Abstract
The origins of benign prostatic diseases, such as benign prostatic hyperplasia (BPH) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), are poorly understood. Patients suffering from benign prostatic symptoms report a substantially reduced quality of life, and the relationship between benign prostate conditions and prostate cancer is uncertain. Epidemiologic data for BPH and CP/CPPS are limited, however an apparent association bet ween BPH symptoms and cardiovascular disease (CVD) has been consistently reported. The prostate synthesizes and stores large amounts of cholesterol and prostate tissues may be particularly sensitive to perturbations in cholesterol metabolism. Hypercholesterolemi, a major risk factor for CVD, is also a risk factor for BPH. Animal model and clinical trial findings suggest that agents that inhibit cholesterol absorption from the intestine, such as the class of compounds known as polyene macrolides, can reduce prostate gland size and improve lower urinary tract symptoms (LUTS). Observational studies indicate that cholesterol-lowering drugs reduce the risk of aggressive prostate cancer, while prostate cancer cell growth and survival pathways depend in part on cholesterol-sensitive biochemical mechanisms. Here we review the evidence that cholesterol metabolism plays a role in the incidence of benign prostate disease and we highlight possible therapeutic approaches based on this concept.
Keywords: BPH, CP/CPPS, cholesterol, statins, ezetimibe (Zetia)
The human prostate is subject to a variety of pathologic conditions and syndromes that are not well understood. The prevalence of benign prostatic hyperplasia (BPH) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) greatly exceeds that of prostate cancer, which is the most common non-cutaneous malignancy among males in the United States. BPH and CP/CPPS have been documented to reduce quality of life to a similar extent as hypertension and heart disease.1, 2 Both conditions affect adults of all ages, yet they can co-exist with prostate cancer, and their mechanistic relationship to prostate cancer, and to other pre-malignant conditions, such as prostatic inflammatory atrophy (PIA)3, is uncertain.
While prostate cancer has been extensively studied and much is now known about this disease at the molecular level, there has been comparatively little study of BPH and CP/CPPS using modern tools, and the etiology and natural history of these conditions are poorly described in mechanistic terms. These limitations are major obstacles preventing rational, targeted strategies for new therapeutic interventions. A recent meta-analysis summarizing the state of the literature on the findings of randomized controlled trials for CP/CPPS4 revealed that no effective therapy currently exists for this condition. Although medical therapies for BPH have resulted in a substantial decrease in traditional surgical approaches5, the effectiveness of the current medical treatments, primarily alpha-blockers and 5α-reductase inhibitors, is difficult to evaluate6. In addition, data are not available on the percentage of men on medical therapy who go on to more invasive therapy at a later time. BPH as a clinical condition is not well defined and histologic confirmation of BPH is rarely attempted. Because objective information about underlying mechanisms in benign prostate disease is largely lacking, much confusion and inconsistency exists in the literature, and in clinical practice, on clinical categories, terminology and therapeutic recommendations7.
Prostate health and metabolism
Despite the lack of basic information and well-defined clinical data, most investigators have concluded that prostate health is highly susceptible to lifestyle and stage-of-life influences8. This association with lifestyle likely reflects a complex interplay between genetic, epigenetic, biochemical and metabolic processes. In the last two decades in Western countries it has become clear that interactions between the genome and lifestyle are rapidly changing the incidence of human disease in unpredictable ways. This is particularly evident in the US where sedentary habits, a high calorie diet and obesity are now widespread. Tissue homeostasis is regulated in part by dietary components that mediate reactions to oxidative stress and inflammation9. For example, type 2 diabetes, which is now appearing for the first time at significant rates in children, is driven by a sedentary lifestyle in combination with dietary behaviors that lead to obesity10. The epidemiologic associations seen in diabetes are partly understood to reflect alterations in the phenotype and endocrine function of adipose tissue, which secretes a number of adipokines that exert potent effects throughout the body, including the promotion of a chronic state of low-level inflammation11. Effects of lifestyle or dietary regimen are likely to emerge in distinctive ways in different organ systems as metabolic and endocrine processes intersect with the genetic and epigenetic programming of specialized cells and tissues.
Cholesterol, signal transduction and gene expression
Our laboratory has focused for a number of years on the role of the neutral lipid, cholesterol, in signal transduction in tissues and cells of the urogenital system. In vertebrate cells, cholesterol represents about one third of the plasma membrane lipids and its concentration in membranes is tightly regulated, even in the face of wide swings in bioavailability. Cholesterol is one of the key regulators of membrane dynamics by its tendency to closely pack the acyl chains of membrane phospholipids, thereby stabilizing local membrane structure12. The effect of cholesterol on membrane lipid packing serves to partition membranes into cholesterol-rich, “liquid-ordered” and cholesterol-poor, “liquid-disordered” microdomains. Liquid-ordered microdomains have been termed “lipid rafts” to evoke the image of relatively stable membrane patches floating in a more dynamic “lipid sea.”13 The membrane segmentation provided by cholesterol, in association with other lipids, exerts major influences on signal transduction. Along with glycosphingolipids and lipidated signaling proteins, such as caveolins, cholesterol facilitates the three-dimensional assembly of multi-protein signaling complexes within cholesterol-rich subcompartments14. This membrane partitioning promotes interactions between potential protein binding partners by segregating protein subunits with—and away from—interacting proteins that process discrete classes of signals. The lipid raft model of membrane organization posits that cholesterol-rich microdomains channel extracellular stimuli down discrete biochemical pathways to the nucleus15.
In addition to the formation of lipid raft complexes, cholesterol can also affect signal transduction and gene expression in other ways. Certain signaling proteins, such as Sonic hedgehog (SHH), a secreted cytokine, are post-translationally modified by covalent addition of cholesterolcholesterol. SHH has been linked to fetal prostate development16-18 as well as prostate cancer19,20, and the cholesterol modification may be involved in formation of bioactive gradients across tissue spaces21. Cholesterol also serves as a metabolic precursor for synthesis of steroid hormones, such as androgen, which are the primary activators of transcription by their cognate nuclear receptors. Many studies using cultured cells have identified substantial effects on gene and protein expression by depleting or adding cholesterol to cellular membranes22-25. Certain signaling proteins, which show sensitivity to manipulations of membrane cholesterol at the level of the plasma membrane, also directly regulate cholesterol and/ or lipid metabolism. An example is the serine theonine kinase, AKT, which localizes to lipid rafts and whose activity can be altered by manipulating membrane cholesterol26, 27. AKT is an important regulator of cell growth and survival and indirectly controls, at the transcriptional level, a large suite of genes involved in cholesterol and lipid biosynthesis28.
Cholesterol and prostate cancer
Recent epidemiologic studies from a number of groups have shown that cholesterol-lowering drugs (primarily 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, known generically as “statins”) may lower prostate cancer risk, and in particular, the risk of advanced disease29-35. However, this literature is complicated to interpret and littered with confusing claims and counter-claims. There are four types of epidemiological studies that shed light on the relationship between prostate cancer and cholesterol: (1) large population studies of cholesterol and mortality, (2) population studies specifically focused on cholesterol and prostate cancer incidence/mortality, (3) randomized studies examining cholesterol-lowering drugs (mostly statins) and cancer risk, and (4) observational studies either examining cholesterol level or cholesterol-lowering drugs and prostate cancer risk. These four study types tend to paint different pictures of the cholesterol-prostate cancer relationship.
Population studies of cholesterol and cancer mortality tend to show that low cholesterol is associated with cancer risk in general, a finding that is almost certainly due to the metabolic activity of tumors, including tumors not yet clinically detected36,37, 38. This metabolic effect of pre-existing tumors on cholesterol levels can confound attempts to address the question of whether high circulating cholesterol might increase risk of specific cancer types. Population studies with a specific focus on prostate cancer have shown that low cholesterol is associated with less aggressive prostate cancer, or an overall reduced risk of prostate cancer-specific mortality39,40-42. Randomized placebo controlled trials of statins that report on cancer of many kinds show no relationship with statin drug use and prostate cancer43,44,45. Finally, as of this writing, many observational studies of prostate cancer and statin use have demonstrated an apparent chemopreventive effect of statin drugs on either overall prostate cancer incidence or the risk of advanced disease30,32,33,46,47.
The reason for the disparate findings in these studies likely originates from study design and the types of data gathered. We will thoroughly explore the differences between these study types, the data they have generated, and the reasons underlying the conclusions that can be drawn from these data sets in an upcoming review of these studies (Solomon and Freeman, manuscript in preparation). Here we will briefly touch on some of the most prominent distinctions. Large inclusive population studies on cancer typically include small numbers of prostate cancers, and few to no prostate cancer deaths. In 52 studies with 7.95 million individuals, only 1,128 prostate cancer cases are recorded and few of these are deaths. Population studies of cholesterol level include many more prostate cancer patients (3,273 prostate cancers in 369,206 patients) and more advanced cases or cases leading to death. Large randomized placebo controlled trials of statins include limited numbers of prostate cancers. We reviewed 49 trials that included 134,516 individuals and identified only 5 prostate cancer deaths and 1,142 incident prostate cancer cases, and these trials were of relatively short duration (4.2 years on average). Observational studies of prostate cancer risk and statin use include many more prostate cancer cases (77,325 in a population of 4,168,049) in studies examining men for up to 14 years. When considered in aggregate, the current literature is consistent with the view that HMG-CoA-reductase inhibitors exert moderate protective effects against prostate cancer progression, while the effect on incident prostate cancer is still uncertain (and given the heterogeneity of the disease, may be impossible to evaluate).
Our group has provided mechanistic evidence in support of potential chemopreventive effects of cholesterol-lowering in prostate cancer. In a series of laboratory studies over the past 9 years, we have shown that a variety of signal transduction mechanisms, including those propagated by the prosurvival kinase AKT23, 24, 27, androgen receptor48, IL-649, STAT349, caveolin-150, and other proteins and pathways relevant to prostate cancer involve constituents that localize to cholesterol-rich microdomains. We and others have shown that the biochemical activity of lipid raft-associated proteins can be re-directed by targeting plasma membrane cholesterol in cell culture22, 25, 49. Our studies have also demonstrated that these membrane-associated proteins, and their functional roles, can be altered by changes in circulating cholesterol in vivo23, 51. From the point of view of therapy or chemoprevention, circulating cholesterol can be effectively reduced by widely used, well-tolerated medications, which also confer additional health benefits. Consistent with population studies showing evidence of inhibition of disease progression with long-term statin therapy, our research has demonstrated that high circulating cholesterol promotes, while cholesterol-lowering retards, the growth of human prostate cancer xenografts in mice23, 51. Taken together, these data point to the possibility that prostatic cells respond to the external cholesterol environment in a manner that alters their potential for growth and possibly other cell activities.
Metabolism and the prostate
Because tumor cells retain programming that reflects the cells and tissues of their origin52, one implication of the cholesterol-sensitivity of prostate cancer is that the normal prostate might also be affected in significant ways by changes in cholesterol metabolism. The prostate synthesizes high levels of cholesterol, at similar rates or in excess of those seen in the liver, and the prostate accumulates cholesterol deposits with age53. BPH, as defined by several criteria, including lower urinary tract symptom (LUTS) score and prostate growth rate, correlates with symptoms of metabolic syndrome, such as low HDL cholesterol levels, peripheral insulin insensitivity, high body mass index (BMI), high triglyceride levels and large waist circumference54. A recent community-based cohort study found a four-fold increased risk of BPH among diabetic men with LDL cholesterol in the highest tertile in comparison to men in the lowest tertile55. There has been very limited study of the effects of statins on BPH, with two studies showing no discernable effect56, 57 and one study showing statin use to be associated with a 6.5-7-year delay in onset of moderate to severe LUTS or benign prostatic enlargement58. Several studies indicate that heart disease, diabetes and metabolic syndrome are associated with increased risk or severity of BPH59-62. To date, there are no reports in humans of the effects of statins on CP/CPPS. These findings are consistent with data from a number of groups indicating a demonstrable relationship between LUTS and metabolic syndrome-like symptoms and/or cardiovascular disease. This potential relationship has led to the suggestion that a healthy heart = a healthy prostate.8
If such a relationship exists, the underlying mechanisms are unknown. Statin drugs inhibit the bioactivity of HMG-CoA-reductase, a proximal enzyme in the mevalonic acid pathway that synthesizes cholesterol. However, HMG-Co-reductase inhibitors also reduce the synthesis of the isoprenoid intermediates, farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are involved in a number of biosynthetic processes, including post-translational modification (isoprenylation) of many signal transduction proteins, such as RAS and RHO family GTPases13. Inhibition of isoprenoid synthesis by statins can therefore disrupt many signal transduction cascades relevant to cell turnover, survival and differentiation. Other complications in interpretation arise from the results of experiments with statins in animal models. In contrast to humans, circulating cholesterol in rodents is largely in the form of HDL, not LDL;63 moreover, statins are ineffective at lowering serum cholesterol in rats and mice,64 the conventional subjects of pre-clinical studies. Thus, reports of statin effects in these species65, 66 are largely due to inhibition of isoprenoid synthesis, most often from typically large, supraphysiologic doses, or from methods of application that bypass the liver, and not from cholesterol lowering. Similarly, while statins potently lower cholesterol levels in humans, their multiple effects complicate attempts to translate epidemiologic findings into mechanistic conclusions.
Cholesterol and inflammation
Pathologic effects of high circulating cholesterol are prominently seen in the formation of atherosclerotic lesions. Accumulation of cholesterol-rich lipid deposits in arterial walls precipitates and sustains inflammatory processes of the innate and adaptive immune systems. In humans, under conditions of high circulating cholesterol, LDL particles accumulate in the arterial intima, where they are enzymatically modified to become stimulants of sustained inflammation. This involves a complex web of interactions between endothelial cells, smooth muscle cells, monocytes, macrophages, lymphocytes, mast cells and neutrophils67. Atherosclerotic changes are progressive, move through successive phases, and develop slowly over decades. Importantly, clinical manifestations of advanced atherosclerotic lesions can be reversed 20-40% by prolonged treatment with HMG-CoA-reductase inhibitors68. Relative reductions in risk are proportional to the extent of LDL cholesterol lowering across a broad range of LDL concentrations. These and other pre-clinical data have identified cholesterol, and its byproducts (such as cholesteryl esters), as principal mediators of local inflammatory changes in the cardiovascular system67.
Many human cancers (~20%) are believed to arise from chronic inflammatory or infectious conditions69. Accumulating evidence now links pathologic or premalignant changes in the prostate, as well as prostate adenocarcinoma, with inflammatory mechanisms3. Most BPH tissues show evidence of an inflammatory reaction. In one study, only 23% of prostate biopsies from 284 patients were free of infiltrating inflammatory cells70. The presence of inflammatory infiltrates in BPH tissues obtained from patients in the Medical Therapy of Prostatic Symptoms (MTOPs) study has been associated with increased rates of disease progression and elevated risk of acute urinary retention5. Human BPH stromal cells isolated from surgical specimens express all of the toll-like receptors (TLRs) of the innate immune system and the TLRs expressed by these cells respond to bacterial or viral agonists by secreting proinflammatory cytokines71. In addition, BPH stromal cells can act as antigen presenting cells (APCs) by activating alloantigen-specific CD4+ T cells to secrete IFN-γ and IL-1771. Our group recently published the results of an unbiased DNA microarray study of BPH-like histomorphologic changes in the rat induced by chronic α(1)-adrenergic receptor activation72. In this report, we used informatics tools to objectively construct a signaling network that identified inflammatory pathways as the most significant gene ontology (GO) processes associated with the experimental treatment, daily phenylephrine injection. We verified aspects of this proposed BPH network in vivo by demonstrating elevated TGFβ signaling, a classical inflammatory mechanism, and by confirming the informatics findings that the signaling protein clusterin, which has been linked to anti-inflammatory mechanisms73, is a prominent node in the network.
The origin of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is essentially completely obscure, however evidence for an autoimmune origin is beginning to emerge. Human CD4+ and CD8+ T cells recognize determinants present in prostate specific antigen (PSA)74, suggesting the possibility that prostatic secretory products, some of which are produced in large amounts, can elicit an autoimmune reaction. Peripheral blood mononuclear cells (PBMC) and CD4+ T cells from CP/CPPS patients proliferate in the presence of seminal plasma and in response to prostate antigens75, 76, indicating that the immune system might identify components of the prostatic secretion as foreign. Consistent with this hypothesis, seminal plasma from CP/CPPS patients has been reported to contain high levels of proinflammatory cytokines, including IL-6, IL-8, IL-1β and TNFα77, 78 IL-8 has been proposed as a biomarker for CP/CPPS as well as BPH79. IL-6 and IL-8, in addition to their roles in inflammatory cell recruitment, are mitogens for prostate cells80, 81, suggesting the possibility that locally elevated concentrations of these inflammatory mediators can alter the balance between cell growth and cell death required to maintain tissue homeostasis.
In human prostate tissues, focal areas of epithelial atrophy can be recognized. These atrophic regions are often accompanied by increased infiltration of inflammatory cells, leading De Marzo and colleagues to propose the term “proliferative inflammatory atrophy” (PIA) for this histologic feature3. Frequent transitions have been reported between areas of PIA, or proliferative atrophy without inflammatory infiltrate, and high-grade prostatic intraepithelial neoplasia (PIN)3. Inflammatory reactions preceding malignant changes are also observed in animal models. For example, neonatal estrogen imprinting of the prostate results in lobe-specific inflammation, hyperplasia, and PIN-like lesions in adult animals82. Genomic searches for prostate cancer susceptibility genes have identified a number of loci involved in innate or acquired immunity, including RNASEL, which encodes a ribonuclease expressed by lymphocytes83; MSR1, encoding macrophage scavenger receptor 1, a homotrimeric protein complex expressed largely by macrophages84; and TLR4, encoding a toll like receptor and a mediator of innate and adaptive immunity85. Other loci that mediate inflammatory processes have also been associated with increased prostate cancer risk85, 86. Although the data are still limited, collectively, these observations suggest that the prostate is susceptible to several types of inflammatory disruptions, particularly with age, and that these reactions lead to pathology. Anti-inflammatory actions of statins (HMG-CoA-reductase inhibitors) could potentially account for reported clinical efficacy of these drugs on benign prostatic enlargement and LUTS58.
Cholesterol targeting and prostate health
Polyene macrolides, such as amphotericin B, candicidin, nystatin and filipin, are antifungal agents that alter membrane permeability and structure. They are extremely effective as antifungals because of their potency, broad spectrum of activity, and because the emergence of resistant fungal strains is infrequent. However, when administered i ntravenously, they are also toxic, causing serious side effects such as renal failure, hypokalemia and thrombophlebitis. Using Mycoplasm a laidlawii grown in either the presence or absence of cholesterol (the organism does not synthesize its own cholesterol), Weber and Kinsky87 demonstrated that sensitivity to filipin required cholesterol, a result that suggested that the bioactivity of polyene macrolides was sterol-dependent. Subsequent s tudies demonstrated that structural requirements of sterols necessary for their optimal interaction with polyenes include the presence of a cholestane ring structure, a Δ 22 double bond, which allows for a more favorable interaction with ergosterol than with cholesterol, and a 3β-OH on the steroid nucleus, likely for membrane orientation88. These studies established that polyene macrolides interact predominantly or specifically with sterols.
Later studies by Gordon and Schaffner,89 which were designed to determine the oral toxicity of several polyene macrolides in a canine model, noted a surprising finding. The prostates of dogs 7-15 years of age regressed after treatment with the compounds below at 5-20 mg/kg day for 30 days. The change in prostate volumes was substantial, with the following average reductions: candicidin 42.1% (100-300 mg/day), nystatin 20.9% (200-400 mg/day), amphotericin B 37.2% (200-500 mg/day), filipin 39.3% (dose 200-400 mg/day) and fungimycin 29.9% (100 mg/day). These same authors also noted that polyenes delivered orally reduced serum cholesterol levels in beagle dogs by 36-50%, depending on the specific polyene and the degree of hypercholesterolemia present. In these experiments, candicidin exhibited the greatest hypocholesterolemic activity, with up to a 36% reduction dependent on dose. Nystatin was the least potent agent, with a maximum 18% reduction in serum cholesterol, including at higher doses,90. Notably, the authors observed no toxicity with any of the compounds. Because polyenes are not absorbed to any significant extent from the gastrointestinal tract, and because their bioavailability is low, the authors suggested that the mechanism of prostatic regression was intimately related to the drug's hypocholesterolemic properties. Results in a hamster model of spontaneous, age-dependent BPH confirmed the candicidin effect and demonstrated that treatment with another hypocholesterolemic compound, the bile-acid binding resin colestipol, also reduced prostate size91. Together these reports provided compelling evidence that the cholesterol-reducing properties of oral polyene macrolides were responsible for the drugs' surprising effects on the prostate.
The pre-clinical studies on the effect of polyenes on the canine prostate led to 10 clinical BPH trials 1982, in 4 countries (US, Soviet Union, Denmark and Japan), from 1970-198292-101. Aalkaer92 tested nystatin in 18 patients for 2 months, and reported an improvement in subjective symptoms in 50% of the subjects, with diminished nocturia and a decrease in residual urine in 29%. However, the effect of nystatin on circulating cholesterol levels was inconsistent. Nystatin was the least potent polyene in the dog studies reported by Gordon and Schaffner89, suggesting that an alternative choice of drug would have produced a more pronounced effect in this trial. Theodorides et al.99 also used nystatin vs. placebo for a 6 week BPH trial and reported that nystatin was ineffective; given the duration of the trial and the drug tested, this may not be surprising. Keshin96 treated 92 patients with candicidin at 300 mg/day for at least 5 months, with up to 18 months of total follow up and observed no toxicity. Moreover, in the patients that were candidates for surgery, 73% improved to the extent that surgery was unnecessary, and improvements were evident using both subjective and objective endpoints. Yamamoto et al.100 treated a small cohort (10 patients) with amphotericin (800-1,200 mg/day) in a short trial (2-10 weeks) and observed a marked effect in one patient, and observable effects in 6 others. Klijucharev et al.97 tested lavorin, which is structurally identical to candicidin,102 on 14 patients with BPH in a 3 month trial and demonstrated a complete resolution of dysuria in 93% and a reduction in prostate size in 57% of the patients. They reported no side effects. Orkin101 performed a placebo controlled trial of 300 mg/day candicidin for ≥ 3 months and reported that 89% of the experimental group had improved subjective symptoms vs. 18% in the placebo group. Residual urine was decreased in 86% of the patients, 89% had improved urine flow rate in the active drug cohort, and >33% had reduced prostate size, based on digital rectal exam, in comparison to none in the placebo cohort. Sporer et al.98 ran a double-blind placebo controlled trial of candicidin (300 mg/day) for 7 months in patients with BPH and demonstrated marked improvement in subjective symptoms (intermittency, dribbling, force of stream, stream size, nocturia, and diurinal frequency) as well as objective symptoms including residual urine. No alteration of serum steroid levels or prostate Abrams93 noted. also performed a placebo controlled randomized trial of candicidin (300 mg/day for 6 months) and found that both the treatment and placebo groups demonstrated improved subjective symptoms. Urodynamic data indicated that the maximum urine flow rate improved in the candicidin group but not in the placebo cohort (p=0.06), as did maximum flow pressure. Residual urine decreased in the treatment group by 24%, while in the placebo group a decrease of 12% was noted. Jensen and Hammen95 also ran a placebo controlled trial of candicidin (300 mg/day for 12 months) and found no statistical difference in subjective symptoms between the candicidin and placebo cohorts, which improved in both groups. Interestingly, the authors confirm the hypocholesterolemic properties of candicidin taken orally by demonstrating a serum cholesterol level drop of≈10% in the candicidin cohort, while the control group demonstrated an increase of approximately 5%. Similarly, Jensen and Madsen94 performed a double blind placebo controlled trial of candicidin (300 mg/day 6 months). Most measures in the treatment group, which included subjective symptoms and urodynamics, improved significantly over pre-treatment values but the differences between the treatment and control cohorts were not significant, except for residual urine and bladder volume, likely owing to a strong placebo effect (some symptoms significantly improved in the placebo group).
The last of these clinical trials of polyene macrolide therapy for BPH were conducted almost 30 years ago. To our knowledge, there have been no trials of these agents in recent times in the context of BPH. Given the promising outcomes of these prospective experiments in humans, one wonders why one or more polyenes did not undergo additional clinical evaluation with the goal of advancing one of them toward standard therapy for BPH. One possibility is that an insufficient distinction was made between the toxicity of these compounds when they are given intravenously and the lack of toxicity seen when they are given orally. In addition, their use as oral agents is not typical because they are poorly absorbed. Another impediment to clinical translation was that controversy emerged over the interpretation of the animal and human data. Robb et al103. suggested that animals treated with polyenes ate less and lost weight; hence the nutritional state of the subjects explained the effect on the prostate. However, data presented in this report does not establish weight loss as the principal mechanism of the polyene effect. The experiments on young rats described in Robb et al.103 are not germane to the study of BPH because they are not a model for this disease. Additionally, if canine experiments also presented in Robb et al103. are analyzed without the lowest (and likely non-therapeutic) dose of candicidin (3 mg/kg/day) the ratio of prostate weight change (mg)/body weight change (kg) is 1.78 mg/kg. If the lowest dose of pimaricin (7 mg/kg/day) is also not included, the ratio of prostate weight change is 4.4 mg/kg body weight. Moreover, even if this interpretation is not compelling, subsequent studies also demonstrated that the change in prostate size was substantially greater than the overall weight loss. For example, when 87.20 strain Syrian hamsters, which exhibit a spontaneous age-dependent prostate enlargement, were treated with candicidin at 40mg/kg/day for 5 months, they exhibited an insignificant body weight loss (124.4 ± 5.0 g control vs. 117 ± 4.5 g candicidin, 6% change), whereas a substantial change in ventral prostate weight was observed (138.4 ± 12 mg control vs. 95.6 ± 9.2 mg candicidin, 31% change)91,101. Prostate weight change in this case was 5.2 mg/kg body weight. In addition, the effects on weight claimed by Robb et al. were not noted in human patients treated with candicidin with candicidin101, or in other canine studies104.
Probably the most damaging of the preclinical studies on the effects of oral polyenes, with respect to the potential for clinical translation of these agents, was one from Texter and coffey104. These authors reported that oral amphotericin B inhibited testicular function, as evaluated by serum testosterone levels and spermatogenesis in dogs. The authors reported 74-98% decrease in serum testosterone after treatment for 30 days, less motile spermatozoa in prostatic secretions, and an absence of spermatogenesis in testicular biopsies taken 1 month after discontinuation of the drug. Notably, these observations were not apparent in other preclinical experiments on dogs and hamsters89-91. A careful analysis of the data in the Texter and Coffey report is difficult to reconcile with the biology and physiology of the systems under investigation. As noted above, polyene macrolides are poorly absorbed from the intestine and exhibit low bioavailability. Consequently, we are skeptical that a small amount of the oral agent could elicit such a massive reduction in serum testosterone. This result also cannot be a function of the cholesterol-lowering properties of polyenes because the blood-testes barrier prevents changes in the circulation from affecting the testes, and because cholesterol reduction does not alter serum testosterone levels105. Given that this is the only study to show a decline in testicular function with oral polyene therapy, we believe that, in all likelihood the results reported by Texter and Coffey are an artifact106. One clue is given in the Discussion of this paper “A few of the control dogs have shown transitory histologic changes in the testes which may be attributed to cage confinement”, but the authors did not report the testosterone levels for these controls, so a comparison of the polyene treated dogs vs. untreated controls is not possible. Given the reported loss of testicular function and the fact that new pharmaceuticals for BPH were soon to become available, it is likely that the claim that polyenes reduced prostate size by severely interfering with testicular function reduced enthusiasm for research into the use of polyene macrolides for the treatment of BPH.
Ezetimibe (Zetia) is an FDA approved hypocholesterolemic drug that blocks cholesterol absorption from the intestine by interfering with the bona fide gut cholesterol transporter NPC1L1107-109. Inhibition of cholesterol absorption by ezetimibe also causes LDL receptor levels to increase, thereby facilitating removal of cholesterol from the circulation. Ezetimibe is believed to be a highly selective cholesterol antagonist. Because the mechanism of action of ezetimibe is similar to that of the polyene macrolides, we sought to replicate the observations made with the polyenes with this new agent that has no apparent toxicity and for which the mechanism of action is well established.
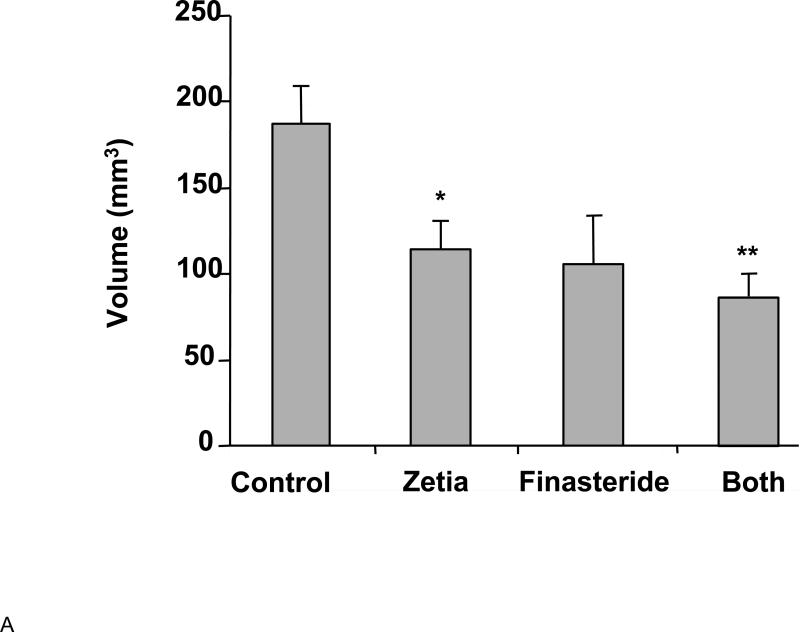
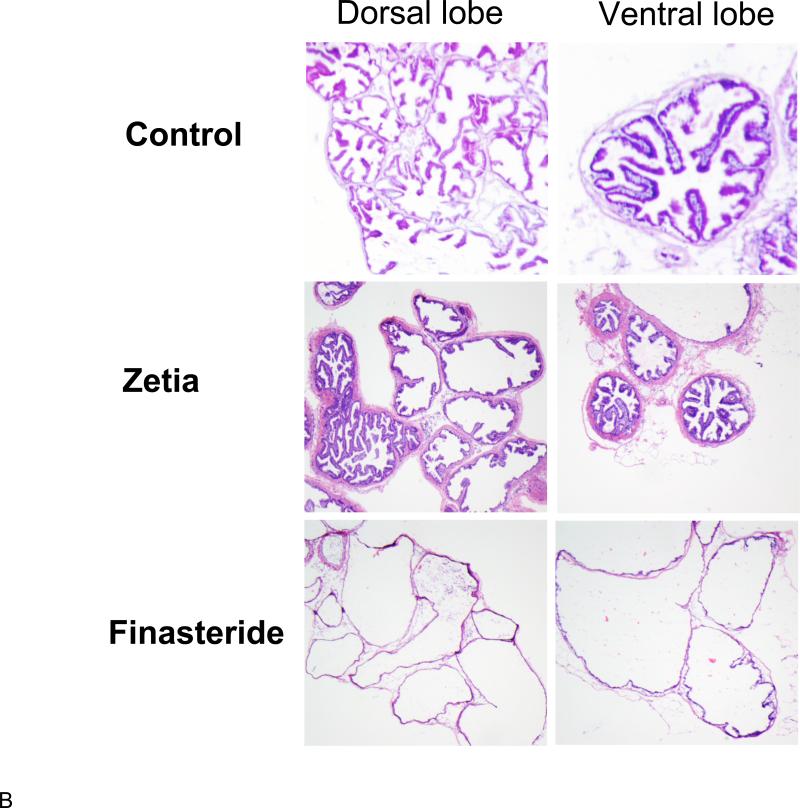
We recently reported on the effects on the prostate of reducing cholesterol levels in Syrian 87.20 hamsters with ezetimibe110. As mentioned above, the males in this strain exhibit a substantial, age-dependent prostatic enlargement. In these experiments, we found that ezetimibe was as effective in reducing prostate size as the 5α-reductase inhibitor, finasteride, a compound that inhibits the production of the most bioactive intraprostatic androgen, dihydrotestosterone (DHT) (Figure 1A). Finasteride and other 5α-reductase inhibitors are widely used to treat BPH in humans. Finasteride and ezetimibe used together evoked the greatest degree of prostatic regression. Histological analysis of prostate tissue indicated that finasteride induced widespread epithelial atrophy, consistent with inhibition of DHT synthesis. Surprisingly, however, normal glandular architecture was preserved in the ezetimibe cohort, implying a distinct mechanism of action (Figure 1B). Surprisingly, we found that initiation of prostate enlargement in these animals was dependent on the presence of cholesterol in the diet, but was no longer required for maintaining the enlargement in older animals. Because of the increase in prostate size with age, the response to finasteride, and the epithelial glandular atrophy resembling a similar response to finasteride in humans111, 112, these studies also confirmed the suitability of the 87.20 strain hamster as a preclinical model for BPH.
Figure 1.
A. Effects of ezetimibe (Zetia) and finasteride on prostate size (volume) in the Syrian 87.20 hamster strain, which shows spontaneous, age-dependent prostate enlargement. Data are presented as mean prostate volume (mm3) vs. drug group ± SD. *p<0.05, **p<0.01 (Students t test) n=4/group. Zetia was as effective as finasteride at reversing prostate enlargement in this model. B. Representative micrographs of hamster prostate frozen sections reveal that finasteride induced prostatic epithelial atrophy, while Zetia did not produce a discernible effect on the prostate epithelium. These data were originally reported in Pelton et al.110 Used with permission.
Although we were not the first to use this hamster strain in preclinical experiments on BPH, the last published studies using this model in this context were reported in the early 1980s106. Our results suggest that ezetimibe might be effective as an alternative to standard medical BPH therapy and, further, that dysregulation of cholesterol metabolism may be an important, and neglected, component of disease etiology113. These results also strongly suggest that the original findings described above with polyene macrolides, published over 30 years ago, were likely correct and that reducing intestinal cholesterol absorption is a viable approach to controlling LUTS in men. Our preclinical data provide support for prospective studies on ezetimibe in men as a novel approach to treating BPH.
Conclusions
The mechanism of prostatic enlargement, and accompanying symptoms defined empirically as LUTS, is poorly understood. We have presented an unusual perspective on benign prostate health and potential novel treatment strategies. Circulating cholesterol has recently emerged as a viable target for chemoprevention and adjuvant therapy in prostate cancer. We have summarized an overview of the literature, going back over 30 years, suggesting that some of the relevant mechanisms that have been proposed as central to the emergence of BPH may be susceptible to cholesterol-targeting approaches. In this regard, we have highlighted a substantial literature on BPH in humans that strongly suggests that cholesterol-targeting is indeed a viable clinical strategy. The pre-clinical and clinical data on cholesterol levels in the context of BPH are in substantial agreement, and systems-level analysis of human BPH tissues and animal models indicate that mechanisms that evoke remodeling of the prostate are broadly shared across great evolutionary distances72, 114, 115. Inflammatory mechanisms seem to be a unifying concept resolvable from the published data114, but much work needs to be undertaken to refine the currently existing models. The literature on polyene macrolides and BPH is largely unknown among basic science investigators and medical practitioners in the area. Consequently, we hope this review will stimulate new research. Phamacologic reductions in cholesterol synthesis or bioavailability may also lower levels of bioactive sterols, such as pregnenolone, testosterone, and estradiol and the physiologic consequences of these changes are uncertain. Although our understanding of the origins of CP/CPPS is even more limited than BPH, we believe the potential for cholesterol-targeting therapy in this context also deserves experimental attention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts to disclose.
References
- 1.Wenninger K, Heiman JR, Rothman I, Berghuis JP, Berger RE. Sickness impact of chronic nonbacterial prostatitis and its correlates. J Urol. 1996;155(3):965–8. [PubMed] [Google Scholar]
- 2.McNaughton Collins M, Pontari MA, O'Leary MP, Calhoun EA, Santanna J, Landis JR, Kusek JW, Litwin MS. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. J Gen Intern Med. 2001;16(10):656–62. doi: 10.1111/j.1525-1497.2001.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimitrakov JD, Kaplan SA, Kroenke K, Jackson JL, Freeman MR. Management of chronic prostatitis/chronic pelvic pain syndrome: an evidence-based approach. Urology. 2006;67(5):881–8. doi: 10.1016/j.urology.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roehrborn CG. Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Rev Urol. 2008;10(1):14–25. [PMC free article] [PubMed] [Google Scholar]
- 6.Emberton M, Fitzpatrick JM, Garcia-Losa M, Qizilbash N, Djavan B. Progression of benign prostatic hyperplasia: systematic review of the placebo arms of clinical trials. BJU Int. 2008;102(8):981–6. doi: 10.1111/j.1464-410X.2008.07717.x. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrakov JD. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354(18):1950–1. author reply 1950-1. [PubMed] [Google Scholar]
- 8.Moyad MA, Lowe FC. Educating patients about lifestyle modifications for prostate health. Am J Med. 2008;121(8 Suppl 2):S34–42. doi: 10.1016/j.amjmed.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 9.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54(6):945–55. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 10.Marcovecchio M, Mohn A, Chiarelli F. Type 2 diabetes mellitus in children and adolescents. J Endocrinol Invest. 2005;28(9):853–63. doi: 10.1007/BF03347581. [DOI] [PubMed] [Google Scholar]
- 11.Qi L, Saberi M, Zmuda E, Wang Y, Altarejos J, Zhang X, Dentin R, Hedrick S, Bandyopadhyay G, Hai T, Olefsky J, Montminy M. Adipocyte CREB promotes insulin resistance in obesity. Cell Metab. 2009;9(3):277–86. doi: 10.1016/j.cmet.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rog T, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M. Ordering effects of cholesterol and its analogues. Biochim Biophys Acta. 2009;1788(1):97–121. doi: 10.1016/j.bbamem.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290(5497):1721–6. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 14.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185(3):381–5. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazarov-Stoica C, Surls J, Bona C, Casares S, Brumeanu TD. CD28 signaling in T regulatory precursors requires p56lck and rafts integrity to stabilize the Foxp3 message. J Immunol. 2009;182(1):102–10. doi: 10.4049/jimmunol.182.1.102. [DOI] [PubMed] [Google Scholar]
- 16.Yu M, Gipp J, Yoon JW, Iannaccone P, Walterhouse D, Bushman W. Sonic hedgehog-responsive genes in the fetal prostate. J Biol Chem. 2009;284(9):5620–9. doi: 10.1074/jbc.M809172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249(2):349–66. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- 18.Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol. 1999;209(1):28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- 19.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431(7009):707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 20.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145(8):3961–70. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Litingtung Y, Chiang C. Region-specific requirement for cholesterol modification of sonic hedgehog in patterning the telencephalon and spinal cord. Development. 2007;134(11):2095–105. doi: 10.1242/dev.000729. [DOI] [PubMed] [Google Scholar]
- 22.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168(4):1107–18. doi: 10.2353/ajpath.2006.050959. quiz 1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115(4):959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–31. [PubMed] [Google Scholar]
- 25.Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278(51):51125–33. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasserre R, Guo XJ, Conchonaud F, Hamon Y, Hawchar O, Bernard AM, Soudja SM, Lenne PF, Rigneault H, Olive D, Bismuth G, Nunes JA, Payrastre B, Marguet D, He HT. Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation. Nat Chem Biol. 2008;4(9):538–47. doi: 10.1038/nchembio.103. [DOI] [PubMed] [Google Scholar]
- 27.Adam RM, Mukhopadhyay NK, Kim J, Di Vizio D, Cinar B, Boucher K, Solomon KR, Freeman MR. Cholesterol sensitivity of endogenous and myristoylated Akt. Cancer Res. 2007;67(13):6238–46. doi: 10.1158/0008-5472.CAN-07-0288. [DOI] [PubMed] [Google Scholar]
- 28.Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24(43):6465–81. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- 29.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123(7):1693–8. doi: 10.1002/ijc.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 31.Flick ED, Habel LA, Chan KA, Van Den Eeden SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry CP, Jr., Sternfeld B, Jacobsen SJ, Whitmer RA, Caan BJ. Statin use and risk of prostate cancer in the California Men's Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2218–25. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 32.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22(12):2388–94. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2226–32. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 34.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. 2008;123(4):899–904. doi: 10.1002/ijc.23550. [DOI] [PubMed] [Google Scholar]
- 35.Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162(4):318–25. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 36.Hiatt RA, Fireman BH. Serum cholesterol and the incidence of cancer in a large cohort. J Chronic Dis. 1986;39(11):861–70. doi: 10.1016/0021-9681(86)90034-2. [DOI] [PubMed] [Google Scholar]
- 37.Knekt P, Reunanen A, Aromaa A, Heliovaara M, Hakulinen T, Hakama M. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. J Clin Epidemiol. 1988;41(6):519–30. doi: 10.1016/0895-4356(88)90056-x. [DOI] [PubMed] [Google Scholar]
- 38.Dyer AR, Stamler J, Paul O, Shekelle RB, Schoenberger JA, Berkson DM, Lepper M, Collette P, Shekelle S, Lindberg HA. Serum cholesterol and risk of death from cancer and other causes in three Chicago epidemiological studies. J Chronic Dis. 1981;34(6):249–60. doi: 10.1016/0021-9681(81)90030-8. [DOI] [PubMed] [Google Scholar]
- 39.Huxley R. The impact of modifiable risk factors on mortality from prostate cancer in populations of the Asia-Pacific region. Asian Pac J Cancer Prev. 2007;8(2):199–205. [PubMed] [Google Scholar]
- 40.Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IM, Jr., Kristal AR. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2807–13. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21(1):61–8. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011;22(2):311–8. doi: 10.1007/s10552-010-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Group, A. O. a. C. f. t. A. C. R. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). Jama. 2002;288(23):2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 44.Group, H. P. S. C. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 45.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large u.s. Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2213–7. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 47.Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, Gaziano JM. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100(2):134–9. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- 48.Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282(40):29584–93. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Adam RM, Solomon KR, Freeman MR. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology. 2004;145(2):613–9. doi: 10.1210/en.2003-0772. [DOI] [PubMed] [Google Scholar]
- 50.Di Vizio D, Adam RM, Kim J, Kim R, Sotgia F, Williams T, Demichelis F, Solomon KR, Loda M, Rubin MA, Lisanti MP, Freeman MR. Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle. 2008;7(14):2257–67. doi: 10.4161/cc.7.14.6475. [DOI] [PubMed] [Google Scholar]
- 51.Solomon KR, Pelton K, Boucher K, Joo J, Tully C, Zurakowski D, Schaffner CP, Kim J, Freeman MR. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174(3):1017–26. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lukes L, Crawford NP, Walker R, Hunter KW. The origins of breast cancer prognostic gene expression profiles. Cancer Res. 2009;69(1):310–8. doi: 10.1158/0008-5472.CAN-08-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91(1):54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 54.Berger AP, Bartsch G, Deibl M, Alber H, Pachinger O, Fritsche G, Rantner B, Fraedrich G, Pallwein L, Aigner F, Horninger W, Frauscher F. Atherosclerosis as a risk factor for benign prostatic hyperplasia. BJU Int. 2006;98(5):1038–42. doi: 10.1111/j.1464-410X.2006.06400.x. [DOI] [PubMed] [Google Scholar]
- 55.Parsons JK, Bergstrom J, Barrett-Connor E. Lipids, lipoproteins and the risk of benign prostatic hyperplasia in community-dwelling men. BJU Int. 2008;101(3):313–8. doi: 10.1111/j.1464-410X.2007.07332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mills IW, Crossland A, Patel A, Ramonas H. Atorvastatin treatment for men with lower urinary tract symptoms and benign prostatic enlargement. Eur Urol. 2007;52(2):503–9. doi: 10.1016/j.eururo.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 57.Stamatiou KN, Zaglavira P, Skolarikos A, Sofras F. The effects of lovastatin on conventional medical treatment of lower urinary tract symptoms with finasteride. Int Braz J Urol. 2008;34(5):555–61. doi: 10.1590/s1677-55382008000500003. discussion 561-2. [DOI] [PubMed] [Google Scholar]
- 58.St Sauver JL, Jacobsen SJ, Jacobson DJ, McGree ME, Girman CJ, Nehra A, Roger VL, Lieber MM. Statin use and decreased risk of benign prostatic enlargement and lower urinary tract symptoms. BJU Int. 107(3):443–50. doi: 10.1111/j.1464-410X.2010.09598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee E, Park MS, Shin C, Lee H, Yoo K, Kim Y, Shin Y, Paik HY, Lee C. A high-risk group for prostatism: a population-based epidemiological study in Korea. Br J Urol. 1997;79(5):736–41. doi: 10.1046/j.1464-410x.1997.00149.x. [DOI] [PubMed] [Google Scholar]
- 60.Hammarsten J, Hogstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39(2):151–8. doi: 10.1159/000052430. [DOI] [PubMed] [Google Scholar]
- 61.Sandfeldt L, Hahn RG. Cardiovascular risk factors correlate with prostate size in men with bladder outlet obstruction. BJU Int. 2003;92(1):64–8. doi: 10.1046/j.1464-410x.2003.04277.x. [DOI] [PubMed] [Google Scholar]
- 62.Karatas OF, Bayrak O, Cimentepe E, Unal D. An insidious risk factor for cardiovascular disease: Benign prostatic hyperplasia. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.03.099. [DOI] [PubMed] [Google Scholar]
- 63.Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol. 2004;55(3):503–17. [PubMed] [Google Scholar]
- 64.Chen Z, Fukutomi T, Zago AC, Ehlers R, Detmers PA, Wright SD, Rogers C, Simon DI. Simvastatin reduces neointimal thickening in low-density lipoprotein receptor-deficient mice after experimental angioplasty without changing plasma lipids. Circulation. 2002;106(1):20–3. doi: 10.1161/01.cir.0000022843.76104.01. [DOI] [PubMed] [Google Scholar]
- 65.Zheng X, Cui XX, Avila GE, Huang MT, Liu Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, Rabson AB, Reddy BS, Conney AH. Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clin Cancer Res. 2007;13(18 Pt 1):5480–7. doi: 10.1158/1078-0432.CCR-07-0242. [DOI] [PubMed] [Google Scholar]
- 66.Reddy BS, Wang CX, Kong AN, Khor TO, Zheng X, Steele VE, Kopelovich L, Rao CV. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66(8):4542–6. doi: 10.1158/0008-5472.CAN-05-4428. [DOI] [PubMed] [Google Scholar]
- 67.Virella G, Lopes-Virella MF. Atherogenesis and the humoral immune response to modified lipoproteins. Atherosclerosis. 2008;200(2):239–46. doi: 10.1016/j.atherosclerosis.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168(1):1–14. doi: 10.1016/s0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 69.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 70.Morote J, Lopez M, Encabo G, de Torres IM. Effect of inflammation and benign prostatic enlargement on total and percent free serum prostatic specific antigen. Eur Urol. 2000;37(5):537–40. doi: 10.1159/000020190. [DOI] [PubMed] [Google Scholar]
- 71.Penna G, Fibbi B, Amuchastegui S, Cossetti C, Aquilano F, Laverny G, Gacci M, Crescioli C, Maggi M, Adorini L. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol. 2009;182(7):4056–64. doi: 10.4049/jimmunol.0801875. [DOI] [PubMed] [Google Scholar]
- 72.Kim J, Yanagihara Y, Kikugawa T, Ji M, Tanji N, Masayoshi Y, Freeman MR. A signaling network in phenylephrine-induced benign prostatic hyperplasia. Endocrinology. 2009 doi: 10.1210/en.2008-1782. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santilli G, Aronow BJ, Sala A. Essential requirement of apolipoprotein J (clusterin) signaling for IkappaB expression and regulation of NF-kappaB activity. J Biol Chem. 2003;278(40):38214–9. doi: 10.1074/jbc.C300252200. [DOI] [PubMed] [Google Scholar]
- 74.Corman JM, Sercarz EE, Nanda NK. Recognition of prostate-specific antigenic peptide determinants by human CD4 and CD8 T cells. Clin Exp Immunol. 1998;114(2):166–72. doi: 10.1046/j.1365-2249.1998.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Motrich RD, Maccioni M, Molina R, Tissera A, Olmedo J, Riera CM, Rivero VE. Presence of INFgamma-secreting lymphocytes specific to prostate antigens in a group of chronic prostatitis patients. Clin Immunol. 2005;116(2):149–57. doi: 10.1016/j.clim.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Batstone GR, Doble A, Gaston JS. Autoimmune T cell responses to seminal plasma in chronic pelvic pain syndrome (CPPS). Clin Exp Immunol. 2002;128(2):302–7. doi: 10.1046/j.1365-2249.2002.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998;52(5):744–9. doi: 10.1016/s0090-4295(98)00390-2. [DOI] [PubMed] [Google Scholar]
- 78.Nadler RB, Koch AE, Calhoun EA, Campbell PL, Pruden DL, Bennett CL, Yarnold PR, Schaeffer AJ. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000;164(1):214–8. [PubMed] [Google Scholar]
- 79.Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51(2):524–33. doi: 10.1016/j.eururo.2006.07.016. discussion 533. [DOI] [PubMed] [Google Scholar]
- 80.Malinowska K, Neuwirt H, Cavarretta IT, Bektic J, Steiner H, Dietrich H, Moser PL, Fuchs D, Hobisch A, Culig Z. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr Relat Cancer. 2009;16(1):155–69. doi: 10.1677/ERC-08-0174. [DOI] [PubMed] [Google Scholar]
- 81.Lee LF, Louie MC, Desai SJ, Yang J, Chen HW, Evans CP, Kung HJ. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene. 2004;23(12):2197–205. doi: 10.1038/sj.onc.1207344. [DOI] [PubMed] [Google Scholar]
- 82.Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Ann N Y Acad Sci. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carpten J, Nupponen N, Isaacs S, Sood R, Robbins C, Xu J, Faruque M, Moses T, Ewing C, Gillanders E, Hu P, Bujnovszky P, Makalowska I, Baffoe-Bonnie A, Faith D, Smith J, Stephan D, Wiley K, Brownstein M, Gildea D, Kelly B, Jenkins R, Hostetter G, Matikainen M, Schleutker J, Klinger K, Connors T, Xiang Y, Wang Z, De Marzo A, Papadopoulos N, Kallioniemi OP, Burk R, Meyers D, Gronberg H, Meltzer P, Silverman R, Bailey-Wilson J, Walsh P, Isaacs W, Trent J. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat Genet. 2002;30(2):181–4. doi: 10.1038/ng823. [DOI] [PubMed] [Google Scholar]
- 84.Xu J, Zheng SL, Komiya A, Mychaleckyj JC, Isaacs SD, Chang B, Turner AR, Ewing CM, Wiley KE, Hawkins GA, Bleecker ER, Walsh PC, Meyers DA, Isaacs WB. Common sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Am J Hum Genet. 2003;72(1):208–12. doi: 10.1086/345802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng SL, Augustsson-Balter K, Chang B, Hedelin M, Li L, Adami HO, Bensen J, Li G, Johnasson JE, Turner AR, Adams TS, Meyers DA, Isaacs WB, Xu J, Gronberg H. Sequence variants of toll-like receptor 4 are associated with prostate cancer risk: results from the CAncer Prostate in Sweden Study. Cancer Res. 2004;64(8):2918–22. doi: 10.1158/0008-5472.can-03-3280. [DOI] [PubMed] [Google Scholar]
- 86.Sun J, Wiklund F, Zheng SL, Chang B, Balter K, Li L, Johansson JE, Li G, Adami HO, Liu W, Tolin A, Turner AR, Meyers DA, Isaacs WB, Xu J, Gronberg H. Sequence variants in Toll-like receptor gene cluster (TLR6-TLR1-TLR10) and prostate cancer risk. J Natl Cancer Inst. 2005;97(7):525–32. doi: 10.1093/jnci/dji070. [DOI] [PubMed] [Google Scholar]
- 87.Weber MM, Kinsky SC. Effect Of Cholesterol On The Sensitivity Of Mycoplasma Laidlawii To The Polyene Antibiotic Filipin. J Bacteriol. 1965;89:306–12. doi: 10.1128/jb.89.2.306-312.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Norman AW, Demel RA, de Kruyff B, van Deenen LL. Studies on the biological properties of polyene antibiotics. Evidence for the direct interaction of filipin with cholesterol. J Biol Chem. 1972;247(6):1918–29. [PubMed] [Google Scholar]
- 89.Gordon HW, Schaffner CP. The effect of polyene macrolides on the prostate gland and canine prostatic hyperplasia. Proc Natl Acad Sci U S A. 1968;60(4):1201–8. doi: 10.1073/pnas.60.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schaffner CP, Gordon HW. The hypocholesterolemic activity of orally administered polyene macrolides. Proc Natl Acad Sci U S A. 1968;61(1):36–41. doi: 10.1073/pnas.61.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang GM, Schaffner CP. Effect of candicidin and colestipol on the testes and prostate glands of BIO 87.20 hamsters. Invest Urol. 1976;14(1):66–71. [PubMed] [Google Scholar]
- 92.Aalkjaer V. [Prostatic hypertrophy treated with antimycotics. An orientation study]. Ugeskr Laeger. 1970;132(34):1556–8. [PubMed] [Google Scholar]
- 93.Abrams PH. A double-blind trial of the effects of candicidin on patients with benign prostatic hypertrophy. Br J Urol. 1977;49(1):67–71. doi: 10.1111/j.1464-410x.1977.tb04526.x. [DOI] [PubMed] [Google Scholar]
- 94.Jensen KM, Madsen PO. Candicidin treatment of prostatism: a prospective double-blind placebo-controlled study. Urol Res. 1983;11(1):7–10. doi: 10.1007/BF00272701. [DOI] [PubMed] [Google Scholar]
- 95.Jensen SK, Hammen S. [Candicidin treatment of benign hypertrophy of the prostate]. Ugeskr Laeger. 1982;144(1):26–7. [PubMed] [Google Scholar]
- 96.Keshin JG. Effect of candicidin on the human benign hypertrophied prostate gland. Int Surg. 1973;58(2):116–22. [PubMed] [Google Scholar]
- 97.Kliucharev BV, Berman NA, Ivanov NM, Margolin AM, Mikhailets GA. [Early results of the use of levorin in prostatic adenoma]. Vopr Onkol. 1972;18(5):36–41. [PubMed] [Google Scholar]
- 98.Sporer A, Cohen S, Kamat MH, Seebode JJ. Candicidin: physiologic effect on prostate. Urology. 1975;6(3):298–304. doi: 10.1016/0090-4295(75)90750-5. [DOI] [PubMed] [Google Scholar]
- 99.Theodorides P, Bourke JB, Griffin JP. Evaluation of a polyene macrolide: nystatin. Proc R Soc Med. 1972;65(2):130–1. [PMC free article] [PubMed] [Google Scholar]
- 100.Yamamoto C, Miyoshi T, Namikawa K, Onoe Y. [Effect of polyene macrolide administration on prostatic hypertrophy]. Hinyokika Kiyo. 1972;18(1):45–51. [PubMed] [Google Scholar]
- 101.Orkin LA. Efficacy of Candicidin in Benign Prostatic Hyperplasia. Urology. 1974;4(1):80–84. doi: 10.1016/0090-4295(74)90113-7. [DOI] [PubMed] [Google Scholar]
- 102.Mechlinski W, Schaffner CP. Characterization of aromatic heptaene macrolide antibiotics by high performance liquid chromatography. J Antibiot (Tokyo) 1980;33(6):591–9. doi: 10.7164/antibiotics.33.591. [DOI] [PubMed] [Google Scholar]
- 103.Robb CA, Carroll PT, Langston JB, Zellers RL. Evidence that nutritional state and well-being are involved in the prostate response to certain polyene macrolides. Invest Urol. 1971;9(1):47–54. [PubMed] [Google Scholar]
- 104.Texter JH, Coffey DS. The effects of amphotericin B on prostatic and testicular function in the dog. Invest Urol. 1969;7(1):90–106. [PubMed] [Google Scholar]
- 105.Hall SA, Page ST, Travison TG, Montgomery RB, Link CL, McKinlay JB. Do statins affect androgen levels in men? Results from the Boston area community health survey. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1587–94. doi: 10.1158/1055-9965.EPI-07-0306. [DOI] [PubMed] [Google Scholar]
- 106.Schaffner CP. Prostatic cholesterol metabolism: regulation and alteration. Prog Clin Biol Res. 1981;75A:279–324. [PubMed] [Google Scholar]
- 107.Davis HR, Veltri EP. Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscler Thromb. 2007;14(3):99–108. doi: 10.5551/jat.14.99. [DOI] [PubMed] [Google Scholar]
- 108.Jurado J, Seip R, Thompson PD. Effectiveness of ezetimibe in clinical practice. Am J Cardiol. 2004;93(5):641–3. doi: 10.1016/j.amjcard.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 109.Knopp RH, Gitter H, Truitt T, Bays H, Manion CV, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;24(8):729–41. doi: 10.1016/s0195-668x(02)00807-2. [DOI] [PubMed] [Google Scholar]
- 110.Pelton K, Di Vizio D, Insabato L, Schaffner CP, Freeman MR, Solomon KR. Ezetimibe reduces enlarged prostate in an animal model of benign prostatic hyperplasia. J Urol. 2010;184(4):1555–9. doi: 10.1016/j.juro.2010.05.083. [DOI] [PubMed] [Google Scholar]
- 111.Rittmaster RS, Norman RW, Thomas LN, Rowden G. Evidence for atrophy and apoptosis in the prostates of men given finasteride. J Clin Endocrinol Metab. 1996;81(2):814–9. doi: 10.1210/jcem.81.2.8636309. [DOI] [PubMed] [Google Scholar]
- 112.Rittmaster RS, Manning AP, Wright AS, Thomas LN, Whitefield S, Norman RW, Lazier CB, Rowden G. Evidence for atrophy and apoptosis in the ventral prostate of rats given the 5 alpha-reductase inhibitor finasteride. Endocrinology. 1995;136(2):741–8. doi: 10.1210/endo.136.2.7835306. [DOI] [PubMed] [Google Scholar]
- 113.Pelton K, Di Vizio D, Insabato L, Schaffner CP, Freeman MR, Solomon KR. Ezetimibe reduces enlarged prostate in an animal model of benign prostatic hyperplasia. J Urol. 184(4):1555–9. doi: 10.1016/j.juro.2010.05.083. [DOI] [PubMed] [Google Scholar]
- 114.Prakash K, Pirozzi G, Elashoff M, Munger W, Waga I, Dhir R, Kakehi Y, Getzenberg RH. Symptomatic and asymptomatic benign prostatic hyperplasia: molecular differentiation by using microarrays. Proc Natl Acad Sci U S A. 2002;99(11):7598–603. doi: 10.1073/pnas.112191399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo J, Dunn T, Ewing C, Sauvageot J, Chen Y, Trent J, Isaacs W. Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate. 2002;51(3):189–200. doi: 10.1002/pros.10087. [DOI] [PubMed] [Google Scholar]