased IL-13/IFN-γ) of the immune response to DS in the TSP-1KO strain.
Other studies have shown proinflammatory effects of TSP-1. Li et al. [33] showed down-regulation of obesity-associated inflammation in mice that lack TSP-1. De Luna et al. [34] similarly showed the role of macrophage-associated TSP-1 to promote and sustain inflammation in muscle tissue. However, data about the effects of TSP-1 on the ocular surface immunoinflammatory response to desiccation are limited. Turpie et al. [11] reported the spontaneous development of Sjg̈ren's syndrome-like ocular pathology with age in TSP-1KO mice. These mice developed lacrimal gland T cell infiltration spontaneously, decreased tear production, and disruption of the corneal barrier. These studies corroborate our results further that TSP-1 deletion attenuates the Th1- and Th17-mediated responses.
Lack of desiccation-induced ocular surface inflammation in the TSP-1KO mice was observed in the cornea and conjunctiva. Characteristics of ocular surface epithelial disease caused by inflammation in dry eye include reduced conjunctival GC density, corneal barrier disruption, and corneal surface irregularity [16]. After desiccation, TSP-1KO mice failed to show any worsening of these ocular surface parameters above their baseline. Infiltration in the conjunctival epithelium with CD4+ T cells is another sign of dry eye-induced inflammation [30]. DS was found previously to increase the density of CD4+ T cells in the conjunctival epithelium of WT mice, and GC density has been shown to correlate inversely with the degree of conjunctival and CD4+ T cell infiltration [30]. In contrast to WT, the density of CD4+ T cells decreased paradoxically in the TSP-1KO mice in response to DS, and this was accompanied by an increase in GC density, suggesting that there is a defective immunoinflammatory response to DS in this strain. The lack of up-regulation of Th cytokines IL-17A and IFN-γ, as well as MMP-3 and MMP-9—mediators that have been shown previously to be major contributors to the pathogenesis of the ocular surface epithelial disease in this model [8, 12, 35–37]—serves as further evidence that these mice have an abnormal response to DS.
We have reported previously that an autoimmune mouse strain, whose CD4+ T cells have a defective TGF-β receptor, develop a Sjg̈ren's syndrome-like disease spontaneously [30]. These mice have a phenotype similar to the TSP-1KO mice: their corneal barrier function improves during desiccation, and it is accompanied by an increase of filled GCs and down-regulation of Th17-related genes [30]. Moreover, local neutralization of TGF-β by subconjunctival injections during DS recapitulated the same phenotype, with improvement of T cell infiltration in the conjunctiva and also decreased expression of IL-17A in the cornea and conjunctiva. Our results are in agreement with Veldhoen and colleagues [29], who reported amelioration of EAE symptoms when immunized mice received local injections of anti-TGF-β. They postulate that local administration of TGF-β interferes with DC-T cell interaction, preventing generation of Th17 and dampening of EAE symptoms.
Our findings indicate that TSP-1 is essential for the generation of pathogenic CD4+ T cells involved in the ocular surface inflammatory response to desiccation. We observed that adoptive transfer of CD4+ T cells from WT mice into naive RAG-1KO recipients resulted in a significant reduction in conjunctival GCs and an increase in inflammatory mediators. In stark contrast, transfer of CD4+ T cells from TSP-1KO donors failed to cause ocular surface immunopathology.
Ocular surface DCs have been found previously to be responsible for the generation of pathogenic CD4+ T cells in this DS model [38]. Therefore, we performed an experiment to determine whether TSP-1+ DCs mediate this process. Adoptively transferred DCs from WT (TSP-1+) mice were observed in the conjunctiva of TSP-1KO mice, confirming that these cells migrated to the ocular surface. Reconstitution of TSP-1-deficient mice with TSP-1+ DCs restored the normal immunoinflammatory response to desiccation that is observed in WT mice. This suggests that the TSP-1+ subset of ocular surface DCs is responsible for priming CD4+ T cells in the afferent arm of this immune response. Our results are consistent with those of Saban and colleagues [39], who reported that TSP-1 expression by DCs regulates their capacity to mediate allergic immune responses in an allergic conjunctivitis model.
Our results suggest the importance of TSP-1, presumably through its role in TGF-β1 activation, in this immunoinflammatory process. These findings have allowed us to dissect further the immunopathogenesis of desiccation-induced ocular surface inflammation and provide potential therapeutic targets for dry-eye disease.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health grants EY11915 (to S.C.P.) and EY018888 (to C.S.d.P.), Research to Prevent Blindness, and Core grant P30-EY002520, by the Cytometry and Cell Sorting Core at Baylor College of Medicine (with funding from the U.S. National Institutes of Health: National Institute of Allergy and Infectious Diseases P30AI036211, National Cancer Institute P30CA125123, and National Center for Research Resources S10RR024574); the Oshman Foundation; William Stamps Farish Fund; and Hamill Foundation.
The authors thank Joel M. Sederstrom for expert assistance with the flow cytometry experiments.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- APC
- allophycocyanin
- CLN
- cervical LN
- CM
- complete medium
- CT
- comparative threshold
- DS
- desiccating stress
- DS5/10
- 5/10 days of desiccating stress
- EAE
- experimental autoimmune encephalomyelitis
- GC
- goblet cell
- HPRT1
- hypoxanthine phosphoribosyltransferase 1
- IC
- isotype control
- LAP
- latency-associated peptide
- LSM
- laser-scanning microscopy
- MGB
- minor groove binder
- MMP
- matrix metalloproteinase
- NS
- nonstressed
- OGD
- Oregon Green dextran
- PAS
- periodic acid-Schiff
- qPCR
- quantitative PCR
- RAG-1KO
- RAG-1 knockout mouse
- rm
- recombinant murine
- TSP-1
- thrombospondin-1
- TSP-1KO
- thrombospondin-1 knockout mouse
AUTHORSHIP
N.B.G., D-Q.L., S.C.P., and C.S.d.P. designed research and analyzed and interpreted data. N.B.G., Z.S., X.Z., E.A.V., F.S.A.P., and S.A.R. performed research. N.B.G., E.A.V., S.C.P., and C.S.d.P. drafted the manuscript.
DISCLOSURES
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This paper was presented, in part, as an abstract at the annual meeting of the Association for Research in Vision and Ophthalmology, May 2–6, 2011 (Fort Lauderdale, FL, USA). The authors have declared that no competing interests exist.
REFERENCES
- 1. Behrens A., Doyle J. J., Stern L., Chuck R. S., McDonnell P. J., Azar D. T., Dua H. S., Hom M., Karpecki P. M., Laibson P. R., Lemp M. A., Meisler D. M., Del Castillo J. M., O'Brien T. P., Pflugfelder S. C., Rolando M., Schein O. D., Seitz B., Tseng S. C., van Setten G., Wilson S. E., Yiu S. C., Dysfunctional Tear Syndrome Study Group (2006) Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea 25, 900–907 [DOI] [PubMed] [Google Scholar]
- 2. Niederkorn J. Y., Stern M. E., Pflugfelder S. C., de Paiva C. S., Corrales R. M., Gao J., Siemasko K. (2006) Desiccating stress induces T cell-mediated Sjogren's syndrome-like lacrimal keratoconjunctivitis. J. Immunol. 176, 3950–3957 [DOI] [PubMed] [Google Scholar]
- 3. Wan Y. Y., Flavell R. A. (2007) ‘Yin-Yang’ functions of transforming growth factor-β and T regulatory cells in immune regulation. Immunol. Rev. 220, 199–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong C. (2008) TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8, 337–348 [DOI] [PubMed] [Google Scholar]
- 5. Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852 [DOI] [PubMed] [Google Scholar]
- 6. Chen Z., O'Shea J. J. (2008) Th17 cells: a new fate for differentiating helper T cells. Immunol. Res. 41, 87–102 [DOI] [PubMed] [Google Scholar]
- 7. Stockinger B., Veldhoen M., Martin B. (2007) Th17 T cells: linking innate and adaptive immunity. Semin. Immunol. 19, 353–361 [DOI] [PubMed] [Google Scholar]
- 8. de Paiva C. S., Chotikavanich S., Pangelinan S. B., Pitcher J. I., Fang B., Zheng X., Ma P., Farley W. J., Siemasko K. S., Niederkorn J. Y., Stern M. E., Li D. Q., Pflugfelder S. C. (2009) IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chauhan S. K., El Annan J., Ecoiffier T., Goyal S., Zhang Q., Saban D. R., Dana R. (2009) Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 182, 1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy-Ullrich J. E., Poczatek M. (2000) Activation of latent TGF-β by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 11, 59–69 [DOI] [PubMed] [Google Scholar]
- 11. Turpie B., Yoshimura T., Gulati A., Rios J. D., Dartt D. A., Masli S. (2009) Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am. J. Pathol. 175, 1136–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Paiva C. S., Corrales R. M., Villarreal A. L., Farley W. J., Li D. Q., Stern M. E., Pflugfelder S. C. (2006) Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 83, 526–535 [DOI] [PubMed] [Google Scholar]
- 13. Rahimy E., Pitcher J. D., III, Pangelinan S. B., Chen W., Farley W. J., Niederkorn J. Y., Stern M. E., Li D. Q., Pflugfelder S. C., de Paiva C. S. (2010) Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am. J. Pathol. 177, 744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abe M., Harpel J. G., Metz C. N., Nunes I., Loskutoff D. J., Rifkin D. B. (1994) An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216, 276–284 [DOI] [PubMed] [Google Scholar]
- 15. Yang T., Mendoza-Londono R., Lu H., Tao J., Li K., Keller B., Jiang M. M., Shah R., Chen Y., Bertin T. K., Engin F., Dabovic B., Rifkin D. B., Hicks J., Jamrich M., Beaudet A. L., Lee B. (2010) E-selectin ligand-1 regulates growth plate homeostasis in mice by inhibiting the intracellular processing and secretion of mature TGF-β. J. Clin. Invest. 120, 2474–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Paiva C. S., Corrales R. M., Villarreal A. L., Farley W., Li D. Q., Stern M. E., Pflugfelder S. C. (2006) Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest. Ophthalmol. Vis. Sci. 47, 2847–2856 [DOI] [PubMed] [Google Scholar]
- 17. Lutz M. B., Kukutsch N., Ogilvie A. L., Rossner S., Koch F., Romani N., Schuler G. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 18. Zheng X., de Paiva C. S., Li D. Q., Farley W. J., Pflugfelder S. C. (2010) Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest. Ophthalmol. Vis. Sci. 51, 3083–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X., Volpe E. A., Gandhi N. B., Schaumburg C. S., Siemasko K. F., Pangelinan S. B., Kelly S. D., Hayday A. C., Li D. Q., Stern M. E., Niederkorn J. Y., Pflugfelder S. C., De Paiva C. S. (2012) NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One 7, e36822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng X., de Paiva C. S., Rao K., Li D. Q., Farley W. J., Stern M., Pflugfelder S. C. (2010) Evaluation of the transforming growth factor-β activity in normal and dry eye human tears by CCL-185 cell bioassay. Cornea 29, 1048–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshino K., Garg R., Monroy D., Ji Z., Pflugfelder S. C. (1996) Production and secretion of transforming growth factor β (TGF-β) by the human lacrimal gland. Curr. Eye Res. 15, 615–624 [DOI] [PubMed] [Google Scholar]
- 22. Gupta A., Monroy D., Ji Z., Yoshino K., Huang A. J. W., Pflugfelder S. C. (1996) Transforming growth factor β-1 and β-2 in human tear fluid. Curr. Eye Res. 15, 605–614 [DOI] [PubMed] [Google Scholar]
- 23. Zamiri P., Masli S., Kitaichi N., Taylor A. W., Streilein J. W. (2005) Thrombospondin plays a vital role in the immune privilege of the eye. Invest. Ophthalmol. Vis. Sci. 46, 908–919 [DOI] [PubMed] [Google Scholar]
- 24. Futagami Y., Sugita S., Vega J., Ishida K., Takase H., Maruyama K., Aburatani H., Mochizuki M. (2007) Role of thrombospondin-1 in T cell response to ocular pigment epithelial cells. J. Immunol. 178, 6994–7005 [DOI] [PubMed] [Google Scholar]
- 25. Yang K., Vega J. L., Hadzipasic M., Schatzmann Peron J. P., Zhu B., Carrier Y., Masli S., Rizzo L. V., Weiner H. L. (2009) Deficiency of thrombospondin-1 reduces Th17 differentiation and attenuates experimental autoimmune encephalomyelitis. J. Autoimmun. 32, 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pflugfelder S. C., Farley W., Luo L., Chen L. Z., de Paiva C. S., Olmos L. C., Li D. Q., Fini M. E. (2005) Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am. J. Pathol. 166, 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wahl S. M., Allen J. B., Costa G. L., Wong H. L., Dasch J. R. (1993) Reversal of acute and chronic synovial inflammation by anti-transforming growth factor β. J. Exp. Med. 177, 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wahl S. M. (1994) Transforming growth factor β: the good, the bad, and the ugly. J. Exp. Med. 180, 1587–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veldhoen M., Hocking R. J., Flavell R. A., Stockinger B. (2006) Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 7, 1151–1156 [DOI] [PubMed] [Google Scholar]
- 30. de Paiva C. S., Volpe E. A., Gandhi N. B., Zhang X., Zheng X., Pitcher J. D., III, Farley W. J., Stern M. E., Niederkorn J. Y., Li D. Q., Flavell R. A., Pflugfelder S. C. (2011) Disruption of TGF-β signaling improves ocular surface epithelial disease in experimental autoimmune keratoconjunctivitis sicca. PLoS One 6, e29017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maxhimer J. B., Shih H. B., Isenberg J. S., Miller T. W., Roberts D. D. (2009) Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast. Reconstr. Surg. 124, 1880–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bige N., Shweke N., Benhassine S., Jouanneau C., Vandermeersch S., Dussaule J. C., Chatziantoniou C., Ronco P., Boffa J. J. (2012) Thrombospondin-1 plays a profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int. 81, 1226–1238 [DOI] [PubMed] [Google Scholar]
- 33. Li Y., Tong X., Rumala C., Clemons K., Wang S. (2011) Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One 6, e26656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Luna N., Gallardo E., Sonnet C., Chazaud B., Dominguez-Perles R., Suarez-Calvet X., Gherardi R. K., Illa I. (2010) Role of thrombospondin 1 in macrophage inflammation in dysferlin myopathy. J. Neuropathol. Exp. Neurol. 69, 643–653 [DOI] [PubMed] [Google Scholar]
- 35. Corrales R. M., Stern M. E., de Paiva C. S., Welch J., Li D. Q., Pflugfelder S. C. (2006) Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest. Ophthalmol. Vis. Sci. 47, 3293–3302 [DOI] [PubMed] [Google Scholar]
- 36. Chotikavanich S., de Paiva C. S., Li D-Q., Chen J. J., Bian F., Farley W. J., Pflugfelder S. C. (2009) Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest. Ophthalmol. Vis. Sci. 50, 3203–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo L., Li D. Q., Corrales R. M., Pflugfelder S. C. (2005) Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 31, 186–193 [DOI] [PubMed] [Google Scholar]
- 38. Schaumburg C. S., Siemasko K. F., de Paiva C. S., Wheeler L. A., Niederkorn J. Y., Pflugfelder S. C., Stern M. E. (2011) Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J. Immunol. 187, 3653–3662 [DOI] [PubMed] [Google Scholar]
- 39. Saban D. R., Masli S., Khandelwal P., Lee H. S., Schelereth S., Blanco T. (2012) Deletion of thrombospondin (TSP)-1 in dendritic cells (DC) of the conjunctiva exacerbates allergic conjunctivitis (AC). The Association for Research in Vision and Ophthalmology Annual Meeting Abstracts 53, 1241 [Google Scholar]