Abstract
BACKGROUND
Leisure-time physical activity (LTPA) is recommended during pregnancy and has been associated with lower risk of delivering a large infant. We sought to characterize the effect of LTPA across the entire birth weight distribution.
METHODS
Women enrolled in the Pregnancy Outcomes and Community Health (POUCH) Study (1998–2004) were followed-up in 2007. Follow-up efforts were extensive for a subcohort and minimal for the remainder (non-subcohort). Thus, 596 subcohort and 418 non-subcohort women who delivered at term participated. Offspring were categorized as small-, appropriate-, or large-for-gestational-age (SGA, AGA, and LGA, respectively) based on gender and gestational age-specific birth weight z-scores (BWz). At follow-up, women recalled pregnancy LTPA and were classified as inactive, insufficiently active or meeting LTPA recommendations. Linear, logistic, and quantile regression analyses were conducted separately by subcohort status.
RESULTS
Meeting LTPA recommendations decreased odds of LGA significantly among the non-subcohort (aOR=0.30, 95%CI: 0.14–0.64) and non-significantly among the subcohort (aOR=0.68, 95%CI: 0.34–1.34). In quantile regression, meeting LTPA recommendations reduced BWz among the upper quantiles in the non-subcohort.
CONCLUSIONS
LTPA during pregnancy lowered odds of LGA and reduced BWz among the upper quantiles, without shifting the entire distribution. LTPA during pregnancy may be useful for reducing risks of large fetal size.
Keywords: exercise, prenatal, maternal, fetal macrosomia, cohort studies
INTRODUCTION
The American College of Obstetricians and Gynecologists (ACOG) recommends that pregnant women participate in at least 30 minutes of moderate leisure-time physical activity (LTPA) on most days of the week in the absence of obstetrical complications.1 Recent governmental LTPA guidelines also recommend at least 150 min/wk of at least moderate LTPA for pregnant women.2 Population-based data indicate that the majority of pregnant women choose to engage in some amount of LTPA, but only 14–22% participate at recommended levels.3
While LTPA during pregnancy has been associated with several maternal health benefits including decreased risk for gestational diabetes, preeclampsia, and preterm delivery, effects on birth weight are still debated.4–9 Research has shown repeatedly that LTPA during pregnancy does not increase risk of delivering a low birth weight infant.6, 8, 10 However, results with mean birth weight have been more varied, with some reporting no changes, others finding significant reductions, and one study showing increased mean birth weight associated with vigorous exercise during pregnancy.8, 11–15
Few have considered the effect of LTPA during pregnancy on high birth weight. One U.S. study found that LTPA later in pregnancy significantly reduced odds of delivering a large-for-gestational-age (LGA) infant without affecting risk of delivering small-for-gestational-age (SGA).16 Results from the Norwegian Mother and Child Cohort Study and the Danish National Birth cohort have also supported inverse associations between LTPA during pregnancy and excessive birth weight.15, 17 However, two other cohort studies in Norway and Denmark failed to show associations among sports/LTPA during pregnancy and high birth weight (≥ 4.2 or 4.5 kg).18, 19 Giving birth to a large infant is associated with several adverse health outcomes including prolonged and complicated deliveries for the mother, as well as increased risk of birth trauma and future childhood obesity for the offspring.20, 21 Thus, if LTPA during pregnancy could decrease the risk of delivering a large infant without shifting the entire birth weight distribution downwards, this could represent a significant health benefit.
We sought to more fully characterize the effect of LTPA during pregnancy on birth size among women who delivered at term (≥ 37 weeks). Specifically, we sought to determine whether LTPA during pregnancy was associated with a mean shift in birth weight, with delivering SGA or LGA, and/or whether there was an isolated effect on the high end of the birth weight distribution.
METHODS
Study Population
We used follow-up data from women enrolled in the Pregnancy Outcomes and Community Health (POUCH) Study. Women were originally recruited in gestational weeks 15–27 from 1998–2004 from 52 clinics in five Michigan communities. Inclusion criteria were singleton pregnancy with no known congenital anomaly, maternal age ≥ 15 years, maternal serum alpha-fetoprotein (MSAFP) screen in gestational weeks 15–22, no pre-existing diagnoses of diabetes mellitus, and proficiency in English. Women with high MSAFP levels (≥ 2 multiples of the median) were oversampled due to a particular interest in this biomarker for the original study aims.22 Originally, 3,019 women were enrolled and followed till birth. The POUCH study was approved from institutional review boards at Michigan State University, Michigan Department of Community Health, and nine community hospitals. Women signed informed consent at enrollment and at follow-up.
After delivery, a “subcohort” of women (n=1371) was selected for more detailed study to maximize resources when evaluating original study aims. The subcohort included all women who delivered preterm (<37 weeks), women with term deliveries but unexplained high MSAFP levels, and a race-stratified sample of women with term deliveries and normal MSAFP levels (i.e., 72% African American women in this category). Women enrolled in the POUCH Study but not meeting these criteria comprised the “non-subcohort” (n=1648). The subcohort was contacted periodically for follow-up studies (2005–2006; 2006–2007), while the non-subcohort received minimal contact.
In fall 2007 (at 3–9 yrs postpartum), follow-up surveys on child health outcomes were sent to all POUCH study participants who had not declined further contact after delivery and whose children were living with them (n=1629 non-subcohort; n=1261 subcohort). Although not part of the primary aims for follow-up, women were asked to recall their POUCH pregnancy physical activity participation on this follow-up survey. Non-subcohort women were sent a single mailing which asked them to complete and return an enclosed survey. No further contact was attempted. Women in the subcohort were sent the same mailing; however, phone contact and additional mailings were attempted to encourage participation.
For the current analyses, women who delivered preterm (< 37 weeks of gestation) were excluded (non-subcohort n=0, subcohort n=335). A total of 1200 non-subcohort and 299 subcohort women failed to return the follow-up survey, and others provided incomplete information on LTPA during pregnancy (non-subcohort n=11, subcohort n=31). Thus, the final sample for this investigation included 418 (26% of eligible) non-subcohort and 596 (66% of eligible) subcohort women.
Study Protocol
The POUCH study has been described elsewhere in detail.22 The enrollment interview and survey provided self-reported maternal pre-pregnancy weight and measured height (pre-pregnancy body mass index (BMI) calculated as kg/m2), race/ethnicity, maternal age, education, relationship status, enrollment in Medicaid, occupational level (Low=Clerical/Sales, Service/Blue Collar, or Homemaker/Other/Unknown; High = Professional/Manager/Technical), and smoking status at mid-pregnancy.
Chart review provided birth weight, child gender, parity, and gestational weight gain. Gestational age was calculated using the last menstrual period unless it disagreed by > 2 weeks with ultrasound conducted prior to 25 weeks gestation, in which case the ultrasound value was used. Sex and gestational-age specific birth weight z-scores (BWz) were calculated as the observed minus the mean birth weight, divided by the population standard deviation using birth weight standards from Kramer et al.23 Offspring were classified as SGA, appropriate-for-gestational-age (AGA), or LGA if their sex and gestational age-specific birth weight was ≤ 10th, between the 10th and 90th, or ≥ 90th percentile, respectively.24 Women were classified into gestational weight gain categories (less than, within, and greater than recommended) based on the 2009 Institute of Medicine recommendations according to their pre-pregnancy BMI.25 For women in the subcohort only, chart review also provided information on diagnoses of gestational diabetes and/or preeclampsia/gestational hypertension.
At follow-up, women recalled whether they had participated in any LTPA during the POUCH pregnancy. If so, they recalled the type, average duration (min/d), and average frequency (d/wk) of up to two activities performed most often during a typical week while pregnant. Metabolic equivalent (MET) values which estimate energy expenditure were assigned to each reported activity using the Compendium for Physical Activities.26 Time spent in activities classified as at least moderate intensity (i.e., ≥3 METs) was summed within each woman and used to classify them as inactive (0 min/wk), insufficiently active (>0–<150 min/wk) or meeting/exceeding LTPA recommendations (≥150 min/wk) during pregnancy. Length of recall was calculated as time from delivery to the follow-up survey (<4, 4–<6, 6–9 years).
Statistical Analyses
Because of the sampling scheme employed to create the original POUCH study cohort and subcohort, and the differing follow-up strategies, analyses were conducted separately for non-subcohort and subcohort participants. Analyses were conducted using SAS version 9.1 with significance set at an alpha level of P ≤ 0.05. Linear, polytomous logistic, and quantile regression analyses were used to assess the relation between LTPA during pregnancy (referent: inactive) and birth size (modeled as BWz or size for gestational age categories). The following variables were considered as covariates in adjusted models based on previous literature: pre-pregnancy BMI, maternal height, gestational weight gain, parity, smoking during pregnancy, Medicaid enrollment, relationship status, maternal age at delivery, educational level, and occupational level. Due to homogeneity and/or missing data for the non-subcohort, maternal race and diagnoses of gestational diabetes and preeclampsia/gestational hypertension were considered as covariates for subcohort models only. Any variable that significantly entered the model or altered parameter estimates for LTPA by more than 10% was examined as a potential confounder or mediator. All multivariate models included length of recall for LTPA during pregnancy.
Linear regression analyses were conducted to determine whether LTPA during pregnancy was associated with a mean shift in the BWz distribution. Adjusted R2 values were used to assess the amount of variance explained. Polytomous logistic regressions estimated associations among LTPA during pregnancy and delivering an SGA or LGA infant (referent: AGA). Likelihood ratio tests were used for significance testing in building adjusted models.
Quantile regressions were used to determine whether LTPA during pregnancy had a constant effect across the entire BWz distribution, or had a variable effect on only certain parts by estimating the association within individual quantiles (i.e. equal groupings) of BWz. Unlike logistic regression, quantile regression does not require the transformation of birth size into somewhat arbitrary categories of SGA, AGA, and LGA, but rather uses all available data to examine the effect on each quantile of the distribution.27 We assessed the association between LTPA during pregnancy and BWz for every 0.05 quantile from the 0.05 to 0.95 quantile of the distribution. Histograms of standardized residuals were evaluated to determine goodness of fit.
RESULTS
Within both the non-subcohort and subcohort, non-responders to the follow-up survey included significantly more women who were African American, younger, had less than high school education, single, enrolled in Medicaid, and smoked during pregnancy, compared to participating women (data not shown). Prevalence of LGA was lower among non-responders in both the non-subcohort (13% vs. 18% for non-responders vs. responders, p=0.06) and the subcohort (8% vs. 11% for non-responders vs. responders, p=0.03). Among the subcohort, prevalences of gestational diabetes (3% vs. 6%) and preeclampsia/gestational hypertension (3% vs. 7%) were lower for non-responders vs. responders, respectively (p<0.05).
Among participants, maternal characteristics were quite different between non-subcohort and subcohort women with the subcohort displaying greater racial and socioeconomic diversity (Table 1). Some differences were expected by design (e.g. 4% non-subcohort vs. 34% subcohort women were African American), while other differences may reflect self-selection of follow-up participation. Mean birth weight was 3.55 ± 0.45 kg among the non-subcohort and 3.42 ± 0.49 kg among the subcohort (p<0.001). While a similar proportion of infants were born AGA in both groups, 18% of non-subcohort infants were born LGA compared to 11% of subcohort infants (p<0.001). A similar proportion of women in each group were classified as meeting LTPA recommendations (~30%); however, a lower percentage of non-subcohort women were classified as inactive (39%) as compared to subcohort women (48%, p=0.005). Average length of recall did not differ between groups (5.3±1.3 vs. 5.5±1.3 yrs for non-subcohort vs. subcohort, respectively) and there was no suggestion of effect modification by length of recall in any analyses.
Table 1.
Participant Characteristics of the POUCH Study Non-Subcohort and Subcohort a
| Non-Subcohort N (%) |
Subcohort N (%) |
Chi-Square p-value |
|
|---|---|---|---|
| N | 418 | 596 | |
| Maternal Race | |||
| White/Other | 401 (96.0) | 394 (66.1) | <0.001 |
| African American | 17 (4.1) | 202 (33.9) | |
| Pre-pregnancy Maternal BMI | |||
| < 25 kg/m2 | 232 (55.5) | 289 (48.5) | |
| 25–<30 kg/m2 | 112 (26.8) | 139 (23.3) | <0.001 |
| ≥ 30 kg/m2 | 74 (17.7) | 168 (28.2) | |
| Maternal Height | |||
| < 65 in | 195 (46.7) | 288 (48.3) | 0.600 |
| Gestational Weight Gainb | |||
| Low | 41 (9.8) | 91 (15.3) | |
| Recommended | 137 (32.8) | 163 (27.4) | 0.018 |
| High | 240 (57.4) | 342 (57.4) | |
| Maternal Age at Delivery | |||
| <20 years | 23 (5.5) | 60 (10.1) | |
| 20–<30 years | 202 (48.3) | 346 (58.1) | <0.001 |
| ≥ 30 years | 193 (46.2) | 190 (31.9) | |
| Maternal Education | |||
| < High School | 34 (8.1) | 89 (14.9) | |
| High School | 77 (18.4) | 154 (25.8) | <0.001 |
| > High School | 307 (73.4) | 353 (59.2) | |
| Medicaid | |||
| Yes | 98 (23.4) | 277 (46.5) | <0.001 |
| Relationship Status | |||
| Single | 54 (12.9) | 173 (29.0) | <0.001 |
| Occupational Statusc | |||
| Low | 281 (67.2) | 457 (76.7) | <0.001 |
| Smoking During Pregnancy | |||
| At Least Some | 33 (7.9) | 92 (15.4) | <0.001 |
| Parity | |||
| Nulliparous | 186 (44.5) | 245 (41.1) | 0.282 |
| Child Gender | |||
| Male | 200 (47.9) | 297 (49.8) | 0.534 |
| Size at Birth | |||
| SGA (≤ 10th %tile) | 19 (4.6) | 63 (10.6) | |
| AGA (>10th–<90th %tile) | 324 (77.5) | 465 (78.0) | <0.001 |
| LGA (≥ 90th %tile) | 75 (17.9) | 68 (11.4) | |
| LTPA During Pregnancyd | |||
| Inactive | 162 (38.8) | 288 (48.3) | |
| Insufficiently Active | 125 (29.9) | 134 (22.5) | 0.005 |
| Meeting LTPA Recs | 131 (31.3) | 174 (29.2) | |
POUCH=Pregnancy Outcomes and Community Health; BMI = Body Mass Index; SGA= Small for Gestational Age; AGA= Appropriate for Gestational Age; LGA = Large for Gestational Age; LTPA Recs= leisure-time physical activity recommendations
Includes only Pregnancy Outcomes and Community Health (POUCH) study participants who gave birth at term and were enrolled in the 2007 follow-up study
Based on 1990 IOM guidelines according to pre-pregnancy BMI
Low Occupational Status = Clerical/Sales, Service/Blue Collar, or Homemaker/Other/Unknown
“Inactive”=no leisure time physical activity during pregnancy, “Insufficiently Active”= leisure time physical activity during pregnancy less than the recommended (<150 min/wk), “Meeting LTPA Recs”= leisure time physical activity during pregnancy at or above the recommended level (≥150 min/wk)
Mean BWz
Being insufficiently active or meeting LTPA recommendations was not associated with changes in mean BWz among either the non-subcohort or the subcohort once adjusted for maternal characteristics (Table 2). Adjusted models for both groups included maternal height, pre-pregnancy BMI, gestational weight gain, parity, smoking and length of recall. The non-subcohort model also included maternal age while the subcohort model also adjusted for maternal education and race.
Table 2.
Linear Regression Analyses for the Association Between Maternal Leisure Time Physical Activity During Pregnancy and Birth Weight Z-Score by Subcohort Status
| Non-Subcohort N=418 |
Subcohort N=596 |
|||||
|---|---|---|---|---|---|---|
| Adjusted R2 | Beta Values | 95% Confidence Intervals | Adjusted R2c | Beta Values | 95% Confidence Intervals | |
|
|
|
|||||
| Model 1: | 0.009 | 0.011 | ||||
| LTPA in Pregnancy (ref: Inactive) a | ||||||
| Insufficiently Active | −0.13 | −0.36, 0.10 | 0.24 | 0.03, 0.45* | ||
| Meeting LTPA Recs | −0.23 | −0.45, 0.00 | −0.05 | −0.24, 0.14 | ||
| Model 2: b | 0.157 | 0.218 | ||||
| LTPA in Pregnancy (ref: Inactive) a | ||||||
| Insufficiently Active | −0.06 | −0.29, 0.16 | 0.11 | −0.08, 0.31 | ||
| Meeting LTPA Recs | −0.10 | −0.31, 0.12 | −0.06 | −0.24, 0.11 | ||
| Smoking in Pregnancy (Ref: none) | −0.40 | −0.73, −0.07* | −0.48 | −0.70, −0.26* | ||
| Nulliparous (ref: Parous) | −0.28 | −0.47, −0.09* | −0.40 | −0.56, −0.24* | ||
| Age at Delivery (Ref: <20y) | ||||||
| 20–<30 years | 0.42 | 0.01, 0.83* | ||||
| ≥ 30 years | 0.48 | 0.05, 0.91* | ||||
| African-American Race (ref: White) | −0.41 | −0.58, −0.24* | ||||
| Education (ref: < High School) | ||||||
| = High School | −0.05 | −0.30, 0.21 | ||||
| > High School | 0.21 | 0.00, 0.45* | ||||
| Maternal Height (inches) | 0.05 | 0.02, 0.08* | 0.06 | 0.03, 0.09* | ||
| Pre-Pregnancy BMI (kg/m2) | 0.03 | 0.02, 0.05* | 0.02 | 0.01, 0.03* | ||
| Gestational Weight Gain (lbs) | 0.02 | 0.01, 0.02* | 0.01 | 0.00, 0.01* | ||
| Length of Recall (Ref: <4 years) | ||||||
| 4–<6 years | 0.03 | −0.21, 0.26 | −0.06 | −0.29, 0.16 | ||
| ≥ 6 years | 0.06 | −0.19, 0.31 | −0.08 | −0.32, 0.15 | ||
BMI=Body Mass Index; LTPA Recs=Leisure Time Physical Activity Recommendations
Significant p-value < 0.05
“Inactive”=no leisure time physical activity during pregnancy, “Insufficiently Active”= leisure time physical activity during pregnancy less than the recommended (<150 min/wk), “Meeting LTPA Recs”= leisure time physical activity during pregnancy at or above the recommended level (≥ 150 min/wk)
Race could not be considered in the non-subcohort model due to homogeneity. Other estimates (i.e., “Education” for the non-subcohort model and “Age at Delivery” for the subcohort model) are missing because they did not significantly enter their respective models.
Extreme Birth Weight
Being insufficiently active was not related to delivering either SGA or LGA in either group (Table 3). Furthermore, meeting LTPA recommendations was not related to delivering SGA. Among the non-subcohort, meeting LTPA recommendations significantly reduced the odds (by 70%) of delivering an LGA infant and adjustment for confounders did not affect the association (aOR=0.30, 95%CI: 0.14–0.64). Among the subcohort, meeting LTPA recommendations also tended to be associated with lower odds (~37%) of giving birth to an LGA infant; however, this association was not statistically significant (aOR=0.68, 95%CI: 0.34–1.34). Diagnoses of gestational diabetes or preeclampsia/gestational hypertension did not significantly enter the subcohort model. However, removing women from the subcohort who were diagnosed with gestational diabetes (n=31), preeclampsia/gestational hypertension (n=40), or both conditions (n=3) strengthened the association between meeting LTPA recommendations and odds of delivering LGA to borderline significance (aOR=0.52, 95%CI: 0.25–1.10) without affecting odds of delivering SGA (aOR=0.90, 95%CI: 0.45–1.79).
Table 3.
Associations Among Maternal Leisure-Time Physical Activity During Pregnancy (Reference: Inactive) and Appropriateness of Size-for-Gestational-Age at Birth (Reference: AGA) by Subcohort Status
| Non-Subcohort (n=418)
|
Subcohort (n=596)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | aORa | 95% CI | OR | 95% CI | aORb | 95% CI | |
|
|
|
|||||||
| SGA | ||||||||
| Insufficiently Activec | 1.92 | 0.70, 5.23 | 1.80 | 0.61, 5.31 | 0.64 | 0.30, 1.34 | 0.84 | 0.38, 1.83 |
| Meeting LTPA Recsc | 0.29 | 0.06, 1.40 | 0.20 | 0.04, 1.08 | 0.95 | 0.53, 1.73 | 1.10 | 0.59, 2.06 |
| LGA | ||||||||
| Insufficiently Activec | 0.98 | 0.56, 1.73 | 1.03 | 0.59, 1.90 | 1.14 | 0.53, 1.73 | 1.08 | 0.57, 2.04 |
| Meeting LTPA Recsc | 0.30* | 0.15, 0.61 | 0.30* | 0.14, 0.64 | 0.63 | 0.33, 1.21 | 0.68 | 0.34, 1.34 |
AGA= Appropriate for Gestational Age; OR=Odds Ratio; aOR = adjusted Odds Ratio; CI=Confidence Interval; SGA = Small for Gestational Age; LGA = Large for Gestational Age; LTPA Recs = Leisure Time Physical Activity Recommendations
p<0.05
Non-Subcohort aOR’s are adjusted for maternal weight gain during pregnancy (below, meeting, or over IOM recommendation based on pre-pregnancy BMI), maternal height (</≥ 65 in), parity (nulliparous/parous), maternal occupational level (Low/High), and years of recall (< 4, 4–<6, ≥ 6 yrs)
Subcohort aOR’s are adjusted for maternal race (black vs white/other), weight gain during pregnancy (below, meeting, or over IOM recommendation based on pre-pregnancy BMI), maternal height (</≥ 65 in), parity (nulliparous/parous), maternal education (less than, equal to, or more than high school), maternal smoking during pregnancy (yes/no), and years of recall (< 4, 4–<6, ≥ 6 yrs)
“Inactive”=no leisure time physical activity during pregnancy, “Insufficiently Active”= leisure time physical activity during pregnancy less than the recommended (<150 min/wk), “Meeting LTPA Recs”= leisure time physical activity during pregnancy at or above the recommended level (≥150 min/wk)
Quantiles of the BWz Distribution
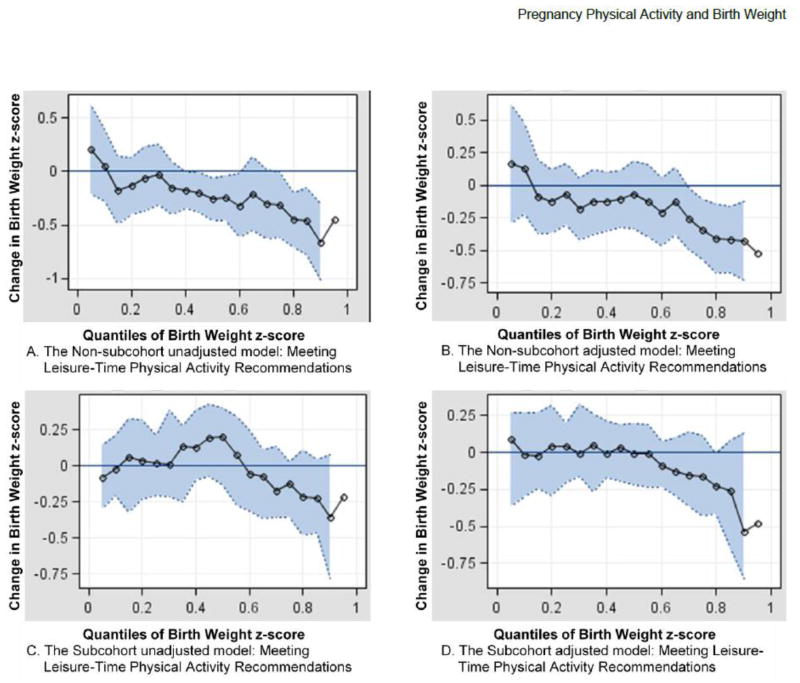
Results of quantile regressions are displayed as quantplots in Figure 1. Black circles represent parameter estimates for the effect of LTPA during pregnancy at every 0.05 quantile of the BWz distribution. The gray shaded region represents the 95% confidence interval. Parameter estimates located below the zero line indicate a reduction in BWz and are considered significant for any quantile where the shaded 95% confidence interval does not include the zero line. A mostly flat line would indicate that LTPA during pregnancy has a constant effect on the entire distribution of BWz and would result in a mean shift of the distribution. In contrast, a curvilinear line would indicate that LTPA during pregnancy has isolated effects on specific portions of the distribution.
Figure 1.
Associations of meeting leisure-time physical activity recommendations (≥ 150 min/wk) during pregnancy and birth weight z-score among non-subcohort (Plots A and B) and subcohort (Plots C and D) participants across quantiles. The black circles represent the estimated effect at every 0.05 quantile while the gray shaded area represents the 95% confidence interval. Plots A and B show the unadjusted and adjusted effect of meeting/exceeding leisure time physical activity recommendations on birth weight z-score within the non-subcohort, respectively (ref: inactive). Plot B is adjusted for gestational weight gain (below, meeting, or over recommended), maternal height (</≥ 65 in), parity (nulliparous/parous), maternal occupational level (Low/High), maternal age at delivery (<20, 20–<30, ≥ 30 yrs), smoking during pregnancy (yes/no), and years of recall (< 4, 4–<6, ≥ 6 yrs). Plots C and D show the unadjusted and adjusted effect of meeting/exceeding leisure time physical activity recommendations on birth weight z-score within the subcohort, respectively (ref: inactive). Plot D is adjusted for race (White and/or Other vs. Black), gestational weight gain, maternal height, parity, education (< High School, =High School, > High School), smoking during pregnancy, and years of recall.
Quantplots for insufficient LTPA during pregnancy indicated a null effect across every BWz quantile in both the non-subcohort and subcohort (data not shown). In contrast, quantplots for meeting/exceeding LTPA recommendations during pregnancy indicated a significant reduction in BWz, but only among the upper quantiles of the distribution for the non-subcohort (Figure 1, Plots A and B). In the non-subcohort adjusted model (Panel B) meeting LTPA recommendations during pregnancy reduced BWz by up to 0.5 units after the 0.70 quantile of the distribution. Among the subcohort, results for meeting/exceeding LTPA recommendations similarly reduced BWz among the upper quantiles; however, the estimated effect was non-significant in both unadjusted (Panel C) and adjusted (Panel D) models.
DISCUSSION
Our results indicate that meeting LTPA recommendations during pregnancy was not associated with a shift in the entire BWz distribution. Rather, LTPA at/above recommended levels decreased odds of delivering an LGA infant and significantly decreased BWz above the 0.70 quantile among the non-subcohort. The subcohort demonstrated similar trends; however, their results were less dramatic and not statistically significant.
The descriptive characteristics of women in the non-subcohort and subcohort varied widely, with the subcohort displaying more racial and socioeconomic diversity. This is likely due to the race-stratified sampling scheme used to create the subcohort, as well as the extra efforts used to follow-up this group. Thus, the methods employed produced essentially two different populations within which to evaluate associations among LTPA during pregnancy and birth size.
The selective descriptive characteristics of the non-subcohort (i.e., majority White, normal pre-pregnancy BMI, higher educated, non-single relationship status, and non-smokers) are similar to participant characteristics of previous studies evaluating LTPA during pregnancy.12,14,15,17–19 Perhaps not surprisingly, results from the non-subcohort are similar to those previously published.12,14,15–17 On the other hand the subcohort displays a greater diversity of participant characteristics not often seen in investigations of LTPA during pregnancy and show a more diluted effect of LTPA on birth size. We believe that our approach of stratifying our sample adds strength to our study as we provide important information regarding the role of LTPA on birth weight in a largely understudied population. It is possible that the effect of LTPA during pregnancy on birth size may be blunted within the subcohort because other factors such as African American race and smoking rates may exert stronger effects on birth size. Unmeasured confounding due to lifestyle factors not assessed may also be greater in diverse samples and could partly explain the attenuated effects of LTPA during pregnancy on birth size in the subcohort vs. non-subcohort. Interestingly, when women with pregnancy complications that may confound the relation between LTPA and birth size (i.e. gestational diabetes or preeclampsia/gestational hypertension) were removed from the subcohort, the effect of meeting LTPA recommendations on reduced odds of delivering LGA became stronger and approached statistical significance. The subcohort also had a lower percentage of LGA births (11%) compared to the non-subcohort (18%), thus differences in the statistical significance of our results may reflect lower power to detect relationships within the subcohort.
Non-responders tended to display greater racial and socioeconomic diversity and have lower rates of LGA deliveries compared to responders in both groups. While most (64%) of the subcohort participated in follow-up, only 26% of the non-subcohort returned the follow-up survey, and they did so in response to a single mailing. The non-subcohort therefore represents a select group of women that are less generalizable to other populations, and their results should be interpreted cautiously. However, previous literature has demonstrated that while non-responders may significantly differ from responders in regard to prevalence of exposure and outcome variables, the exposure-outcome associations are often similar in both groups if the study hypotheses are not suspected 28, 29. In this case, the association between recalled physical activity during pregnancy and birth weight was a secondary analyses of existing data, thus women had no reason to suspect our hypotheses while responding to the survey. Encouragingly, despite more selective follow-up in the non-subcohort, both groups displayed similar trends in results.
Very few studies have examined LTPA during pregnancy in relation to risk of delivering LGA. Similar to our results for the non-subcohort, Alderman et. al. found that participation in ≥ 120 min/wk of at least moderate LTPA during pregnancy reduced risk of delivering an LGA infant (OR=0.3, 95%CI=0.2–0.7) with no effect on delivering an SGA infant among a group of 291 Coloradan women.16 Recent reports from the Norwegian Mother and Child Cohort Study and the Danish National Birth Cohort also showed inverse associations between prospectively measured LTPA during mid to late pregnancy and risk of LGA (HR=0.93, 95%CI: 0.89–0.98) or excessive birth weight (aOR=0.77, 95%CI: 0.61–0.96), even after adjusting for confounding factors.15, 17
In contrast, when studying modifiable determinants of high birth weight (≥4.2 kg) among 553 Norwegian women, Voldner et. al. found that LTPA during pregnancy was unrelated to birth weight, but that low LTPA pre-pregnancy (<1 hr/wk) increased risk of high birth weight (aOR=2.9, 95%CI: 1.2–7.3).18 Additionally, a different prospective cohort study among 4458 Danish women found no association between participation in sports/LTPA during the second or third trimesters of pregnancy and high birth weight (≥4.5 kg).19 Discrepancies in results from these studies as compared to ours may be due to methodological differences in defining birth weight outcomes and/or LTPA during pregnancy. Both the Voldner et. al. and the Danish study used arbitrary birth weight cutpoints to define “high birth weight” without regard for gestational age at delivery, rather than using population-specific growth curves to define infants who were born too large for their gestational age. Thus, their “high birth weight” categories may represent different groups of infants than those included in our LGA group. Additionally, the Voldner et. al. study classified women as participating in low or high (< or ≥ 1 hr/wk) levels of LTPA while the Danish study classified LTPA by categories of hours/week spent in sport or by categories of any vs. no participation in types of sport-activity (i.e., weight bearing, non-weight bearing).18, 19 It is possible that these LTPA categorizations which often combined inactive and low active women as the referent group were unable to detect associations with extreme birth size that we found when comparing women who met LTPA recommendations to inactive women.
The novel use of quantile regression analyses allowed us to refine existing knowledge of the association between LTPA during pregnancy and birth size. Our results showed a dramatic reduction in BWz associated with meeting LTPA recommendations during pregnancy, but only among the upper quantiles (>0.70). Higher birth weight is associated with short- and long-term health risks including birth trauma for both the mother and infant and increased risk for obesity and components of the metabolic syndrome during childhood.20, 21, 30 Thus, our results suggest that LTPA during pregnancy may improve maternal/child health outcomes by helping limit excessive fetal growth without reducing normal fetal growth.
Normalization of fetal growth with LTPA participation may occur by normalizing maternal blood glucose, reducing insulin resistance, and altering placental blood flow and nutrient delivery.7, 9, 31, 32 Placental blood flow decreases intermittently during the exercise bout, but is increased at rest due to training adaptations.31, 33 While LTPA during the first and second trimesters appears to improve placentation and vascularization, LTPA during the third trimester may have the most direct effect on fetal growth.31 Unfortunately, trimester-specific estimates of LTPA were not available for this study.
Other limitations should be noted. Information on LTPA during pregnancy was recalled by our participants 3–9 years postpartum, thus recall bias cannot be discounted and results should be interpreted with caution. Questions used to recall LTPA in this study were based on physical activity questions used in the 2000 Behavioral Risk Factor Surveillance Survey, which have demonstrated moderate reliability and validity when used to recall physical activity done in the past month.34 Our recall period was much longer. Encouragingly, other work has demonstrated strong correlations (r=0.57–0.85) among recalled and originally measured LTPA during pregnancy at six years postpartum using a form of the Modifiable Activity Questionnaire.35 Thus, reliable historical recall of LTPA performed during pregnancy is possible. Other studies have shown poor reliability for recalling moderate LTPA (r=0.1–0.2) but stronger reliability for recalling vigorous LTPA (r=0.3–0.5) occurring 1–5 years previously.36, 37 Construct-validity for recalled LTPA appears to be stronger than reliability of recall. Data from the Aerobics Center Longitudinal Study (ACLS) found that recalled amounts of LTPA for up to 10 years in the past were significantly correlated with indices of cardiovascular fitness originally measured during the recalled year (r=0.40–0.61), and that associations did not diminish with longer length of recall.38 Additionally, women recalling LTPA amounts that met/exceeded LTPA guidelines had significantly higher cardiovascular fitness and lower measured BMI values in the recalled year compared to those recalling lower amounts of physical activity. Thus, recalled LTPA data can be used to separate women into meaningful groups in terms of health.
Recall bias in our study may have been somewhat reduced by categorizing women into groups of Inactive, Insufficiently Active, and Meeting/Exceeding LTPA Guidelines. Additionally, differential bias is unlikely because women were not told of the study aims when they were asked to recall LTPA. However, there is still potential for unmeasured confounding because LTPA is correlated with other lifestyle factors, such as diet, that were not measured in this study. It is important to note that our results were not significantly altered when stratified by the presence or absence of intervening pregnancies, indicating that recall ability was not influenced by parity (data not shown). Others have also shown that the accuracy of maternal recall is not influenced by intervening pregnancies.39 Finally, our analyses considered only LTPA, but other modes of physical activity, such as work, household, and/or care giving activities may also be important.40, 41 We tried to control for time spent standing or lifting objects at work during mid-pregnancy (recorded at enrollment), but neither of these variables significantly altered any analyses (data not shown).
Despite limitations, our study adds significantly to the existing literature. While a handful of previous studies have shown that LTPA during pregnancy reduces the risk of delivering LGA, this is the first time a quantile regression technique has been used to more clearly determine where the threshold of effect with BWz occurs.15–18 Additionally, the majority of previous work has been done among Scandinavian populations, which may have different population and birth characteristics compared to the U.S.15, 17–19 Our results indicate that participating in LTPA during pregnancy at or above recommended levels may lower risk for delivering an LGA infant, and reduce BWz among the upper quantiles of the distribution without reducing mean BWz. While results were significant only among the select non-subcohort, the same trends were apparent within the more diverse subcohort. Due to the adverse maternal and child health effects associated with higher birth weight, our results may indicate a substantial health benefit for LTPA during pregnancy. Future studies with prospectively ascertained, detailed measures of LTPA during each trimester of pregnancy are needed to test and refine our findings within diverse populations of pregnant women.
Acknowledgments
FUNDING SOURCE: This work was supported by the Perinatal Epidemiological Research Initiative Program Grant from the March of Dimes Foundation [Grants 20FY01-38 and 20-FY04-37], the National Institute of Child Health and Human Development and the National Institute of Nursing Research [Grant R01 HD34543], the Thrasher Research Foundation [Grant 02816-7], the Centers for Disease Control and Prevention [Grants U01 DP000143-01 and R36 DP001322-01], and the Blue Cross Blue Shields of Michigan Foundation [Grant 1416 Student Award Program].
Contributor Information
Lanay M. Mudd, Department of Epidemiology, Michigan State University, East Lansing, MI
Jim Pivarnik, Department of Kinesiology, Michigan State University, East Lansing, MI.
Claudia B. Holzman, Department of Epidemiology, Michigan State University, East Lansing, MI
Nigel Paneth, Department of Epidemiology, Michigan State University, East Lansing, MI.
Karin Pfeiffer, Department of Kinesiology, Michigan State University, East Lansing, MI.
Hwan Chung, Department of Epidemiology, Michigan State University, East Lansing, MI.
References
- 1.ACOG committee opinion #267. Exercise during pregnancy and the postpartum period. Int J Gynaecol Obstet. 2002;77:79–81. doi: 10.1016/s0020-7292(02)80004-2. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Physical activity guidelines for Americans. U.S. Department of Health and Human Services. [Accessed June 10, 2010.]; http://www.health.gov/paguidelines: ODPHP Publication No. U0036, 2008.
- 3.Evenson KR, Wen F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Prev Med. 2010;50:123–128. doi: 10.1016/j.ypmed.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Damm P, Breitowicz B, Hegaard H. Exercise, pregnancy, and insulin sensitivity--what is new? Appl Physiol Nutr Metab. 2007;32:537–540. doi: 10.1139/H07-027. [DOI] [PubMed] [Google Scholar]
- 5.Pivarnik JM, Chambliss HO, Clapp JF, et al. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med Sci Sports Exerc. 2006;38:989–1006. doi: 10.1249/01.mss.0000218147.51025.8a. [DOI] [PubMed] [Google Scholar]
- 6.Hegaard HK, Pedersen BK, Nielsen BB, Damm P. Leisure time physical activity during pregnancy and impact on gestational diabetes mellitus, pre-eclampsia, preterm delivery and birth weight: a review. Acta Obstet Gynecol Scand. 2007;86:1290–1296. doi: 10.1080/00016340701647341. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey JC, Butler CL, Williams MA. No need for a pregnant pause: physical activity may reduce the occurrence of gestational diabetes mellitus and preeclampsia. Exerc Sport Sci Rev. 2005;33:141–149. doi: 10.1097/00003677-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Gavard JA, Artal R. Effect of exercise on pregnancy outcome. Clin Obstet Gynecol. 2008;51:467–480. doi: 10.1097/GRF.0b013e31816feb1d. [DOI] [PubMed] [Google Scholar]
- 9.Weissgerber TL, Wolfe LA, Davies GA, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: A review of the literature. Appl Physiol Nutr Metab. 2006;31:661–674. doi: 10.1139/h06-060. [DOI] [PubMed] [Google Scholar]
- 10.Pivarnik JM. Potential effects of maternal physical activity on birth weight: brief review. Med Sci Sports Exerc. 1998;30:400–406. doi: 10.1097/00005768-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Perkins CC, Pivarnik JM, Paneth N, Stein AD. Physical activity and fetal growth during pregnancy. Obstet Gynecol. 2007;109:81–87. doi: 10.1097/01.AOG.0000249605.11458.ac. [DOI] [PubMed] [Google Scholar]
- 12.Leet T, Flick L. Effect of exercise on birthweight. Clin Obstet Gynecol. 2003;46:423–431. doi: 10.1097/00003081-200306000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;3:CD000180. doi: 10.1002/14651858.CD000180.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatch MC, Shu XO, McLean DE, et al. Maternal exercise during pregnancy, physical fitness, and fetal growth. Am J Epidemiol. 1993;137:1105–1114. doi: 10.1093/oxfordjournals.aje.a116614. [DOI] [PubMed] [Google Scholar]
- 15.Juhl M, Olsen J, Andersen PK, Nohr EA, Andersen AM. Physical exercise during pregnancy and fetal growth measures: a study within the Danish National Birth Cohort. Am J Obstet Gynecol. 2010;202:63.e61–63.e68. doi: 10.1016/j.ajog.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Alderman BW, Zhao H, Holt VL, Watts DH, Beresford SA. Maternal physical activity in pregnancy and infant size for gestational age. Ann Epidemiol. 1998;8:513–519. doi: 10.1016/s1047-2797(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 17.Owe KM, Nystad W, Bo K. Association between regular exercise and excessive newborn birth weight. Obstet Gynecol. 2009;114:770–776. doi: 10.1097/AOG.0b013e3181b6c105. [DOI] [PubMed] [Google Scholar]
- 18.Voldner N, Froslie KF, Bo K, et al. Modifiable determinants of fetal macrosomia: role of lifestyle-related factors. Acta Obstet Gynecol Scand. 2008;87:423–429. doi: 10.1080/00016340801989825. [DOI] [PubMed] [Google Scholar]
- 19.Hegaard HK, Petersson K, Hedegaard M, et al. Sports and leisure-time physical activity in pregnancy and birth weight: a population-based study. Scand J Med Sci Sports. 2010;20:e96–102. doi: 10.1111/j.1600-0838.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- 20.Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188:1372–1378. doi: 10.1067/mob.2003.302. [DOI] [PubMed] [Google Scholar]
- 21.Hediger ML, Overpeck MD, McGlynn A, Kuczmarski RJ, Maurer KR, Davis WW. Growth and fatness at three to six years of age of children born small- or large-for-gestational age. Pediatrics. 1999;104:e33. doi: 10.1542/peds.104.3.e33. [DOI] [PubMed] [Google Scholar]
- 22.Holzman C, Bullen B, Fisher R, Paneth N, Reuss L. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr Perinat Epidemiol. 2001;15 (Suppl 2):136–158. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 23.Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence EJ. A Matter of Size: Part 2 Evaluating the Large-for-Gestational-Age Neonate. Adv Neonatal Care. 2007;7:187–197. doi: 10.1097/01.ANC.0000286335.06047.28. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21:521–526. doi: 10.1097/GCO.0b013e328332d24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Koenker R, Hallock K. Quantile Regression. J Econ Perspect. 2001;51:143–156. [Google Scholar]
- 28.Osler M, Kriegbaum M, Christensen U, Holstein B, Nybo Andersen AM. Rapid report on methodology: does loss to follow-up in a cohort study bias associations between early life factors and lifestyle-related health outcomes? Ann Epidemiol. 2008;18:422–424. doi: 10.1016/j.annepidem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Martikainen P, Laaksonen M, Piha K, Lallukka T. Does survey non-response bias the association between occupational social class and health? Scand J Public Health. 2007;35:212–215. doi: 10.1080/14034940600996563. [DOI] [PubMed] [Google Scholar]
- 30.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 31.Clapp JF. Influence of Endurance Exercise and Diet on Human Placental Development and Fetal Growth. Placenta. 2006;27:527–534. doi: 10.1016/j.placenta.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Bung P, Artal R, Khodiguian N, Kjos S. Exercise in gestational diabetes. An optional therapeutic approach? Diabetes. 1991;40 (Suppl 2):182–185. doi: 10.2337/diab.40.2.s182. [DOI] [PubMed] [Google Scholar]
- 33.Pivarnik JM, Mauer MB, Ayres NA, Kirshon B, Dildy GA, Cotton DB. Effects of chronic exercise on blood volume expansion and hematologic indices during pregnancy. Obstet Gynecol. 1994;83:265–269. [PubMed] [Google Scholar]
- 34.Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS) Soz Praventivmed. 2001;46 (Suppl 1):S3–42. [PubMed] [Google Scholar]
- 35.Bauer PW, Pivarnik JM, Feltz DL, Paneth N, Womack CJ. Validation of a historical physical activity recall tool on postpartum women. J Phys Act Health. 2010;7:658–661. doi: 10.1123/jpah.7.5.658. [DOI] [PubMed] [Google Scholar]
- 36.Chasan-Taber L, Erickson JB, Nasca PC, Chasan-Taber S, Freedson PS. Validity and reproducibility of a physical activity questionnaire in women. Med Sci Sports Exerc. 2002;34:987–992. doi: 10.1097/00005768-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 37.DuBose KD, Edwards S, Ainsworth BE, Reis JP, Slattery ML. Validation of a historical physical activity questionnaire in middle-aged women. J Phys Act Health. 2007;4:343–355. doi: 10.1123/jpah.4.3.343. [DOI] [PubMed] [Google Scholar]
- 38.Bowles HR, FitzGerald SJ, Morrow JR, Jr, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160:279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- 39.Buka SL, Goldstein JM, Spartos E, Tsuang MT. The retrospective measurement of prenatal and perinatal events: accuracy of maternal recall. Schizophr Res. 2004;71:417–426. doi: 10.1016/j.schres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Croteau A, Marcoux S, Brisson C. Work activity in pregnancy, preventive measures, and the risk of delivering a small-for-gestational-age infant. Am J Public Health. 2006;96:846–855. doi: 10.2105/AJPH.2004.058552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takito MY, Benicio MH. Physical activity during pregnancy and fetal outcomes: a case-control study. Rev Saude Publica. 2010;44:90–101. doi: 10.1590/s0034-89102010000100010. [DOI] [PubMed] [Google Scholar]