Abstract
Objective
To assess the effectiveness of sodium nitroprusside (SNP)-“enhanced” cardiopulmonary resuscitation (SNPeCPR) on 24-hr survival rates compared to standard CPR in animals after cardiac arrest. SNPeCPR consists of large intravenous SNP bolus doses during CPR enhanced by active compression-decompression CPR, an inspiratory impedance threshold device (ITD), and abdominal binding (AB). The combination of active compression-decompression CPR+ITD+AB without SNP will be called “enhanced” or eCPR.
Design
Randomized, blinded, animal study.
Setting
Preclinical animal laboratory.
Subjects
Twenty-four female farm pigs (30 ± 1 kg).
Interventions
Isoflurane anesthetized and intubated pigs were randomized after 8 mins of untreated ventricular fibrillation to receive either standard CPR (n = 8), SNPeCPR (n = 8), or eCPR (n = 8) for 25 mins followed by defibrillation.
Measurements and Main Results
The primary end point was carotid blood flow during CPR and 24-hr survival with good neurologic function defined as an overall performance category score of ≤2 (1 = normal, 5 = brain dead or dead). Secondary end points included hemodynamics and end-tidal CO2. SNPeCPR significantly improved carotid blood flow and 24-hr survival rates with good neurologic function compared to standard CPR or eCPR (six of eight vs. zero of eight vs. one of eight, p < .05). The improved survival rates were associated with higher coronary perfusion pressure and ETCO2 during CPR.
Conclusion
In pigs, SNPeCPR significantly improved hemodynamics, resuscitation rates, and 24-hr survival rates with good neurologic function after cardiac arrest when compared with standard CPR or eCPR alone.
Keywords: vasodilators, cardiopulmonary resuscitation, neurological function, resuscitation rates, carotid blood flow
The vast majority of patients who suffer an out-of-hospital cardiac arrest die despite receiving cardiopulmonary resuscitation (CPR) for 20 –30 mins by emergency medical personnel at the scene (1–4). Such patients, when treated with the standard of care as recommended by the American Heart Association guidelines, typically receive epinephrine every 3–5 mins (5). While the standard of care for decades, this practice has recently been challenged by animal and clinical trials showing that use of epinephrine may actually have more detrimental than beneficial effects (6–9).
In stark pharmacologic contrast to epinephrine, sodium nitroprusside (SNP) is a potent vasodilator. SNP is used to treat hypertensive emergencies and heart failure and it primary mode of action is the release of nitric oxide (10, 11). Use of SNP would typically be considered an anathema for the treatment of the profound hypotension associated with cardiac arrest and manual CPR.
Contrary to current thinking, large doses of SNP during CPR do not cause a significant decrease in central aortic pressure (12). This can be explained by the way aortic pressure is generated during CPR. With chest compression, the resultant increase in intrathoracic pressure results in an instantaneous increase in the pressure of all of the intrathoracic vascular structures (13). Therefore, when arterial vascular resistance is decreased by SNP, forward blood flow can be significantly increased with each chest compression. The new approach, SNPeCPR or SNP-“enhanced” CPR (pronounced “snappy CPR”), incorporates three components: 1) active compression-decompression CPR with the use of an impedance threshold device (ACD CPR+ITD), which by actively decreasing intrathoracic pressure during the decompression phase decreases right atrial and intracranial pressures and improves vital organ perfusion (14, 15); 2) a potent vasodilator, SNP, which by decreasing vascular resistance promotes forward blood flow; and 3) lower abdominal binding (AB) that limits descending aortic blood flow and redirects the augmented cardiac output to the heart and brain, where it is needed the most. For simplicity, the combination of ACD CPR+ITD+AB without SNP will be called from here thereafter as eCPR.
SNPeCPR consists of easily deployable components that can be readily applied during CPR. ACD CPR+ITD is currently clinically practiced. AB can be performed easily either manually or with a belt/ pneumatic bladder/compression clamp. SNP can be given intravenously or intraosseously as any other drug that is currently delivered during CPR without further complexity.
We hypothesize that SNPeCPR will improve carotid blood flow, resuscitation rates, and 24-hr survival rates compared to standard CPR (S-CPR) plus epinephrine.
MATERIALS AND METHODS
The study was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center. All animal care was compliant with the National Research Council's 1996 Guidelines for the Care and Use of Laboratory Animals. All studies were performed by a qualified, experienced research team in Yorkshire female farm pigs weighing 30 ± 1.5 kg. A certified and licensed veterinarian provided a blinded neurologic assessment in the 24-hr survival experiments.
Preparatory Phase
The anesthesia, surgical preparation, data monitoring, and recording procedures used in this study have been described previously by our group (16). Briefly, we employed aseptic surgical conditions, using initial sedation with intramuscular ketamine (7 mL of 100 mg/mL, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) followed by inhaled isoflurane at a dose of 0.8% to 1.2%. Pigs were intubated with a size 7.0 endotracheal tube. The animal's temperature was maintained at 37.5 ± 0.5°C, with a warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, MN). Central aortic blood pressure was recorded continuously with a micromanometer-tipped (Mikro-Tip Transducer, Millar Instruments, Houston, TX) catheter placed at the beginning of the descending thoracic aorta. A second Millar catheter was inserted in the right atrium via the right external jugular vein. All animals received an intravenous heparin bolus (100 units/kg). A ultrasound flow probe (Transonic 420 series multichannel, Transonic Systems, Ithaca, NY) was placed to the left internal carotid to record blood flow (mL/min). The animals were then ventilated with room air, using a volume-control ventilator (Narcomed, Telford, PA), with a tidal volume of 10 mL/kg and a respiratory rate adjusted to continually maintain a PaCO2 of 40 Torr and PaO2 of 80 Torr (blood oxygen saturation >95%), as measured from arterial blood (Gem 3000, Instrumentation Laboratory, Lexington, MA) to adjust the ventilator as needed. Surface electrocardiographic tracings were continuously recorded. All data were recorded with a digital recording system (Superscope II version 1.295, GW Instruments, Somerville, MA). End-tidal CO2 (ETCO2), tidal volume, minute ventilation, and blood oxygen saturation were continuously measured with a respiratory monitor (CO2SMO Plus, Novametrix Medical Systems, Wallingford, CT). Right atrial pressure was adjusted between 2 and 5 mm Hg with saline infusion as needed before induction of ventricular fibrillation.
Measurements and Recording
Aortic pressure, right atrial pressure, ETCO2, and carotid blood flow were continuously recorded. Coronary perfusion pressure during CPR was calculated from the mean arithmetic difference between right atrial pressure and aortic pressure during the decompression phase. Doppler derived common carotid blood flow was reported in mL/sec.
Experimental Protocol
After the surgical preparation was complete, oxygen saturation on room air was >95% and ETCO2 was stable between 35 and 42 mm Hg for 5 mins. Ventricular fibrillation was induced by delivering direct current via a temporary pacing wire (Daig Division, St Jude Medical, Minnetonka, MN) positioned in the right ventricle. The ventilator was disconnected from the endotracheal tube. S-CPR and ACD CPR were performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark) as previously described (17). Uninterrupted chest compressions at a rate of 100 compressions/min, with a 50% duty cycle and a compression depth of 25% of the anteroposterior chest diameter, were provided. During CPR, asynchronous positive-pressure ventilations were delivered to simulate advanced life support with a manual resuscitator bag. The fraction of inspired oxygen was 1.0, the tidal volume was maintained at ~10 mL/kg, and the respiratory rate was 10 breaths/min.
Protocol
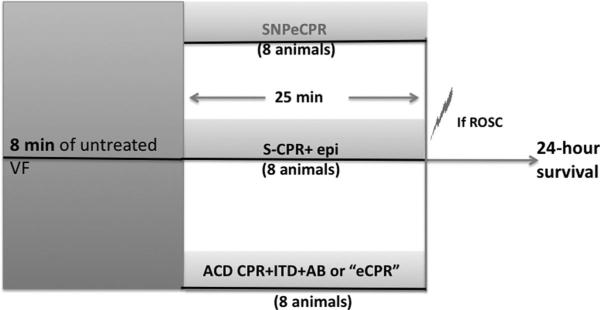
Following 8 mins of untreated ventricular fibrillation, 24 pigs were randomized to 25 mins of S-CPR (n = 8), ACD CPR+ITD+AB (n = 8), or SNPeCPR (n = 8). Epinephrine was administered to the S-CPR control group in 0.5-mg boluses every 5 mins, whereas SNP was delivered in the SNPeCPR group in 1-mg bolus every 5 mins (timetable is shown in Fig. 1). SNPeCPR and eCPR groups did not receive epinephrine during CPR. Defibrillation was delivered after 25 mins of CPR. Earlier defibrillations were not attempted because the study was designed to assess the effectiveness of SNPeCPR after 25 mins of CPR compared to S-CPR (since most of the patients require support for that period of time clinically). Animals that had a return of spontaneous circulation (ROSC) were then observed under general anesthesia with isoflurane until hemodynamics were stable. Hemodynamic stability was defined as a mean aortic pressure of >55 mm Hg without epinephrine for 10 mins, carotid blood flow equal or higher than baseline, and normalization of ETCO2 and acidosis. At that point, vascular repair of the internal jugular and the left common femoral artery was then performed.
Figure 1.
Protocol timelines. ROSC, return of spontaneous circulation; S-CPR, standard cardiopulmonary resuscitation, ACD+ITD, active compression-decompression CPR with an inspiratory impedance threshold device; AB, abdominal binding; SNP, sodium nitroprusside; VF, ventricular fibrillation; epi., epinephrine. (The combination of ACD CPR+ITD+AB is called “enhanced” eCPR.) Electric bolt sign, DC cardioversion.
Animals that had a stable post-ROSC rhythm but were hypotensive (mean arterial pressure of <50 mm Hg) received 250–500 mL of intravenous normal saline bolus. If mean arterial pressure was still <50 mm Hg, they received increments of 0.1–0.2 mg of epinephrine every 5 mins until mean arterial pressure rose above 50 mm Hg. Survivors were given intramuscular analgesic injections of nonsteroidal anti-inflammatory drugs as previously described (16) and had free access to water and food. There was no other post-ROSC medical care provided after the vascular repair. Animals were not treated with hypothermia. All surviving pigs with stable circulation for at least 30 mins received intravenous antibiotics with a third-generation cephalosporin and a repeat dose at 12 hrs. At 24 hrs, a certified veterinarian blinded to the intervention assessed the pig's neurologic function based on an overall performance category (OPC) scoring system. The veterinarian used clinical signs such as response to opening the cage door, response to noxious stimuli if unresponsive, response to trying to lift the pig, whether the animal could stand, walk, eat, urinate, and defecate, and appropriate response to the presence of a person walking the cage. The following scoring system was used: 1 = normal; 2 = slightly disabled; 3 = severely disabled but conscious; 4 = vegetative state; or 5 = dead (16).
Postresuscitation care was not blinded, since the same team performed CPR and provided post-ROSC care.
Echocardiographic Evaluation of Left Ventricular Function
After 1 hr, all survivors had a transthoracic echocardiogram to evaluate their left ventricular ejection fraction. Images were obtained from the right parasternal window, which provides similar views as the long and short parasternal windows for humans (18). The ejection fraction was assessed with trace planimetry and was reported by a blinded independent echocardiographer both for baseline and 1 hr post-ROSC.
Statistical Analysis
Values were expressed as mean ± SD. Baseline data were compared using a t test. Hemo-dynamics and blood gases during CPR were analyzed with two-way ANOVA. A two-tailed Fisher's exact test was used to compare ROSC rates and 24-hr survival as well as OPC proportions, which were dichotomized to OPC 1 and OPC 2 (good outcome) and OPC 3, 4, and 5 (bad outcome). A p value of <0.05 was considered statistically significant.
RESULTS
There were no baseline differences between the three treatment groups in any hemodynamic or respiratory parameters.
S-CPR vs. SNPeCPR or eCPR
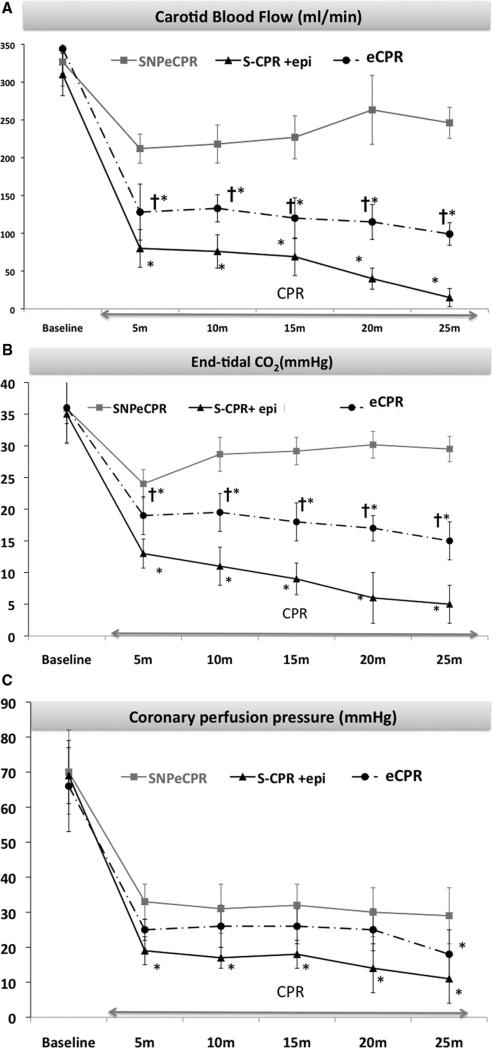
Pigs treated with S-CPR with epinephrine demonstrated significantly low aortic and coronary perfusion pressures compared with SNPeCPR or eCPR. There was profound progressive acidosis and lower ETCO2 with progressive decrease in carotid blood flow despite repetitive epinephrine doses (Table 1 and Fig. 2).
Table 1.
Hemodynamics and resuscitation rates
| Resuscitation Type | Baseline | CPR 10 min | CPR 20 min | 1-hr Return of Spontaneous Circulation | 24-hr Survival |
|---|---|---|---|---|---|
| SNPeCPR | |||||
| SBP | 100 ± 12 | 72 ± 16 | 73 ± 13 | 7 of 8 | 7 of 8 |
| DBP | 72 ± 12 | 38.6 ± 6 | 40 ± 5 | ||
| RA | 1.8 ± 1 | 7 ± 6 | 10 ± 7 | ||
| CPP | 70 ± 12 | 31 ± 7 | 30 ± 5 | ||
| eCPR | |||||
| SBP | 93 ± 15 | 77 ± 13 | 81 ± 5 | 2 of 8a | 2 of 8a |
| DBP | 68 ± 11 | 35 ± 5 | 36 ± 3 | ||
| RA | 1.5 ± 1 | 9 ± 7 | 11 ± 5 | ||
| CPP | 66 ± 13 | 26 ± 7 | 25 ± 8 | ||
| S-CPR | |||||
| SBP | 98 ± 11 | 58 ± 8a | 51 ± 5a | 0 of 8a | 0 of 8a |
| DBP | 71 ± 9 | 22 ± 5a | 18 ± 3a | ||
| RA | 1.5 ± 2 | 5 ± 3 | 4 ± 3a | ||
| CPP | 69 ± 8 | 17 ± 5a | 14 ± 8a |
Values are shown as mean ± sd. Cardiopulmonary resuscitation (CPR) for 25 mins with SNPeCPR, eCPR, and S-CPR (see text for definitions). All pressures are in mm Hg and carotid blood flow is in ml/min. SBP, systolic blood pressure; DBP, diastolic blood pressure; RAP, right atrial pressure; CPP, coronary perfusion pressure.
Statistically significant difference compared to SNPeCPR with a p value of <.05.
Figure 2.
Carotid blood flow and end-tidal CO2. Carotid blood flow (mL/min) (A), end-tidal CO2 (Torr) (B), and coronary perfusion pressure (mm Hg) (C) with sodium nitroprusside-enhanced cardiopulmonary resuscitation (SNPeCPR), CPR enhanced by active compression-decompression CPR, an inspiratory impedance threshold device, and abdominal binding without SNP (eCPR), and standard CPR (S-CPR) over 25 mins of CPR. *Statistically significant difference with a p value of <.05 compared to SNPeCPR. †Significant difference with a p value of <.05 between eCPR and S-CPR. epi., epinephrine.
SNPeCPR vs. eCPR
With the exception of carotid blood flow and ETCO2, the other measured hemodynamic parameters did not differ significantly between the two groups. This was not the case for arterial blood gases: there was a progressive metabolic acidosis in the eCPR group but not the SNPeCPR group (Tables 1 and 2 and Fig. 2). It is noteworthy that carotid blood flows after 20 and 25 mins of SNPeCPR were similar to baseline pre-ventricular fibrillation values.
Table 2.
Arterial blood gases during cardiopulmonary resuscitation and after return of spontaneous circulation
| Resuscitation Type | Baseline | CPR |
Return of Spontaneous Circulation (30 min) | ||
|---|---|---|---|---|---|
| 5 min | 10 min | 20 min | |||
| SNPeCPR | |||||
| pH | 7.38 ± 0.2 | 7.35 ± 0.1 | 7.28 ± 0.06 | 7.24 ± 0.07 | 7.38 ± 0.06 |
| Pco2 | 44 ± 3 | 42 ± 7 | 40 ± 46 | 43 ± 7 | 36 ± 46 |
| Po2 | 90 ± 13 | 172 ± 32 | 188 ± 63 | 145 ± 45 | 102 ± 13 |
| HCO3 | 24 ± 2 | 24 ± 2 | 19.8 ± 2 | 16 ± 4 | 21 ± 2a |
| Sao2 | 96 ± 2 | 100 | 100 | 100 | 100 |
| eCPR | |||||
| pH | 7.42 ± 0.2 | 7.33 ± 0.1 | 7.28 ± 0.07 | 7.15 ± 0.04a | 7.21 ± 0.05a |
| Pco2 | 44 ± 4 | 42 ± 6 | 42 ± 7 | 47 ± 5 | 40 ± 5 |
| Po2 | 92 ± 8 | 195 ± 65 | 203 ± 77 | 131 ± 59 | 108 ± 25 |
| HCO3 | 22 ± 2 | 22 ± 4 | 18 ± 5 | 11 ± 3a | 16 ± 3 |
| Sao2 | 95 ± 3 | 100 | 100 | 100 | 100 |
| S-CPR | |||||
| pH | 7.39 ± 0.1 | 7.23 ± 0.1a | 7.21 ± 0.07a | 7.11 ± 0.04a | N/A |
| Pco2 | 42 ± 3 | 44 ± 6 | 40 ± 7 | 44 ± 5 | N/A |
| Po2 | 89 ± 9 | 195 ± 51 | 176 ± 59 | 121 ± 45 | N/A |
| HCO3 | 22 ± 3 | 14 ± 3a | 13 ± 5a | 9 ± 3a | N/A |
| Sao2 | 94 ± 5 | 100 | 100 | 100 | N A |
See text for definitions of SNPeCPR, eCPR, and S-CPR.
Mean ± sd. Arterial blood gas measurements during cardiopulmonary resuscitation (CPR) and 30 mins after return of spontaneous circulation. Partial pressures in Torr. Sao2, % arterial oxygen saturation. HCO3, bicarbonate (mEq/L).
Statistically significant difference compared to SNPeCPR with a p value of <.05.
ROSC and 24-hr Survival
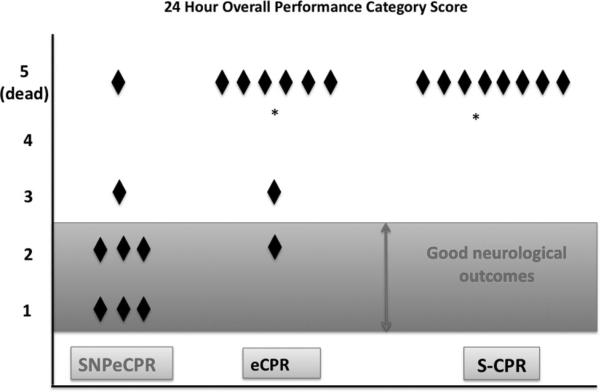
No animal treated with S-CPR with epinephrine group had successful ROSC and therefore could not be assessed for 24-hr survival. Eight of eight animals that received SNPeCPR had a ROSC, and seven of eight survived for both 1 and 24 hrs. A total of five of eight animals with eCPR had an initial ROSC; two of eight survived to 1 hr and two of eight survived 24 hrs (p = .04 for 1-hr and 24-hr survival compared with SNPeCPR). Good outcomes at 24 hrs (OPC 1 or 2) were significantly better in the SNPeCPR group than in the eCPR (six of eight vs. one of eight) (p = .04) (Fig. 3).
Figure 3.
Overall performance category score and 24-hr survival. Overall performance category score at 24 hrs after 25 mins with sodium nitroprusside-enhanced cardiopulmonary resuscitation (SNPeCPR), CPR enhanced by active compression-decompression CPR, an inspiratory impedance threshold device, and abdominal binding without SNP (eCPR), and standard CPR (S-CPR). *p < .05 compared to SNPeCPR.
Left Ventricular Function
There was no baseline difference between groups in the left ventricular ejection fraction (SNPeCPR: 64% ± 7%, S-CPR: 66% ± 8%, eCPR: 64% ± 9%). An echocardio-graphic evaluation performed 1 hr after cardiac arrest revealed that the SNPeCPR animals had an ejection fraction of 58% ± 12%. The two animals in the eCPR group had ejection fractions of 38% and 44%, respectively.
In addition, it was observed that six of eight animals in the eCPR and six of eight animals in the S-CPR group developed severe pulmonary edema that started after 15 mins of CPR. Only one of eight animals developed mild pulmonary edema in the SNPeCPR group, which self-terminated once the animals was connected to the ventilator (six of eight vs. one of eight, p < .05). The one animal that died in the SNPeCPR group developed intractable ventricular tachycardia/ fibrillation 30 mins after successful ROSC. No antiarrhythmic drugs were used.
Postresuscitation Care
SNPeCPR animals received an average of 0.2 ± 0.1 mg and 358 ± 128 mL of epinephrine and saline, respectively. The two survivors in the eCPR group received 0.4 and 0.3 mg of epinephrine and 500 mL of saline, respectively.
DISCUSSION
Results from this first series of experiments with SNPeCPR demonstrate the feasibility of using SNP in the treatment of cardiac arrest to enhance brain flow and increase 24-hr survival with favorable neurologic outcomes. When SNP was combined with the “mechanical platform” of eCPR, a high level of cardiac and cerebral perfusion pressure as well as carotid blood flow was maintained for 25 mins, and 24 hrs later most of the animals were alive with good neurologic function.
By contrast, none of the pigs treated with traditional means of S-CPR and epinephrine survived for 24 hrs, and only two of eight treated with eCPR survived for 24 hrs. Thus, SNP was an essential element of this novel therapeutic approach. The combination of hemodynamic findings, pH and ETCO2 measurements, and 24-hr survival outcomes supports the hypothesis that SNPeCPR provided superior hemodynamics and especially carotid blood flow than either S-CPR or eCPR.
In this study, S-CPR could not sustain forward blood flow for the duration of the protocol and carotid blood flow, and ETCO2 deteriorated over time. This contributed to the inability to successfully defibrillate the animals. Coronary perfusion pressure also significantly decreased after the first 5 mins of S-CPR. In the clinical setting, where most patients receive CPR for up to 30 mins and few are resuscitated, the pathophysiology is likely to be similar to what was observed in this study, The temporal deterioration of SCPR efficiency could explain the grim prognosis of patients with out-of-hospital cardiac arrest (1). The significant deterioration of ETCO2 in the S-CPR treated animals could also be due to the development of pulmonary edema that was commonly observed in this group.
By contrast, the addition of SNP to eCPR significantly increased carotid blood flow and resulted in improved survival rates with more favorable neurologic outcomes. Many clinical resuscitation efforts last even longer than the protocol used in these pig studies. As such, the findings underscore the criticality of using a CPR method that can have a sustainable effect on vital organ perfusion until ROSC is finally achieved. Superior blood flow appears to be essential for an improved neurologic function. In addition, the absence of hypotension during SNPeCPR and the immediate post-ROSC period, as well as the minimal need for fluid or vasopressor administration during the recovery phase, suggests that intra-CPR SNP administration is also safe.
The mechanisms underlying the beneficial effects of SNP during CPR observed in this study remain speculative but are likely due to the significant increase in blood flow secondary to nitric oxide, generated by the metabolism of SNP, and also by endothelial donation, as it has been recently described in a smaller animal model (19). Exogenous nitric oxide donation offered by SNP has been shown to alleviate ischemia reperfusion injury in a number of organs such as the heart, kidney, and liver (20).
Animal studies have also documented the protective effect of nitric oxide in the early stages of cerebral ischemia and point to the therapeutic potential of SNP in the management of brain ischemic damage (21). We speculate that the global ischemia of cardiac arrest promotes a systemic microcirculatory dysfunction and possibly a “low-reflow” or “no-reflow” phenomenon similar to coronary flow after percutaneous revascularization for ST elevation myocardial infarction, where intracoronary infusion of SNP has been shown to be beneficial (22). In addition, the presence of pulmonary edema during S-CPR and eCPR suggests that the left ventricular compliance is significantly altered in a favorable manner with SNP. This has been previously shown in patients when global intracoronary infusion of SNP significantly improved left ventricular diastolic distensibility and significantly increased compliance (23).
Our study has several limitations. First, we did not investigate the biochemical mechanism responsible for the improved outcomes with SNP. While we presume the observed physiologic benefit was largely secondary to the vasodilatory effects of this drug, it could also be due in part to theoretically protective effects of nitric oxide donation. Levels of cyanide, a metabolic byproduct of SNP, and its potentially protective or detrimental effect on mitochondrial metabolism were not studied. There was no evidence of cyanide toxicity, since none of the animals showed evidence of persistent metabolic acidosis. All SNPeCPR animals had almost normal arterial pH and acid base balance within 30 mins. Regardless of the exact mechanism that SNP offers its benefits in prolonged CPR, when coadministered with a mechanical means to maintain adequate coronary perfusion and aortic pressures, it appears appropriate for use in the setting of CPR. Second, we did not rigorously study the combination of SNP and S-CPR. However, we did perform preliminary studies, not reported herein, that demonstrated that the hemo-dynamics of S-CPR and SNP were equal to or less than S-CPR with epinephrine, described above for the control group (12). Those findings are the subject of a separate report. Finally, we continued to treat animals in ventricular fibrillation with CPR but without defibrillation for a total of 25 mins in an effort to simulate the duration of CPR reported in most clinical trials and effectively assess the ability of the CPR method to sustain heart and brain viability for that duration.
CONCLUSION
Use of SNPeCPR significantly improved carotid blood flow, hemodynamics, acid-base status, ROSC, and 24-hr survival rates with good neurologic outcomes compared with either S-CPR with epinephrine or eCPR. SNPeCPR can be delivered with currently available drugs and devices.
Acknowledgments
Supported, in part, by an Institutional Division of Cardiology grant at the University of Minnesota to Dr. Yannopoulos.
Footnotes
See also p. 000.
Presented, in part, as preliminary data at the Resuscitation Science Symposium in Chicago, IL, ●●●, 2010; published in an abstract form: Yannopoulos et al. Circulation. 2010; 122: A164
Dr. Lurie is the founder of Advance Circulatory Systems Incorporated (ACSI), and coinventor of the inspiratory impedance threshold device and ACD CPR technique used in this study. Dr. Yannopoulos received funding from the Division of Cardiology at the University of Minnesota. Dr. Lurie received a patent and holds stock ownership with ACD and ITD CPR (ACSI). The remaining authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation. 2010;121:709–729. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aufderheide TP, Pirrallo RG, Provo TA, et al. Clinical evaluation of an inspiratory impedance threshold device during standard cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest. Crit Care Med. 2005;33:734–740. doi: 10.1097/01.ccm.0000155909.09061.12. [DOI] [PubMed] [Google Scholar]
- 4.Thayne RC, Thomas DC, Neville JD, et al. Use of an impedance threshold device improves short-term outcomes following out-of-hospital cardiac arrest. Resuscitation. 2005;67:103–108. doi: 10.1016/j.resuscitation.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(Suppl 24):IV1–IV203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 6.Voelckel WG, Lurie KG, McKnite S, et al. Effects of epinephrine and vasopressin in a piglet model of prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2002;30:957–962. doi: 10.1097/00003246-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardio-pulmonary resuscitation. Crit Care Med. 2007;35:2145–2149. doi: 10.1097/01.ccm.0000280427.76175.d2. [DOI] [PubMed] [Google Scholar]
- 8.Berg RA, Otto CW, Kern KB, et al. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: A prospective, randomized study. Crit Care Med. 1994;22:282–290. doi: 10.1097/00003246-199402000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel V, Krismer AC, Arntz HR, et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350:105–113. doi: 10.1056/NEJMoa025431. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN, Burke LP. Nitroprusside. Ann Intern Med. 1979;91:752–757. doi: 10.7326/0003-4819-91-5-752. [DOI] [PubMed] [Google Scholar]
- 11.Guiha NH, Cohn JN, Mikulic E, et al. Treatment of refractory heart failure with infusion of nitroprusside. N Engl J Med. 1974;291:587–592. doi: 10.1056/NEJM197409192911201. [DOI] [PubMed] [Google Scholar]
- 12.Yannopoulos D, Matsuura T, Kotsifas K, et al. Use of sodium nitroprusside in combination with active enhancement of venous return improves cerebral blood flow during prolonged CPR. Circulation. 2009;120:S1450. [Google Scholar]
- 13.Halperin HR, Tsitlik JE, Guerci AD, et al. Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation. 1986;73:539–550. doi: 10.1161/01.cir.73.3.539. [DOI] [PubMed] [Google Scholar]
- 14.Lurie KG, Coffeen P, Shultz J, et al. Improving active compression-decompression cardiopulmonary resuscitation with an inspira-tory impedance valve. Circulation. 1995;91:1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 15.Yannopoulos D, Nadkarni VM, McKnite SH, et al. Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation. 2005;112:803–811. doi: 10.1161/CIRCULATIONAHA.105.541508. [DOI] [PubMed] [Google Scholar]
- 16.Yannopoulos D, Matsuura T, McKnite S, et al. No assisted ventilation cardiopulmonary resuscitation and 24-hour neurological outcomes in a porcine model of cardiac arrest. Crit Care Med. 2010;38:254–260. doi: 10.1097/CCM.0b013e3181b42f6c. [DOI] [PubMed] [Google Scholar]
- 17.Shultz JJ, Coffeen P, Sweeney M, et al. Evaluation of standard and active compression-decompression CPR in an acute human model of ventricular fibrillation. Circulation. 1994;89:684–693. doi: 10.1161/01.cir.89.2.684. [DOI] [PubMed] [Google Scholar]
- 18.Marino BS, Yannopoulos D, Sigurdsson G, et al. Spontaneous breathing through an inspiratory impedance threshold device augments cardiac index and stroke volume index in a pediatric porcine model of hemorrhagic hypovolemia. Crit Care Med. 2004;32(Suppl 9):S398–S405. doi: 10.1097/01.ccm.0000139950.39972.68. [DOI] [PubMed] [Google Scholar]
- 19.Dezfulian C, Shiva S, Alekseyenko A, et al. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips L, Toledo AH, Lopez-Neblina F, et al. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22:46–55. doi: 10.1080/08941930802709470. [DOI] [PubMed] [Google Scholar]
- 21.Salom JB, Ortí M, Centeno JM, et al. Reduction of infarct size by the NO donors sodium nitroprusside and spermine/NO after transient focal cerebral ischemia in rats. Brain Res. 2000;865:149–156. doi: 10.1016/s0006-8993(00)02095-3. [DOI] [PubMed] [Google Scholar]
- 22.Tesic MB, Stankovic G, Vukcevic V, et al. The use of intracoronary sodium nitroprusside to treat no-reflow after primary percutaneous coronary intervention in acute myocardial infarction. Herz. 2010;35:114–118. doi: 10.1007/s00059-010-3243-4. [DOI] [PubMed] [Google Scholar]
- 23.Paulus WJ, Vantrimpont PJ, Shah AM. Acute effects of nitric oxide on left ventricular relaxation and diastolic distensibility in humans. Assessment by bicoronary sodium nitroprusside infusion. Circulation. 1994;89:2070–2078. doi: 10.1161/01.cir.89.5.2070. [DOI] [PubMed] [Google Scholar]