Abstract
Background
Individuals with a family history of alcoholism (FH+) are at enhanced risk of developing alcohol or other substance use disorders relative to those with no family history (FH−). Alcoholics and FH+ subjects have greater interference scores on the Stroop color-word task, suggesting these impairments may be a component of the cognitive phenotype of at-risk individuals.
Methods
In the present study, we examined whole brain activations in 24 FH+ and 28 FH-young adults performing the counting Stroop task, a variant of the Stroop task adapted for neuroimaging studies.
Results
Across all subjects, incongruent versus congruent comparisons showed activations in regions including parietal lobe areas, frontal eye fields, premotor areas, the anterior cingulate cortex, dorsolateral prefrontal cortex, and bilateral insula, indicating typical regions of activation involved in conflict-resolution tasks. Compared to FH− participants, FH+ participants had greater activations in the left superior parietal lobule and precuneus (BA 7 and 19), inferior parietal lobule (BA 40), and middle temporal gyrus (BA 39 and 19), indicating a predominance of greater left hemisphere activity among FH+ in temporoparietal regions. There were no regions showing greater activations in the FH− group compared to the FH+ group.
Conclusions
These results are consistent with less efficient cognitive functioning potentially due to poorer communication over long pathways connecting temporoparietal regions to prefrontal brain regions that participate in a distributed network involved in cognitive processing and working memory necessary for conflict resolution.
Keywords: Counting Stroop task, family history, temporoparietal, cognition, vulnerability
Introduction
Individuals with a family history of alcohol and other drug use disorders (FH+) are at increased risk for developing these same disorders compared to those with no such history (FH−) (Finn et al., 1990, Lieb et al., 2002, Merikangas et al., 1998). Although the FH+ associated risk has a strong genetic component (Reich et al., 1998, Slutske et al., 2002, Merikangas, 1990, Cloninger et al., 1981), the specific biological mechanisms mediating this risk remain elusive. In real world settings, FH+ is linked with a pattern of “behavioral undercontrol” (Sher et al., 2004, Sher and Trull, 1994) or “neurobehavioral disinhibition” (Tarter et al., 2003), patterns that include increased sensation seeking, risk-taking, aggressiveness, and antisocial behaviors. Similarly, laboratory behavioral assessments reveal that FH+ is associated with subtle deficits on measures of executive functioning, impulse control, decision-making, and attention (e.g., Stevens et al., 2003, Deckel, 1999, Corral et al., 2003, Acheson et al., 2011a, Acheson et al., 2011b, Lovallo et al., 2006). Emerging functional imaging research indicates that FH+ appear to have altered patterns of brain activity during such tasks (e.g., Schweinsburg et al., 2004, Acheson et al., 2009, Silveri et al., 2011, Heitzeg et al., 2010, Cservenka et al., 2012, Glahn et al., 2007). Improved understanding of how neural functioning is altered in FH+ individuals could substantially advance our understanding of underlying biological vulnerabilities to alcohol and other substance use disorders.
We have shown in a large sample study that FH+ subjects have modest performance impairments relative to FH− on the traditional Stroop color-word task, as indicated by their greater interference scores (longer times to name text colors in incongruent word-color pairings vs. reading words) (Stroop, 1935, Lovallo et al., 2006). Given that Stroop deficits are also seen in alcoholics and other populations with addictive disorders (Vadhan et al., 2007), these impairments may be a component of the cognitive phenotype of at-risk individuals. Recently, Silveri and colleagues (2011) reported brain activations differences within a frontolimbic region of interest in FH+ versus FH+ adolescents while performing the classic Stroop Color-Word task. However, by restricting the analyses to these regions, this study was not able to examine other brain regions implicated in Stroop task performance such as the parietal cortex and precuneus (BA 7) (Bush et al., 1998, Matthews et al., 2004). In the present study, we sought to extend these findings by examining whole brain activations in FH+ and FH− young adults performing a counting version of the Stroop task (Bush et al., 1998). This task induces comparable activation patterns as traditional Stroop tasks, however it is less prone to data loss due to excessive head movement from speaking. We hypothesized that FH+ individuals would also show altered activity in both forebrain as well as non-forebrain regions known to be involved in Stroop task performance.
Methods
Subjects
28 FH− individuals and 24 FH+ were recruited from a larger cohort of 450 volunteers participating in the Oklahoma Family Health Patterns (OFHP) project intended to identify cognitive, psychological, behavioral, and physiological characteristics of healthy young adults at elevated risk of alcoholism. Characteristics of the FH groups are given in Table 1.
Table 1.
Sample Characteristics
| Family History | FH− | FH+ | p Values |
|---|---|---|---|
| N (M/F) | 28 (12/16) | 24 (5/19) | |
| Age (Years) | 24.0 (2.6) | 23.6 (4.0) | 0.7 |
| Education (Years) | 15.6 (1.4) | 15.4 (2.1) | 0.6 |
| SES | 51 (12) | 42 (13) | 0.01 |
| Shipley Vocabulary | 31 (4.2) | 31 (3.5) | 0.5 |
| Ethnicity | 0.006 | ||
| Caucasian | 28 | 18 | |
| African American | 0 | 6 | |
| Other | 0 | 0 | |
| Hispanic | 1 | 2 | 0.6 |
| CPI-So | 31.6 (4.2) | 30.8 (4.0) | 0.5 |
| BDI | 4.4 (5.8) | 4.0 (4.4) | 0.8 |
| EPI-Neuroticism | 5.5 (4.1) | 4.0 (3.5) | 0.8 |
| Narcissism | 1 | 1 | 0.9 |
| Histrionic | 0 | 0 | . |
| Borderline | 1 | 0 | 0.9 |
| Antisocial | 0 | 3 | 0.09 |
| AUDIT | 4.9 (3.0) | 4.0 (2.6) | 0.4 |
| Age at First Drink | 16.8 (2.8) | 15.2 (4.0) | 0.1 |
| Alcohol Intake (oz/mo) |
34 (47) | 19 (30) | 0.2 |
| Past Depression | 3 | 2 | 0.7 |
| Past Alcohol Abuse | 2 | 1 | 0.6 |
| Caffeine (mg/day) | 167 (177) | 167 (255) | 0.9 |
| Tobacco (N Using Weekly) |
1 | 1 | 0.9 |
Entries (mean ± SD) unless given otherwise. SES scores shown are considered “Middle Class.” FH−, negative family history; FH+, positive family history; M, male; F, female; SES, Hollingshead & Redlich Socioeconomic Status Score; Shipley Vocabulary, Shipley Institute of Living Vocabulary Score; CPI-So, California Personality Inventory Sociability Scale; BDI, Beck Depression Inventory; EPI, Eysenck Personality Inventory; AUDIT, Alcohol Use Disorders Identification Test.
Inclusion and exclusion criteria
Prospective volunteers were excluded if they had: a history of alcohol or drug dependence; met criteria for substance abuse within the past 2 months; failed a urine drug screen or a breath-alcohol test on days of testing; or who had a history of any Axis I disorder other than past depression (> 60 days), as defined by the Diagnostic and Statistical Manual of Mental disorders, 4th ed. (APA, 2000). Subjects were also excluded if they met criteria for an Axis II disorder in clusters A or C. Cluster B diagnoses were not exclusionary due to the known overlap between risk for alcoholism and antisocial tendencies. Psychiatric history was obtained by the computerized Diagnostic Interview Schedule-IV (CDIS-IV) conducted by a certified research assistant under the direction of a licensed clinical psychologist, and through the Beck Depression Inventory II (Beck et al., 1996). Women were required to have a negative urine pregnancy test on each day of testing. All participants were in good physical health, had a body mass index < 30, were not taking prescription medications, had no reported history of serious medical disorder, and were right handed as assessed with the Toronto Handedness Scale. Smoking and smokeless tobacco use were not exclusionary.
All participants signed consent forms approved by the Institutional Review Boards of the University of Oklahoma Health Sciences Center and the Veterans Affairs Medical Center in Oklahoma City, OK and at the University of Texas Health Sciences Center, San Antonio, TX and were paid for their participation.
Family history of alcohol and other drug use disorders
Family history classification was established using the Family History Research Diagnostic Criteria (FH-RDC; Andreasen et al., 1977). The FH-RDC has a high degree of interrater reliability (.95) for reports of substance use disorders (Andreasen et al., 1977, Zimmerman et al., 1988). In the neuroimaging studies emerging from the OFHP, family history reports by volunteers were all confirmed by parent report. All FH+ participants reported that at least one biological parent met at least 2 of the possible 6 criteria for alcohol or substance abuse. Participants were excluded if either they or the parent reported possible fetal exposure to alcohol or other drugs.
Procedure
Stroop task
We used a counting version of the Stroop task that did not require reading aloud and that was adapted for use in the MRI environment administered as a block design task (Bush et al., 1998). This version consisted of a 6 second welcome screen with task instructions followed by 5 congruent trial blocks alternating with 5 incongruent trial blocks. Each trial block had 20 trials, and each trial lasted 1.5 seconds. Each trial in congruent trial blocks showed 1–4 semantically related words (e.g. “lion, tiger, cheetah”) and the subject pressed a button indicating how many words appeared. Trials on the incongruent trial blocks were identical except that the words presented were incompatible number words (e.g., the word “one” printed two times).
Imaging Acquisition
Imaging was carried out on a research-dedicated Siemens 3T MRI (Siemens, Munich, Germany) housed in the Research Imaging Institute at UTHSCSA. Functional imaging used a gradient-echo, echo-planar sequence, acquiring 30 slices (4 mm thick, 1 mm gap) parallel to the anterior commissure-posterior commissure (AC-PC) plane (repetition time/echo time [TR/TE] = 3000/30 msec, 128 × 128 × 5 mm, and field of view [FOV] = 256 mm). For anatomical reference, we acquired a high-quality three-dimensional (3-D) image (TR/TE = 2200/2.83 msec, and flip angle = 13°, 0.8 mm isotropic). The imaging session was completed in one hour.
Analysis of fMRI Data
Analysis of functional images was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl; Smith et al., 2004). Prior to statistical modeling, data were motion corrected (Woolrich et al., 2001), non-brain tissue was removed (Jenkinson et al., 2002, Jenkinson and Smith, 2001), spatially smoothed with a Gaussian kernel of FWHM 5mm and a high-pass temporal filtering was applied (Gaussian-weighted least-squares straight line fitting, with sigma=50.0s). Time-series statistical analysis was carried out using local autocorrelation correction (Beckmann et al., 2003, Woolrich et al., 2004). Registration to high resolution and/or standard images was carried out using FLIRT (Worsley et al., 1992). Higher-level analysis was carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) with a mixed-effects model (Beckmann et al., 2003, Woolrich et al., 2004) to generate z statistical images for the incongruent versus congruent conditions. Group maps for all subjects and FH− versus FH+ were generated using conservative cluster thresholds (corrected p < 0.05, z ≥ 1.96; Woolrich et al., 2005) .
Results
Behavioral results
The FH groups performances on the congruent and incongruent trial blocks over the course of the task were not significantly different (Table 2),
Table 2.
Performance on the Stroop task
| Reaction Time (msec) | FH+ | FH − | p-value |
| Interference | 1437 (391) | 1362 (144) | 0.4 |
| No interference | 1260 (350) | 1227 (144) | 0.7 |
| Difference | 176 (228) | 135 (162) | 0.5 |
|
Accuracy (% correct
responses) |
FH+ | FH − | p-value |
| Interference | 78 (22) | 86 (14) | 0.2 |
| No interference | 82 (23) | 90 (13) | 0.1 |
| Difference | −3.17 (6) | −3.89 (4) | 0.6 |
Entries show M ± SD
Imaging results
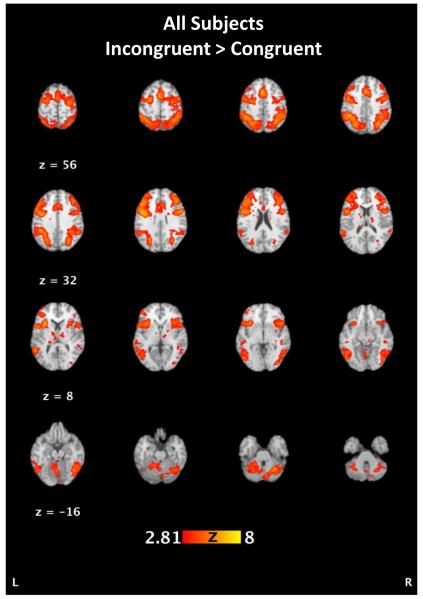
Across all subjects, incongruent versus congruent comparisons showed activations in regions including parietal lobe areas (BA 7, 19, and 40 including bilateral precuneous, left inferior parietal lobule, and right superior parietal gyrus), frontal eye fields (precentral gyrus, BA 6), premotor areas (BA 8 and 9) the anterior cingulate cortex (BA 24, 32, 33), dorsolateral prefrontal cortex (BA 46), and bilateral insula (BA 13) (Figure 1, Table 3). This pattern indicates significant involvement in parietal regions, anterior cingulate, and dorsolateral prefrontal cortex in accord with the demands of the task, involving resolution of response competition during incongruent trial blocks.
Figure 1.
Areas activated across all subjects map for the incongruent vs congruent contrast. See Table 3 for details.
Table 3.
Activation across all subjects
| Talaraich space (mm) | Region | Brodmann Area | z score | Cohen’s d |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left Hemisphere | ||||||
| −22 | −62 | 40 | Precuneus | 7 | 6.22 | 1.73 |
| −38 | −44 | 38 | Inf Parietal Lobule | 40 | 6.71 | 1.86 |
| −56 | −46 | 10 | Sup Temporal Gyrus | 22 | 5.42 | 1.50 |
| −42 | −58 | 6 | Fusiform Gyrus | 37 | 5.66 | 1.57 |
| −8 | 6 | 28 | Ant Cingulate Gyrus | 24 | 4.95 | 1.37 |
| −2 | 10 | 46 | “ | 32 | 6.8 | 1.89 |
| −50 | 12 | 32 | “ | 9 | 6.24 | 1.73 |
| −16 | 4 | 60 | “ | 6 | 4.8 | 1.33 |
| −24 | −4 | 58 | “ | 6 | 5.15 | 1.43 |
| −24 | −8 | 56 | “ | 6 | 5.19 | 1.44 |
| −26 | −6 | 48 | “ | 6 | 6.23 | 1.73 |
| −34 | −10 | 40 | “ | 6 | 5.51 | 1.53 |
| −36 | 32 | 22 | “ | 46 | 6.41 | 1.78 |
| −40 | 38 | 14 | “ | 46 | 5.29 | 1.47 |
| −44 | 44 | 2 | Inf Frontal Gyrus | – | 4.66 | 1.29 |
| −34 | −2 | 30 | Precentral Gyrus | 6 | 7.19 | 1.99 |
| −40 | 0 | 28 | “ | 6 | 7.16 | 1.99 |
| −48 | 6 | 6 | Insula | 13 | 5.52 | 1.53 |
| −42 | 14 | 6 | “ | 13 | 5.97 | 1.66 |
| −28 | 20 | −4 | “ | 47 | 5.65 | 1.57 |
| −30 | 12 | 8 | “ | – | 6.35 | 1.76 |
| −14 | −59 | −24 | Cerebellum | – | 5.37 | 1.49 |
| Right Hemisphere | ||||||
| 32 | −60 | 42 | Sup Parietal Lobule | 7 | 6.42 | 1.78 |
| 26 | −66 | 54 | “ | 7 | 5.32 | 1.48 |
| 16 | −72 | 48 | Precuneus | 7 | 4.72 | 1.31 |
| 30 | −72 | 32 | “ | 19 | 5.94 | 1.65 |
| 44 | −76 | 14 | Fusiform Gyrus | 19 | 5.5 | 1.53 |
| 50 | 6 | 26 | Inf Frontal Gyrus | 9 | 6.24 | 1.73 |
| 44 | 32 | 26 | Middle Frontal Gyrus | 9 | 6.36 | 1.76 |
| 40 | 0 | 28 | Precentral Gyrus | 6 | 5.21 | 1.44 |
| 44 | −2 | 38 | “ | 6 | 5.33 | 1.48 |
| 50 | 2 | 36 | “ | 6 | 4.99 | 1.38 |
| 0 | 2 | 48 | Ant Cingulate Gyrus | 24 | 5.99 | 1.66 |
| 4 | 22 | 30 | “ | 32 | 5.37 | 1.49 |
| 32 | 16 | 10 | Insula | 13 | 5.69 | 1.58 |
| 0 | −64 | −16 | Cerebellum | – | 5.23 | 1.45 |
| 12 | −74 | −26 | “ | – | 5.36 | 1.49 |
| 26 | −64 | −30 | “ | – | 5.83 | 1.62 |
| 32 | −58 | −28 | “ | – | 5.89 | 1.63 |
| 42 | −62 | −16 | “ | – | 5.63 | 1.56 |
p< 0.0001, z ≥ 4.00
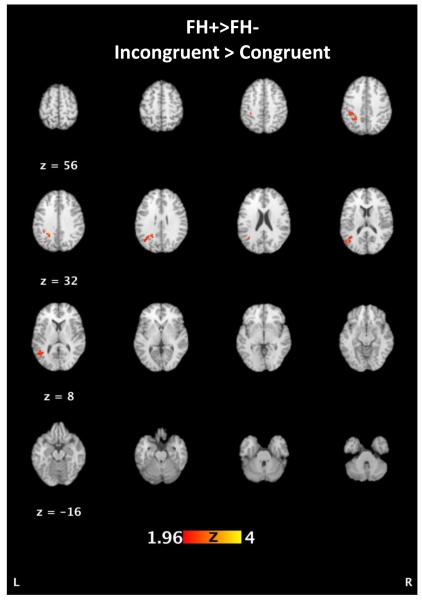
We next examined statistical activation maps contrasting FH+ vs. FH− groups. These contrasts demonstrated relatively greater activations for FH+ in the left superior parietal lobule and precuneus (BA 7 and 19), inferior parietal lobule (BA 40), and middle temporal gyrus (BA 39 and 19) (Figure 2, Table 4). This pattern shows a predominance of greater left hemisphere activity among FH+ in temporoparietal regions. There were no regions showing greater activations in the FH− group compared to the FH+ group. Cohen’s d effect sizes were calculated for the z-scores of the significant clusters from the group differences.
Figure 2.
Activation differences in FH− and FH+ individuals for the incongruent vs congruent contrast. FH+ participants had greater activations in the left superior parietal lobule and precuneus (BA 7 and 19), inferior parietal lobule (BA 40), and middle temporal gyrus (BA 39 and 19; see Table 4).
Table 4.
Group Activation Differences, FH+ > FH−
| Talaraich | space | (mm) | Region | Brodmann Area | z score | Cohen’s d |
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| −24 | −58 | 42 | L Superior Parietal Lobule | 7 | 2.16 | 0.60 |
| −20 | −58 | 40 | Precuneus | 7 | 2.18 | 0.61 |
| −22 | −50 | 40 | “ | 7 | 2.98 | 0.83 |
| −24 | −46 | 38 | “ | 7 | 3.05 | 0.85 |
| −32 | −70 | 30 | “ | 19 | 2.16 | 0.60 |
| −30 | −36 | 40 | L Inferior Parietal Lobule | 40 | 3.07 | 0.85 |
| −32 | −52 | 40 | “ | 40 | 2.09 | 0.58 |
| −36 | −36 | 38 | “ | 40 | 2.93 | 0.81 |
| −38 | −68 | 28 | L Middle Temporal Gyrus | 39 | 2.29 | 0.63 |
| −34 | −58 | 22 | “ | 39 | 3.25 | 0.90 |
| −50 | −68 | 14 | “ | 39 | 2.69 | 0.74 |
| −40 | −66 | 14 | “ | 39 | 2.59 | 0.72 |
| −36 | −56 | 12 | “ | 19 | 2.83 | 0.79 |
| −40 | −60 | 10 | “ | 19 | 2.90 | 0.80 |
p< 0.05, z ≥ 1.96
Discussion
In the present study we observed that a family history of alcoholism (FH+ vs. FH−) was positively related to greater activation in the left parietal lobe and a closely associated extension of the left temporal lobe. The FH groups did not significantly differ on reaction time change during incongruent trials or in reduced percent correct, suggesting the greater parietal and temporal lobe activations in this group may imply a lower level of efficiency in performing the task. The results for the whole group indicated that the pattern of cerebral activation is consistent with other imaging studies of the Stroop task suggesting that the task activations were typical for the demands imposed by the interference condition (Laird et al., 2005). The differences in activation patterns in the FH groups suggest potential for future work on functional differences associated with risk for alcoholism.
The classic Stroop Color-Word interference effect on incongruent trials reflects the time required to suppress a prepotent response (reading the word) and choosing the correct response modality (ink color) and then providing that response (speaking the ink color) (Stroop, 1935). Smith and Jonides (1999) have shown that the executive processes necessary for overcoming Stroop interference engage the anterior cingulate gyrus and dorsolateral prefrontal cortex. The counting Stroop (Bush et al., 1998) is a variation of the task developed for neuroimaging studies that avoids the use of speech and induces similar activation contrasts on incongruent vs. congruent trial blocks, including the anterior cingulate cortex (BA 24 and 32), dorsolateral prefrontal cortex (BA 9 and 46), frontal eye fields (BA 6, 8), higher level visual association cortex (BA 19), and the superior parietal lobule and precuneus (BA 7) (Bush et al., 1998, Matthews et al., 2004). Our findings in the present study also accord with findings from imaging studies of other conflict tasks, indicating that the dorsal anterior cingulate is involved with conflict identification and engages the dorsolateral prefrontal cortex to resolve the conflict (Carter and van Veen, 2007, Peterson et al., 1999).
To date, one previous neuroimaging study has examined Stroop-task activations in FH groups. Silveri and colleagues (2011) compared activations within a frontolimbic region of interest in adolescents performing a variant of the color-word Stroop, and found that FH+ adolescents averaging 13 years of age, had greater activation in left middle frontal gyrus (BA 6) and insula (BA 13). In contrast, in the present study, our whole-brain analysis of counting Stroop activations in young adult FH groups revealed that FH+ adults, averaging 24 years of age, had greater activity in temporoparietal regions (BA 7, 19, 39, 40). It is possible that Silveri et. al. may have observed results similar to ours had they also examined structures outside of their region of interest. Alternatively, these differences in the studies may result from the age differences in the subject samples and specific differences in task requirements, as the Silveri task called for spoken responses and the use of colored words, while ours avoided the use of speech and but required tracking numbers of objects appearing on the screen and manual responding. A number of studies show that the precuneus and superior parietal lobule become engaged in tasks calling for hand-eye coordination and object matching and grasping (Culham and Kanwisher, 2001).
While both our and the Silveri et. al (2011) study found increased activations in FH+ individuals during Stoop task performance, studies of brain activity during Stroop interference comparing controls to persons with impulse control and substance use disorders have shown hypoactivations in regions such as parietal areas and anterior cingulate gyrus, along with dorsolateral and ventromedial prefrontal cortex (Bush et al., 1999, Potenza et al., 2003, Barros-Loscertales et al., 2011). The lack of significant hypoactivations in FH+ individuals may be due to excluding individuals with comorbid psychiatric conditions and thus examining individuals with more normative brain functioning. The left parietal and temporoparietal hyperactivations we observed in the present study do suggest that circuits involving processing the language components of the task were affected, perhaps due to less efficient regulation of these circuits by frontal cortical regions.
The present study and the Silveri et al. study (Silveri et al., 2011) implicate a constellation of temporoparietal and prefrontal areas seen as encompassing a network of regions involved in control of attention, decision-making, and working memory necessary for successful Stroop performance. In an influential review of the primate literature involving anatomical tracing techniques, Goldman-Rakic (1998) showed that cognitive processing utilizes a network of regions with the parietal association cortex at the center of a reciprocal distributed network including: visual association cortex, superior temporal gyrus, parahippocampal gyrus, anterior and posterior cingulate cortex, and dorsolateral prefrontal cortex (terms translated into human neuroanatomical features). The foregoing review of the Stroop imaging literature encompasses this same set of regions. We may speculate that in both our study and the Silveri et al. study FH+ individuals had less efficient functioning within given regions or poorer communication across the distributed cognitive network as described by Goldman-Rakic. It is noteworthy that the concept of cognitive efficiency has been used to account for neuropsychological performance deficits in studies of abstinent alcoholics (Lawton-Craddock et al., 2003, Nixon et al., 2002). The potential for poorer efficiency in carrying out tasks of working memory in both substance abusers and nonabusing FH+ suggests the possible conclusion that some cognitive deficits seen in alcohol and other-substance abusers may represent a risk factor rather than a consequence of the disorders.
There are strengths and limitations to the present study. Strengths include a well-characterized subject population with confirmed family histories, no current co-morbid conditions and little problem drinking as indicated by the low AUDIT scores. Limitations include that we were only able to scan a subset of participants from the full cohort of subjects the OFHP project due to constraints in budget and subject availability. Furthermore, both subject groups contained more women than men, and there were socioeconomic and racial differences between the groups as well. Controlling for socioeconomic status in preliminary analyses did not affect the FH group differences, however attempting to control for race and gender did affect these outcomes. We did not attempt to model the influence of race and gender in our final analyses because we were not adequately powered to examine the contributions of these factors. While we do not feel that race would influence our findings beyond that accounted for by socioeconomic differences, it is possible that gender effects such influences of sex hormones may have affected our results. Additionally given evidence for altered default mode activity in FH+ individuals (Spadoni et al., 2008), it is possible that differences in baseline brain activity may have influenced our outcomes. Finally, we did not observe Stroop task performance differences, but the Stroop task differences we reported previously were of modest effect size and unlikely to be detected with the sample size of the present study (Lovallo et al., 2006). Additionally while the differences observed between groups was not statistically significant, they were all in the same direction with our previously observed findings in the larger sample and suggest a tendency towards poorer Stroop task performance for FH+ adults.
At present it is difficult to tightly relate the brain activation differences seen here to FH classification and to a behavioral phenotype associated with risk for alcoholism. Although Stroop performance was similar for the two FH groups, we were able to detect impairments in FH+ subjects on Stroop performance in a much larger sample of subjects (Lovallo et al., 2006), and the activation differences seen here may relate to these relatively subtle behavior differences. Furthermore, differences in processing information that call for greater cerebral resource utilization by FH+ may indicate a reduced level of cognitive control over behavior or the potential for greater lapses in attention that may result in a long-term pattern of disinhibited behavior. Studies replicating the present one are therefore called for as well as studies of white matter integrity in pathways connecting distant brain regions involved in the distributed network of regions involved in cognition generally. We have consistently found a pattern of antisocial and disinhibitory behavior in FH+ persons in the OFHP cohort (Acheson et al., 2011b, Saunders et al., 2008, Yechiam et al., 2006), and these differences in temperament may similarly reflect a less tightly regulated pattern of behavior, again involving a need for greater allocation of effort to maintain behavioral control.
In conclusion, we report that while undergoing fMRI scanning, FH+ adults relative to FH− controls showed greater activations in temporoparietal regions while performing a counting Stroop-task. These results are consistent with less efficient cognitive functioning or poorer communication over long pathways connecting these posterior regions to more anterior brain areas that participate in a distributed network of regions involved in cognitive processing and working memory.
Acknowledgments
Supported by the Department of Veterans Affairs Medical Research Service, Merit Award CX000252, the National Institutes of Health, NIAAA, grants AA12207 and AA19691. The content is solely the view of the authors and does not necessarily represent the official view of the National Institutes of Health or the VA.
References
- Acheson A, Richard DM, Mathias CW, Dougherty DM. Adults with a family history of alcohol related problems are more impulsive on measures of response initiation and response inhibition. Drug Alcohol Depend. 2011a;117:198–203. doi: 10.1016/j.drugalcdep.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater Discounting of Delayed Rewards in Young Adults with Family Histories of Alcohol and Drug Use Disorders: Studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2011b;35(9):1607–13. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Stitzer RL, Winokur G. The family history method using diagnostic criteria. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. 4th ed American Psychiatric Association; Washington, D.C: 2000. [Google Scholar]
- Barros-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res. 2011;194:111–118. doi: 10.1016/j.pscychresns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: Cross fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Corral M, Holguin SR, Cadaveira F. Neuropsychological characteristics of young children from high-density alcoholism families: a three-year follow-up. J Stud Alcohol. 2003;64:195–199. doi: 10.15288/jsa.2003.64.195. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug Alcohol Depend. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Deckel AW. Tests of executive functioning predict scores on the MacAndrew Alcoholism Scale. Progress in neuro-psychopharmacology & biological psychiatry. 1999;23:209–223. doi: 10.1016/s0278-5846(98)00108-0. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kleinman I, Pihl RO. The lifetime prevalence of psychopathology in men with multigenerational family histories of alcoholism. The Journal of Nervous and Mental Disease. 1990;178:500–504. [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biological psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Annual Reviews of Neuroscience. 1998;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zucker RA, Zubieta JK. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton-Craddock A, Nixon SJ, Tivis R. Cognitive efficiency in stimulant abusers with and without alcohol dependence. Alcohol Clin Exp Res. 2003;27:457–464. doi: 10.1097/01.ALC.0000056620.98842.E6. [DOI] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychological medicine. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcoholism, Clinical and Experimental Research. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage. 2004;22:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Merikangas KR. The genetic epidemiology of alcoholism. Psychological medicine. 1990;20:11–22. doi: 10.1017/s0033291700013192. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Archive of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Progress in neuro-psychopharmacology & biological psychiatry. 2002;26:919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. The American journal of psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr., Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. American Journal of Medical Genetics. 1998;81:207–215. [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task in persons with a positive family history of alcoholism Alcoholism Clinical and Experimental Research. 2008;32(5):888–94. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Reviews of Clinical Psychology. 2004;22:1–22. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during Stroop performance. Alcohol Clin Exp Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. Journal of Abnormal Psychology. 2002;111:124–133. [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kaplan RF, Hesselbrock VM. Executive-cognitive functioning in the development of antisocial personality disorder. Addict Behav. 2003;28:285–300. doi: 10.1016/s0306-4603(01)00232-5. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American Journal of Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Carpenter KM, Copersino ML, Hart CL, Foltin RW, Nunes EV. Attentional bias towards cocaine-related stimuli: relationship to treatment-seeking for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:727–736. doi: 10.1080/00952990701523722. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Smith SM. Mixture models with adaptive spatial regularization for segmentation with an application to FMRI data. Ieee Transactions on Medical Imaging. 2005;24:1–11. doi: 10.1109/tmi.2004.836545. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yechiam E, Goodnight J, Bates JE, Busemeyer JR, Dodge KA, Pettit GS, Newman JP. A formal cognitive model of the go/no-go discrimination task: evaluation and implications. Psychol Assess. 2006;18:239–249. doi: 10.1037/1040-3590.18.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Pfohl B, Stangl D. The reliability of the family history method for psychiatric diagnoses. Arch. Gen. Psychiatry. 1988;45:320–322. doi: 10.1001/archpsyc.1988.01800280030004. [DOI] [PubMed] [Google Scholar]