Abstract
Despite high rates of early revascularization with intra-arterial stroke therapy, the clinical efficacy of this approach has not been clearly demonstrated. Neuroimaging biomarkers will be useful in future trials for patient selection and for outcomes evaluation. To identify patients who are likely to benefit from intra-arterial therapy, the combination of vessel imaging, infarct size quantification and degree of neurologic deficit appears critical. Perfusion imaging may be useful in specific circumstances, but requires further validation. For measuring treatment outcomes, surrogate biomarkers that appear suitable are angiographic reperfusion as measured by the modified Thrombolysis in Cerebral Infarction scale and final infarct volume.
Keywords: Acute ischemic stroke, CT, Intra-arterial, Endo-vascular, MRI, Neuroimaging, Reperfusion therapy
INTRODUCTION
In acute ischemic stroke patients, early revascularization is the only proven beneficial therapy.31,82 Intra-arterial treatment (IAT) improves revascularization of proximal artery occlusions compared to intravenous tissue plasminogen activator (IV tPA).10 Despite this fact, three recent randomized controlled trials (RCTs) have shown equivalent clinical outcomes between intervention and standard medical management.13,21,46 In this context, neuroimaging biomarkers may play a critical role in future studies. This article will discuss the utility of neuroimaging for enhancing patient selection and improving outcome evaluation.
NEUROIMAGING BIOMARKERS FOR PREDICTING THE CLINICAL RESPONSE TO INTRA-ARTERIAL TREATMENT
Is Advanced Neuroimaging Worth the Time Expense?
The chance of a good outcome after IAT decreases with longer time from stroke onset to revascularization.45 Data from the recent Interventional Management of Stroke (IMS) III trial (Table 1) reveal a 10% relative reduction in the probability of good outcome [90-day modified Rankin Scale (mRS) score 0–2] for every 30-min delay in reperfusion, after adjusting for other predictors (Dr. Pooja Khatri, 2013 International Stroke Conference presentation). This has placed an emphasis on rapid treatment, leading some physicians to forego advanced neuroimaging due to fear of treatment delay.
TABLE 1.
Patient selection criteria and primary results of the major recent trials of intra-arterial therapy
| Trial | No. of patients | Design | Treatment arms | Clinical criteria | Imaging criteria |
|---|---|---|---|---|---|
| IMS III | 656 (stopped enrollment due to futility; target was 900) | Randomized controlled trial | Combined IV tPA + IAT vs. IV tPA | NIHSS ≥ 10*; anterior and posterior circulation; IV tPA < 3 h; start IAT < 5 h and completed < 7 h; Age 18–82 | NCCT: Infarct (clear hypodensity) ≤ 1/3 of the MCA territory£, no ICH, midline shift or tumor§ |
| SYNTHESIS Expansion | 362 | Randomized controlled trial | IAT vs. IV tPA | NIHSS ≤ 25; anterior and posterior circulation; IV tPA < 4.5 h; start IAT < 6h; Age 18–80 | NCCT: no evidence of acute infarction (i.e., well-defined hypodensity), no ICH. no tumor |
| MR-RESCUE | 127 (analysis restricted to 118) | Randomized controlled trial | IAT vs. standard care¥ | NIHSS 6–29; anterior circulation only: randomization < 8 h after LSW; Age 18–85 | Multimodal CT and/or MRI: large-vessel proximal anterior circulation occlusion (ICA, M1, or M2 MCA) |
| DEFUSE 2 | 138 (mismatch assignment and endovascular therapy in 104) | Prospective cohort study | IAT in entire cohort | NIHSS ≥ 5; start IAT < 12 h; Age > 17 | Baseline MRI within 90 min of treatment (GRE, MRA, DWI and PWI) |
| Primary outcome | Prespecified imaging analysis | Primary outcome (IAT vs. control) | Symptomatic ICH (IAT vs. control) |
Mortality (IAT vs. control) |
|---|---|---|---|---|
| mRS score ≤ 2 at 90 days | N/A | 40.8 vs. 38.7% | 6.2 vs. 5.9% | 19.1 vs. 21.6% |
| mRS score ≤ 1 at 90 days | N/A | 30.4 vs. 34.8% | 6 vs. 6% | 14.4 vs. 9.9% |
| Shift analysis across 90-day mRS score 0–6 (higher scores indicating greater disability† | Analysis stratified by presence of a favorable penumbral pattern: predicted infarct core ≤90 cm3 and occupying ≤70% of at-risk region | 3.9 vs. 3.9&’ Penumbral: 3.9 vs. 3.4&’ Nonpenumbral: 4.0 vs. 4.4& |
4.7 vs. 3.7% Penumbral: 8.8 vs. 5.9% Nonpenumbral: 0 vs. 0% |
18.8 vs. 24.1% Penumbral: 17.6 vs. 20.6% Nonpenumbral: 20 vs. 30% |
| Improvement of 8 or more on the NIHSS score between baseline and day 30, or a score of 0–1 at day 30¶ | Analysis stratified by target mismatch profile: ratio between the volumes of Tmax > 6 s perfusion lesion and ischemic core ≥ 1.8, with an absolute difference of ≥15 cm3; ischemic core volume < 70 cm3; and tissue with a severe delay in bolus arrival (Tmax > 10 s) < 100 cm3 | Adjusted odds ratio for favorable clinical response associated with reperfusion€ was 8.8 (95% CI 2.7–29.0) in the target mismatch group and 0.2 (0.0–1.6) in the no target mismatch group (p = 0.003) | 11.5 vs. 9.5%^ | 19.2 vs. 14.3%^ |
This table is adapted from Morais et al. 63
IMS III Interventional Management of Stroke III, SYNTHESIS Expansion Intra-arterial Versus Systemic Thrombolysis for Acute Ischemic Stroke, MR RESCUE Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy, DEFUSE 2 Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2, IAT intra-arterial therapy, NIHSS National Institutes of Health Stroke Scale, LSW last seen well, IV tPA intravenous tissue plasminogen activator, MRI magnetic resonance imaging, GRE gradient recalled echo, MRA magnetic resonance angiography, DWI diffusion-weighted imaging, PWI perfusion-weighted MRI imaging, NCCT noncontrast computed tomography, MCA middle cerebral artery, ICH intracranial hemorrhage, mRS Modified Rankin Scale, N/A not applicable, ICA intracranial internal carotid artery, M1 stem of the middle cerebral artery, M2 a second-order division of the middle cerebral artery.
The secondary clinical endpoint was good functional outcome, defined as mRS score ≤ 2 at day 90.
Reperfusion was assessed on a follow-up MRI scan (within 12 h of the revascularization procedure) and defined as more than 50% reduction in the volume of the lesion from baseline on PWI.
Or a NIHSSS 8 or 9 with a CT angiography-documented occlusion seen in M1, ICA, or basilar artery.
An Alberta Stroke Program Early CT score (ASPECTS) of <4 could be used as a guideline when evaluating > 1/3 region of territory involvement.
CT angiography was obtained in 306 patients, of whom 92% demonstrated vascular occlusions.
43.8% of patients in the endovascular group and 29.6% of patients in the standard care group initially received IV t-PA.
For secondary analyses, patients with mRS score ≤2 at day 90 were classified as having a good functional outcome.
mean mRS (no statistically significant differences in all comparisons).
Target mismatch group vs. no target mismatch (including patients without reperfusion).
However, this approach ignores cerebrovascular physiology.32 Specifically, individual differences in collateral strength exert a profound effect on the rate of neuronal loss (Fig. 1)56, and better collateral grade is a strong predictor of improved tissue and clinical outcomes after IAT.5,20,49 Moreover, there are additional factors that determine brain viability including tissue tolerance to ischemia (i.e., preconditioning) and blood pressure fluctuations. Advanced imaging, therefore, may be useful for excluding patients who have sustained a large volume of irreversible tissue injury and for whom treatment entails more harm than benefit, and may allow for safe and effective treatment of patients who are outside of current time windows but have evidence of good collaterals.1,41 It should also be noted that advanced neuroimaging does not necessarily preclude rapid treatment. Increased experience decreases imaging-related delays,75 such that advanced imaging can be performed during the time required to mobilize the interventional and anesthesia teams and to set up the interventional suite.61
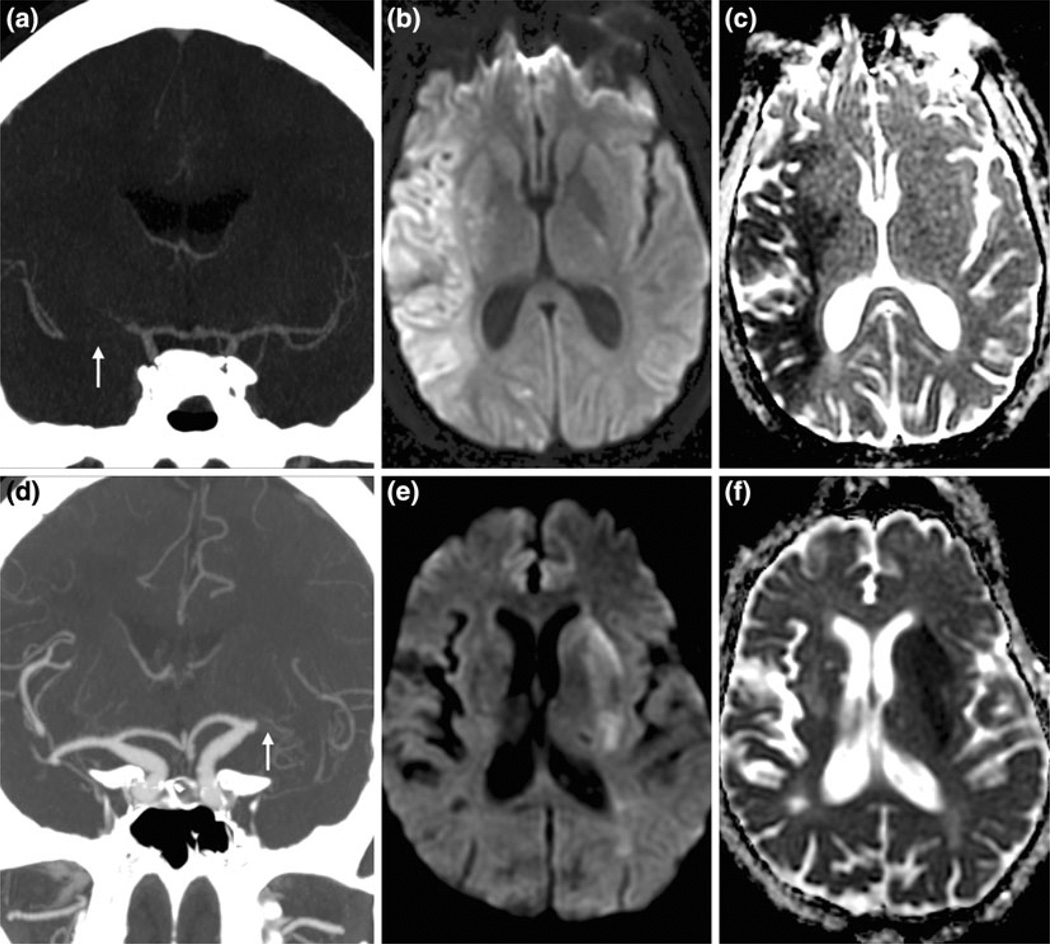
FIGURE 1.
27-Year-old male with NIHSS score of 25 secondary to right middle cerebral artery (MCA) M1 segment occlusion (arrow) as shown on coronal CT angiography (CTA) maximum intensity projection (MIP) image (a). Diffusion weighted (DWI; b) and apparent diffusion coefficient (ADC; c) images, performed 1 h, 48 min after stroke onset, revealed a large infarct volume of 93.1 ml. 88-Year-old female with NIHSS score of 22 secondary to left MCA M1 segment occlusion (arrow) as shown on coronal CTA MIP (d). She received intravenous tissue plasminogen activator 2 h after stroke onset at outside hospital, and was transferred to our center where DWI (e) and ADC (f) images, performed 5 h, 20 min after stroke onset, revealed a small infarct volume of 12 ml.
Penumbral Imaging Biomarkers for IAT
The foundational principle of reperfusion therapy is to halt infarct growth within the penumbra. Neuroimaging seeks to identify patients who have a significant and clinically relevant amount of ‘‘penumbra,’’ or hypoperfused, nonfunctioning brain tissue that will go on to infarction without timely flow restoration. However, various approaches to penumbral imaging have emerged, and for intra-arterial therapy, are represented by two main methods: the clinical-core infarct mismatch and the perfusion-core infarct mismatch.
Clinical-Core Infarct Mismatch
In this approach, the overall size of the combined infarct and territory-at-risk is estimated by the presence of a proximal artery occlusion and a substantial neurologic deficit as measured by the NIH Stroke Scale (NIHSS) score. For IAT decision making, noninvasive vascular imaging is critical for identifying a proximal artery occlusion, which is the direct target of catheter-based therapy. The IMS III and SYNTHESIS Expansion trials underscored the value of vessel imaging (Table 1).13,21 In these studies, pretreatment vessel imaging was not required for patient selection, which may help to account for their negative results regarding the benefit of IAT. In IMS III, CT angiography (CTA) was performed in approximately half of the cohort, of whom 8% had no occlusion.13 Consistent with other studies that have shown a greater revascularization benefit among patients with a visible occlusion,25 IMS III patients with a documented CTA occlusion demonstrated a significant benefit from IAT (p = 0.01).27
In patients with proximal occlusions, a significant neurological deficit serves as a sign of a substantial territory-at-risk (‘‘clinical penumbra’’) and predicts poor outcome without treatment. Recent studies have shown that patients with lower NIHSS scores (e.g., less than 8) are very likely to have a good outcome regardless of treatment.26,91 Approximately 30% of patients with MCA M1 segment occlusions have an NIHSS score <10.59 In this population with less severe deficit, the risk of treatment likely outweighs the benefit. In a prespecified subgroup analysis of the Prolyse in Acute Cerebral Thromboembolism (PRO-ACT) II trial, there was no difference in the rate of good outcome (63%) between the treatment and control arms among patients with NIHSS score 4–10.31 In patients with NIHSS scores of 11–20, there was a significant benefit of IAT, with a similar but nonsignificant effect in scores above 20.
Among patients with a moderate-severe neurologic deficit (i.e., NIHSS score > 10) secondary to a proximal occlusion, the infarct size at presentation is helpful for identifying those patients whose infarcts are already large and will not benefit from IAT. In support of this idea, relevant studies have shown that pretreatment infarct size is strongly associated with the clinical response to intervention.36,37,42 Data from the PROACT II trial have shown that patients with ASPECTS 8–10 (i.e., small infarcts) demonstrate a significant IAT benefit, while none is observed among patients with ASPECTS 0–7 (i.e., larger infarcts).37 Moreover, a pooled subset analysis from the Penumbra Pivotal trial and the Penumbra Imaging Collaborative Study has demonstrated that patients with ASPECTS 0–4 have a particularly poor clinical response to IAT (<5% rate of 90-day mRS 0–2).92 Similarly, DEFUSE 2 and other studies have shown that a large DWI lesion volume at baseline (i.e., >70– 100 cm3) identifies patients who are unlikely to benefit from IAT.51,96 These populations are also at heightened risk of symptomatic intracranial hemorrhage (sICH).77,92 Singer et al.77 demonstrated a 16.1% sICH risk among patients with pretreatment diffusion lesion volume >100 mL. Furthermore, this risk is enhanced with subsequent reperfusion, as demonstrated by a post hoc analysis of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study.52
Taken together, the combination of vessel status, baseline infarct size and neurologic deficit appears sufficient for treatment selection in anterior circulation large artery occlusions. The two main noninvasive approaches to vessel imaging are CTA and MR angiography (MRA). Of the two, CTA is the optimal test, demonstrating >95% sensitivity and specificity compared to digital subtraction angiography (DSA).8,55 It is also rapid and widely available. Three-dimensional time-of-flight MRA of the intracranial circulation can detect proximal occlusions with suitable sensitivity (84–87%) and specificity (85–98%) compared to DSA.8,83 Compared to CTA, MRA requires longer imaging times, which can lead to patient motion artifact, and suffers from lower spatial resolution and increased susceptibility to flow artifacts.8
For pretreatment infarct imaging, the most commonly used techniques are noncontrast CT (NCCT) and MRI diffusion weighted imaging (DWI). A NCCT scan was the only required imaging technique prior to enrollment in both the SYNTHESIS Expansion and IMS III trials (Table 1).13,21 In addition to hemorrhage, an imaging exclusion criterion for SYNTHESIS Expansion was the presence of clear hypoattenuation as a marker of potentially longer stroke duration. In IMS III, it was the presence of clear hypodensity involving more than one-third of the middle cerebral artery (MCA) territory. Hypoattenuation is due to vasogenic edema which results in a net increase in water content,85 and is highly specific for infarction.14,15,87 NCCT is much less sensitive than DWI, however, for the detection of ‘‘hyperacute’’ cytotoxic edema (sensitivity 75%6), particularly when the infarct is large (>33% MCA territory, 14–43%50). This poor sensitivity likely leads to many large strokes being treated in trials employing NCCT-only selection.
If NCCT is utilized for parenchymal evaluation, narrow window-width and variable center-level settings should be used to maximize lesion contrast.54 A rigorous and reliable approach to quantifying infarct burden is the Alberta Stroke Program Early CT Score (ASPECTS).7 In this grading scheme, ten regions (four deep and six cortical structures) within the MCA territory are evaluated for ischemic changes, with a point deducted for each involved region. As opposed to the original ASPECTS system, currently only regions of hypoattenuation (rather than focal cortical swelling) are scored,14 and all available images are inspected rather than the two slices specified in the original scheme.
MRI diffusion imaging is the most accurate technique for delineating hyperacute infarct size (class I, level of evidence A), with high sensitivity (91–100%) and specificity (86–100%) in the first 6 h from onset.29,53,74 DWI lesions have strong inter-rater reliability for quantifying lesion extent due to high contrast-to-noise ratio.4,58 A potential limitation is diffusion reversibility, but recent reports have shown that this phenomenon is uncommon, involves negligible tissue volumes, and appears unlikely to be clinically relevant.18,88
Contrast-based CT methods for estimating acute infarction include static and dynamic techniques. CTA source imaging (CTA-SI) is a static method that is more sensitive than NCCT for detecting acute ischemic change.9,15 The degree of tissue hypoattenuation on CTA-SI depends on the amount of contrast that has arrived into the tissue via pial collaterals. Although CTA-SI lesion volume has been shown to approximate concurrent DWI lesion volume in an early study,76 more recent work reveals that lesion size on CTA-SI is technique dependent.67 Specifically, the size of the critically hypoperfused region can be overestimated using faster protocols which image before the contrast has traversed the pial collaterals. CTA-SI hypodensity, therefore, is not specific for tissue injury.
Like CTA source imaging, CT perfusion (CTP) also has limitations. First, CTP measurements reflect a single ‘‘snapshot in time’’. Second, thresholds for infarction depend on ischemic duration as well as the degree, timing, and location of subsequent reperfusion, variables which are difficult to know with great precision. Moreover, a recent systematic review reported a large variety of perfusion parameters and a considerable heterogeneity of tissue definitions in CT and MRI studies evaluating perfusion threshold values for tissue characterization.22 The authors found inconsistencies between threshold values reported in CT/MRI studies and those reported in PET imaging studies. Another issue is that postprocessing may be performed in several different ways, and may yield varying results for the same patient.19,48 Consequently, recent studies using different postprocessing algorithms have disagreed over the optimal CTP parameter to define the infarct core (i.e., CBV vs. CBF).11,43,73,90 Similarly, Fahmi et al.28 have shown that two different commercial software packages result in large differences in infarct size for the same patient data. Further standardization and validation are required before CTA-SI and CTP techniques can reliably estimate infarct size.53,89
Perfusion-Core Infarct Mismatch
This alternative approach to penumbral selection utilizes dynamic perfusion imaging to directly image the region of hypoperfusion. Several trials have implemented numerous operational definitions of penumbral tissue based on CTP and MR perfusion imaging with varying success.24,34,65
A recent phase 2b, multicenter RCT, the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) trial (Table 1)46, assessed whether endovascular thrombectomy can improve stroke outcome, specifically in patients with a perfusion-core infarct mismatch, against standard medical management. In the 58% of patients with a favorable penumbral pattern (predicted infarct volume of ≤90 cm3 and occupying ≤70% of the at-risk territory), there was no outcome improvement (90-day mRS 0–2) with IAT. The failure of MR RESCUE to support the imaging selection hypothesis may be related to multiple factors. First, the pretreatment infarct volume threshold of 90 cm3 used to define a favorable penumbral pattern is much higher than the 70-cm threshold that has been demonstrated to be highly specific for poor outcomes.3,71 In addition, CTP was used in approximately 20% of patients, and may have influenced the results given the concerns regarding the accuracy of perfusion imaging to evaluate infarct size. Also, there are nonimaging related factors that may account for the failure of MR RESCUE. The success of imaging selection is predicated on an effective therapy, and the majority of the interventions in the trial were performed with the first-generation Merci retriever, which was recently demonstrated to have poor recanalization efficacy.64,72 This resulted in a very low rate (27%) of substantial reperfusion [modified Thrombolysis in Cerebral Infarction (mTICI) grades 2B-3: reperfusion in greater than half of the ischemic territory (Table 3)]. Current generation stent-retrievers yield mTICI 2B-3 rates of between 70 and 85%.23,64, Moreover, times from stroke onset to procedure initiation were long (mean: 6 h, 21 min) with long delays from the start of imaging to groin puncture (mean: 2 h, 4 min). Such delays between imaging and treatment may negate the advantage of imaging selection if there has been significant infarct growth during this interval.
TABLE 3.
Major angiographic reperfusion scales.
| Angiographic criteria | |
|---|---|
| TIMI | |
| 0 | No perfusion |
| 1 | Minimal flow past the occlusion with little to no perfusion |
| 2 | Antegrade partial perfusion of the downstream ischemic territory |
| 3 | Antegrade complete perfusion of the downstream ischemic territory |
| mTICI | |
| 0 | No perfusion |
| 1 | Minimal flow past the occlusion with little to no perfusion |
| 2A | Antegrade partial perfusion of less than half of the downstream ischemic territory |
| 2B | Antegrade partial perfusion of half or greater of the downstream ischemic territory |
| 3 | Antegrade complete perfusion of the downstream ischemic territory |
Perfusion signifies capillary opacification or blush angiographically.
TIMI thrombolysis in myocardial infarction, mTICI modified thrombolysis in cerebral infarction.
Support for the imaging selection hypothesis is provided by the DEFUSE 2 study, which was a multicenter prospective cohort study that tested whether patients with a target mismatch profile would have better clinical and tissue outcomes after endovascular reperfusion than patients without a target mismatch (Table 1)51. Of the 99 study patients in the main analysis, 78 had a target mismatch (infarct volume < 70 cm3; ratio of Tmax > 6 s volume to infarct volume ≥1.8 with absolute difference ≥15 cm ; and Tmax > 10 s volume < 100 cm3). Target mismatch patients had a significantly higher odds for a favorable clinical response with reperfusion (adjusted odds ratio 8.8 vs. 0.2; p = 0.003). Also, reperfusion resulted in less infarct growth only in this patient subset. DEFUSE 2 strongly supports the imaging selection hypothesis when there is substantial (i.e., >50%) reperfusion, which was achieved in 56% of the study cohort.
Despite the validation of the perfusion-core infarct mismatch in DEFUSE 2, closer examination suggests that the results may be due to the influence of the baseline infarct volume alone. There was a significant difference between target and nontarget mismatch groups in the median volume of the baseline diffusion lesion (13 vs. 45 cm3, respectively; p = 0.02); however, there was no difference in the volume of the perfusion lesion (Tmax >6 s) (80 vs. 74 cm3, respectively; p = NS). Based on these results, perfusion imaging may add little incremental value over knowledge of infarct volume alone. This can be explained by the fact that when there is a proximal occlusion, the territory-at-risk is so extensive that an imaging mismatch is virtually guaranteed, unless the infarct is prohibitively large.35,42
Perfusion imaging may provide useful information in more distal occlusions (i.e., M2 segment), where the territory-at-risk is smaller and highly variable. In this context, an imaging match between the infarct and the hypoperfused territory would be an important indicator of no tissue-at-risk. Furthermore, perfusion imaging may potentially delineate regions that do not demonstrate DWI abnormality but have critical ischemia and are highly likely to go on to infarction despite rapid reperfusion. These questions require further investigation.
Finally, there are significant questions regarding the reliability of perfusion imaging to identify territory-at-risk. A recent systematic review reveals no well-validated CT or MRI perfusion thresholds for characterizing tissue status.22 Moreover, recent studies have shown that thresholds for infarction and at-risk tissue are dependent on the postprocessing software,43,44 and this variability alone can result in major differences in lesion volumes.28 Finally, concerns have been raised regarding the errors of perfusion quantification using dynamic contrast methods.17,30,81 Perfusion imaging should be further investigated prior to widespread clinical application for penumbral characterization.33
Imaging Biomarkers of IV tPA Resistance (Recanalization Benefit of Added IAT)
Clot Imaging
Recent work has investigated the use of imaging to depict clot burden and composition in hopes of predicting recanalization efficiency and the relative value of IV and IA approaches. Using thin-section NCCT with slice widths of 2.5 mm or less, approximately 90% of occlusions are visualized as hyperdense clots, and the length of these clots can be measured accurately (Fig. 2)69,70,94. With smaller slice widths, there is less volume averaging with adjacent cerebrospinal fluid and brain tissue, resulting in improved clot contrast. With respect to clot composition, studies have shown that hyperdense clots are red blood cell predominant, whereas low density clots are fibrin-rich.57,97
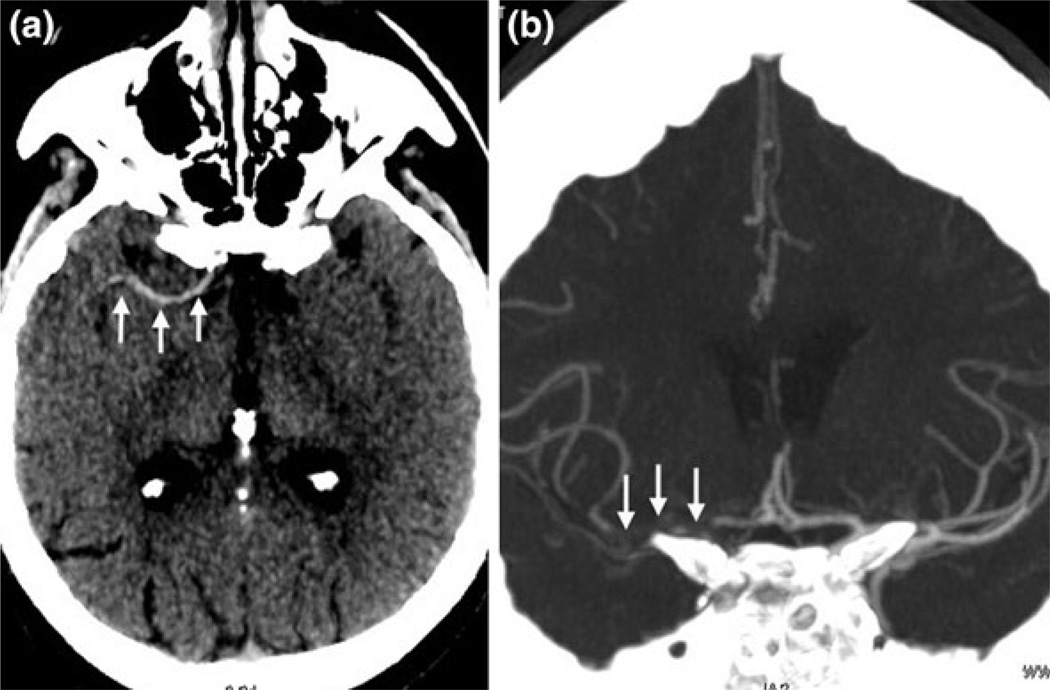
FIGURE 2.
82-Year-old female with NIHSS score of 10 presents 3 h after stroke onset. Thin-section noncontrast CT MIP data (a) demonstrate a long hyperdense clot (arrows) within the right internal carotid and middle cerebral arteries. The occlusion (arrows) is confirmed on the coronal CTA MIP (b). The hyperdense clot measured 30.7 mm.
Prediction of IV tPA Recanalization
Riedel et al.70 demonstrated that in patients with NCCT-determined clot lengths of 8 mm or greater, there is a low chance of recanalization with IV tPA alone. The majority (95%) of ICA terminus clots are ≥8 mm,94 which explains the very low rate (4.4%) of early recanalization after IV tPA.10 The added benefit of a bridging approach in this population is supported by data from IMS III. For ICA terminus occlusions confirmed with CTA (n = 58) in this study, the rate of good outcome (90-day mRS 0–2) with bridging therapy was 4 times higher than for IV tPA alone (23 vs. 5.3%; p = 0.14).27 For CTA-documented M1 occlusions, there was no difference in good outcome rates between the treatment arms (48 vs. 51% for bridging therapy vs. IV tPA). Therefore, in Ml occlusion patients, clot length may be an important biomarker to identify patients who will derive an added benefit from IAT by excluding short clots that have a high chance of opening with IV tPA alone—approximately 20% of Ml clots are <8 mm in length.94 This idea is being tested in the ongoing Penumbra THERAPY trial (Table 2). Finally, studies have shown that lower density clots are resistant to IV thrombolysis.47,62 However, they also appear to be refractory to IAT, although the stent-retrievers were not evaluated in these studies.47,62
TABLE 2.
Ongoing randomized controlled trials of intra-arterial therapy
| Trial | Estimated enrollment (no. patients) |
Treatment arms | Clinical criteria | Imaging criteria | Intra-arterial treatment modality |
Primary outcome | Estimated completion date |
|---|---|---|---|---|---|---|---|
| THRACE (enrolling) | 480 | Combined IV tPA + IAT vs. IV tPA | Eligible for IV tPA; NIHSS score 10–25; Presentation within 4 h from LSW | Occlusion of ICA, M1 MCA or basilar terminus | Any modality | Shift analysis across 90-day mRS score 0–6 | End of 2012 |
| ESCAPE (enrolling) | 250 | IAT vs. standard care | NIHSS > 5 at the time of randomization; LSW to randomization time <12h; groin puncture within 60 min of CT/CTA with target CTA to first recanalization of 90 min | CTA one or more of the following locations: ICA, M1 MCA, or M1-MCA equivalent (2 or more M2-MCAS); ASPECTS score on NCCT > 5¢ | Any modality | NIHSS score ≤ 2 or mRS score ≤ 2 at 90 days | December 2014 |
| EXTEND IA (enrolling) | 100 | Combined IV tPA + IAT vs. IV tPA | Eligible for IV tPA therapy within 4.5 h; groin puncture within 6 h of stroke onset | Arterial occlusion (ICA, M1, or M2) on CTA or MRA; Mismatch‡ ratio of greater than 1.2, with absolute mismatch volume > 10 cm3 and infarct core lesion volume < 70 cm3 | Solitaire FR | Reperfusion at 24 h (CTP or PWI); NIHSS reduction ≥8 points or reaching 0–1 at 3 days | December 2014 |
| THERAPY (enrolling) | 692 | Combined IV tPA + IAT vs. IV tPA | Eligible for IV tPA therapy; NIHSS score ≥ 8 | Evidence of a large clot burden (clot length≥ 8 mm) in the anterior circulation; NCCT infarct < 1/3 of MCA territory | Penumbra System | m mRS score ≤ 2 at 90 days | January 2015 |
| MR CLEAN (enrolling) | 500 | IAT vs. standard care | NIHSS score ≥ 2; possibility to start treatment within 6 h from LSW | Occlusion of the distal ICA or M1/M2 MCA or A1/A2 ACA, demonstrated with CTA, MRA, DSA, or TCD | Any modality | Shift analysis across 90-day mRS score 0–6 | January 2015 |
| REVASCAT (enrolling) | 690 | IAT vs. standard care | Patients ineligible for IV tPA or without recanalization after a minimum of 30 min from start of the IV tPA infusion; NIHSS score ≥ 6; treatable within 8 h from LSW | Occlusion of the ICA and/or M1 MCA, as evidenced by CTA, MRA, or DSA (cervical ICA occlusion or stenosis allowed); ASPECTS > 6 on NCCT, CTP-CBV or CTA-SI, or >5 on DWI | Solitaire FR | Shift analysis across 90-day mRS score 0–6 | December 2015 |
| PISTE (planned) | 800 | Combined IV tPA + IAT vs. IV tPA | IV tPA <4.5 h after LSW; NIHSS score ≥ 6; groin puncture within 90 min of the start of IV tPA | Large-vessel occlusion demonstrated on CTA, MRA, or DSA (ICA, M1 or single proximal M2 branch); NCCT without extensive established hypodensity | Any modality | mRS score ≤ 2 at 90 days | August 2017 |
| BASICS (enrolling) | 750 | Combined IV tPA + IAT vs. IV tPA | Eligible for IV tPA therapy initiated within 4.5 h; NIHSS > 10 at the time of randomization; start IAT ≤6 h of estimated time of basilar artery occlusion. | Basilar artery occlusion confirmed by CTA/MRA | Any modality | mRS score ≤ 3 at 90 days | October 2017 |
| SWIFT PRIME (enrolling) | 833 | Combined IV tPA + IAT vs. IV tPA | Eligible for IV tPA therapy within 4.5 h; NIHSS 8–30; treatment within 1.5 h from imaging to groin puncture | TICI 0–1 flow in the ICA and M1 MCA confirmed by CTA or MRA; baseline CTP or DWI/PWI | Solitaire FR | Shift analysis across 90-day mRS score 0–6 | September 2018 |
This table is adapted from Morais et al.63
WASSABI Wake up Symptomatic Stroke in Acute Brain Ischemia, THRACE Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke, ESCAPE Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke, EXTEND IA, Extending the Time for Thrombolysis in Emergency Neurological Deficits—Intra-Arterial, THERAPY The Randomized Controlled Trial to Assess the Penumbra System’s Safety and Effectiveness in Acute Stroke Treatment, MR CLEAN Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands, REVASCATEndovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours, PISTE Pragmatic Ischaemic Stroke Thrombectomy Evaluation, BASICS Basilar Artery International Cooperation Study, SWIFT PRIME Solitaire FR as Primary Treatment for Acute Ischemic Stroke, IV tPA intravenous tissue plasminogen activator, IAT intra-arterial therapy, NIHSS National Institutes of Health Stroke Scale, NCCT noncontrast computed tomography, MCA middle cerebral artery, mRS Modified Rankin Scale, TICIThrombolysis in Cerebral Infarction classification, ICA intracranial internal carotid artery, M1 stem of the middle cerebral artery, CTA computed tomography angiography, MRA magnetic resonance angiography, CTP computed tomography perfusion, DWI diffusion-weighted imaging, PWIperfusion-weighted MRI imaging, LSW last seen well, DSA digital subtraction angiography, M2a second-order division of the middle cerebral artery, ACA anterior cerebral artery, A1 and A2 divisions of the anterior cerebral artery, TCD transcranial Doppler, ASPECTS Alberta Stroke Program Early CT score, CBV cerebral blood volume, CTA-SI computed tomography angiography source images.
Using CT or MRI with a Tmax > 6 s delay perfusion volume and either CT-rCBF or DWI infarct core volume.
Other than using NCCT ASPECTS score, confirmation of a moderate to large core can also be defined by one of three ways: no or minimal collaterals in a region greater than 50% of the MCA territory when compared to pial filling on the contralateral side (CTA); or a low CBV and very low CBF ASPECTS < 6 in the symptomatic MCA territory (on CTP > 8 cm coverage) or a region of low CBV and very low CBF > 1/3 of the CTP imaged symptomatic MCA territory (on CTP > 8 cm coverage).
IMAGING BIOMARKERS AS SURROGATE ENDPOINTS IN TRIALS OF INTRA-ARTERIAL THERAPY
Outcomes after stroke are affected by a host of variables that are unrelated to the disease process itself, including access to acute medical care and rehabilitation services, and social and cultural factors. The “noise” related to these variables is often difficult to control for in trial design, and may overwhelm the “signal” of treatment benefit. For this reason, surrogate biomarkers are important as primary endpoints in early phase studies and as auxiliary endpoints to confirm the direction of treatment effects in phase III trials.
A surrogate biomarker should satisfy several criteria prior to use.68 First, it must be strongly linked to clinical outcome, and should be specific to the population, treatment and outcome of interest. Ideally, this validation should be confirmed in multiple datasets. Moreover, a surrogate biomarker should be easy to measure and have high inter-rater reproducibility. Finally, it should be cost-effective, either by reducing sample size or shortening time to follow up.
Angiographic Revascularization Endpoints
Angiographic biomarkers of revascularization have been used as surrogate endpoints in device trials performed for U.S. Food and Drug Administration clearance.64,66,72,78 Unfortunately, in these trials, numerous operational definitions have been applied to various grading scales, which has limited comparisons across trials and contributed to the heterogeneous clinical response that is seen with “successful revascularization.”79
Revascularization is a broad term that applies to all treatment-related improvements in blood flow, and encompasses both recanalization and reperfusion. Recanalization scales evaluate the patency at the site of the target arterial lesion, and provide a direct measure of device effectiveness at the site of action. The only angiographic scale designed to measure recanalization is the arterial occlusive lesion (AOL) scale.84 Angiographic reperfusion scales, on the other hand, measure the extent of capillary-level opacification (i.e., tissue blush or staining) that is restored to the downstream ischemic territory in an antegrade fashion, and include the Thrombolysis in Myocardial Infarction (TIMI) and mTICI scales (Table 3).
As mentioned, a significant problem is the lack of standardization in revascularization grading. For instance, numerous studies have utilized TIMI to measure recanalization rather than reperfusion.72,78 Although they are closely related, these endpoints do not always correspond. Some degree of vessel recanalization is required for tissue reperfusion, yet recanalization may not produce reperfusion when there is distal clot embolization or no reflow phenomenon related to a well-established infarct. Of the two measures, reperfusion is a stronger predictor of clinical and tissue outcomes.80,84
Among the major angiographic reperfusion scales, the primary difference between TIMI and mTICI is that the latter scale further categorizes partial reperfusion (TIMI 2) as minor (TICI 2A) vs. major (TICI 2B) (Table 3). Numerous studies have shown that using a 50% threshold, as employed in the mTICI scale, major reperfusion yields significantly better outcomes than minor reperfusion.2,39,60,95 Moreover, in a retrospective study of 313 M1 occlusions treated with IAT, mTICI was better than TIMI (c-statistic: 0.74 vs. 0.68; p < 0.0001) for predicting good outcome at 90 days,95 with the optimal threshold at mTICI 2B or greater. In further support of mTICI as a surrogate endpoint, good inter-rater agreement has been demonstrated for distinguishing <50 vs. ≥50% reperfusion of the downstream territory (k = 0.95).2
Final Infarct Volume
The goal of reperfusion therapy is to limit final infarct size. Therefore, it is not surprising that among anterior circulation stroke patients treated with IAT, final infarct volume is the single best predictor of 90-day good outcome (mRS 0–2). This has been demonstrated in two independent datasets.93,98 In these studies, the c-statistic for predicting a good outcome with final infarct volume was approximately 0.8, which is the accepted benchmark for surrogate biomarker validation.40 Both studies found that a final infarct size of between 40 and 50 cm3 optimally differentiated good vs. poor outcome. Moreover, age modifies the effect of final infarct volume such that older patients require smaller infarcts in order to achieve functional independence.38,93
Infarct volume offers key advantages as a surrogate endpoint. First, it can be measured within the first 24 to 48 h because no appreciable infarct growth occurs beyond this time.16 This prevents loss to follow up in trials, and facilitates early clinical prognostication. In addition, final infarct volume measurements are highly reproducible between readers.16,58,86 An automated method for measuring final infarct volume has recently been described,12 which would further facilitate its use in clinical trials and practice.
CONCLUSIONS
Advanced neuroimaging provides critical information which can help in treatment decision making for intra-arterial therapy. For identifying a clinically significant penumbra as a marker of potential treatment response, there are two main imaging approaches. For intracranial internal carotid artery and MCA M1 segment occlusions, there are strong data to support the use of a small infarct in the context of a substantial neurologic deficit for IAT selection. The alternative approach based on a perfusion-core infarct mismatch may add value in specific scenarios but requires further validation. In addition, clot length determined on thin-section NCCT may be an important imaging biomarker for predicting the failure of IV tPA revascularization and the added benefit of a bridging approach. With protocol optimization, pretreatment advanced imaging can be performed with little time expense. Multiple ongoing randomized trials will provide further data regarding the added value of advanced neuroimaging-based selection (Table 2).
After stroke intervention, neuroimaging endpoints provide a direct measure of treatment effects, and may improve our evaluation of future devices and therapeutic approaches. Currently, the best validated surrogate biomarkers are the modified TICI scale and final infarct volume.
REFERENCES
- 1.Abou-Chebl A. Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke. 2010;41(9):1996–2000. doi: 10.1161/STROKEAHA.110.578997. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Nedeltchev K, Remonda L, et al. Recanalisation of middle cerebral artery occlusion after intra-arterial thrombolysis: different recanalisation grading systems and clinical functional outcome. J. Neurol. Neurosurg. Psychiatry. 2005;76(10):1373–1376. doi: 10.1136/jnnp.2004.055160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arsava E, Ay H, Singhal AB, Ona W, Furie KL, Sorenson AG. International Stroke Conference. New Orleans: Louisiana; 2008. An infarct volume threshold on early DWI to predict unfavorable clinical outcome. [Google Scholar]
- 4.Ay H, Arsava EM, Vangel M, et al. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke. 2008;39(4):1171–1176. doi: 10.1161/STROKEAHA.107.502104. [DOI] [PubMed] [Google Scholar]
- 5.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42(3):693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber PA, Darby DG, Desmond PM, et al. Identification of major ischemic change: diffusion-weighted imaging versus computed tomography. Stroke. 1999;30(10):2059–2065. doi: 10.1161/01.str.30.10.2059. [DOI] [PubMed] [Google Scholar]
- 7.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 8.Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am. J. Neuroradiol. 2005;26(5):1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia R, Bal SS, Shobha N, et al. CT angiographic source images predict outcome and final infarct volume better than noncontrast CT in proximal vascular occlusions. Stroke. 2011;42(6):1575–1580. doi: 10.1161/STROKEAHA.110.603936. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):2254–2258. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- 11.Bivard A, McElduff P, Spratt N, et al. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovasc. Dis. 2011;31(3):238–245. doi: 10.1159/000321897. [DOI] [PubMed] [Google Scholar]
- 12.Boers AM, Marquering HA, Jochem JJ, et al. Automated cerebral infarct volume measurement in follow-up noncontrast CT scans of patients with acute ischemic stroke. AJNR Am. J. Neuroradiol. 2013 doi: 10.3174/ajnr.A3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N. Engl. J. Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butcher KS, Lee SB, Parsons MW, et al. Differential prognosis of isolated cortical swelling and hypoattenuation on CT in acute stroke. Stroke. 2007;38(3):941–947. doi: 10.1161/01.STR.0000258099.69995.b6. [DOI] [PubMed] [Google Scholar]
- 15.Camargo EC, Furie KL, Singhal AB, et al. Acute brain infarct: detection and delineation with CT angiographic source images versus nonenhanced CT scans. Radiology. 2007;244(2):541–548. doi: 10.1148/radiol.2442061028. [DOI] [PubMed] [Google Scholar]
- 16.Campbell BC, Tu HT, Christensen S, et al. Assessing response to stroke thrombolysis: validation of 24-hour multimodal magnetic resonance imaging. Arch. Neurol. 2012;69(1):46–50. doi: 10.1001/archneurol.2011.232. [DOI] [PubMed] [Google Scholar]
- 17.Carroll TJ, Teneggi V, Jobin M, et al. Absolute quantification of cerebral blood flow with magnetic resonance, reproducibility of the method, and comparison with H2(15)O positron emission tomography. J. Cereb. Blood Flow Metab. 2002;22(9):1149–1156. doi: 10.1097/00004647-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Chemmanam T, Campbell BC, Christensen S, et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology. 2010;75:1040–1047. doi: 10.1212/WNL.0b013e3181f39ab6. [DOI] [PubMed] [Google Scholar]
- 19.Christensen S, Mouridsen K, Wu O, et al. Comparison of 10 perfusion MRI parameters in 97 sub-6-hour stroke patients using voxel-based receiver operating characteristics analysis. Stroke. 2009;40(6):2055–2061. doi: 10.1161/STROKEAHA.108.546069. [DOI] [PubMed] [Google Scholar]
- 20.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am. J. Neuroradiol. 2005;26(7):1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 21.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N. Engl. J. Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dani KA, Thomas RG, Chappell FM, et al. Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: definitions and thresholds. Ann. Neurol. 2011;70(3):384–401. doi: 10.1002/ana.22500. [DOI] [PubMed] [Google Scholar]
- 23.Davalos A, Pereira VM, Chapot R, et al. Retrospective multicenter study of Solitaire FR for revascularization in the treatment of acute ischemic stroke. Stroke. 2012;43(10):2699–2705. doi: 10.1161/STROKEAHA.112.663328. [DOI] [PubMed] [Google Scholar]
- 24.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 25.De Silva DA, Fink JN, Christensen S, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET) Stroke. 2009;40(8):2872–2874. doi: 10.1161/STROKEAHA.108.543595. [DOI] [PubMed] [Google Scholar]
- 26.DeGraba TJ, Hallenbeck JM, Pettigrew KD, et al. Progression in acute stroke: value of the initial NIH stroke scale score on patient stratification in future trials. Stroke. 1999;30(6):1208–1212. doi: 10.1161/01.str.30.6.1208. [DOI] [PubMed] [Google Scholar]
- 27.IMS III Investigators. IMS III: comparison of outcomes between IV and IV/IA treatment in baseline CTA confirmed ICA, M1, M2 and basilar occlusions. In: Demchuk AM, editor. International Stroke Conference. Honolulu: Hawaii; 2013. [Google Scholar]
- 28.Fahmi F, Marquering HA, Streekstra GJ, et al. Differences in CT perfusion summary maps for patients with acute ischemic stroke generated by 2 software packages. AJNR Am. J. Neuroradiol. 2012;33(11):2074–2080. doi: 10.3174/ajnr.A3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiebach JB, Schellinger PD, Jansen O, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002;33(9):2206–2210. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 30.Fiorella D, Heiserman J, Prenger E, et al. Assessment of the reproducibility of postprocessing dynamic CT perfusion data. AJNR Am. J. Neuroradiol. 2004;25(1):97–107. [PMC free article] [PubMed] [Google Scholar]
- 31.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282(21):2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez RG. Imaging-guided acute ischemic stroke therapy: from ‘‘time is brain’’ to ‘‘physiology is brain’’. AJNR Am. J. Neuroradiol. 2006;27(4):728–735. [PMC free article] [PubMed] [Google Scholar]
- 33.Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology. 2013;266(1):16–21. doi: 10.1148/radiol.12112134. [DOI] [PubMed] [Google Scholar]
- 34.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8(2):141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakimelahi R, Yoo AJ, He J, et al. Rapid identification of a major diffusion/perfusion mismatch in distal internal carotid artery or middle cerebral artery ischemic stroke. BMC Neurol. 2012;12(1):132. doi: 10.1186/1471-2377-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill MD, Demchuk AM, Tomsick TA, et al. Using the baseline CT scan to select acute stroke patients for IV-IA therapy. AJNR Am. J. Neuroradiol. 2006;27(8):1612–1616. [PMC free article] [PubMed] [Google Scholar]
- 37.Hill MD, Rowley HA, Adler F, et al. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using ASPECTS. Stroke. 2003;34(8):1925–1931. doi: 10.1161/01.STR.0000082483.37127.D0. [DOI] [PubMed] [Google Scholar]
- 38.Jadhav AP, Nanduri S, Aghaebrahim A, Zaidi S, Jumaa M, Linares G, Reddy V, Hammer M, Jankowitz B, Wechsler L, Jovin TG. Octogenarians have high rates of favorable outcomes after endovascular therapy for acute stroke due to M1 occlusions if final infarct volumes are small. Stroke. 2013;44 ATP6. [Google Scholar]
- 39.Jayaraman MV, Grossberg JA, Meisel KM, et al. The clinical and radiographic importance of distinguishing partial from near-complete reperfusion following intra-arterial stroke therapy. AJNR Am. J. Neuroradiol. 2013;34(1):135–139. doi: 10.3174/ajnr.A3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston KC, Barrett KM, Ding YH, et al. Clinical and imaging data at 5 days as a surrogate for 90-day outcome in ischemic stroke. Stroke. 2009;40(4):1332–1333. doi: 10.1161/STROKEAHA.108.528976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jovin TG, Liebeskind DS, Gupta R, et al. Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well: retrospective multicenter analysis of 237 consecutive patients. Stroke. 2011;42(8):2206–2211. doi: 10.1161/STROKEAHA.110.604223. [DOI] [PubMed] [Google Scholar]
- 42.Jovin TG, Yonas H, Gebel JM, et al. The cortical ischemic core and not the consistently present penumbra is a determinant of clinical outcome in acute middle cerebral artery occlusion. Stroke. 2003;34(10):2426–2433. doi: 10.1161/01.STR.0000091232.81947.C9. [DOI] [PubMed] [Google Scholar]
- 43.Kamalian S, Kamalian S, Maas MB, et al. CT cerebral blood flow maps optimally correlate with admission diffusion-weighted imaging in acute stroke but thresholds vary by postprocessing platform. Stroke. 2011;42(7):1923–1928. doi: 10.1161/STROKEAHA.110.610618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamalian S, Konstas AA, Maas MB, et al. CT per-fusion mean transit time maps optimally distinguish benign oligemia from true ‘‘at-risk’’ ischemic penumbra, but thresholds vary by postprocessing technique. AJNR Am. J. Neuroradiol. 2012;33(3):545–549. doi: 10.3174/ajnr.A2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73(13):1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N. Engl. J. Med. 2013;368(10):914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EY, Heo JH, Lee SK, et al. Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT. Neurology. 2006;67(10):1846–1848. doi: 10.1212/01.wnl.0000244492.99737.a8. [DOI] [PubMed] [Google Scholar]
- 48.Konstas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: technical implementations. AJNR Am. J. Neuroradiol. 2009;30(5):885–892. doi: 10.3174/ajnr.A1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kucinski T, Koch C, Eckert B, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003;45(1):11–18. doi: 10.1007/s00234-002-0881-0. [DOI] [PubMed] [Google Scholar]
- 50.Lansberg MG, Albers GW, Beaulieu C, et al. Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology. 2000;54(8):1557–1561. doi: 10.1212/wnl.54.8.1557. [DOI] [PubMed] [Google Scholar]
- 51.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11(10):860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lansberg MG, Thijs VN, Bammer R, et al. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007;38(8):2275–2278. doi: 10.1161/STROKEAHA.106.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40(11):3646–3678. doi: 10.1161/STROKEAHA.108.192616. [DOI] [PubMed] [Google Scholar]
- 54.Lev MH, Farkas J, Gemmete JJ, et al. Acute stroke: improved nonenhanced CT detection—benefits of soft-copy interpretation by using variable window width and center level settings. Radiology. 1999;213(1):150–155. doi: 10.1148/radiology.213.1.r99oc10150. [DOI] [PubMed] [Google Scholar]
- 55.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J. Comput. Assist. Tomogr. 2001;25(4):520–528. doi: 10.1097/00004728-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin. N. Am. 2005;15(3):553–573. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42(5):1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luby M, Bykowski JL, Schellinger PD, et al. Intra-and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke. 2006;37(12):2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maas MB, Furie KL, Lev MH, et al. National Institutes of Health Stroke Scale score is poorly predictive of proximal occlusion in acute cerebral ischemia. Stroke. 2009;40(9):2988–2993. doi: 10.1161/STROKEAHA.109.555664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marks MP, Lansberg MG, Mylnash M, Straka M, Kemp S, Inoue M, Tipirneni A, McTaggart R, Zahar-chuk G, Bammer R, Albers GW DEFUSE2 Investigators. Correlation of AOL recanalization, TIMI reperfusion and TICI reperfusion with infarct growth and clinical outcome in the DEFUSE 2 trial. Stroke. 2013;44:A63. [Google Scholar]
- 61.Mehta BP, Leslie-Mazwi TM, Chandra RV, Bell DL, Rabinov JD, Ogilvy CS, Hirsch JA, Goldstein JN, Rost NS, Schwamm LH, Yoo AJ. Reducing time to intra-arterial therapy in acute ischemic stroke. Stroke. 2013;44:A19. [Google Scholar]
- 62.Moftakhar P, English JD, Cooke DL, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44(1):243–245. doi: 10.1161/STROKEAHA.112.674127. [DOI] [PubMed] [Google Scholar]
- 63.Morais LT, Leslie-Mazwi TM, Lev MH, Albers GW, Yoo AJ. Imaging-based selection for intra-arterial stroke therapies. J. Neurointerv. Surg. 2013 doi: 10.1136/neurintsurg-2013-010733. [DOI] [PubMed] [Google Scholar]
- 64.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380(9849):1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N. Engl. J. Med. 2012;366(12):1099–1107. doi: 10.1056/NEJMoa1109842. [DOI] [PubMed] [Google Scholar]
- 66.Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40(8):2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 67.Pulli B, Schaefer PW, Hakimelahi R, et al. Acute ischemic stroke: infarct core estimation on CT angiography source images depends on CT angiography protocol. Radiology. 2012;262(2):593–604. doi: 10.1148/radiol.11110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puntmann VO. How-to guide on biomarkers: biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad. Med. J. 2009;85(1008):538–545. doi: 10.1136/pgmj.2008.073759. [DOI] [PubMed] [Google Scholar]
- 69.Riedel CH, Jensen U, Rohr A, et al. Assessment of thrombus in acute middle cerebral artery occlusion using thin-slice nonenhanced Computed Tomography reconstructions. Stroke. 2010;41(8):1659–1664. doi: 10.1161/STROKEAHA.110.580662. [DOI] [PubMed] [Google Scholar]
- 70.Riedel CH, Zimmermann P, Jensen-Kondering U, et al. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42(6):1775–1777. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 71.Sanak D, Nosal V, Horak D, et al. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuro-radiology. 2006;48(9):632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 72.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 73.Schaefer PW, Barak ER, Kamalian S, et al. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke. 2008;39(11):2986–2992. doi: 10.1161/STROKEAHA.107.513358. [DOI] [PubMed] [Google Scholar]
- 74.Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75(2):177–185. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schellinger PD, Jansen O, Fiebach JB, et al. Feasibility and practicality of MR imaging of stroke in the management of hyperacute cerebral ischemia. AJNR Am. J. Neuroradiol. 2000;21(7):1184–1189. [PMC free article] [PubMed] [Google Scholar]
- 76.Schramm P, Schellinger PD, Fiebach JB, et al. Comparison of CT and CT angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke. 2002;33(10):2426–2432. doi: 10.1161/01.str.0000032244.03134.37. [DOI] [PubMed] [Google Scholar]
- 77.Singer OC, Humpich MC, Fiehler J, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63(1):52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- 78.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39(4):1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 79.Soares BP, Chien JD, Wintermark M. MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: everything that recanalizes does not necessarily reperfuse! Stroke. 2009;40(3 Suppl):S24–S27. doi: 10.1161/STROKEAHA.108.526814. [DOI] [PubMed] [Google Scholar]
- 80.Soares BP, Tong E, Hom J, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using ct in acute ischemic stroke patients. Stroke. 2009;41(1):e34–e40. doi: 10.1161/STROKEAHA.109.568766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takasawa M, Jones PS, Guadagno JV, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke. 2008;39(3):870–877. doi: 10.1161/STROKEAHA.107.500090. [DOI] [PubMed] [Google Scholar]
- 82.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 83.Tomanek AI, Coutts SB, Demchuk AM, et al. MR angiography compared to conventional selective angiography in acute stroke. Can. J. Neurol. Sci. 2006;33(1):58–62. doi: 10.1017/s0317167100004704. [DOI] [PubMed] [Google Scholar]
- 84.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am. J. Neuroradiol. 2008;29(3):582–587. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Unger E, Littlefield J, Gado M. Water content and water structure in CT and MR signal changes: possible influence in detection of early stroke. AJNR Am. J. Neuroradiol. 1988;9(4):687–691. [PMC free article] [PubMed] [Google Scholar]
- 86.van der Worp HB, Claus SP, Bar PR, et al. Reproducibility of measurements of cerebral infarct volume on CT scans. Stroke. 2001;32(2):424–430. doi: 10.1161/01.str.32.2.424. [DOI] [PubMed] [Google Scholar]
- 87.von Kummer R, Bourquain H, Bastianello S, et al. Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology. 2001;219(1):95–100. doi: 10.1148/radiology.219.1.r01ap0695. [DOI] [PubMed] [Google Scholar]
- 88.Wheeler HM, Mlynash M, Inoue M, et al. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke. 2013;44(3):681–685. doi: 10.1161/STROKEAHA.111.000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wintermark M, Albers GW, Alexandrov AV, et al. Acute stroke imaging research roadmap. AJNR Am. J. Neuroradiol. 2008;29(5):e23–e30. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wintermark M, Flanders AE, Velthuis B, et al. Perfu-sion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37(4):979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 91.Yoo AJ, Barak ER, Copen WA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010;41(8):1728–1735. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]
- 92.Yoo AJ, Chaudhry ZA, Gonzalez RG, Goyal M, Demchuk A, Mualem E, Buell H, Sit SP, Bose A The Penumbra Pivotal and PICS Investigators. Refining the pre-treatment noncontrast CT ASPECTS threshold for IAT selection: pooled analysis of the penumbra pivotal study and PICS registry. Stroke. 2012;43:A72. [Google Scholar]
- 93.Yoo AJ, Chaudhry ZA, Nogueira RG, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke. 2012;43(5):1323–1330. doi: 10.1161/STROKEAHA.111.639401. [DOI] [PubMed] [Google Scholar]
- 94.Yoo AJ, Heck DV, Frei D, Aceves M, Buell H, Kuo S, Kamalian S, Morais L, Sit SP, Bose A The Penumbra THERAPY Investigators. Preliminary clot length analysis of anterior circulation large vessel occlusions. Stroke. 2013;44:A193. [Google Scholar]
- 95.Yoo AJ, Simonsen CZ, Prabhakaran S, Chaudhry ZA, Issa M, Fugate JE, Linfante I, Kallmes DF, Dabus G, Zaidat OO. Refining angiographic biomarkers of reperfusion: modified TICI is superior to TIMI for predicting clinical outcomes after intra-arterial therapy. Stroke. 2013;44:A62. doi: 10.1161/STROKEAHA.113.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoo AJ, Verduzco LA, Schaefer PW, et al. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40(6):2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuki I, Kan I, Vinters HV, et al. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. AJNR Am. J. Neuroradiol. 2012;33(4):643–648. doi: 10.3174/ajnr.A2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zaidi SF, Aghaebrahim A, Urra X, et al. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke. 2012;43(12):3238–3244. doi: 10.1161/STROKEAHA.112.671594. [DOI] [PubMed] [Google Scholar]