Abstract
Rationale
Activated nuclear factor (NF)-κB signaling in the vascular endothelium promotes the initiation and progression of atherosclerosis. Targeting endothelial NF-κB may provide a novel strategy to limit chronic inflammation.
Objective
To examine the role of microRNA-181b (miR-181b) in endothelial NF-κB signaling and effects on atherosclerosis.
Methods and Results
MiR-181b expression was reduced in the aortic intima and plasma in apolipoprotein E–deficient mice fed a high-fat diet. Correspondingly, circulating miR-181b in the plasma was markedly reduced in human subjects with coronary artery disease. Systemic delivery of miR-181b resulted in a 2.3-fold overexpression of miR-181b in the aortic intima of apolipoprotein E–deficient mice and suppressed NF-κB signaling revealed by bioluminescence imaging and reduced target gene expression in the aortic arch in apolipoprotein E–deficient/NF-κB-luciferase transgenic mice. MiR-181b significantly inhibited atherosclerotic lesion formation, proinflammatory gene expression and the influx of lesional macrophages and CD4+ T cells in the vessel wall. Mechanistically, miR-181b inhibited the expression of the target gene importin-α3, an effect that reduced NF-κB nuclear translocation specifically in the vascular endothelium of lesions, whereas surprisingly leukocyte NF-κB signaling was unaffected despite a 7-fold overexpression of miR-181b. Our findings uncover that NF-κB nuclear translocation in leukocytes does not involve importin-α3, but rather importin-α5, which miR-181b does not target, highlighting that inhibition of NF-κB signaling in the endothelium is sufficient to mediate miR-181b's protective effects.
Conclusions
Systemic delivery of miR-181b inhibits the activation of NF-κB and atherosclerosis through cell-specific mechanisms in the vascular endothelium. These findings support the rationale that delivery of miR-181b may provide a novel therapeutic approach to treat chronic inflammatory diseases such as atherosclerosis.
Keywords: atherosclerosis, endothelial cells, inflammation, karyopherins, microRNAs, NF-κB
Atherosclerosis is recognized as a chronic inflammatory disease of the arterial wall.1,2 Nuclear factor-κB (NF-κB)–mediated vascular inflammation plays a critical role in the initiation and progression of atherosclerosis. The transcriptional activity of NF-κB can be induced by a variety of atherogenic stimuli, including inflammatory cytokines, type 2 diabetes mellitus, oxidized low-density lipoprotein, angiotensin II, and hemodynamic forces.3–8 In the canonical NF-κB signaling pathway, NF-κB heterodimers exist in an inactive form in the cytoplasm bound to an inhibitor such as IκBα. On stimulus-mediated activation, the IκB kinase (IKK) complex rapidly phosphorylates IκBα, which results in IκBα degradation by the proteasome.9,10 Once NF−κB heterodimers are released from IκBα, importin proteins (also known as karyopherins) direct NF-κB translocation to the nucleus where it controls a wide range of gene expression by binding to various κB elements. In the vascular endothelium, NF-κB activation induces the expression of proinflammatory genes, including those encoding adhesion molecules, cytokines, and chemoattractant proteins that collectively play critical roles in the initiation and progression of atherosclerosis.8,11,12 Consistent with this premise, endothelial cell (EC)–specific NF-κB inhibition reduces atherosclerosis in 3 different mouse models, IKKγ EC knockout, IKKγ EC–inducible knockout, and dominant-negative IκBα EC transgenic, in apolipoprotein E–deficient (ApoE−/−) mice.13 Furthermore, genetic inhibition of several NF-κB target genes, including vascular cell adhesion molecule-1, intercellular adhesion molecule-1, E- and P-selectins, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β also reduces various aspects of atherosclerotic lesion formation.14–18 Thus, targeting NF-κB–mediated EC activation holds promise for the development of novel anti-inflammatory therapies for acute and chronic inflammatory diseases.
In This Issue, see p 2 Editorial, see p 3
MicroRNAs (miRNAs) are single-stranded, noncoding, small RNAs that regulate gene expression by destabilizing target mRNAs or inhibiting translation. For example, in the context of vascular inflammation, miR-126, miR-31, and miR-17-3p were reported to reduce the expression of vascular cell adhesion molecule-1, E-selectin, and intercellular adhesion molecule-1, respectively, by directly targeting the 3′ untranslated region of these genes.19,20 MiR-10a targeted 2 proteins, MAP3K7 (TAK1) and β-TRC, that regulate IκBα degradation.21 MiR-146a can repress the proinflammatory NF-κB pathway and the MAP kinase pathway in ECs by targeting TNF receptor–associated factor 6 and HuR.22 Recently, we identified that miR-181b inhibits NF-κB–mediated endothelial activation by reducing the expression of importin-α3 (IPOA3), a protein critical for NF-κB translocation from cytoplasm to nucleus.23 However, the role of miRNA-181b in chronic inflammatory disease states, such as atherosclerosis, has not been examined.
In this study, we investigate the role of miR-181b in the development of atherosclerosis in ApoE−/− mice. Our findings reveal cell-specific mechanisms by which miR-181b exerts its protective effect in the vascular endothelium via importin isoform targeting and provide the rationale for the potential clinical use of miR-181b mimetics to treat chronic vascular inflammatory diseases such as atherosclerosis.
Methods
Pre-miR miRNA precursor molecules negative (nonspecific) control #1 (AM17110) and Hsa-miR-181b-5p Pre-miR miRNA precursor (PM12442) were used from Ambion. Real-time quantitative polymerase chain reaction was performed with the Mx3000P real-time polymerase chain reaction system (Stratagene) following the manufacturer's instructions. NF-κB promoter with GFP/luciferase fusion reporter (NGL) mice fully backcrossed into C57BL/6 were crossed with homozygous ApoE−/− mice to generate ApoE−/−/NGL transgenic mice. To induce atherosclerosis, 8-week-old male ApoE−/− mice were fed a high-fat diet (HFD) from Research Diets Inc (D12108Ci) for 12 weeks. Aortas were carefully excised from mice and examined for immunohistology and characterization of atherosclerotic lesions.
For detailed experimental methods, please see the Online Data Supplement.
Results
MiR-181b Expression Is Reduced in Aortic Intima of ApoE−/− Mice or in Human Plasma From Patients With Coronary Artery Disease
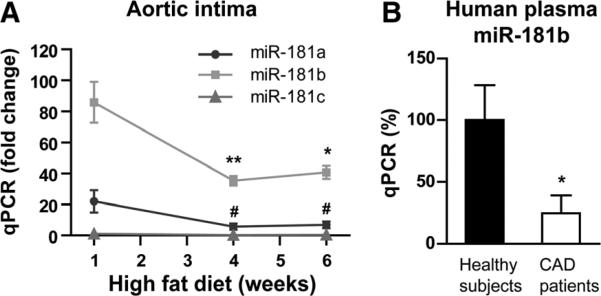
In response to acute proinflammatory stimuli (eg, TNF-α or lipopolysaccharide for 4 hours), we previously demonstrated that miR-181b expression is reduced in the aortic intima of mice.23 To examine whether miR-181b expression is reduced in chronic inflammation, aortic intima was harvested from ApoE−/− mice fed a HFD. As shown in Figure 1A, miR-181b expression was reduced by ≈59% and ≈53% in the aortic intima of ApoE−/− mice after HFD for 4 or 6 weeks. The second most dominantly expressed miR-181 family member in the aortic intima is miR-181a, which was also reduced in aortic intima at the time of 4 or 6 weeks of HFD (≈74% and ≈69%). The level of miR-181c is much lower than that of miR-181a and miR-181b and was not significantly changed. In human subjects with coronary artery disease, circulating miR-181b levels were also reduced by ≈76% in plasma compared with subjects without angiographically defined focal obstructive coronary artery disease (Figure 1B). Consistently, circulating plasma miR-181b levels were reduced by ≈44% in ApoE−/− mice after 4 weeks of HFD (Online Figure IA). In contrast, circulating miR-146a levels increased by 3.5- and 3.1-fold after 4 and 6 weeks of HFD, respectively (Online Figure IB), suggesting a specific effect for the miR-181b reduction. To characterize the components of the aortic intima, quantitative polymerase chain reaction analysis was performed for cell-specific markers. As shown in Online Figure IIA, endothelial mRNA markers von Willebrand factor and Tie-2 were robustly enriched in the intima and were barely detectable in the media plus adventitia (2.2% and 2.3% compared with the intima, respectively). Conversely, the expression of smooth muscle cell myosin heavy chain mRNA was much lower (1.4%) in the aortic intima compared with the media plus adventitia (Online Figure IIB). Finally, the macrophage marker, CD68, and T-cell markers, CD3 and CD4, were also detected at very low levels (1.8%, 4.8%, and 3.1%, respectively) in the aortic intima (Online Figure IIC and IID) compared with peripheral blood-derived macrophages and T cells, respectively. These data indicate that the isolated aortic intima contained >90% RNA enriched from ECs. These data demonstrate that miR-181b is reduced by chronic inflammatory stimuli in the vascular endothelium and plasma of mice, suggesting it may be involved in the early pathogenesis of atherosclerosis.
Figure 1. MiR-181b expression is reduced in the aortic intima of apolipoprotein E–deficient mice (ApoE−/−) mice fed a high-fat diet (HFD) or in plasma from human subjects with coronary artery disease.
A, MiR-181a, miR-181b, and miR-181c expression was detected by quantitative polymerase chain reaction (qPCR) in the aortic intima from ApoE−/− mice fed a HFD for 1, 4, or 6 weeks (n=5–8 per group). The expression of miR-181 was normalized to small RNA U6 expression and compared with the expression of miR-181c at 1 week of HFD that was subsequently set to a value of 1. B, Circulating miR-181b expression was detected by qPCR in human plasma samples from control human subjects without (n=14) or with coronary artery disease (n=26). Data show mean±SEM. *P<0.05; **P<0.01; and #P<0.001. CAD indicates coronary artery disease.
Rescue of MiR-181b Expression in the Aortic Intima
To rescue the expression of miR-181b in the aortic intima under chronic inflammatory conditions, we systemically delivered liposomally encapsulated miR-181b mimics (181b-m) or nonspecific control mimics by tail vein injection. As shown in Figure 2A, the expression of miR-181b in the aortic intima from mice injected with miR-181b was 2.3-fold higher than that in mice injected with the miRNA nonspecific control (Figure 2A). No overexpression of miR-181b was observed in the aortic media/adventitia (Figure 2B). However, the expression of miR-181b in peripheral blood mononuclear cells (PBMCs) from mice injected with miR-181b was ≈10.6-fold higher than that in PBMCs from mice injected with the miRNA nonspecific control (Figure 2C). Systemic delivery of miR-181b did not alter the endogenous expression of miR-181a and miR-181c in the aortic intima, media/adventitia, or PBMCs (Figure 2A–2C). Basal endogenous levels of miR-181b expression in the media/adventitia and PBMCs were 3.2-fold and 5-fold higher, respectively, than that in the aortic intima (Online Figure III). However, intravenous injection of miRNA nonspecific control did not change endogenous miR-181b expression (Figure 2A–2C), indicating that the fold differences in the aortic intima, media/adventitia, and PBMCs represent exogenous miR-181b. These data indicate that exogenous miR-181b is able to accumulate in the aortic intima and PBMCs but not the aortic media/adventitia after intravenous administration.
Figure 2. Systemic delivery of miR-181b reduces nuclear factor (NF)-κB activity in the aortic arch of apolipoprotein E–deficient (ApoE−/−)/NF-κB GFP-luciferase reporter (NGL) mice.
A to E, ApoE−/−/NGL or NGL transgenic (Tg) mice (NF-κB promoter with GFP/luciferase fusion reporter) were fed a high-fat diet and tail vein injected with miRNA nonspecific control (NS-m), miR-181b (181b-m) mimics, or vehicle (Veh) twice a week for 4 weeks as described in the Methods section. MiR-181a, miR-181b, and miR-181c expression was examined by quantitative polymerase chain reaction (qPCR) in the aortic intima (A), media/adventitia (B), and peripheral blood mononuclear cells (PBMCs; C). Data shown are mean±SEM (n=3). The expression of miR-181 was normalized to small RNA U6 expression and compared with the expression of miR-181a in mice injected with vehicle that was subsequently set to a value of 1. D, Bioluminescence imaging of luciferase activity (represents NF-κB activity) is shown in excised aortas from NGL Tg mice that received vehicle (n=8) and from ApoE−/−/NGL Tg mice that received vehicle (n=5), NS-m (n=8), or 181b-m (n=12), respectively. E, qPCR analysis of NF-κB target gene expression in the aortic arch. The expression of NF-κB target gene was normalized to mouse β-actin expression and compared with its expression in mice received NS-m that was subsequently set to a value of one hundred. Data shown are mean±SEM (n=3–12). *P<0.05; and **P<0.01. ICAM indicates intercellular adhesion molecule; NS, nonsignificant; and VCAM, vascular cell adhesion molecule.
Systemic Delivery of MiR-181b Inhibits NF-κB Signaling and Gene Expression
NF-κB–mediated EC activation and vascular inflammation play a critical role in the initiation and progression of atherosclerosis.13 MiR-181b has been shown to inhibit these events in an acute inflammatory disease state such as sepsis.23 To explore whether miR-181b is able to suppress NF-κB signaling in the context of atherosclerosis, we generated compound ApoE−/−/NGL mice by crossing ApoE−/− mice with transgenic NGL reporter mice. After 4 weekly injections (IV) of 181bm, miRNA nonspecific control mimics, or vehicle control in the ApoE−/−/NGL and NGL mice, NF-κB activity in the aortic arch was quantified by bioluminescence imaging. As shown in Figure 2D, the intensity of NF-κB activity increased by ≈3-fold in the aortic arch in ApoE−/−/NGL mice compared with NGL mice after 4 weeks of HFD. However, in the presence of exogenous miR-181b, NF-κB activity was reduced by 31%. There were no differences observed in ApoE−/−/NGL mice treated with either the vehicle control or the nonspecific control mimics. In addition, the induction of expression of NF-κB target genes, such as vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin, were significantly reduced in the aortic arch in ApoE−/−/NGL mice by 32%, 20%, and 43%, respectively, in the presence of exogenous 181b-m group (Figure 2E). Collectively, these data demonstrate that systemic delivery of miR-181b inhibits NF-κB activation and target gene expression primarily in the vessel wall of ApoE−/−/NGL mice.
Systemic Delivery of MiR-181b Protects ApoE−/− Mice From Atherosclerosis
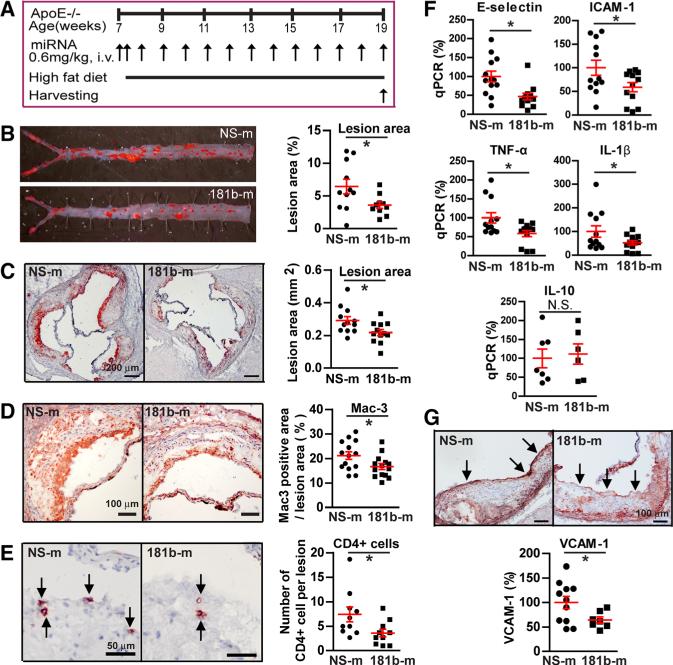
To explore the role of systemically delivered miR-181b in atherosclerosis, ApoE−/− mice were fed a HFD for 12 weeks and administered weekly injections of 181b-m or miRNA nonspecific control as shown in Figure 3A. After 12 weeks on HFD, there were no differences observed in body weight, total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride levels between ApoE−/− mice injected with miR-181b mimics or miRNA nonspecific control (Table). Analyses of atherosclerotic lesion formation by Oilred O staining revealed a 44% reduction in lesion area in the descending thoracic and abdominal aorta (Figure 3B) and a 25% reduction in lesion size at the level of the aortic sinus (Figure 3C). Histological assessment of atherosclerotic lesions at the aortic sinus revealed a 21% reduction of macrophages by Mac3 staining (Figure 3D) and a 50% reduction of CD4+ T cells by CD4 staining (Figure 3E). The miR-181b–mediated inhibition of atherosclerotic lesion formation was associated with decreased expression of proinflammatory markers, including adhesion molecules intercellular adhesion molecule-1 and E-selectin by 41% and 53%, respectively, and cytokines TNF-α and IL-β by 42% and 48%, respectively, in the aortic arch of ApoE−/− mice (Figure 3F). The expression of IL-10 in the aortic arch was not different between 2 groups (Figure 3F). As shown by immunohistochemistry in Figure 3G, ApoE−/− mice that received miR-181b mimics exhibited a reduction in vascular cell adhesion molecule-1 expression by 36% compared with miRNA nonspecific control–treated ApoE−/− mice. To test whether systemic delivery of miR-181b would lead to changes in liver function, we examined liver NF-κB activity and measured blood levels of aspartate aminotransferase and alanine transaminase (Online Figure IV). No differences were observed in liver NF-κB activity or plasma alanine transaminase and aspartate aminotransferase concentrations between miRNA nonspecific control mimics and miR-181b treatment (Online Figure IV). These data suggest that liver NF-κB activity or liver toxicity is not likely contributory in the miR-181b–mediated inhibition of atherosclerotic lesion formation. Taken together, these data indicate that systemic delivery of miR-181b inhibits NF-κB activity and proinflammatory gene expression in the vessel wall and results in reduced leukocyte accumulation and atherosclerotic lesion formation in ApoE−/− mice.
Figure 3. Systemic delivery of miR-181b mimics inhibits atherosclerosis in apolipoprotein E–deficient (ApoE−/−) mice.
ApoE−/− mice were fed a high-fat diet and received weekly tail vein injections of miRNA nonspecific control (NS-m) or miR-181b (181b-m) mimics for 12 weeks as described in the Method section. A, Schema of experimental procedure.B, Lesion areas shown were quantified using Oil-red O (ORO) staining of the thoracoabdominal aorta. Data represent ORO-stained areas as a percentage of aorta areas. C, Lesion areas shown were quantified as areas between the lumen and tunica media on ORO-stained aortic sinus sections. D and E, Representative images and quantification show Mac3-positive macrophages (D) and CD4-positive T cells (E) in the aortic root lesions. Mac3-stained areas as a percentage of lesion area are shown in D. F, Quantitative polymerase chain reaction (qPCR) analysis of the indicated proinflammatory genes in the aortic arch. The expression of inflammatory genes was normalized to mouse β-actin expression and compared with its expression in mice received NS-m that was subsequently set to a value of one hundred. G, Images and quantification of vascular cell adhesion molecule-1 (VCAM-1) staining in the intima layer at the aortic root. Data shown are mean±SEM (n=6–14 mice per group). *P<0.05. ICAM indicates intercellular adhesion molecule; IL, interleukin; NS, nonsignificant; and TNF, tumor necrosis factor.
Table.
Systemic Delivery of miR-181b Did Not Affect Lipid Profiles or Body Weight of ApoE−/− Mice Fed a HFD
| Ctl Mimics | miR-181b | |
|---|---|---|
| Total cholesterol, mg/dL | 1076.8±93.6 | 994.7±41.3 |
| Triglycerides, mg/dL | 120.3±10.2 | 108.8±6.9 |
| LDL cholesterol, mg/dL | 993.2±97.3 | 905.9±40.1 |
| HDL cholesterol, mg/dL | 51.6±6.7 | 63.7±7.5 |
| Body weight, g | 31.8±1.0 | 29.9±0.7 |
All values Ctl mimics vs miR-181b, P=NS; mean±SEM (n=10 to 12). ApoE−/− mice indicates apolipoprotein E–deficient mice; HDL, high-density lipoprotein; HFD, high-fat diet; and LDL, low-density lipoprotein.
MiR-181b Reduces the Expression of IPOA3 and NF-κB p65 Nuclear Translocation in the Vascular Endothelium of Lesions
We previously demonstrated that miR-181b directly targeted IPOA3 and reduced its expression and subsequently inhibited NF-κB nuclear translocation.23 Interestingly, IPOA3 mRNA expression increased in the aortic intima by ≈20% in ApoE−/− mice after 4 weeks of HFD (Online Figure V). To further validate the hypothesis that miR-181b inhibits endothelial NF-κB in the context of atherosclerosis, IPOA3 expression and nuclear translocation of NF-κB were directly evaluated in the endothelium of atherosclerotic lesions by immunostaining with antibodies against IPOA3 and CD31, or p65 and CD31, in sections from the aortic sinus. As shown in Figure 4A, ApoE−/− mice that received 181b-m exhibited a reduction in IPOA3 expression by 35% in the endothelium compared with miRNA nonspecific control–treated ApoE−/− mice. Multiple genes have been identified as direct targets of miR-181a or miR-181b in different cell types.24–31 In addition to IPOA3, the expression of these genes was also examined in the aortic arch (Online Figure VI). Systemic delivery of miR-181b significantly reduced the expression of neuropilin-1, but not other reported target genes. Consistent with reduced expression of IPOA3 expression in the vascular endothelium in the presence of systemically delivered miR-181b, the accumulation of NF-κB p65 in endothelial nuclei within lesions was reduced by 33% compared with mice injected with miRNA nonspecific control (Figure 4B). Interestingly, we found that systemically delivered miR-181b did not reduce NF-κB p65 nuclear accumulation in lesional Mac3-positive macrophages by immunofluorescent staining (Figure 4C). Taken together, these data indicate that systemic delivery of miR-181b inhibits IPOA3 expression and NF-κB p65 nuclear translocation in vascular endothelium, but not in lesional macrophages.
Figure 4. MiR-181b inhibits importin-α3 expression and nuclear factor (NF)-κB activation in aortic endothelial cells in lesions.
Apolipoprotein E–deficient (ApoE−/−) mice were fed a high-fat diet and weekly tail vein injected with miRNA nonspecific control (NS-m) or miR-181b (181b-m) mimics for 12 weeks. Frozen sections of aortic root were stained for anti-importin-α3 (IPOA3; red) and CD31 (green; A), or anti-p65 (red), anti-CD31 (green), and 4′,6-diamidino-2-phenylindole (DAPI; blue; B), or anti-IPOA3 (red), Mac3 (green), and DAPI (blue; C). Arrows indicate differential IPOA3 expression (A), p65 accumulation in nuclei of endothelial cells (ECs; B), or nuclear p65 in macrophage (C). Importin-α3 expression and nuclear p65 expression were quantified in vascular ECs reflecting NS-m (n=40 or 42 ECs) and 181b-m (n=41 or 43 ECs). Nuclear p65 expression in lesional macrophages was quantified reflecting NS-m (n=41 cells) and 181b-m (n=42 cells). Data shown are mean±SEM (n=8–10 mice each group). *P<0.05. NS indicates nonsignificant.
Differential Expression of IPOA3 and Importin-α5 in ECs and Leukocytes Account for the Cell-Specific Effect of MiR-181b on NF-κB Inhibition
The role of myeloid NF-κB signaling in atherogenesis remains controversial,32–36 which prompted us to ask whether systemic delivery of miR-181b regulates NF-κB signaling in leukocytes. We found that systemic delivery of miR-181b does not inhibit p65 nuclear translocation in lesional macrophages by immunostaining of sections at the aortic sinus from ApoE−/− mice fed a HFD for 12 weeks (Figure 4C). Further examination revealed that miR-181b was ≈7.2-fold higher in PBMCs of ApoE−/− mice injected with miR-181b compared with mice injected with the miRNA nonspecific control (Figure 5A). Surprisingly, systemic delivery of miR-181b had no significant effect on NF-κB activity in PBMCs (Figure 5B) or on the expression of NF-κB target genes in PBMCs, such as COX-2, IL-1β, and IL-10 (Figure 5C). Overexpression of miR-181b was not able to reduce NF-κB activation as measured by NF-κB–induced luciferase activity in bone marrow–derived macrophages isolated from ApoE−/−/NGL mice (Figure 5D) or p65 nuclear translocation in peritoneal macrophages in response to lipopolysaccharide (Online Figure VII). Previous studies demonstrate that several importin-α molecules, including IPOA3 and importin-α5 (IPOA5), may be involved in NF-κB nuclear translocation.37,38 We previously identified that miR-181b directly targets IPOA3, a protein that is critical for nuclear translocation of NF-κB in ECs.23 As shown in Figure 5E, the expression of IPOA3 was also reduced in mouse PBMCs by systemically delivered miR-181b. In contrast, the expression of IPOA5 was not changed (Figure 5F). To examine why miR-181b was capable of inhibiting NF-κB in ECs and aortic intima, but not in PBMCs despite the reduction of IPOA3 by miR-181b in both cell types, we assessed the relative expression of the miR-181b target IPOA3 and other importin-α proteins in the aortic intima, human and mouse ECs, and mouse PBMCs. As shown in Figure 5G, IPOA3 mRNA expression was 4.4-fold higher in the aortic intima compared with PBMCs. IPOA3 was also the dominantly expressed member of the importin-α family in the aortic intima at the mRNA level and is ≈3-fold higher than the second most abundant importin-α molecule (importin-α7). Consistently, the expression of IPOA3 was higher in ECs (8-fold in mouse aortic endothelial cells) at the protein level compared with mouse PBMCs (Figure 5H). The expression of importin-α4 was also higher in ECs than in PBMCs at the protein level. In contrast, the expression of IPOA5 in PBMCs was ≈3-fold higher compared with the aortic intima at the mRNA level and ≈4-fold higher compared with mouse aortic endothelial cells at the protein level, respectively (Figure 5G and 5H). IPOA5 was the dominantly expressed member of the importin-α family in mouse PBMCs at the mRNA level and is 3.5-fold higher than the second most abundant importin-α molecule (importin-α7). Importantly, miR-181b does not target IPOA523 (Figure 5F), and knockdown of IPOA5 expression by siRNA transfection (Figure 5I) was able to inhibit lipopolysaccharide-induced NF-κB activation (Figure 5J) and NF-κB target gene expression (Figure 5K and 5L) in cultured PBMCs. Furthermore, systemic delivery of IPOA5 siRNA inhibited its expression in vivo in PBMCs (Figure 5M) and inhibited lipopolysaccharide-induced NF-κB activation by 31% (Figure 5N). In summary, these data demonstrate that systemic delivery of miR-181b specifically inhibited NF-κB activation and NF-κB target gene expression in the aortic intima of the vessel wall but not in leukocytes of ApoE−/− mice likely because of distinct expression patterns of importin-α molecules—IPOA3 (a miR-181b target) is expressed significantly higher than IPOA5 in ECs, whereas IPOA5 (a non-miR-181b target) is expressed higher than IPOA3 in leukocytes thereby allowing for NF-κB activation to proceed in this cell type. Furthermore, these findings highlight that inhibition of NF-κB activity in the vascular endothelium is sufficient to confer miR-181b's protective effects on atherosclerotic lesion formation.
Figure 5. MiR-181b does not inhibit nuclear factor (NF-κB) activation in leukocytes because of dominant expression of importin-α5.
A to C, Apolipoprotein E–deficient (ApoE−/−) or ApoE−/−/NF-κB GFP-luciferase reporter Tg mice were fed a high-fat diet and intravenously injected with miRNA nonspecific control (NS-m) or miR-181b (181b-m) mimics once a week for 12 weeks. A, Real-time quantitative polymerase chain reaction (qPCR) analysis of miR-181b in peripheral blood mononuclear cells (PBMCs). B, Luciferase activity (reflecting NF-κB activity) was measured in PBMCs (n=4–6 per group). C, Real-time qPCR analysis of NF-κB target genes in PBMCs. D, Luciferase activity in bone marrow–derived macrophages (BMDM) from ApoE−/−/NGL mice transfected with NS-m or 181b-m, and treated with 10 ng/mL lipopolysaccharide (LPS) for 12 hours. E and F, Western blot analysis of importin-α3 (IPOA3) and importin-α5 (IPOA5) in PBMCs from mice injected with NS-m or 181b-m. G, qPCR analysis of importin-α molecules in PBMCs or aortic intima. The expression of importin-α genes was normalized to mouse β-actin expression and compared with importin-α1 gene expression in PBMCs that was subsequently set to a value of 1 (n=3). H, Western blot analysis of IPOA3, importin-α4 (IPOA4), and importin-α5 (IPOA5) in endothelial cells and PBMCs. I and J, PBMCs from ApoE−/−/NGL mice were transfected with ctl siRNA or IPOA5 siRNA. Western blot analysis of IPOA5 is shown in I and NF-κB luciferase activity in J. K and L, Real-time qPCR analysis of COX-2 (K) and interleukin (IL-1β; L) expression in PBMCs (n=3). M and N, qPCR analysis of IPOA5 and NF-κB luciferase activity in PBMCs from ApoE−/−/NGL mice tail vein injected with ctl siRNA or IPOA5 siRNA (n=4–5). Data shown are mean±SEM. *P<0.05; **P<0.01; and #P<0.001. HAECs indicates human aortic endothelial cells; HUVECs, human umbilical vein endothelial cells; MAEC, mouse aortic endothelial cell; and NS, nonsignificant.
Discussion
The current study demonstrates that systemic delivery of liposomally encapsulated miR-181b mimetics in ApoE−/− mice inhibits NF-κB activation, NF-κB-responsive proinflammatory gene expression, leukocyte accumulation, and atherosclerotic lesion formation. Furthermore, these studies highlight for the first time to our knowledge that miRNA mimetics penetrate the intima lining of the atherosclerotic plaque in amounts sufficient to limit NF-κB activation, inflammatory gene expression, and leukocyte accumulation. Moreover, these miR-181b–mediated effects occurred independent of NF-κB inhibition in lesional macrophages or PBMCs. These findings are consistent with prior studies showing that inhibition of NF-κB signaling specifically in ECs (by ablating IKKγ/NEMO or expression of a dominant-negative IκBα) confers an atheroprotective effect in ApoE−/−mice.13
The NF-κB signaling pathway centrally integrates multiple signal inputs in the pathogenesis of atherosclerosis, and emerging studies suggest that inhibition of NF-κB in specific cell types may have divergent effects. For example, although EC-specific inhibition of NF-κB resulted in reduced lesion formation in ApoE−/− mice,13 the effect of altering NF-κB activation in myeloid cells is more complex. For example, low-density lipoprotein receptor–deficient mice transplanted with IKK2/IKKβ-deficient macrophages had increased atherosclerosis associated with higher numbers of apoptotic cells within the plaque.34 In contrast, a recent study showed that myeloid-specific IKKβ deficiency decreases atherosclerosis in low-density lipoprotein receptor–deficient mice.35 In another study, myeloid IκBα deficiency promoted atherogenesis by enhancing leukocyte recruitment to the developing plaques.32 Furthermore, bone marrow deficiency of NF-κB1 resulted in reduced atherosclerotic lesion size and macrophage foam cells but caused increased plaque inflammation.33 Finally, conditional targeting of TNF receptor–associated factor 6 revealed opposing functions of Toll-like receptor signaling in endothelial and myeloid cells in a mouse model of atherosclerosis.36 Collectively, these studies illustrate that cell-type–specific inhibition of upstream NF-κB effectors may exert varying effects on plaque composition and athero-sclerotic lesion formation.
In our study, miR-181b was overexpressed 2.3-fold in the aortic intima and ≈7- to 10-fold in PBMCs of ApoE−/− mice after intravenous injection of miR-181b mimics for up to 12 weeks. Despite the higher levels achieved for miR-181b overexpression in PBMCs, there were no significant effects on PBMC expression of the NF-κB–regulated inflammatory genes COX-2, IL-1β, or IL-10 (Figure 5C). MiR-181b also had no effect of NF-κB activity in the liver (Online Figure IV). These data suggest that the protective role of miR-181b on vascular inflammation may be independent of effects of miR-181b in PBMCs or liver. As outlined above, IPOA3 is a protein involved in NF-κB translocation from the cytoplasm to nucleus.37,38 We previously demonstrated that IPOA3 is a bona fide direct target of miR-181b in ECs.23 Indeed, systemic delivery of miR-181b significantly reduced the expression of IPOA3 in the aortic intima of lesions by immunohistochemical staining (Figure 4A). Thus, miR-181b seems to suppress NF-κB activation and target gene expression in the vascular wall by reducing IPOA3 expression in the vascular endothelium. In addition to IPOA3, the expression of neuropilin-1, but not other previously identified miR-181b target genes, was reduced by miR-181b in the aortic arch (Online Figure VI). Neuropilin-1 is a single spanning transmembrane glycoprotein, which plays versatile roles in angiogenesis, cell survival, migration, and has been identified as a direct target of miR-181b in ECs in the context of arsenic-induced angiogenesis.24 It remains unknown whether neuropilin-1 exerts anti-inflammatory effects on NF-κB signaling or other relevant proinflammatory pathways in ECs or atherosclerosis.
An interesting question arising from these studies is why did miR-181b overexpression in PBMCs fail to inhibit NF-κB activity and NF-κB–regulated gene expression? An emerging paradigm from several studies indicates that miRNA-mediated effects in a specific cell type are dependent on the relative expression of the proteins that are regulated by the miRNAs.39–41 Consistent with this premise is the finding that the expression of IPOA3 is higher in the aortic intima (4.4-fold at the mRNA level) and ECs (8-fold in mouse aortic endothelial cells at the protein level) compared with PBMCs (Figure 5G and 5H). Interestingly, a similar gradient of cellular expression was noted in the investigation that originally reported cloning for the IPOA3 gene.42 Surprisingly, the expression pattern of importin-α molecules is strikingly different in PBMCs. The expression of IPOA5 is higher in PBMCs than in ECs (3- and 4-fold at the mRNA and protein levels, respectively; Figure 5G and 5H). Furthermore, IPOA5 participates in NF-κB activation in PBMCs as verified by siRNA knockdown of IPOA5 in PBMCs both in vitro and in vivo (Figure 5I–5N). Therefore, the distinct expression pattern of importin-α molecules in ECs and leukocytes with a dominant expression pattern of IPOA3 in ECs and IPOA5 in leukocytes likely accounts for the cell-specific effects of miR-181b on NF-κB signaling. As miR-181b cannot inhibit IPOA5 expression (Figure 5F) or its 3′-untranslated region,23 these findings further support other studies demonstrating that endothelial-specific NF-κB inhibition is sufficient to confer atheroprotection.
MiR-181b–mediated targeting of downstream NF-κB signaling in the vascular endothelium may offer several advantages to inhibit this pathway. First, previous targeting of upstream NF-κB signaling effectors, including IKKs or IκBα, may lead to off-target effects by virtue of the large number of interdependent signaling pathways that they may affect (eg, MAPK signaling, insulin signaling, and p53).43–46 Second, miR-181b–mediated inhibition of IPOA3 provides targeting of a focused downstream event of preventing nuclear translocation of NF-κB heterodimers. Third, because of the differential expression of IPOA3 and IPOA5 in ECs and leukocytes, respectively, miR-181–mediated targeting of IPOA3 only limits NF-κB activation in the vascular endothelium of lesions, thereby providing a means of cell-specific targeting of inflammation. For example, it may be advantageous to avoid inhibition of myeloid NF-κB to maintain protection in response to various infectious pathogens.
In summary, our study in ApoE−/− mice demonstrates that miR-181b mimetics decrease arterial NF-κB activation and NF-κB–regulated gene expression in the vascular endothelium resulting in reduced leukocyte accumulation and atherosclerosis. MiR-181b–mediated effects occurred primarily in the vascular endothelium and independent of NF-κB inhibition in lesional macrophages or PBMCs because of the use of different importin-α isoforms in ECs (IPOA3) and PBMCs (IPOA5; Online Figure VIII). These data indicate that strategies aimed at restoring miR-181b expression may provide a novel therapeutic approach for chronic inflammatory disease states such as atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
Atherosclerosis is a chronic inflammatory disease of the arterial wall.
Endothelial nuclear factor (NF)-κB activation contributes to the initiation and progression of atherosclerosis.
We previously showed that microRNA-181b inhibits NF-κB–mediated endothelial activation during endotoxin-induced acute inflammation by reducing the expression of importin-α3, a protein critical for NF-κB translocation from cytoplasm to nucleus. However, the role of miRNA-181b in chronic inflammatory disease states, such as atherosclerosis, has not been examined.
What New Information Does This Article Contribute?
MiR-181b expression in the aortic intima is markedly reduced after high-fat diet feeding in Apoe−/− mice.
Intravenous delivery of miR-181b inhibited NF-κB activation, NF-κB-responsive proinflammatory gene expression, leukocyte accumulation, and atherosclerotic lesion formation.
MiR-181b inhibited NF-κB only in the vascular endothelium of athero-sclerotic lesions, and not in leukocytes, reflecting high expression of importin-α3 in endothelial cells, but not leukocytes.
NF-κB activation in the vascular endothelium promotes the initiation and development of atherosclerosis. In this study, we found that miR-181b expression is reduced in Apoe−/− mice and in humans with coronary artery disease. Rescue of miR-181b expression by systemic delivery of miR-181b mimetics to the vessel wall inhibited NF-κB activation in vascular ECs, but not leukocytes. This reduced leukocyte accumulation and atherosclerotic lesion formation. These findings suggest that miR-181b mimetics may provide a novel therapeutic approach to treat chronic inflammatory diseases such as atherosclerosis.
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health (HL091076, HL115141, and HL117994 to M.W. Feinberg), a Watkins Cardiovascular Medicine Discovery Award (to M.W. Feinberg), a State Scholarship Fund of the China Scholarship Council (to S. He), and a Jonathan Levy Research Fund (to M.W. Feinberg).
Nonstandard Abbreviations and Acronyms
- 181b-m
microRNA-181 b mimics
- EC
endothelial cell
- HFD
high-fat diet
- IKK
IκB kinase
- IPOA3
importin-α3
- IPOA5
importin-α5
- miRNA
microRNA
- miR-181b
microRNA-181b
- NGL
NF-κB GFP-luciferase reporter
- PBMCs
peripheral blood mononuclear cells
- TNF
tumor necrosis factor
- IL
interleukin
Footnotes
Disclosures
Mark W. Feinberg, Xinghui Sun, and The Brigham and Women's Hospital have a patent pending related to the work that is described in the present study. The other authors report no conflicts.
References
- 1.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 5.Hernández-Presa M, Bustos C, Ortego M, Tuñon J, Renedo G, Ruiz-Ortega M, Egido J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 6.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies PF, Polacek DC, Handen JS, Helmke BP, DePaola N. A spatial approach to transcriptional profiling: mechanotransduction and the focal origin of atherosclerosis. Trends Biotechnol. 1999;17:347–351. doi: 10.1016/s0167-7799(99)01348-7. [DOI] [PubMed] [Google Scholar]
- 8.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 11.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 12.Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005;33:5308–5319. doi: 10.1093/nar/gki836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Bourdillon MC, Poston RN, Covacho C, Chignier E, Bricca G, McGregor JL. ICAM-1 deficiency reduces atherosclerotic lesions in double-knockout mice (ApoE(−/−)/ICAM-1(−/−)) fed a fat or a chow diet. Arterioscler Thromb Vasc Biol. 2000;20:2630–2635. doi: 10.1161/01.atv.20.12.2630. [DOI] [PubMed] [Google Scholar]
- 15.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for vcam-1, but not icam-1, in early atherosclerosis. J ClinI Investig. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 18.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Suárez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010;184:21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5:949–966. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM. Feinberg MW; MICU Registry. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui Y, Han Z, Hu Y, Song G, Hao C, Xia H, Ma X. MicroRNA-181b and microRNA-9 mediate arsenic-induced angiogenesis via NRP1. J Cell Physiol. 2012;227:772–783. doi: 10.1002/jcp.22789. [DOI] [PubMed] [Google Scholar]
- 25.de Yébenes VG, Belver L, Pisano DG, González S, Villasante A, Croce C, He L, Ramiro AR. miR-181b negatively regulates activation-induced cytidine deaminase in B cells. J Exp Med. 2008;205:2199–2206. doi: 10.1084/jem.20080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limon P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. The microrna mir-181 is a critical cellular metabolic rheostat essential for nkt cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013;38:984–997. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood. 2010;116:2395–2401. doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- 32.Goossens P, Vergouwe MN, Gijbels MJ, Curfs DM, van Woezik JH, Hoeksema MA, Xanthoulea S, Leenen PJ, Rupec RA, Hofker MH, de Winther MP. Myeloid IκBα deficiency promotes atherogenesis by enhancing leukocyte recruitment to the plaques. PLoS One. 2011;6:e22327. doi: 10.1371/journal.pone.0022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanters E, Gijbels MJ, van der Made I, Vergouwe MN, Heeringa P, Kraal G, Hofker MH, de Winther MP. Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood. 2004;103:934–940. doi: 10.1182/blood-2003-05-1450. [DOI] [PubMed] [Google Scholar]
- 34.Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Förster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park SH, Sui Y, Gizard F, Xu J, Rios-Pilier J, Helsley RN, Han SS, Zhou C. Myeloid-specific IκB kinase β deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2869–2876. doi: 10.1161/ATVBAHA.112.254573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polykratis A, van Loo G, Xanthoulea S, Hellmich M, Pasparakis M. Conditional targeting of tumor necrosis factor receptor-associated factor 6 reveals opposing functions of Toll-like receptor signaling in endothelial and myeloid cells in a mouse model of atherosclerosis. Circulation. 2012;126:1739–1751. doi: 10.1161/CIRCULATIONAHA.112.100339. [DOI] [PubMed] [Google Scholar]
- 37.Fagerlund R, Melén K, Cao X, Julkunen I. NF-kappaB p52, RelB and c-Rel are transported into the nucleus via a subset of importin alpha molecules. Cell Signal. 2008;20:1442–1451. doi: 10.1016/j.cellsig.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Fagerlund R, Kinnunen L, Köhler M, Julkunen I, Melén K. NF-{kappa} B is transported into the nucleus by importin {alpha}3 and importin {alpha}4. J Biol Chem. 2005;280:15942–15951. doi: 10.1074/jbc.M500814200. [DOI] [PubMed] [Google Scholar]
- 39.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 40.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Cheng Y, Yang J, Xu L, Zhang C. Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J Mol Cell Cardiol. 2012;52:245–255. doi: 10.1016/j.yjmcc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Köhler M, Ansieau S, Prehn S, Leutz A, Haller H, Hartmann E. Cloning of two novel human importin-alpha subunits and analysis of the expression pattern of the importin-alpha protein family. FEBS Lett. 1997;417:104–108. doi: 10.1016/s0014-5793(97)01265-9. [DOI] [PubMed] [Google Scholar]
- 43.Tergaonkar V, Perkins ND. p53 and NF-kappaB crosstalk: IKKalpha tips the balance. Mol Cell. 2007;26:158–159. doi: 10.1016/j.molcel.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 45.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 46.Ashida N, Senbanerjee S, Kodama S, Foo SY, Coggins M, Spencer JA, Zamiri P, Shen D, Li L, Sciuto T, Dvorak A, Gerszten RE, Lin CP, Karin M, Rosenzweig A. IKKβ regulates essential functions of the vascular endothelium through kinase-dependent and -independent pathways. Nat Commun. 2011;2:318. doi: 10.1038/ncomms1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.