Abstract
Background
Cationic liposome (CL)-DNA complexes are promising gene delivery vectors with potential applications in gene therapy. A key challenge in creating CL-DNA complexes for applications is that their transfection efficiency (TE) is adversely affected by serum. In particular, little is known about the effects of high serum contents on TE even though this may provide design guidelines for applications in vivo.
Methods
We prepared CL-DNA complexes in which we varied the neutral lipid (DOPC, glycerol-monooleate (GMO), cholesterol), the headgroup charge and chemical structure of the cationic lipid, and the ratio of neutral to cationic lipid; we then measured the TE of these complexes as a function of serum content and assessed their cytotoxicity. We tested selected formulations in two human cancer cell lines (M21/melanoma and PC-3/prostate cancer).
Results
In the absence of serum, all CL-DNA complexes of custom-synthesized multivalent lipids show high TE. Certain combinations of multivalent lipids and neutral lipids, such as MVL5(5+)/GMO-DNA complexes or complexes based on the dendritic-headgroup lipid TMVLG3(8+) exhibited high TE both in the absence and presence of serum. Although their TE still dropped to a small extent in the presence of serum, it reached or surpassed that of benchmark commercial transfection reagents, in particular at high serum content.
Conclusions
Two-component vectors (one multivalent cationic lipid and one neutral lipid) can rival or surpass benchmark reagents at low and high serum contents (up to 50%, v/v). We suggest guidelines for optimizing the serum resistance of CL-DNA complexes based on a given cationic lipid.
Keywords: gene delivery, serum, cationic liposomes, multivalent cationic lipid, glycerol monooleate
Introduction
Cationic liposome (CL)-DNA complexes are one of the most promising nonviral vectors for both in vitro and in vivo therapeutic applications [1-8]. However, their efficiency still lags behind that of viral vectors, especially in vivo. It is highly desirable to develop more efficient nonviral vectors because of the safety concerns associated with engineered viruses [9-12]. Additional advantages of nonviral vectors include facile and variable preparation, low potential immunogenicity, and the ability to transfer very large pieces of nucleic acids [13].
For in vitro applications, progress in the development of nonviral vectors has yielded multivalent cationic lipid vectors that exhibit transfection efficiencies (TEs; the ability to transfer DNA into cells followed by expression) competitive with those of viral vectors. Beyond the chemical structure of the lipids, properties of the self-assembled CL-NA complexes profoundly impact transfection [14-16]. Salient examples are the nano-scale structures of CL-DNA and CL-siRNA complexes (lamellar LαC; inverted and regular hexagonal HIIC and HIC; gyroid cubic QIIG, siRNA; see Fig. 1A-D) [17-21] and the lipid membrane charge density [22,23]. Custom synthesis of multivalent lipids enabled the discovery that membrane charge density is a predictive chemical parameter for transfection by LαC CL-DNA complexes [23,24], while HIC complexes from a dendritic MVL (+16e charge) significantly improved TE in hard-to-transfect mouse embryonic fibroblast cells [19]. Bicontinuous gyroid cubic CL-siRNA complexes (Fig. 1D) exhibit high silencing efficiency because the cubic phase facilitates fusion of the membranes of complexes and endosomes leading to efficient cytosol delivery [20,25].
Figure 1.
Schematics showing the local nanoscale structures of CL-NA complexes, which result spontaneously when CLs are mixed with nucleic acids. Structures were derived from synchrotron X-ray scattering data. (A) Lamellar (LαC), (B) inverted hexagonal (HIIC), and (C) hexagonal (HIC) CL-DNA complex structure. (D) Gyroid cubic CL-siRNA complex structure (QIIG, siRNA) with siRNA incorporated in the water channels. Parts A and B reprinted from [18] with permission. Part C reprinted with permission from [19]. Copyright 2006 American Chemical Society. Part D reprinted with permission from [20]. Copyright 2010 American Chemical Society.
The transfection efficiency of nonviral vectors is reduced, often drastically, by the presence of serum in cell culture media [1,26-28]. Vectors that maintain high TE in the presence of low amounts of serum (5 to 10% of culture medium, v/v) are desirable for ease of use and because serum starvation can affect the cell cycle of cultured cells. More importantly, high TE in the presence of high serum content may help predict high TE in vivo [27-29], the main current challenge for nonviral vectors.
Numerous attempts have been made to determine how serum decreases the TE of CL-DNA complexes, with limited success. Serum is a complex mixture of components, and several processes, some of them affecting TE in opposite ways, take place simultaneously when complexes are exposed to it. For example, negatively charged serum components bind to the positively charged CL-DNA complexes. This can reduce the interactions between the complexes and the cell membrane, leading to reduced uptake and inefficient endosomal escape. The binding of serum components can also cause structural reorganization of the complexes, colloidal instability, e.g. aggregation and dissociation of the complexes, and rapid clearance by the reticuloendothelial system (RES) [27-29,30-32].
A variety of strategies to improve the serum resistance of CL-DNA complexes have been investigated. Steric stabilization of CL-DNA complexes by incorporation of poly(ethylene glycol) (PEG)-lipids (PEGylation) increases their circulation time [33-35] and may improve their stability in serum, but it also reduces transfection efficiency [36-38]. Additional strategies include the design of novel cationic lipids [39,40], development of new formulations, [30,31,41,42], and using mixtures of cationic polymers with liposomes [43-45]. Multivalent cationic lipids, such as those used in our present study, typically yield DNA complexes with high TE and lower toxicity than univalent lipids [46-48]. Addition of a neutral (“helper”) lipid can affect transfection efficiency by controlling the membrane charge density (σM) of CL-DNA complexes [23], the structure of lipid self-assemblies [17,18,20,25], the thickness of the hydration layer, and the nucleic acid secondary and tertiary structures [49,50]. For example, the inclusion of fusogenic 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) can increase TE in vitro [2,22,51,52], and cholesterol can enhance the colloidal stability and TE of CL-DNA complexes in the absence or the presence of serum [43,53-56].
While we are pursuing strategies to increase the efficiency of PEGylated complexes [57], the focus of this work was on finding formulation strategies for serum-stable complexes based solely on cationic and neutral lipids. A number of commercially available reagents have achieved this goal (high TE at low serum), but their compositions are proprietary. More importantly, CL-DNA complexes have often been claimed to be serum-resistant without examining their TE at the high serum concentrations (≥50%) potentially relevant to gene delivery in vivo.
We measured transfection efficiency in the presence of varied amounts of serum for CL-DNA complexes prepared with a variety of cationic and neutral lipids, using a luciferase assay in mouse L-cells. We employed five different cationic lipids with varied headgroup charge and structure: DOTAP(1+) (2,3-dioleoyloxy-propyl-trimethylammonium chloride) and the custom synthesized lipids (Fig. 2) MVL5(5+) [24], TMVLG3(8+), TMVLBG1(8+), and TMVLBG2(16+) [19,58,59]. TMVLG3, TMVLBG1, and TMVLBG2 are lipids with dendritic headgroups (DLs). As the neutral lipid (NL), we employed DOPC (1,2-dioleoyl-sn-glycerophosphatidylcholine), cholesterol, or GMO (glycerol monooleate), at a number of different cationic lipid to neutral lipid molar ratios. Varying this ratio (1:0, 3:1, 1:1, and 1:3; mol/mol) allowed us to assess the effect of the membrane charge density (σM, the average charge per unit area of membrane) [23]. For selected efficient lipid formulations, we further measured TE in the human PC-3 (prostate cancer) and M21 (melanoma) cell lines. We also assessed the cytotoxicity of the complexes. We compare our results with the benchmark commercial transfection reagent, Lipofectamine2000. Serum-stable combinations of a single multivalent cationic and neutral lipid were able to rival or outperform Lipofectamine2000, especially at high serum content.
Figure 2.
The chemical structures of the multivalent cationic lipid MVL5 and the multivalent lipids with dendritic headgroups used in this work [19,24,58]. All lipids are based on a dioleoyltail moiety (top). The branching ornithine moieties in the headgroups of TMVLG3, TMVLBG1 and TMVLBG2 are highlighted in green, and the charge-bearing groups are highlighted in pink (ornithine for TMVLG3, carboxyspermine for TMVLBG1 and TMVLBG2, and aminopropylated carboxyspermine for MVL5).
Materials and Methods
Lipids and Liposome Preparation
Lipofectamine2000 was purchased from Invitrogen and used as per the manufacturer’s instructions. DOTAP and DOPC were purchased as solutions in chloroform from Avanti Polar Lipids (Alabaster, AL). Cholesterol and GMO were purchased from Sigma-Aldrich and Nu-Check-Prep, respectively, and dissolved in chloroform to prepare stock solutions. MVL5, TMVLBisG1, TMVLBisG2 and TMVLG3 (Fig. 2) were synthesized as described previously [19,24,58] and dissolved in chloroform/methanol (9:1, v/v) to prepare stock solutions. Measurements of the headgroup charge of these cationic lipids in complexes with DNA via an ethidium bromide displacement assay indicated complete protonation of the headgroups, i.e., headgroup charges of +5e (MVL5), +8e (TMVLBisG1 and TMVLG3), and +16e (TMVLBisG2) [58]. Liposomes were prepared at varied mol fraction of neutral lipid, ΦNL, where ΦNL= NNL/(NCL+NNL) (with NCL and NNL the amount (in mol) of cationic and neutral lipid, respectively). The relationship between the membrane charge density, σM, and ΦNL is σM=eZNCL/(NCLACL+NNLANL)=[1−ΦNL/(ΦNL+rΦCL)]σCL. Here, r=ACL/ANL is the ratio of the headgroup areas of the cationic and the neutral lipid; σCL=eZ/ACL is the charge density of the cationic lipid with valence Z; and ΦCL=1−ΦNL is the mol fraction of the cationic lipid. To prepare liposomes, lipid stock solutions were mixed at the appropriate ratios in glass vials and then dried, first under a stream of nitrogen and then in a vacuum (rotary vane pump) for 8-12 h. To the resulting thin lipid film, sterile high-resistivity (18.2 MΩ cm) water was added, and the mixture was incubated at 37 °C for at least 12 h. The final total lipid concentration was 1 mM. All aqueous lipid solutions were sonicated (tip sonicator) prior to use and stored at 4 °C.
Cell Culture and Transfection
Luciferase plasmid DNA (pGL3 Control Vector, Promega) was propagated in E. coli and isolated using a Qiagen Giga Kit. Mouse fibroblast L-cells (ATCC number: CCL-1), M21 cells (human melanoma; a gift from the group of E. Ruoslahti), and PC-3 cells (ATCC number: CRL-1435; human prostate cancer) were maintained in supplemented culture medium (Dulbecco’s Modified Eagle’s Medium (DMEM), containing 1% penicillin (Invitrogen) and 5% or 10% fetal bovine serum (Thermo Scientific) for mouse and human cell lines, respectively) at 37 °C in a humidified atmosphere with 5% CO2. Cells were reseeded approximately every 72 h to maintain subconfluency. For transfection, about 80 000 cells/well were seeded in 24-well plates and incubated for 18-24 h prior to transfection. A solution of pGL3 plasmid DNA (4 μg/mL in Opti-MEM) was prepared from a DNA stock solution at 1.0 mg/mL. Appropriate volumes of liposome solutions (to yield the desired lipid to DNA charge ratio, ρ) were diluted with Opti-MEM. The charge ratio ρ=N+/N-=ZNCL/Nnt, where N+ and N- are the numbers of positive (lipid) and negative (DNA) charges, Z is the valence of the cationic lipid, and NCL and Nnt are the amounts (in mol) of cationic lipids and nucleotides, respectively. Equal volumes of liposome and DNA solutions were combined and after incubation for 20 min at room temperature, 200 μL of this mixture (containing 0.4 μg DNA) were added per well. Thus, the amount of DNA added was identical for all TE measurements. For transfection in serum, complexes were first prepared as described above. To the complex suspension in Opti-MEM, the appropriate volume of serum to achieve the desired final serum concentration of 0%, 10%, 20%, or 50% (v/v) was then added, and the total volume of the mixture was made up to 400 μL with Opti-MEM. The resulting mixture was added to the cells. After 6 h of incubation at 37 °C, the transfection medium was removed, each well was washed once with PBS, and fresh culture medium was added to each well. Cells were harvested in 150 μL of Passive Lysis Buffer (Promega) after another 18-20 h (mouse fibroblasts) or 40-48 h (human cell lines). Luciferase expression was measured according to the assay manufacturer’s (Promega) instructions. A multilabel counter (Perkin-Elmer 1420 Victor3 V) was used to measure the relative light units (RLU) from the luminescence assay. Data points shown are the average of duplicate (and in some cases quadruplicate) measurements, with error bars showing the standard deviation. All experiments were repeated 2 to 3 times to ascertain reproducibility.
Cytotoxicity
Cytoxicity was assessed using a commercial, tetrazolium-salt-based assay (CellTiter 96 Aqueous One assay, Promega) for cell viability. Mouse fibroblast L-cells were seeded in 96-well plates (15 000 cells/well) in maintenance medium. After 18-20 hours incubation, the cells were washed once with PBS, and 40 μL of a mixture containing medium and complexes were added (prepared as for transfection; 0.08 μg DNA per well, i.e., transfection concentration). After 6 hours of incubation, the complex-containing medium was replaced by a mixture of 60 μL of Opti-MEM and 20 μL of the Cell Proliferation Assay. Following 3 h of incubation, absorbance at 490 nm was measured using a scanning multi-well spectrophotometer (Perkin-Elmer 1420 Victor3 V). The experiment was performed simultaneously for all lipids and the results were normalized to control wells, which differed from the experimental wells only in that they were treated with Opti-MEM instead of the medium/complexes mixture. Each data point represents the average of at least quadruplicate measurements, with error bars showing the standard deviation.
Results
The transfection efficiency of CL-DNA complexes in vitro typically decreases, often drastically, in the presence of serum. Our goal was to better understand this phenomenon by looking for correlations between TE and lipid structure or composition. We used a luciferase reporter gene expression assay in mouse fibroblast L-cells to evaluate the TE of a variety of lipid formulations in the presence of increasing amounts of serum. We systematically varied the neutral lipid, the membrane charge density (via the ratio of neutral to cationic lipid), the headgroup charge of cationic lipids and the chemical structure of those headgroups. We also evaluated the TE of selected formulations in two human cell lines: M21 (melanoma) and PC-3 (prostate cancer). Fig. 2 shows the chemical structures of the investigated multivalent cationic lipids, MVL5 (5+) [24], TMVLBisG1 (8+), TMVLG3 (8+), and TMVLBisG2 (16+) [19,58]. These lipids have identical hydrophobic moieties of two oleyl tails attached to a spacer based on 3,4-dihydroxybenzoic acid. The TMVL lipids have a slightly longer spacer and dendritic headgroups with ornithine cores and ornithine or carboxyspermine endgroups. The headgroup of MVL5 is an aminopropylated carboxyspermine. In serum-free transfection medium, DNA complexes of mixtures of all of the investigated multivalent lipids with neutral DOPC (1,2-dioleoyl-sn-glycero phosphatidylcholine) efficiently transfect cells over a broad range of composition [19,23,24,58]. As a univalent cationic lipid to compare with the multivalent lipids, we chose the commercially available univalent lipid DOTAP (1,2-dioleoyl-3-trimethyammonim propane). (See Fig. S1 in the Supporting Information for the structures of DOTAP and DOPC.)
The effect of neutral lipids for univalent DOTAP and multivalent MVL5
The inclusion of a neutral lipid in CL-DNA complexes frequently increases the transfection efficiency over that of cationic lipid alone in serum-free medium [2,51,52], e.g. by tuning the membrane charge density [22,23] or CL-NA complex structure [7,19,20,22,25,59]. To assess how the choice of neutral lipid affects TE in the presence of serum, we investigated CL-DNA complexes based on the commercially available cationic lipids DOTAP (univalent) and MVL5 (multivalent) as well as the neutral lipids DOPC, cholesterol (Chol), and GMO. While much of our prior work has used DOPC, Chol has been employed by others to confer serum resistance and high TE in vivo [43,53-55], and GMO displays intriguing phase behavior (including gyroid cubic structures) and high silencing efficiency in CL-siRNA complexes [20,25]. The chosen formulations—with a charge ratio (ρ) of 3 and neutral lipid mol fraction (ΦNL) of 0.25 for DOTAP-based complexes and ρ=10 and ΦNL=0.5 for MVL5-based complexes—exhibit high TE in the absence of serum. The TE of DOTAP-based complexes containing DOPC and cholesterol is nearly unaffected by 10% serum (Fig. 3A). In contrast, the efficiency of complexes containing GMO as well as of those without neutral lipid moderately drops at this low serum content. As serum content increases, the TE of all DOTAP-based complexes drops strongly. Their TE at a serum content of 50% (TE≈106 RLU/mg protein) is as low as that of uncomplexed DNA in serum-free medium and in some cases even lower (for DOTAP/GMO).
Figure 3.
Transfection efficiency as a function of serum content for CL-DNA complexes based on DOTAP and MVL5 in combination with different neutral lipids (NLs). (A) TE of DOTAP/NL-DNA complexes at ρ=3, without NL and with DOPC, cholesterol, or GMO at ΦNL=0.25. (B) TE of MVL5/NL-DNA complexes at ρ=10, without NL and with DOPC, cholesterol, or GMO at ΦNL=0.5. Also shown are the TE of the benchmark commercial reagent Lipofectamine2000 and uncomplexed DNA. Note the difference in shape of the curves for monovalent DOTAP (gradual decline) and multivalent MVL5 (initial step drop to nearly flat slope). See the text for further discussion.
MVL5-based CL-DNA complexes are as efficient (TE≈109 (RLU/mg protein) as the benchmark commercial reagent Lipofectamine2000 in the absence of serum (Fig. 3B). MVL5/GMO-DNA complexes continue to rival Lipofectamine2000 at 10 and 20% serum and even surpass it at 50% serum. Their TE remains very high after an initial small drop at 10% serum. The TE of the three other MVL5-based complexes (MVL5/DOPC, MVL5/Chol, and MVL5 only) drops strongly at 10% serum but then stays constant (MVL5/Chol) or only drops slightly for 20% and 50% serum. This behavior is markedly different from that of DOTAP-based complexes. As a function of serum content, TE declines gradually for complexes of monovalent DOTAP. However, for complexes of multivalent MVL5, TE shows an initial step drop and then remains nearly constant as serum content increases. As a result, all MVL5-based complexes surpass the DOTAP-based complexes in efficiency at no serum and 50% serum, while the TE of MVL5- and DOTAP-based complexes is comparable at lower (10 and 20%) serum contents. The notable exception are MVL5/GMO-DNA complexes, which show higher TE throughout.
To assess the effect of membrane charge density (σM), we varied ΦNL for the MVL5-based complexes and the DOTAP/Chol-DNA complexes (again using ρ=3 for DOTAP-based complexes and ρ=10 for MVL5-based complexes). With the one exception of ΦChol=0.75, MVL5-based complexes exhibit very high TE (TE>109 RLU/mg protein) in the absence of serum, independent of ΦNL (and equivalently σM) (Fig. 4A-C). As a function of increasing serum content, the MVL5-based complexes exhibit the behavior already seen in Fig. 3B: the largest drop in TE occurs at 10% serum, with little to no change (especially compared to the initial drop) at higher serum contents (Fig. 4A-C). (The largest changes in TE at high serum content (10% serum to 50% serum) are seen for complexes with ΦDOPC=0.75 and ΦGMO=0.25.)
Figure 4.
Transfection efficiency as a function of mol fraction of neutral lipid (ΦNL) for CL-DNA complexes based on MVL5 and DOTAP, in the presence of varying amounts of serum (0%, 10%, 20%, and 50%). Parts A, B, and C show TE for MVL5-based complexes containing DOPC, Cholesterol, and GMO, respectively. All these complexes were prepared at ρ=10. Part D shows the TE of DOTAP/Chol-DNA complexes at ρ=3.
Differences between the neutral lipids are evident when comparing the TE of MVL5-based complexes as a function of ΦNL. The TE of MVL5/GMO-DNA complexes at ΦGMO=0.5 is the least sensitive to the addition of serum (Fig. 4C) and thus remains exceptionally high even at high serum contents (as already seen in Fig. 3B). Similarly, the initial drop in TE (0 to 10% serum) for complexes at ΦGMO=0.25 and 0.75 is smaller than for most other MVL5-based complexes (all but MVL5/Chol-DNA complexes at ΦChol=0.75, which however start at a lower TE). The TE of MVL5/DOPC-DNA complexes (Fig. 4A) decreases slightly with increasing ΦNL, both in the absence and in the presence of serum. The TE of MVL5/Chol-DNA complexes (Fig. 4C) in the absence of serum also decreases with increasing ΦNL, but the drop in TE from ΦChol=0 to ΦChol=0.25 and 0.5 is very small while that from ΦChol=0.5 to ΦChol=0.75 is larger than for DOPC. However, in the presence of serum, TE is remarkably unaffected by ΦChol. This reflects a comparatively small initial drop in TE (when moving from no serum to 10% serum) at ΦChol=0.75, where TE in the absence of serum is lower than at the other values of ΦChol. In other words, increasing cholesterol content reduces the serum sensitivity of TE for MVL5/Chol-DNA complexes. This effect is even more pronounced for DOTAP/Chol-DNA complexes (Fig. 4D). While the TE of these complexes in serum-free medium decreases with ΦChol, it stays essentially constant (10% serum) or even increases (20% and in particular 50% serum) with ΦChol in the presence of serum. As a consequence, the lipid composition with the highest TE in the absence of serum exhibits the lowest TE at higher serum content (20 and 50%) and vice versa. (There is little change in TE with ΦChol at 10% serum.) Others have observed previously that complexes optimized for in vitro transfection in serum-free medium performed poorly at high serum contents or in vivo, and that complexes which transfected efficiently in vivo were sub-optimal for transfection in vitro [27,43,54].
Highly charged cationic lipids with dendritic headgroups
The data displayed in Figs. 3 and 4 shows that multivalent MVL5 consistently led to higher TE than univalent DOTAP and that Chol and GMO but not DOPC are NLs that can improve serum resistance. Thus, we investigated the TE of complexes of other multivalent lipids with Chol and GMO as a function of serum content. We previously prepared a series of multivalent cationic lipids with dendritic headgroups (DLs; headgroup charges of +4 e to + 16 e). In the absence of serum, DNA complexes of these lipids exhibited high TE over a broad range of composition and nonlamellar structures at higher content of DLs [7,19,58,59]. For the present study with serum, we investigated three of these DLs (Fig. 2): the highly charged TMVLBG2(16+) and two lipids (TMVLBG1 and TMVLG3) with eight charges in their headgroup but different headgroup architectures.
Figure 5 shows TE for DNA complexes of TMVLG3, TMVLBG1, or TMVLBG2 mixed with cholesterol or GMO as a function of serum content and ΦNL (at fixed ρ=10). In the absence of serum, all of these complexes transfect very efficiently (TE≈109 RLU/mg protein, comparable to Lipofectamine2000) over the investigated range of membrane charge density.
Figure 5.
Transfection efficiency of CL-DNA complexes based on the highly charged DLs TMVLG3 (8+), TMVLBG1 (8+), and TMVLBG2 (16+), in the presence of varying amounts of serum (0%, 10%, 20%, and 50%). All complexes were prepared at ρ=10. The plots show the effect of different mol fractions of neutral lipid (ΦNL). The first column (parts A (TMVLG3), C (TMVLBG1), and E (TMVLBG2)) shows the TE of DL/Chol-DNA complexes. The second column (parts B (TMVLG3), D (TMVLBG1), and F (TMVLBG2)) shows TE of DL/GMO-DNA complexes.
As seen for MVL5, increasing serum content from 0 to 10% causes a steep drop in the TE of the DL-based complexes. After that initial drop, the TE drops only slightly or even remains constant (for serum contents of 20 and 50%). The initial drop is a little over one order of magnitude for most complexes containing TMVLG3 (8+) (Fig. 5A,B). Because serum contents of 20 and 50% do not reduce their TE further and because their TE in the absence of serum is very high, these complexes are efficient (TE≈108 RLU/mg protein) even at high serum content. In noteworthy contrast, the initial drop in TE is much larger for complexes based on the other two DLs, TMVLBG1 (8+) and TMVLBG2 (16+) (Fig. 5C-F). For most of these complexes, there is also a further but much smaller drop as serum content is increased to 20 and 50%.
Neither the choice of neutral lipid nor the mol fraction of neutral lipid (which controls σM) has a large effect on the serum resistance of DL-based complexes. In fact, complexes with and without neutral lipid behave essentially the same. This is unlike what we observed for MVL5, in particular for MVL5/GMO-DNA complexes. Whether this is because of differences in σM or because the complexes have differing nanostructures is a subject of ongoing investigation.
Transfection of Human Cell Lines
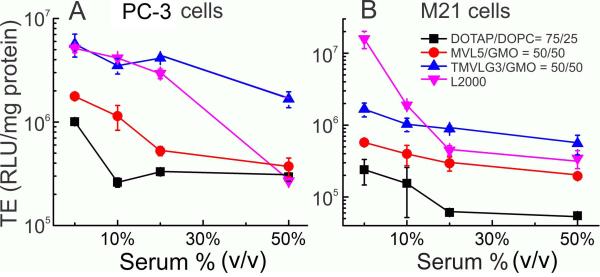
Transfection efficiency, including the relative efficiency of transfection agents, can vary widely between cell lines. To assess if our findings on serum-resistant formulations are broadly applicable, we measured the TE of selected vectors in two human cell lines, M21 (melanoma) and PC-3 (prostate cancer). As vectors based on multivalent lipids, we chose MVL5/GMO-DNA complexes and TMVLG3/GMO-DNA complexes, both at ΦGMO=0.5 and ρ=10 (see Fig. 3B and Fig. 5B). As commercially available benchmarks, we used DOTAP/DOPC-DNA complexes, and Lipofectamine2000.
In the human cell lines, the absolute levels of luciferase expression are low: TE never exceeded 2×107 RLU/mg protein even in the absence of serum (Fig. 6). Most notable, however, is the high performance of TMVLG3/GMO-DNA complexes. Their TE in the absence of serum is as high as that of Lipofectamine2000 in PC-3 cells (Fig. 6A) and then only drops slighly with increasing contents of serum, even as the TE of Lipofectamine2000 drops drastically at 50% serum. In M21 cells, the TE of TMVLG3/GMO-DNA complexes in the absence of serum is higher than that of DOTAP/DOPC-DNA complexes and MVL5/GMO-DNA complexes but about an order of magnitude below that of Lipofectamine2000 (Fig. 6B). Nonetheless, because their TE stays nearly constant with increasing serum content, it exceeds that of all other vectors, including Lipofectamine 2000, at 20 and 50% serum. Lipofectamine2000 exhibits the highest TE in both human cell lines in the absence of serum (together with TMVLG3/GMO-DNA complexes in PC-3 cells). However, it also shows the steepest drop with increasing serum content. In contrast, the TE of both vectors based on multivalent lipids exhibits only a small drop with increasing serum content. Because of this, the TE of MVL5/GMO-DNA complexes rivals that of Lipofectamine2000 at high (20 and 50%, M21 cells) or very high (50%, PC-3 cells) serum content, despite of its comparably low value in the absence of serum. The TE of the DOTAP/DOPC-DNA complexes is the lowest of all vectors, for all data points. It starts low in the absence of serum and then drops moderately with increasing serum content.
Figure 6.
Transfection efficiencies of selected serum-resistant multivalent lipid/GMO-DNA complexes (ΦGMO= 0.5; ρ=10) in two human cell lines (part A: PC-3 (prostate cancer) cells; part B: M21 (melanoma) cells) as a function of serum content. Also shown are the transfection efficiencies of DOTAP/DOPC-DNA complexes (ρ=3, ΦDOPC=0.25) and the benchmark commercial transfecting agent Lipofectamine2000.
Cytotoxicity
Vector toxicity remains a concern for most cationic transfection agents both in vitro and in vivo. Thus, we measured cell viability after incubation with DNA complexes of the investigated cationic lipids alone and of 1:1 mixtures with the investigated neutral lipids. We used the charge ratios employed in the transfection experiments (ρ=10 for multivalent lipids, ρ=3 for DOTAP). As shown in Fig. 7, cell viability remained high at 75% or higher for all data points, with the lowest viabilities observed for Lipofectamine2000 as well as TMVLG3 and TMVLBisG2 alone and in combination with cholesterol.
Figure 7.
Assessment of the cytotoxicity of representative CL-DNA complexes. The plots show cell viability after treatment with CL-DNA complexes prepared with different neutral lipids: DOPC (part A; ΦDOPC=0.5, except for DOTAP (ΦDOPC=0.25)), cholesterol (part B; ΦChol=0.5), GMO (part C; ΦGMO=0.5), and no neutral lipid (part D). Complexes were prepared and added to the cells as for transfection (ρ=10 for multivalent lipids, ρ=3 for DOTAP). Toxicity is negligible in many cases, and never worse than that of the commercial benchmark reagent Lipofectamine2000 (L2000).
Discussion
The TE of many synthetic vectors drops in the presence of serum. While some formulations remain efficient at the level of serum content typically used in cell culture (≤ 10%), the effect of higher serum levels has been investigated less thoroughly in prior studies, despite its possible relevance to choosing viable formulations for transfection in vivo. Varying lipid structure and composition in CL-DNA complexes, we sought to establish guideline for developing serum-resistant vectors from trends in TE. In the following, we will refer to the TE in the absence of serum as the “starting TE” and define serum resistance as a weak dependence of TE on the content of serum (i.e., complexes are called “serum resistant” if their TE remains close to the starting TE as serum content increases).
Neutral Lipids
DOPC as the neutral lipid yields complexes with very poor serum resistance in combination with monovalent (DOTAP, Figs. 3A and 6) as well as multivalent (MVL5, Figs. 3A and 4A) lipid. We therefore did not study mixtures of DOPC with the lipids with dendritic headgroups (DLs, Fig. 2). (We did not investigate formulations with DOPE because of their relatively high toxicity and reported poor efficiency in vivo [21,27,43].)
Cholesterol (Chol) has been reported to increase the serum resistance of DOTAP-based CL-DNA complexes [53,55]. Our data confirms this: cholesterol was the only neutral lipid (out of the three tested) able to preserve relatively high TE in serum (TE≈107 RLU/mg protein at 50% serum, TE≈108 RLU/mg protein at 10% and 20% serum) with univalent DOTAP (Fig. 4D). Chol-based complexes become more serum resistant with decreasing σM (increasing ΦChol) for DOTAP and MVL5 (Fig. 4C,D), but their starting TE drops with σM. This is one of the few trends shared by complexes based on monovalent DOTAP and multivalent MVL5. Indeed, our data is consistent with σM being a key parameter governing the starting TE and serum resistance of Chol-containing complexes: a drop in starting TE and increase in serum resistance occurs at ΦChol of 0.25 for DOTAP, at ΦChol=0.75 for MVL5, while no drop in starting TE and no increased serum resistance is observed for the even more highly charged DLs at the investigated ΦChol. For the DLs, both starting TE and serum resistance essentially do not change with ΦChol over the investigated range. Whether σM has a direct effect on TE and serum resistance or whether these findings are related to the structure of the complexes is a subject of ongoing investigation.
GMO showed the most diverse and cationic lipid-dependent effects of the investigated neutral lipids. A poor choice in combination with DOTAP, GMO is an excellent helper lipid for MVL5 with a strong effect of ΦGMO on serum resistance (Fig. 4B). Serum resistance is maximized at ΦGMO=0.5, where TE—even at 50% serum—remains nearly as high as in serum-free medium. The serum resistance of MVL5/GMO-DNA complexes with ΦGMO=0.25 and ΦGMO=0.75 is also increased compared to ΦGMO=0.
For the more highly charged DLs, however, ΦGMO hardly affects starting TE or serum resistance (Fig. 5B,D,F); the biggest effect is seen at ΦGMO=0.75. Given the success of GMO in combination with the multivalent lipids, the poor TE and serum resistance of DOTAP/GMO-DNA complexes (Fig. 3A) was surprising. This highlights once again that monovalent and multivalent lipids can show fundamentally different behavior as we discuss in more detail in the next section (see also [21,60]).
Further work is needed to elucidate the cause of the uniquely high serum resistance of MVL5/GMO-DNA complexes. The membrane charge densities of MVL5- and DL-based complexes with GMO as the neutral lipid overlap widely. Thus, differences in σM are unlikely to be the cause of the differences in serum resistance. Differing complex nanostructures (lamellar (MVL5 [24,25,60]) vs. nonlamellar (DLs [7,19,58,59])) or specific interactions between neutral and cationic lipids remain as possible explanations which are the subject of ongoing investigation.
It is intriguing that for MVL5/NL-DNA complexes, the serum resistance as a function of ΦNL is distinctly different for the three NLs. It decreases with ΦDOPC, increases with ΦChol, and exhibits a maximum as a function of ΦGMO. This diversity of observed behaviors and their dependence on the cationic lipid makes it challenging to rationalize the differences between neutral lipids based on their structures or properties. Nonetheless, the large, well-hydrated headgroup of DOPC appears to be a feature unfavorable for serum resistance, whereas the small (hydroxy) headgroup of cholesterol (which results in a thinner hydration layer and less permeable membranes) appears favorable. GMO, too, has a fairly small headgroup consisting of two hydroxy groups. Both cholesterol and DOPC have been shown to interact with serum albumin [61-64], the main protein component of serum, but the nature of these interactions may be different and contribute to the difference in serum stability.
Cationic Lipids
We observed a number of differences between monovalent and multivalent lipids. For example, GMO reduced the starting TE and serum resistance only for complexes based on monovalent DOTAP. An important and consistent observation is that the response to different levels of serum is also distinctly different for monovalent and multivalent cationic lipids: while the decline of the TE of DOTAP-based complexes with increasing serum content is gradual, the TE of complexes based on multivalent lipids shows the biggest drop from 0% to 10% serum but generally remains very stable beyond that. Interestingly, that holds even when the drop in TE from 0% to 10% serum is small (Figs. 4B and 6), and these are the complexes that performed better than the commercial benchmark Lipofectamine2000. All DOTAP-based complexes have very low TE at 50% serum and thus are not good candidate vectors for in vivo gene delivery application.
The difference in TE behavior between DOTAP and multivalent lipids with increasing serum content may be related to differences in the efficiency of attachment of anionic serum components. The initial drop at 10% serum and following plateau behavior (where TE is nearly constant between 10 and 50% serum content) that we observe for multivalent lipids suggests that the main interactions between complexes and serum components (e.g., adhesion of anionic serum components) occur already at a serum content between 0 and 10% and that additional interactions between serum components and complexes at higher serum contents are minimal (i.e., for multivalent lipids, ≤10% serum may be the concentration where anionic serum components have essentially neutralized the complexes). In other words, facile neutralization of complexes due to stronger binding by serum components to multivalent lipid-based complexes causes the initial drop in TE that is followed by a plateau. In contrast, higher concentrations of serum are required for complex neutralization for univalent lipids, which exhibit weaker binding. Stronger binding of serum components to multivalent lipid-based complexes is expected because cationic membranes containing multivalent lipids are more efficient at counterion condensation at the complex-solution interface than those containing univalent lipids [65]. Efficient counterion condensation leads to a large adhesion energy for binding of anionic serum components to the complex because of the gain in solution free energy from the release of counterions upon binding [66,67].
The most striking feature in the data for DL-based complexes is how TE is nearly independent of the choice of NL and ΦNL (Chol vs GMO; we did not investigate DOPC-containing complexes of DLs in detail because of the poor serum resistance of both DOTAP/DOPC- and MVL5/DOPC-DNA complexes). This feature of robustness of TE with respect to ΦNL may prove beneficial for applications that require the addition of neutral lipids to perform distinct functions, such as PEG-lipids. This is why we used TMVLG3/GMO-DNA complexes rather than the equally serum-resistant TMVLG3-DNA complexes (Fig. 5A,B) for the experiments with the human cell lines.
Intriguingly, the TE of TMVLG3(8+)-based CL-DNA complexes in the presence of serum is higher than both that of complexes based on TMVLBG2(16+) and TMVLBG1(8+). This is because the initial drop in TE (from 0% to 10% serum) is very large (around two orders of magnitude) for TMVLBG2 and TMVLBG1 but much less (about one order of magnitude) for TMVLG3. This finding is especially surprising considering that TMVLG3 and TMVLBisG1 have the same headgroup charge, exhibit the same very high starting TE at all investigated ΦNL (independent of the neutral lipid), and form the same DL/DOPC-DNA complex structures as a function of ΦDOPC [58]. Thus, not only the headgroup charge of the cationic lipid affects the serum compatibility, but also (and to a large extent) the chemical structure of the headgroup. The DL headgroups are based on internal branching units (ornithine; green background in Fig. 2). In the case of TMVLG3, ornithine (with two amino groups, i.e., two positive charges) also serves as the charge-bearing unit attached to the branching units. In the case of TMVLBG1 and TMVLBG2, carboxyspermine (with four amino groups, i.e., four positive charges), is the charge-bearing unit. The branched core of the TMVLG3 headgroup (1+2 ornithine moieties) is thus larger than that of the TMVLBG1 headgroup (a single ornithine), while their overall headgroup size and charge density is comparable. The additional ornithine moieties in the TMVLG3 headgroup provide more hydrogen bond donors and acceptors (9 vs. 3 amide bonds in the headgroup). These increased capabilities for hydrogen bonding may stabilize the membrane and complex against the detrimental effects of serum. In addition, it is possible that the very high headgroup charge of TMVLBG2 is unfavorable for serum resistance, i.e., there may be an optimal range of headgroup charge.
In our transfection experiments with human cell lines, the robust and high performance of TMVLG3/GMO-DNA complexes with respect to both serum resistance and level of TE in the different cell lines stands out. They transfect more efficiently than Lipofectamine2000 at higher serum content (20 and 50%) in both cell lines. At 10% serum (and without serum for PC3 cells), the TE of these complexes is near-identical to that of Lipofectamine2000. MVL5/GMO-DNA complexes maintained their high serum resistance in human cell lines, but had a surprisingly low starting TE. Thus, our data suggest that the serum resistance of complexes based on multivalent cationic lipids and GMO is independent of the transfected cell line. However, one may have to optimize the choice of cationic lipid to achieve high absolute TE.
Conclusions
Our data shows that optimized combinations of a multivalent lipid and a neutral lipid can outperform benchmark commercial transfection reagents such as Lipofectamine2000 (equal TE at low serum content, higher TE at high serum content). Below, we summarize the findings from this work in the form of guidelines for optimizing the TE of CL-DNA complexes in the presence of serum (with a focus on high serum content). If designing a novel cationic lipid, (a) a multivalent headgroup is desirable (intermediate valency, i.e., +5e and +8e, gave the most favorable results), and (b) incorporating a large number of hydrogen bonding donor and acceptor groups in the headgroup appears favorable. Synthesizing a small library of structurally diverse headgroups according to these design principles increases the likelihood of finding a lipid that is efficient in a variety of cell lines. If optimizing a formulation starting from a given cationic lipid, the starting TE of a formulation (TE in the absence of serum) will not predict the TE in the presence of serum. If the cationic lipid is monovalent, (a) cholesterol should be the first choice of neutral lipid (at medium to high ΦChol) and (b) all serum contents of interest should be tested. If the cationic lipid is multivalent, (a) cholesterol and GMO are the preferred neutral lipids, (b) the TE at 10% serum will likely predict the TE at higher serum contents, and (c) even if the starting TE varies little with ΦNL, this parameter should be varied. In our experience, testing a single, appropriate charge ratio (ρ=3 for monovalent and ρ=10 for multivalent lipids) suffices to assess the potential of a given cationic lipid. In general, optimization may have to be performed separately for each cell line, but this is not necessarily the case: certain vectors, such as the TMVLG3/GMO-DNA complexes investigated in this work, perform extremely well in a variety of cell lines, especially at high serum content. A future challenge is to increase the absolute values of TE for human cell lines.
Supplementary Material
Acknowledgment
This work was supported by NIH GM-59288. CLC was supported by Academia Sinica, Taiwan. We thank the group of Prof. E. Ruoslahti for providing the M21 and PC-3 cell lines.
Footnotes
Conflicts of Interest Statement
There are no conflicts of interest.
References
- 1.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felgner JH, Kumar R, Sridhar CN, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- 3.Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;9:E92–104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortunati E, Bout A, Zanta MA, Valerio D, Scarpa M. In vitro and in vivo gene transfer to pulmonary cells mediated by cationic liposomes. Biochim Biophys Acta. 1996;1306:55–62. doi: 10.1016/0167-4781(95)00217-0. [DOI] [PubMed] [Google Scholar]
- 5.Yang JP, Huang L. Time-dependent maturation of cationic liposome-DNA complex for serum resistance. Gene Ther. 1998;5:380–387. doi: 10.1038/sj.gt.3300596. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Liggitt D, Zhong W, Tu G, Gaensler K, Debs R. Cationic Liposome-mediated Intravenous Gene Delivery. J Biol Chem. 1995;42:24864–24870. doi: 10.1074/jbc.270.42.24864. [DOI] [PubMed] [Google Scholar]
- 7.Ewert KK, Zidovska A, Ahmad A, Bouxsein NF, Evans HM, McAllister CS, Samuel CE, Safinya CR. Cationic Lipid-Nucleic Acid Complexes for Gene Delivery and Silencing: Pathways and Mechanisms for Plasmid DNA and siRNA. Topics Curr Chem. 2010;296:191–226. doi: 10.1007/128_2010_70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 – an update. J Gene Med. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 9.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in an ornithine transcarbamylase-deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nature Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 11.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S-D, Huang L. Non-viral is superior to viral gene delivery. J Controlled Release. 2007;123:181–183. doi: 10.1016/j.jconrel.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Harrington JJ, VanBokkelen G, Mays RW, Gustashaw K, Willard HF. Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nature Genet. 1997;15:345–355. doi: 10.1038/ng0497-345. [DOI] [PubMed] [Google Scholar]
- 14.Safinya CR, Ewert K, Ahmad A, et al. Cationic liposome-DNA complexes: from liquid crystal science to gene delivery applications. Philosoph Trans R Soc London Ser A. 2006;364:2573–2596. doi: 10.1098/rsta.2006.1841. [DOI] [PubMed] [Google Scholar]
- 15.Safinya CR, Ewert KK, Leal C. Cationic liposome-nucleic acid complexes: liquid crystal phases with applications in gene therapy. Liq Cryst. 2011;38:1715–1723. doi: 10.1080/02678292.2011.624364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safinya CR, Deek J, Beck R, et al. Liquid crystal assemblies in biologically inspired systems. Liq Cryst. 2013;40:1748–1758. doi: 10.1080/02678292.2013.846422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rädler JO, Koltover I, Salditt T, Safinya CR. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997;275:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 18.Koltover I, Salditt T, Rädler JO, Safinya CR. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 19.Ewert KK, Evans HM, Zidovska A, Bouxsein NF, Ahmad A, Safinya CR. A columnar phase of dendritic lipid-based cationic liposome-DNA complexes for gene delivery: hexagonally ordered cylindrical micelles embedded in a DNA honeycomb lattice. J Am Chem Soc. 2006;128:3998–4006. doi: 10.1021/ja055907h. [DOI] [PubMed] [Google Scholar]
- 20.Leal C, Bouxsein NF, Ewert KK, Safinya CR. Highly efficient gene silencing activity of siRNA embedded in a nanostructured gyroid cubic lipid matrix. J Am Chem Soc. 2010;132:16841–16847. doi: 10.1021/ja1059763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouxsein NF, McAllister CS, Ewert KK, Samuel CE, Safinya CR. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry. 2007;46:4785–4792. doi: 10.1021/bi062138l. [DOI] [PubMed] [Google Scholar]
- 22.Lin AJ, Slack NL, Ahmad A, George CX, Samuel CE, Safinya CR. Three-dimensional imaging of lipid gene-carriers: membrane charge density controls universal transfection behavior in lamellar cationic liposome-DNA complexes. Biophys J. 2003;84:3307–3316. doi: 10.1016/S0006-3495(03)70055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad A, Evans HM, Ewert K, George CX, Samuel CE, Safinya CR. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: lipid-DNA complexes for gene delivery. J Gene Med. 2005;7:739–748. doi: 10.1002/jgm.717. [DOI] [PubMed] [Google Scholar]
- 24.Ewert KK, Ahmad A, Evans HM, Schmidt HW, Safinya CR. Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J Med Chem. 2002;45:5023–5029. doi: 10.1021/jm020233w. [DOI] [PubMed] [Google Scholar]
- 25.Leal C, Ewert KK, Shirazi RS, Bouxsein NF, Safinya CR. Nanogyroids incorporating multivalent lipids: enhanced membrane charge density and pore forming ability for gene silencing. Langmuir. 2011;27:7691–7697. doi: 10.1021/la200679x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simberg D, Weisman S, Talmon Y, Barenholz Y. DOTAP (and other cationic lipids): chemistry, biophysics, and transfection. Crit Rev Ther Drug Carrier Syst. 2004;21:257–317. doi: 10.1615/critrevtherdrugcarriersyst.v21.i4.10. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–594. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 28.Zelphati O, Uyechi LS, Barron LG, Szoka FC., Jr. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim Biophys Acta. 1998;1390:119–133. doi: 10.1016/s0005-2760(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Qi H, Huang L, Liu D. Factors controlling the efficiency of cationic lipid-mediated transfection in vivo via intravenous administration. Gene Ther. 1997;4:517–523. doi: 10.1038/sj.gt.3300424. [DOI] [PubMed] [Google Scholar]
- 30.Lewis JG, Lin KY, Kothavale A, et al. A serum-resistant cytofectin for cellular delivery of antisense oligodeoxynucleotides and plasmid DNA. Proc Natl Acad Sci USA. 1996;93:3176–3181. doi: 10.1073/pnas.93.8.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Anchordoquy TJ. The role of lipid charge density in the serum stability of cationic lipid/DNA complexes. Biochim Biophys Acta. 2004;1663:143–157. doi: 10.1016/j.bbamem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 33.Nicolazzi C, Mignet N, de la Figuera N, et al. Anionic polyethyleneglycol lipids added to cationic lipoplexes increase their plasmatic circulation time. J Controlled Release. 2003;88:429–443. doi: 10.1016/s0168-3659(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 34.Ambegia E, Ansell S, Cullis P, Heyesa J, Palmera L, MacLachlan I. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim Biophys Acta. 2005;1669:155–163. doi: 10.1016/j.bbamem.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Tam P, Monck M, Lee D, et al. Stabilized plasmid-lipid particles for systemic gene therapy. Gene Ther. 2000;7:1867–1874. doi: 10.1038/sj.gt.3301308. [DOI] [PubMed] [Google Scholar]
- 36.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 37.Harvie P, Wong FM, Bally MB. Use of poly(ethylene glycol)-lipid conjugates to regulate the surface attributes and transfection activity of lipid-DNA particles. J Pharm Sci. 2000;89:652–663. doi: 10.1002/(SICI)1520-6017(200005)89:5<652::AID-JPS11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Herranz A, Ahmad A, Evans HM, Ewert K, Schulze U, Safinya CR. Surface Functionalized Cationic Lipid-DNA Complexes for Gene Delivery: PEGylated Lamellar Complexes Exhibit Distinct DNA-DNA Interaction Regimes. Biophys J. 2004;86:1160–1168. doi: 10.1016/S0006-3495(04)74190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi T, Harada A, Emi N, Kono K. Preparation of Efficient Gene Carriers Using a Polyamidoamine Dendron-Bearing Lipid: Improvement of Serum Resistance. Bioconjugate Chem. 2005;16:1160–1165. doi: 10.1021/bc050012f. [DOI] [PubMed] [Google Scholar]
- 40.Bajaj A, Kondaiah P, Bhattacharya S. Synthesis and gene transfer activities of novel serum compatible cholesterol-based gemini lipids possessing oxyethylene-type spacers. Bioconjugate Chem. 2007;18:1537–1546. doi: 10.1021/bc070010q. [DOI] [PubMed] [Google Scholar]
- 41.Yang JP, Huang L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1997;4:950–960. doi: 10.1038/sj.gt.3300485. [DOI] [PubMed] [Google Scholar]
- 42.Hofland HE, Shephard L, Sullivan SM. Formation of stable cationic lipid/DNA complexes for gene transfer. Proc Natl Acad Sci USA. 1996;93:7305–7309. doi: 10.1073/pnas.93.14.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong K, Zheng W, Baker A, Papahadjopoulos D. Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. FEBS Lett. 1997;400:233–237. doi: 10.1016/s0014-5793(96)01397-x. [DOI] [PubMed] [Google Scholar]
- 44.Matsuura M, Yamazaki Y, Sugiyama M, et al. Polycation liposome-mediated gene transfer in vivo. Biochim Biophys Acta. 2003;1612:136–143. doi: 10.1016/s0005-2736(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Rizzo MA, Bhattacharya S, Huang L. Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5:930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- 46.Byk G, Dubertret C, Escriou V, et al. Synthesis, Activity, and Structure-Activity Relationship Studies of Novel Cationic Lipids for DNA Transfer. J Med Chem. 1998;41:224–235. doi: 10.1021/jm9704964. DOI: 10.1021/jm9704964. [DOI] [PubMed] [Google Scholar]
- 47.Cooper RG, Etheridge CJ, Stewart L, et al. Polyamine Analogues of DC-Chol as Agents for Gene Delivery. Chem Eur J. 1998;4:137–151. DOI: 10.1002/(SICI)1521-3765(199801)4:1<137::AID-CHEM137>3.0.CO;2-2. [Google Scholar]
- 48.Behr J-P, Demeneix B, Leffler J-P, Perez-Mutul J. Efficient Gene Transfer into Mammalian Primary Endocrine Cells with Lipopolyamine-Coated DNA. Proc Natl Acad Sci USA. 1989;86:6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirsch-Lerner D, Barenholz Y. Hydration of lipoplexes commonly used in gene delivery: follow-up by laurdan fluorescence changes and quantification by differential scanning calorimetry. Biochim Biophys Acta. 1999;1461:47–57. doi: 10.1016/s0005-2736(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 50.Zuidam NJ, Hirsch-Lerner D, Margulies S, Barenholz Y. Lamellarity of cationic liposomes and mode of preparation of lipoplexes affect transfection efficiency. Biochim Biophys Acta. 1999;1419:207–220. doi: 10.1016/s0005-2736(99)00069-3. [DOI] [PubMed] [Google Scholar]
- 51.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 52.Hui SW, Langner M, Zhao YL, Ross P, Hurley E, Chan K. The role of helper lipids in cationic liposome-mediated gene transfer. Biophys J. 1996;71:590–599. doi: 10.1016/S0006-3495(96)79309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crook K, Stevenson BJ, Dubouchet M, Porteous DJ. Inclusion of cholesterol in DOTAP transfection complexes increases the delivery of DNA to cells in vitro in the presence of serum. Gene Ther. 1998;5:137–143. doi: 10.1038/sj.gt.3300554. [DOI] [PubMed] [Google Scholar]
- 54.Templeton NS, Lasic DD, Frederik PM, Strey HH, Roberts DD, Pavlakis GN. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol. 1997;15:647–652. doi: 10.1038/nbt0797-647. [DOI] [PubMed] [Google Scholar]
- 55.Xu L, Anchordoquy TJ. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochim Biophys Acta. 2008;1778:2177–2181. doi: 10.1016/j.bbamem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Zidovska A, Evans HM, Ahmad A, Ewert KK, Safinya CR. The Role of Cholesterol and Structurally Related Molecules in Enhancing Transfection by Cationic Liposome-DNA Complexes. J Phys Chem B. 2009;113:5208–5216. doi: 10.1021/jp809000e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan CL, Majzoub RN, Shirazi RS, et al. Endosomal Escape and Transfection Efficiency of PEGylated Cationic Liposome-DNA Complexes Prepared with an Acid-Labile PEG-Lipid. Biomaterials. 2012;33:4928–4935. doi: 10.1016/j.biomaterials.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ewert KK, Evans HM, Bouxsein NF, Safinya CR. Dendritic cationic lipids with highly charged headgroups for efficient gene delivery. Bioconjugate Chem. 2006;17:877–888. doi: 10.1021/bc050310c. [DOI] [PubMed] [Google Scholar]
- 59.Zidovska A, Evans HM, Ewert KK, et al. Liquid crystalline phases of dendritic lipid-DNA self-assemblies: lamellar, hexagonal, and DNA bundles. J Phys Chem B. 2009;113:3694–3703. doi: 10.1021/jp806863z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farago O, Ewert KK, Ahmad A, Evans HM, Grønbech-Jensen N, Safinya CR. Transitions between distinct compaction regimes in complexes of multivalent cationic lipids and DNA. Biophys J. 2008;95:836–846. doi: 10.1529/biophysj.107.124669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Marcel YL. Serum Albumin Is a Significant Intermediate in Cholesterol Transfer between Cells and Lipoproteins. Biochemistry. 1996;35:7174–7180. doi: 10.1021/bi952242v. [DOI] [PubMed] [Google Scholar]
- 62.Liu P, He M, Chen F, Li X, Zhang C. The Interaction Between Cholesterol and Human Serum Albumin. Protein Pept Lett. 2008;15:360–364. doi: 10.2174/092986608784246542. [DOI] [PubMed] [Google Scholar]
- 63.Meierhofer T, Elsen JH, Cameron P, Muñoz-Berbel X, Jenkins AT. The Interaction of Serum Albumin with Cholesterol Containing Lipid Vesicles. J Fluoresc. 2010;20:371–376. doi: 10.1007/s10895-009-0522-7. [DOI] [PubMed] [Google Scholar]
- 64.Churchman AH, Wallace R, Milne SJ, Brown AP, Brydson R, Beales PA. Serum albumin enhances the membrane activity of ZnO nanoparticles. Chem Comm. 2013;49:4172–4174. doi: 10.1039/c3cc37871c. [DOI] [PubMed] [Google Scholar]
- 65.Lukatsky DB, Safran SA, Lau AWC, Pincus P. Enhanced counterion localization induced by surface charge modulation. Europhys Lett. 2002;58:785–791. [Google Scholar]
- 66.DeHaseth PL, Lohman TM, Record MT. Nonspecific interaction of lac repressor with DNA: an association reaction driven by counterion release. Biochemistry. 1977;16:4783–4790. doi: 10.1021/bi00641a004. [DOI] [PubMed] [Google Scholar]
- 67.Gelbart WM, Bruinsma RF, Pincus PA, Parsegian VA. DNA inspired electrostatics. Phys Today. 2000;53:38–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.