Abstract
Exposure to chemical contaminants is often invoked to explain recruitment failures to populations of sturgeon worldwide, but there is little empirical evidence to support the idea that young sturgeon are sensitive at environmentally relevant concentrations. The authors used shortnose sturgeon (Acipenser brevirostum) and Atlantic sturgeon (Acipenser oxyrinchus) as models to investigate the sensitivities of sturgeon to early-life-stage toxicities from embryonic exposures to graded doses of polychlorinated biphenyl 126 (PCB126) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Survival to hatching of shortnose sturgeon decreased with increasing dose, although the duration of the embryonic period was not significantly altered by exposure in either species. Morphometric features of larvae of both species were affected by dose, including shortening of the body, reduction in head size, reduction in quantity of yolk reserves, and reduction in eye size. Eye development in both species was delayed with increasing dose for both chemicals. The persistence of larvae in a food-free environment decreased inversely with dose in both species, with sharp declines occurring at PCB126 and TCDD doses of ≥1 ppb and ≥0.1 ppb, respectively. Dose-responsive early-life-stage toxicities reported here are among the more sensitive found in fish and occurred at burdens similar to those found in situ in a sympatric bottom-dwelling bony fish in the Hudson River Estuary. The present study is among the first demonstrating the sensitivity of any sturgeon to the hallmark early-life-stage toxicities induced by aryl hydrocarbon receptor agonists.
Keywords: Hudson River, early-life-stage toxicities, larval survivorship, eye development, morphometrics
INTRODUCTION
Twenty-six species of sturgeon exist worldwide, and all or some populations of each are threatened with extinction because of anthropogenic impacts [1]. Three reasons are commonly provided to explain the declines in sturgeon abundance: overharvest, habitat loss, and exposure to chemical toxicants [2]. Direct relationships between levels of harvest (landings) and population status are evident in some species [3]. The presence of dams on rivers precludes access to historical spawning habitats for some populations, which has likely diminished recruitment. However, few if any studies have empirically evaluated the effects on sturgeon populations of chemical pollutants, although this is often cited as a reason for their declines [4].
Studies in fish have demonstrated the extreme sensitivity of some species to early-life-stage toxic effects of dioxin-like chemicals. For example, early life-stage lake trout Salvelinus namaycush exhibited lesions and reduced survival at concentrations of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) as low as 34 to 100 parts per trillion [5]. For lake trout, vulnerability to dioxin-like chemicals has been linked to recruitment failure, population decline, and extirpation in some of the Great Lakes [6]. Hallmark toxic endpoints in the early life stages of fish include craniofacial malformations, abnormal spinal curvature, yolk-sac and pericardial edema, hemorrhaging, peripheral ischemia, altered hatching rate, and reduced survivorship [7]. It is believed that many of these toxic responses to dioxin-like chemicals result from impaired structure and function of the cardiovascular system [8,9]. Further studies have demonstrated a high level of variation in the vulnerabilities to these toxicants among fish species. For example, the mortality risk from TCDD exposure during early life stages of seven species of freshwater fish (normalized for egg lipid concentrations) was 16 to 180 times less than the same risks for lake trout [10].
Studies of a variety of vertebrate taxa, including fish [11], have demonstrated that the most toxic effects of TCDD and coplanar polychlorinated biphenyl (PCB) congeners are mediated by activation of the aryl hydrocarbon receptor (AHR) pathway. Although only a single form of AHR exists in mammals, at least two forms (AHR1 and AHR2) have been identified in fish [12], among which AHR2 is more active in binding TCDD [13] and in mediating early-life-stage toxic responses to TCDD [14], including cardiac impairment [15]. Aryl hydrocarbon receptor 2 is needed to activate transcription of genes in the AHR battery, such as cytochrome P4501 (CYP1A), but the role of induced expression of these genes in higher level toxic responses remains unclear [16].
Eight species of sturgeon are found in North America. Based on their life histories and habitats, all are thought to be at risk to chemical pollutants. Shortnose sturgeon (Acipenser brevirostrum) and Atlantic sturgeon (Acipenser oxyrinchus) co-occur along the Atlantic coast of North America from the Saint John River, New Brunswick, Canada, and St. Lawrence River, Quebec, Canada, respectively, to Georgia, USA. Both species of sturgeon are now listed as endangered under the U.S. Endangered Species Act. Additionally, shortnose sturgeon are listed in Appendix I and Atlantic sturgeon in Appendix II of the Convention on International Trade in Endangered Species (CITES).
With regard to life history features relevant to exposure risk, both species feed on a variety of benthic organisms as juveniles and older fish, mature after a protracted juvenile stage, and are long-lived (up to 67 and 75 years for shortnose and Atlantic sturgeon, respectively). The frequency of spawning among individuals varies from 1 to 3 years in shortnose sturgeon and from 1 to 5 years in Atlantic sturgeon. Spawning occurs between the fall line and salt front of large rivers, which is a common location for urbanization and industrial activities. Spawning progresses seasonally from early spring to summer with increasing latitude. Both species are modestly fecund for their body size compared with the fecundity of other marine and estuarine fish taxa, and their relatively large eggs sink to the river bottom. Large egg size is associated with protracted embryonic periods in fish [17], and the embryonic periods of shortnose and Atlantic sturgeon at 15° are 12 to 14 and 7 to 8 d, respectively. These size and buoyancy attributes of sturgeon eggs increase the risk of sturgeon embryo exposure to lipophilic sediment-borne contaminants such as PCBs.
Shortnose sturgeon are amphidromous and are believed to be largely restricted to their natal estuaries, although seasonal forays through coastal waters may be more frequent and extended than previously thought for populations at the extremes of their distribution [18]. Adult shortnose sturgeon mature at older ages (~3–11 years) in northern compared with southern populations and spawn later in the spring–summer season with increasing latitude [19]. Spawning commences in mid-February to mid-March in the southern portions of the geographic range (Georgia–South Carolina, USA), in late April to early May in the mid-Atlantic Bight (Delaware River to Connecticut River, USA), and not until mid-May to early June in the Saint John River [19].
Atlantic sturgeon are anadromous, but they return to their natal estuaries for spawning only once sexual maturation is attained (~7–29 years, increasing with latitude). In the Hudson River, females mature at age 14 years or older (mean = 20 years) and males at age 12 years or older (mean = 15 years) [20]. Subadult and adult Atlantic sturgeon undertake extensive coastal migrations ranging from at least North Carolina, USA, to the Minas Basin of the Bay of Fundy, Canada [21]. Spawning in Atlantic sturgeon also progresses from south to north during the first seven months of the calendar year, and typically occurs after spawning by shortnose sturgeon in any given locale [22]. The spawning period for Atlantic sturgeon in the Hudson River extends from late May through early July and peaks in early to mid-June [20]. Although the geographic extent of Atlantic sturgeon spawning in the Hudson River has yet to be delineated, spawning is believed to extend in the mid-Hudson from at least Stony Point upstream to Catskill, New York (K. Hattala, NYSDEC, personal communication). The likely spawning habitat in the Hudson River Estuary overlaps with areas of elevated concentrations of PCBs in sediment and fish tissues [23].
The life history and ecological attributes of both species— long life, generalized bottom feeding, large and demersal eggs, and inhabiting inshore to riverine habitats that are especially vulnerable to industrial pollutants—place both sturgeon species at risk to the effects of a suite of toxicants. These vulnerabilities have likely contributed to both species’ precarious status in many of their resident ecosystems. Indeed, shortnose sturgeon have been listed as federally endangered in the United States since the original passage of the federal Endangered Species Act in 1973, and the Hudson River population of Atlantic sturgeon and those farther to the south have recently been federally listed as endangered.
The Hudson River has a long history of contamination with PCBs, dioxins/furans (PCDD/Fs), and polycyclic aromatic hydrocarbons (PAHs) [24]. Because of its history of PCB contamination and downstream transport, the Hudson River PCB Superfund site is the largest in the United States, extending for nearly 320 km from Hudson Falls, New York, downstream to the southern tip of Manhattan. Contamination by PCDD/Fs, and particularly for the most toxic congener, TCDD, is greatest in Newark Bay, New Jersey, and its tributaries, which are linked to and just west of the Hudson River. Polycyclic aromatic hydrocarbon contamination is greatest in the sediments of the lower estuary near New York City.
Many surveys have documented high levels of PCBs in the fillets of Hudson River resource fish species and in livers of some other fish [25] (http://response.restoration.noaa.gov/querrymanager). In some instances, tissue burdens of coplanar PCBs and PCDD/Fs in fish from the Hudson River Estuary, expressed as toxic equivalency quotients (TEQs) with respect to TCDD, exceed those known to induce early-life-stage toxicities in sensitive species. For example, Fernandez et al. [26] reported levels of hepatic TCDD TEQs in livers of Atlantic tomcod Microgadus tomcod from the Hudson River Estuary (up to 736 pg/g TCDD TEQs in the Hackensack River and 69 pg/g TCDD TEQs in the main-stem Hudson River) that exceed those shown to induce early-life-stage toxicities in sensitive salmonid species (as low as 40 pg/g TCDD) [5]. Reports have also chronicled elevated tissue levels of total PCBs and total PCDD/Fs in shortnose sturgeon and Atlantic sturgeon from the Hudson River compared with tissues from conspecifics from cleaner estuaries (http://response.restoration.noaa.gov/querry-manager). No published study has yet to evaluate the sensitivities of sturgeon to the early-life-stage toxic effects of coplanar PCBs or TCDD.
The Delaware River Estuary also has a long history of chemical pollution from PCBs and PCDD/Fs, among other toxicants [27]. The Delaware River supports a robust population of shortnose sturgeon, but its Atlantic sturgeon population is greatly reduced compared with historic levels. The rarity in recent decades of spawning adult Atlantic sturgeon and fish in their early life stages led to the supposition that they had been extirpated [28]. Several reports have documented high tissue burdens of both PCBs and PCDD/Fs in shortnose sturgeon from the Delaware River [29]. Concern exists regarding proposed dredging projects in the Delaware River, particularly at the recently discovered Atlantic sturgeon nursery area, and the potential for resuspension of damaging levels of sediment-borne toxicants. Unfortunately, no study has calculated PCB or PCDD/F tissue burdens in either sturgeon species on a congener-specific basis from these or other populations, so total TCDD TEQs burdens have yet to be determined.
The AHRs have yet to be identified in sturgeon, but we recently demonstrated that AHR-mediated CYP1A expression is inducible in early life stages of shortnose sturgeon and Atlantic sturgeon by their exposure to environmentally relevant graded doses of TCDD and PCB126 [30]. Our results are consistent with the expectation that AHR-mediated toxic effects are conserved in sturgeon. Because CYP1A transcription is activated through the AHR pathway, we would expect these sturgeon to express toxic effects to dioxin-like chemicals at higher biological levels (e.g., physiological, organismal, and population). No published studies, however, have provided data with which to evaluate this expectation.
The primary goal of this study was to evaluate experimentally whether shortnose sturgeon and Atlantic sturgeon are vulnerable under controlled laboratory conditions to early-life-stage toxic effects of coplanar PCB126 and TCDD and, if so, whether the effects are dose responsive and occur at environmentally relevant concentrations. We used PCB126 and TCDD as focal toxicants because of their high level of occur-rence in tissues of other Hudson River fish [26,31], their high toxicities in fish, and their relatively wide use in toxicological studies in other taxa. Several of the macrophenotypic responses that we measured (e.g., body size and eye development in recently hatched larvae) are closely linked to a fish's ability to persist and thrive in nature. We view these metrics as sublethal effects and as components of fitness. Prior to addressing this primary study goal, and because of the limited information on culture protocols appropriate for toxicological experiments on early life stages of sturgeon, we first evaluated culture techniques appropriate for controlled and replicated toxicological experiments, and then assessed via uptake studies the efficacy of our protocol for exposing embryos to the contaminants.
MATERIALS AND METHODS
Sources of fertilized eggs and general maintenance
Sturgeon embryos were obtained from programs that spawn and culture both species for research and aquaculture. Fertilized shortnose sturgeon eggs of Connecticut River origin were obtained from the USGS Conte Lab (Turners Falls, MA) in mid-May. Additional fertilized eggs of shortnose sturgeon were of Saint John River origin and were obtained in late May, as were Atlantic sturgeon eggs that were obtained in July. The Saint John River sturgeon eggs were provided by Acadian Sturgeon and Caviar. The Saint John River is not thought to have major inputs of PCBs (sediment PCB concentrations of <10 mg/kg; K. Munkittrick, Canadian Rivers Institute, personal communication); however, at least six pulp and paper mills have been sited historically along the river. Tissue burdens of PCBs and PCDD/Fs have yet to be empirically evaluated in sturgeon from the Saint John River.
At each source location, ova and milt were obtained by strip spawning at the hatchery. Gametes were mixed and then inundated with a mixture of local river water and fine silt (Fuller's earth). Shortnose and Atlantic sturgeon eggs are strongly adhesive and the fine constituent particles of silt in this de-adhesion solution provide an ephemeral coating that prevents the eggs from sticking to one another and to the container in which they are housed [32]. The silt coating is lost after multiple water changes during the first few days after fertilization and before use in our uptake and toxicity studies. Fertilized eggs were shipped to our location (NOAA Fisheries Howard Marine Sciences Laboratory, Highlands, NJ) within 1 to 3 d after fertilization. On arrival, eggs were transferred to modified MacDonald's jars plumbed into a recirculating system that included inline biofiltration media, ultraviolet sterilization, and daily water replacement of half of the system volume. The eggs were incubated throughout the study in 0.1 parts per thousand (ppt) salt water and initially resided in the Mac-Donald's jars for up to 24 h while eggs were counted into groups of 30, which were allocated to the various experimental treatments. Except when noted below, shallow glass dishes (15 × 11 × 4 cm), containing 250 ml of water changed twice daily, were used to house embryos for experimentation. With each water change, eggs were inspected visually for mortalities, and dead eggs were removed and discarded. Most mortalities occurred early in the embryonic period and were detected by discoloration or marbling of the yolk. Mortality of more developmentally advanced embryos was based on inspection of the embryo by eye, with the aid of magnification. As larvae hatched, they were removed from their egg dish, counted, and transferred to a holding dish to be processed immediately (details below). All eggs and larvae were maintained in an environmental control chamber with a water temperature of 15° and a 14-h-light to 10-h-dark regime. All live-animal experimentation was conducted at NOAA's NEFSC Howard Laboratory, the protocols of which conform to U.S. federal animal-care standards.
Evaluation of culture techniques
Sturgeon eggs are large (2.5–3 mm diameter) for marine and estuarine taxa, negatively buoyant, adhesive, and prone to fungal infestations. Years of culture trials in hatcheries and production facilities have shown that high densities of sturgeon eggs survive well when an egg de-adhesion method is applied and the eggs are kept in continuous motion by water circulation using MacDonald's jars or modifications thereof [32,33].
A primary goal of our study was to assess and quantify the early-life-stage toxicities in sturgeon when exposed as embryos to PCB and TCDD. This goal requires experimental designs appropriate for the statistical evaluation of multiple experimental treatments applied to controlled, standardized, and replicated sets of embryos and larvae. Hence, a prerequisite for our assessment of toxicities in these sturgeon was the identification and testing of reliable and cost-effective methods for incubating large numbers of groups of small numbers of embryos in a highly replicated manner that would result in healthy larvae if not subjected to contaminants.
We performed these evaluations by allocating groups of 30 eggs to each of six container types that differed in size, shape, and materials and seven egg maintenance protocols. These tests included our base culture conditions, which consisted of 30 fertilized and de-adhesed eggs placed into static 0.1-ppt saltwater with twice daily water change, maintained at 15° in a 14 to 10-h light–dark regime and placed into a walk-in environmental chamber. In addition, modifications were made to the following: (1) container design, materials, shapes, and water volumes, including the use of MacDonald's jars; (2) thermal environment (±5° of base); (3) water change frequency; (4) aeration; (5) exchange to newly clean containers at each water change; and (6) lack of application of de-adhesion materials to eggs with Fuller's earth (i.e., eggs were allowed to adhere to glass slides). All evaluations of container types and maintenance protocols were replicated three times except, for the MacDonald's jar, for which one replicate was used.
These evaluations were applied to the first cohort of short-nose sturgeon available to us, which were the offspring of Connecticut River broodstock. We scored the efficacy of the various container types and egg maintenance protocols by percentage survival to hatching.
Quantification of uptake levels of PCB126
Shortnose and Atlantic sturgeon embryos were exposed to radiolabeled 3H-PCB126 (American Radiolabeled Chemicals; 5 Ci/mmol, 99% purity in hexane) to estimate whole-body burdens of PCB126 in our toxicity experiments. The 3H-PCB126 was diluted in acetone and nonlabeled PCB126 to working stocks of 20 ng PCB126/μl, 2 ng PCB126/μl, and 0.2 ng PCB126/μl, and 25 μl of each was used in exposure solutions. Exposure conditions were identical to those in subsequent toxicity experiments except for duration and nominal PCB126 concentrations. Exposures of shortnose sturgeon embryos were for 2, 8, and 24 h and were performed in duplicate. Atlantic sturgeon embryos were exposed for 2, 8, and 24 h in triplicate. In both cases, final exposure concentrations of 0.1, 1, and 10 ppb PCB126 were used. Each beaker contained either ten, 7-d-old (shortnose sturgeon), or ten, 5-d-old (Atlantic sturgeon) embryos just beyond the midpoint of the embryonic period (late neurulation to elongation of the propnephros and protrusion of embryos above yolk). Embryos were exposed in 50 ml of 0.1-ppt artificial seawater in 100-ml glass beakers at 14°. At the conclusion of exposures, embryos were washed three times with distilled water, weighed, solubilized in 2 ml Soluene-350 (PerkinElmer Life and Analytical Science) overnight at 50°, cooled to room temperature, flooded with 10 ml Hionic Fluor (Packard Bioscience), and counted for 10 min on a Tri-Carb 2900TR Liquid Scintillation Analyzer (Packard Bioscience).
Toxicity experiment exposures and fish maintenance
Embryos from 56 to 72 h old (early neurulation stage and before organogenesis) were counted into groups of 30 and exposed to graded doses (multiples of 10) of PCB126 (Accu-Standard; 99.7% purity) ranging from 0.01 to 1,000 ppb to graded doses of TCDD (AccuStandard; 99.1% purity) ranging from 0.001 to 100 ppb (both chemicals dissolved in 0.05–0.1% acetone) in 25 μl of acetone vehicle, to 25 μl of acetone alone, or were maintained in base water conditions. Embryos of this age were used because of logistical constraints in obtaining mature eggs from a sufficient number of females to ensure representative genetic variation in the population and the international transport of CITES-listed fish from Canada to the United States. Each treatment combination (species × contaminant × dose) was replicated three times. Exposures were in 50 ml of 0.1 ppt salt water in 100-ml glass beakers for 26 to 27 h at 14°. After exposures, embryos were transferred to egg incubation containers (Pyrex dishes or PVC–nylon mesh baskets) and maintained under our base protocol as described above until hatching. Containers were checked for dead eggs and hatchlings just prior to twice-daily water changes.
Hatched larvae were removed from their incubation containers, counted, and allocated to one of three uses. One set of up to five larvae per source container drawn from the interquartile portion of the hatch-frequency distribution was used for imaging. A second set of up to five larvae per source container, also drawn from the interquartile of hatching, was used to estimate posthatching life span in a food-free environment, which we use as a measure of fish condition at hatching. A third set was archived for potential future chemical analysis. The remainder was preserved in 5% buffered formalin in vials corresponding to each source container (i.e., treatment × replicate) for future analysis.
Larvae used for imaging were transferred to 100-ml beakers, in which they were held in 0.1 ppt salt water for 24 h prior to photography. This 1-d lag time allowed the egg membranes to slough off fully and resulted in much clearer images of larvae for morphometric analysis. Hence, photographs of larvae were taken at ages between 24 and 36-h posthatching. Larvae were anesthetized with MS222 in 150 mg/L 0.1 ppt salt water, placed in a beveled watch glass, and kept chilled prior to and during photography. Photographs of larvae were taken at ×6 or ×12 magnification on a Wild M8 dissecting microscope outfitted with a Zeiss AxioCam CCD color camera. Images were exported to jpg format and analyzed for a suite of morphometric and developmental measures.
Groups of larvae used for analysis of posthatching life span in a food-free environment were transferred to 250-ml beakers filled to 200 ml with 0.1 ppt salt water and maintained under the same temperature and light regimes as described above. These beakers were monitored daily for up to 35 d for determination of age at death. At every census, each group of larvae was examined for evidence of mortality in a stepped fashion with an increasingly intrusive evaluation. Young larvae of both species tend to be very active. If a larva was inactive, the beaker was gently swirled back-and-forth to elicit a response. If none occurred, the suspect larva received a gentle jet of water from a Pasteur pipet. If still no response was elicited, the larva was drawn into a wide-bore glass pipet and visually examined. If the larva exhibited visible red coloration around the heart area it was examined at ×6 magnification to confirm status. If each step resulted in no evidence of life (i.e., lack of motion or circulation), the larva was removed from the group and its day of death recorded.
Response variables
We measured and analyzed a group of macrophenotypic responses of embryos and young larvae that consisted of the embryo period duration; hatchability of embryos; morphometric measures of size, shape, and developmental state of young larvae; and posthatching life span of larvae in a food-free environment. Hatchability (survival to hatching) was the proportion alive at hatch of the 30 embryos originally allocated to each incubation container. The embryo period duration was quantified as the time to hatching in days from fertilization to the day and time of the twice-daily census of the incubation container at which the hatch event was recorded.
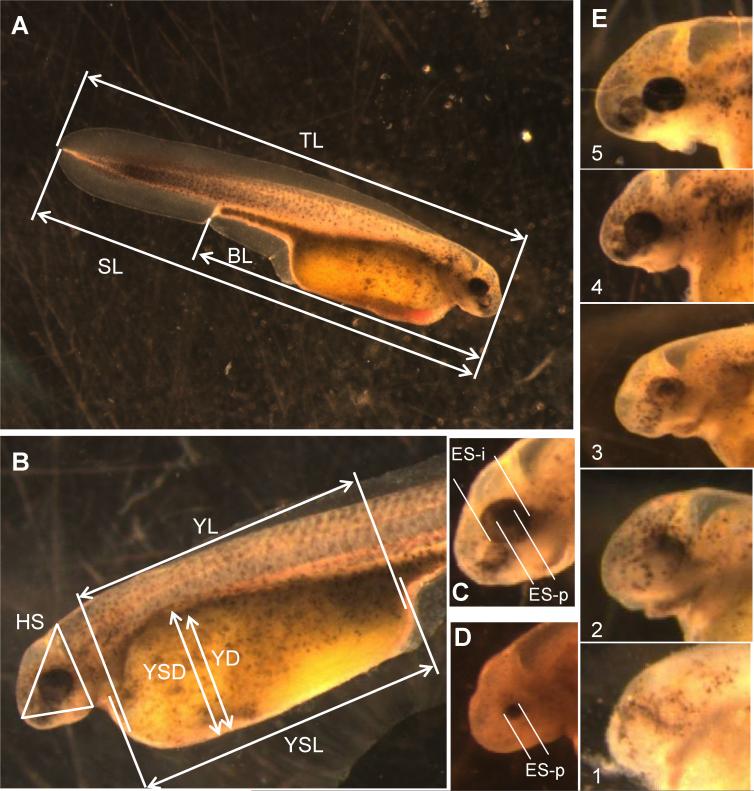
Up to 11 morphometric measures were extracted from saved larval images (Table 1). These measures captured variations among young larvae in their size, shape, and developmental stage. In all, 10 of 11 measures were continuous quantitative variables and included total length, standard length, body length, yolk-sac length and depth, yolk length and depth, head size, and eye size (Fig. 1). Eye size was quantified in shortnose sturgeon by two measures, the diameter of the pupil and the diameter of the outer edge of the iris. For both estimates, the average of two orthogonal diameters was the datum used. The edge of the iris was not usually visible in 1-d-old Atlantic sturgeon larvae, so only the pupil diameter was measured. The morphometric distances extracted from images were scored and converted to millimeters (nearest 0.01 mm) using UTHSCSA Image Tool software. An additional measure was taken for both species that reflected the developmental state of the eye. This qualitative variable ranged from 1 (least developed) to 5 (most developed) based on the degree of eye pigmentation (Fig. 1E).
Table 1.
Morphometric variables collected from images of recently hatched (26–36 h) shortnose and Atlantic sturgeon in toxicity experimentsa
| Variable | Abbreviation | Definition |
|---|---|---|
| Total length | TL | Distance from the anteriormost tip of snout to posterior tip of fin fold |
| Standard length | SL | Distance from the anteriormost tip of snout to posterior tip of notochord |
| Head size | HS | Distance of the perimeter of a triangle with vertices at (1) the anteriormost point of snout, (2) the crease between the head and yolk sac, and (3) a point on the dorsum directly above the head–yolk sac crease (line 3 is perpendicular to the body axis) |
| Body length | BL | Distance from the anteriormost tip of snout to notch at vent opening |
| Yolk sac length | YSL | Distance from the anteriormost point of the yolk sac to the posteriormost point of yolk sac parallel to the body axis |
| Yolk length | YL | Distance from the anteriormost point of the yolk to the posteriormost point of yolk parallel to the body axis |
| Yolk sac depth | YSD | Distance from the dorsalmost point of the yolk sac to the ventralmost point of yolk sac at maximum depth and perpendicular to the body axis |
| Yolk depth | YD | Distance from the dorsalmost point of the yolk to the ventralmost point of yolk at maximum depth and perpendicular to the body axis |
| Eye size (pupil) | ES-p | Average of distance of two perpendicular lines that span across the pupil of eye |
| Eye size (iris)b | ES-i | Average of distance of two perpendicular lines that span across the iris of the eye |
| Eye development | ED | Qualitative index of the degree of development and pigmentation of the pupil of the eye with values ranging from least (1) to most (5) well-developed |
Depictions of each variable shown in Figure 1.
Applicable only to shortnose sturgeon.
Fig. 1.
Morphological measurements taken on shortnosesturgeon(Acipenser brevirostrum) and Atlantic sturgeon (Acipenser oxyrinchus) larvae at 24 to 36 h after hatchingtoassesssublethaleffectsof contaminants.(A,B)Setof linearmeasurements takenonlivelarvae. (C,D)Measuresof eye size(iris andpupildiameters).All images were taken at ×6 magnification. (E) Gradation in eye development was given a qualitative scale from least (1) to most (5) pigmentation. (A–C,E) Shortnose sturgeon, (D) Atlantic sturgeon. TL = total length; SL = standard (notochord) length; BL = body length; HS = head area; YSL= yolk-sac length; YL = yolk length; YSD = yolk-sac depth; YD = yolk depth; ES-i = diameter of eye iris; ES-p = diameter of eye pupil. Definitions of measures given in Table 1. [Color figure can be seen in the online version of this article, available at wileyonlinelibrary.com]
Two persons were involved in measuring the quantitative morphometric variables. One person was responsible for scoring the developmental state of the eye. In all cases and prior to processing the images for the experimental data, each person proceeded through two blind passes of sample images (N 30) for each morphometric response variable to confirm the repeat-ability of the measurements taken. In all cases, the repeatability of the measures was highly significant (r2 > 0.7 and usually r2 > 0.9).
The posthatching life span in a food-free environment was quantified as the duration between hatch time (day and time from twice daily census) and the day on which an individual was scored as dead.
Statistical analyses
All hypothesis testing addressed the null hypothesis of no effect of treatment on response variables. By design, the treatment for each species and each toxicant included each of the six doses, acetone alone, and water only. The statistical analyses applied to these data were predicated on the analysis of variance (ANOVA) assumption of independence of observations. To meet this assumption, either only one datum was analyzed per group from a shared container (e.g., the treatment × replicate mean, median, or summary statistic such as survival), and the interdependence of observations from a group that shared a container was explicitly accounted for by applying multivariate statistical methods (e.g., multivariate analysis of variance [MANOVA]).
Survival to hatching was assessed as the proportion of the original N = 30 that successfully hatched. Those data were transformed (arcsine square root) prior to analyses. The duration of the embryonic period was based on day-of-hatch scores. Those data were used to find the median day of hatch for the treatment group in the experimental container and the latter was used for analyses.
All morphometric analyses were conducted on mean values per treatment combination (i.e., replicate means within species, contaminant, and dose). Data were subjected to a three-step process for statistical analysis. The overall test of effects was a MANOVA applied to the morphometric data. However, prior to the MANOVA and because of the lack of independence of morphometric variables describing size and shape and the expectation of high correlations among the various morpho-metric variables, the morphometric data were reduced by principal component analysis (PCA). The resulting PCA scores were the data employed in a series of MANOVAs with the main effect of dose (i.e., null hypothesis of no effect of dose on outcome). These MANOVAs were run for each of the two species with each contaminant. Follow-up univariate tests for effects, for graphic display, and for interpretation in the context of the original variables were conducted by using ANOVAs.
Posthatching life span was based on day-at-death data and was treated similarly to the age-at-hatch data (both are event data). The median day of death was determined for each experimental population, and the medians were used in ANOVAs.
RESULTS
Evaluation of culture protocols
Survival to hatching of populations assigned to the various container types and incubation protocols varied widely, ranging from 0 to 80% (treatment averages 4–69%). Average survival to hatching was comparable and high for eggs maintained under baseline conditions and housed in glass or plastic containers, ranging from 46 to 63%, and exceeded survival of eggs from the modified MacDonald's jar (17%) as long as the water was changed twice per day. Consistently high average survival was achieved whether or not the beaker or dish was changed during the water change (59 and 57%, respectively), the water was aerated (69%), or larger volume PVC cylinder–nylon mesh systems were employed (50 and 58%; Table 2). The poorest average survival occurred in containers in which water was changed only once per day (37%) and in which eggs were affixed to glass, that is, with no Fuller's earth (4 and 14%). Average embryo survival was low when eggs were housed at water temperatures of 10° (9 and 10%) or 20° (33 and 43%) compared with the same containers at the 15° baseline temperature (62 and 63%).
Table 2.
Survival to hatching of shortnose sturgeon embryos under various container types and egg maintenance protocols
| Container |
Container parameters |
Survival to hatchinga |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Type | Dimensions (dia. × h, or L × W × H) |
Water vol. (ml) |
Bottom area (cm) |
Water depth (cm) |
Base conditionsb |
Low temp (15°C) |
High temp (20°C) |
Daily water change |
Air | Replace container |
No Fuller's earth |
| 1 | Modifiedc MacDonald's jars | 13.8 × 40 | 6,000 | 150 | 40.0 | 0.17 (0.000) | ||||||
| 2 | Nylon mesh-bottom cylinder (3.8-cm-diameter PVC) in pan | 5.3 × 6.5 | 4,000d | 22 | 2.0 | 0.50 (0.088) | ||||||
| 3 | Nylon mesh-bottom cylinder (7.6-cm-diameter PVC) in pan | 7.6 × 5.2 | 4,000d | 45 | 2.0 | 0.58 (0.102) | ||||||
| 4 | Glass beaker (250 ml) | 6.3 × 9.5 | 150 | 31 | 4.75 | 0.46 (0.117) | ||||||
| 5 | Glass beaker (100 ml) | 4.5 × 7 | 80 | 16 | 5.0 | 0.63 (0.088) | 0.09 (0.069) | 0.43 (0.173) | 0.37 (0.133) | 0.69 (0.096) | 0.59 (0.107) | 0.14 (0.164) |
| 6 | Glass dish | 15 × 11 × 4 | 250 | 171 | 1.5 | 0.62 (0.077) | 0.10 (0.067) | 0.33 (0.033) | 0.57 (0.058) | 0.04 (0.038) | ||
Entries are average (± SD) survival of three replicate groups per protocol except for the MacDonald's jar (n = 1).
Base conditions: 30 fertilized and de-adhesion eggs in 0.1 ppt salt water, twice daily water change, no aeration, 15°C, 14 h light per day.
MacDonald's jar base conditions: water recirculated through ultraviolet lamp and biofilter with 50% water replacement daily.
Volume is for water pan (38 × 30 × 9 cm) into which mesh baskets were placed.
Uptake of radiolabeled3 H-PCB126
The PCB126 was bioaccumulated by embryos of both sturgeon species (Table 3). The uptake estimates provide approximate tissue burdens to expect in our toxicity experiments. Our limit of detection in these experiments was approximately 10 pg PCB126. The concentration of PCB126 in shortnose sturgeon embryos approximated that of the nominal water concentrations and was less than in Atlantic sturgeon. Levels of PCB126 in Atlantic sturgeon embryos exposed to the two highest doses (1.0 and 10 ppb) of PCB126 for the longest durations (8 and 24 h) exceeded that of the nominal water concentration by a factor of approximately 3. There was little difference in body burdens at any of the three doses between the 8- and 24-h exposure durations, suggesting that equilibrium was reached by 8 h.
Table 3.
Average (SD) uptake of three nominal doses (0.1, 1.0, and 10 ppb) of radiolabeled 3H-PCB126 in shortnose sturgeon and Atlantic sturgeon embryos after waterborne exposure for 2, 8, or 24 h
| Nominal water dose of PCB126 (ppb) | Duration of exposure (h) | Shortnose sturgeon embryo burden (SD) (ng/g) | Atlantic sturgeon embryo burden (SD) (ng/g) |
|---|---|---|---|
| 0.1 | 2 | 0.114 (0.070) | 0.000 (0.000) |
| 8 | 0.722 (0.451) | 1.041 (0.200) | |
| 24 | 0.246 (0.098) | 0.634 (0.355) | |
| 1 | 2 | 0.195 (0.013) | 0.800 (0.251) |
| 8 | 1.761 (0.157) | 5.409 (2.423) | |
| 24 | 1.236 (0.419) | 3.927 (0.482) | |
| 10 | 2 | 0.155 (0.395) | 9.847 (3.954) |
| 8 | 5.818 (0.372) | 28.890 (7.677) | |
| 24 | 11.891 (1.013) | 27.020 (4.288) |
PCB126 = polychlorinated biphenyl 126.
Toxicity experiments
Survival to hatching.
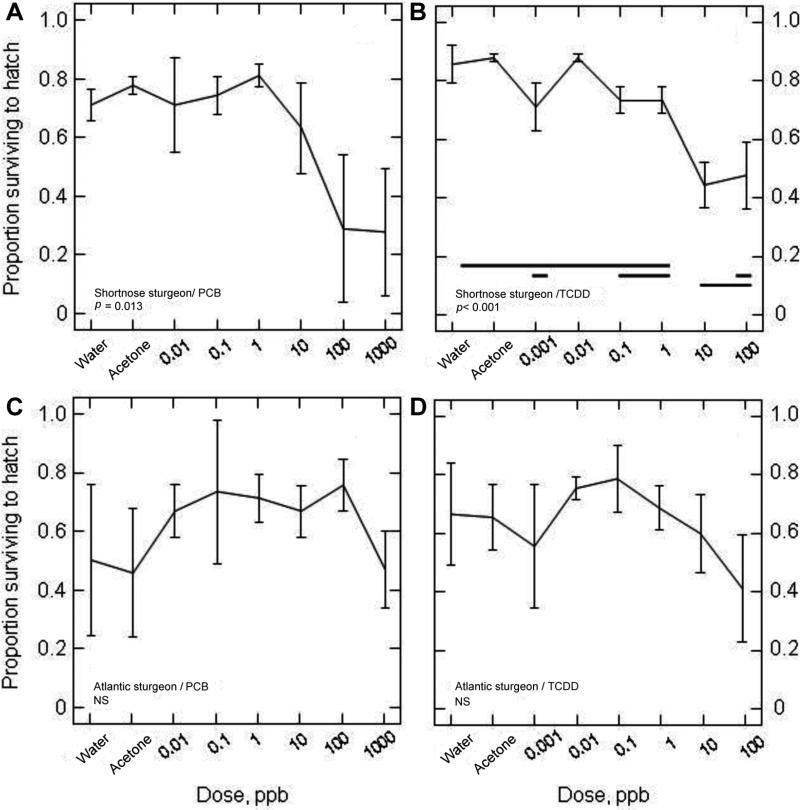
Survival to hatching varied widely between species, between contaminants, and across the range of doses and controls. Exposures to PCB126 and TCDD significantly decreased survival to hatching in shortnose sturgeon (p = 0.013 and p < 0.001, respectively) but not in Atlantic sturgeon (Fig. 2). With respect to PCB126, survival to hatching of shortnose sturgeon embryos averaged between ~0.7 and 0.8 at PCB126 doses ≤1 ppb and for controls (water and acetone did not differ when all control groups were contrasted, p = 0.92), but decreased precipitously to 0.63 at 10 ppb and to <0.3 at concentrations >10 ppb (Fig. 2A). The PCB126 exposure series (including controls) explained >62% of the variation among the experimental groups (i.e., r2 0.628). Having a highly significant effect on egg mortality, TCDD also explained ~84% of the variation among experimental groups in that series. The response to TCDD in shortnose sturgeon largely paralleled that of PCB126, but with a decline in mortality at approximately tenfold lower doses (Fig. 2B). The survival of embryos when exposed to TCDD was consistently high up to and including 1 ppb (average survival of ~0.71–0.88 among doses) and decreased to ~0.45 to 0.50 at 10 and 100 ppb. Although not statistically significant, a similar negative trend in survivorship was observed in Atlantic sturgeon for doses of TCDD >1 ppb (Fig. 2D).
Fig. 2.
Survival to hatching of shortnose sturgeon (A,B) and Atlantic sturgeon (C,D) in response to graded nominal water doses of polychlorinated biphenyl 126 (PCB126) (A,C) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (B,D) administered during the midembryonic period. See Table 3 for relationship between nominal water doses and approximate body burdens of PCB126. Each panel displays the proportion hatching (mean ±SD) of three replicate treatments of each species. Homogeneous subsets, determined by Tukey posterior tests, are denoted by horizontal lines. NS not significant (p > 0.05) as determined by a one-way univariate analysis of variance (ANOVA) with treatment of “dose.” Water and acetone (solvent) were¼used as controls.
Embryonic period duration
The median duration of the embryonic period was greater and more variable in shortnose sturgeon than in Atlantic sturgeon. Shortnose sturgeon hatched in 11.5 to 15.5 d (controls averaged 13.4 d) versus 7 to 8 d (controls 7.8 d) for Atlantic sturgeon. Neither species exhibited a significant effect of PCB126 or TCDD exposure, but there was a tendency for exposed larvae of both species to hatch earlier than controls, especially as doses increased, with a suggestion of a delay in hatch date age at some of the highest doses (data not shown).
Morphological variation
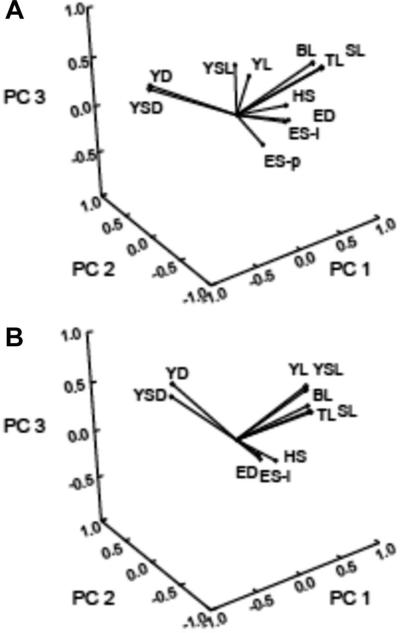
The two sets of morphological variables (11 and 10 variables for shortnose and Atlantic sturgeon, respectively) showed high degrees of both positive and negative pairwise correlations, which lends further support to our treating the variables as an interdependent set of responses. The covariation among variables is reflected in the magnitude of the principal component (PC) weights applied to the original morphological variables to reduce the dimensionality of the response sets. Over 75% of the variation in the original morphological variables measured on larvae of each species was accounted for by the first three PCs, with 49.7, 17.6, and 9.9% of the variation accounted for by PCs 1 through 3 for shortnose sturgeon and 46.9, 18.3, and 11.7% of the variation accounted for by PCs 1 through 3 for Atlantic sturgeon (Table 4). The predominance of a set of correlated variables associated with body length is evident in the PC weightings and in the redistribution of the original variables in the new PC space. Lesser but still significant contributions to PC 2 and PC 3 were related to yolk sac size and yolk quantity (especially the depths) and for eye size and development (Fig. 3).
Table 4.
Weightings applied to morphometric variables of recently hatched shortnose sturgeon and Atlantic sturgeon exposed as embryos to graded doses of PCB126 and TCDD using principal component analysisa
| Shortnose sturgeon |
Atlantic sturgeon |
|||||
|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 3 | PC 1 | PC 2 | PC 3 | |
| Variable | (49.7) | (17.6) | (9.9) | (46.9) | (18.3) | (11.7) |
| Total length | 0.159 | –0.110 | 0.332 | 0.199 | 0.014 | 0.065 |
| Standard length | 0.157 | –0.118 | 0.334 | 0.201 | 0.003 | 0.060 |
| Head size | 0.128 | 0.053 | –0.093 | 0.127 | 0.070 | –0.363 |
| Body length | 0.156 | –0.049 | 0.349 | 0.191 | 0.002 | 0.130 |
| Yolk sac length | 0.090 | 0.363 | 0.194 | 0.166 | –0.051 | 0.320 |
| Yolk length | 0.110 | 0.313 | 0.083 | 0.146 | –0.107 | 0.391 |
| Yolk sac depth | –0.108 | 0.318 | 0.189 | –0.037 | 0.486 | 0.189 |
| Yolk depth | –0.102 | 0.342 | 0.201 | –0.039 | 0.461 | 0.316 |
| Eye size (pupil) | 0.100 | 0.147 | –0.524 | 0.126 | 0.224 | –0.393 |
| Eye size (iris) | 0.138 | 0.092 | –0.299 | |||
| Eye development | 0.143 | 0.081 | –0.280 | 0.114 | 0.170 | –0.393 |
The principal component analysis scores computed with these weights were used subsequently in analysis of variance. Weights (eigenvectors) shown are for significant eigenvalues only (percentage of variance explained in parentheses). Variable definitions are listed in Table 1.
PCB126 = polychlorinated biphenyl 126; TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin; PC = principal component.
Fig. 3.
The set of 11 (shortnose sturgeon) and 10 (Atlantic sturgeon) morphometric variables replotted in factor space to show loadings (contributions) of each variable to the first three principal components (PCs). The reduced dimensioned PC scores were subjected to multivariate analysis of variance. (A) Shortnose sturgeon. (B) Atlantic sturgeon. For abbreviations see Table 1.
A significant effect of the concentration of each contaminant on morphological variation was evident for both species. The pattern of the changes in size and shape of larvae was similar in both species and usually included a shortening of the body, a reduction in head size, and an increased inflation around the yolk sac with increasing concentrations of contaminants. In every case (both species, both contaminants), the overall MANOVA was significant. The primary contributor to this result was typically (three or four cases) from the length variables, that is, the primary contributing variables to PC1. For example, the overall significance of MANOVA for the effects of PCB126 and TCDD on morphometric features in shortnose sturgeon was p 0.003 (df = 3,20) and p < 0.001 (df = 3,18), respectively (Table 5).
Table 5.
Effects of PCB126 (A) and TCDD (B) on morphological features of recently hatched shortnose sturgeon larvaea
| Source | Sums of squares | df | Mean square | Test statistic | p |
|---|---|---|---|---|---|
| A) MANOVA | 3,20 | 0.971 | 0.003 | ||
| PCA1 | 2.392 | 7 | 0.832 | 1.767 | 0.163 |
| Error | 7.535 | 16 | 0.471 | ||
| PCA2 | 3.319 | 7 | 0.474 | 1.803 | 0.156 |
| Error | 4.206 | 16 | 0.263 | ||
| PCA3 | 3.782 | 7 | 0.540 | 2.303 | 0.079 |
| Error | 3.754 | 16 | 0.235 | ||
| B) MANOVA | 3,18 | 0.681 | 0.001 | ||
| PCA1 | 15.708 | 7 | 2.244 | 14.208 | <0.001 |
| Error | 2.211 | 14 | 0.158 | ||
| PCA2 | 0.932 | 7 | 0.133 | 0.163 | 0.989 |
| Error | 11.456 | 14 | 0.818 | ||
| PCA3 | 2.829 | 7 | 0.404 | 0.775 | 0.618 |
| Error | 7.304 | 14 | 0.522 |
Responses are summarized by their principal component scores and those scores subjected to multivariate analysis of variance (MANOVA). The test statistic was a Hotelling–Lawley trace (MANOVA) or F (univariate ANOVA). Treatment was six concentrations of each polychlorinated biphenyl 126 (PCB126) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and two controls (water only and water plus acetone), with two or three replicates of each.
PCA = principal component analysis.
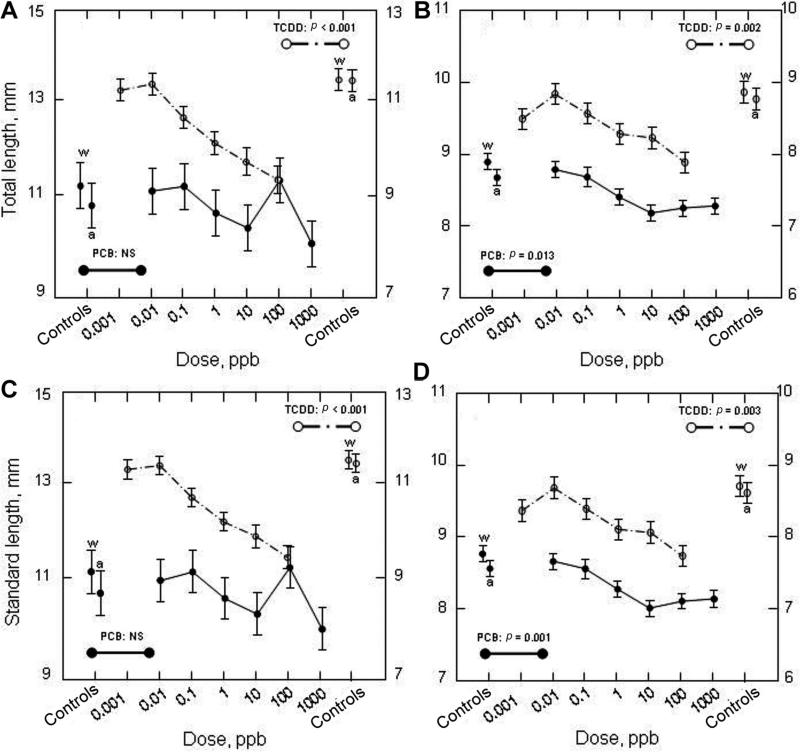
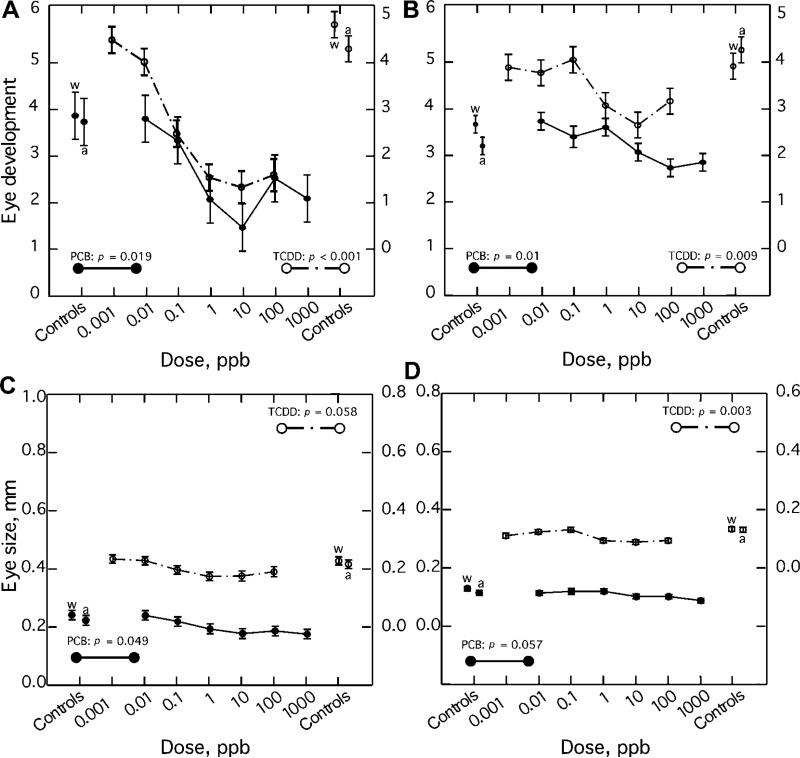
With regard to the pattern of morphological responses of shortnose sturgeon, there was an overall tendency toward smaller sizes (shorter lengths) at higher concentrations of TCDD (Fig. 4A and C). This was especially evident in the reduction in the length of the yolk sac and yolk within it and likely the energy reserves available to the hatchlings at high concentrations (data not shown). The developmental state and size (pupil and iris diameter) and developmental state of the eye of the hatchlings were also significantly reduced relative to controls and were evident at doses as low as 1 and 0.1 ppb for PCB126 and TCDD, respectively (Fig. 5).
Fig. 4.
Total length (A,B) and standard length (C,D) of recently hatched (24–36 h old) shortnose sturgeon (A,C) and Atlantic sturgeon (B,D) larvae that were exposed to graded nominal water doses of polychlorinated biphenyl 126 (PCB126) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). See Table 3 for relationship between nominal water doses and approximate body burdens of PCB126. Mean ±SE. NS = not significant as determined by a one-way univariate analysis of variance (ANOVA) with dose as the treatment. Water (w) and acetone (a; solvent) were used as controls.
Fig. 5.
Eye development and size in recently hatched (24–36 h old) shortnose sturgeon (A,C) and Atlantic sturgeon (B,D) larvae that had been exposed as embryos to graded nominal water doses of polychlorinated biphenyl 126 (PCB126) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). See Table 3 for relationship between nominal water doses and approximatebody burdens of PCB126. Eye development index score of 1 represents a less well-developed eye and a score of 5 represents a more well-developed eye. Eye size was quantified as pupil diameter. Mean ±SE. p value as determined by a one-way univariate analysis of variance (ANOVA) with dose as the treatment. Water (w) and acetone (a; solvent) were used as controls.
Atlantic sturgeon also exhibited a pattern of dose responses to both contaminants and appeared to be more sensitive than shortnose sturgeon. The effects of PCB126 and TCDD on morphometric features as evaluated by the MANOVA test were both significant, p 0.010 (df = 3, 19) and p 0.001 (df = 3, 20), respectively (Table 6). More of the individual morphological features of Atlantic sturgeon displayed significant responses than in shortnose sturgeon to contaminant concentration. The size at hatching (total and standard lengths, Fig. 4 B, D) as well as body length and yolk lengths and the developmental state and size of the eye (Figs. 5) were dose-responsive to PCB126. Indeed, the only features that did not show a significant or nearly significant dose response were the depths of the yolk and yolk sac. Furthermore, the magnitude of the explained variation in responses due to PCB126 and TCDD was comparably high (r2 ≥0.6) across most morphological features that exhibited a dose response. As with shortnose sturgeon, the magnitude of response was greater to TCDD than to PCB126 and was typically elicited by at least one order of magnitude lower concentrations of TCDD than the dose of PCB126 with a comparable effect (i.e., at 0.01 to 0.1 ppb TCDD vs 0.1 to 1 ppb PCB126).
Table 6.
Effects of PCB126 (A) and TCDD (B) on morphological features of recently hatched Atlantic sturgeon larvaea
| Source | Sums of squares | df | Mean square | Test statistic | p |
|---|---|---|---|---|---|
| A) MANOVA | 3,19 | 4.346 | 0.010 | ||
| PCA1 | 7.959 | 7 | 1.137 | 7.603 | 0.001 |
| Error | 2.243 | 15 | 0.150 | ||
| PCA2 | 1.304 | 7 | 0.186 | 0.476 | 0.837 |
| Error | 5.864 | 15 | 0.391 | ||
| PCA3 | 0.578 | 7 | 0.083 | 0.321 | 0.933 |
| Error | 3.866 | 15 | 0.258 | ||
| B) MANOVA | 3,20 | 0.681 | 0.001 | ||
| PCA1 | 10.976 | 7 | 1.568 | 5.729 | 0.002 |
| Error | 4.379 | 16 | 0.274 | ||
| PCA2 | 2.389 | 7 | 0.341 | 1.148 | 0.383 |
| Error | 4.755 | 16 | 0.297 | ||
| PCA3 | 5.439 | 7 | 0.777 | 1.942 | 0.129 |
| Error | 6.403 | 16 | 0.400 |
Responses are summarized by their principal component scores and those scores subjected to multivariate analysis of variance (MANOVA). The test statistic was a Hotelling-Lawley trace (MANOVA) or F (univariate ANOVA). Treatment was six concentrations of each polychlorinated biphenyl 126 (PCB126) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and two controls (water only and water plus acetone), with two or three replicates of each.
PCA = principal component analysis.
Eye development index proved to be a sensitive and dose-responsive indicator of exposure in both species to PCB126 and TCDD. Scores of the index ranged from 1 to 5, with 1 representing a less well-developed eye and 5 a more well-developed eye. The effects of PCB126 and TCDD were both dose responsive and highly significant in shortnose sturgeon, p = 0.019 and p < 0.001, respectively (Fig. 5A). Similarly, the effects of PCB126 and TCDD on the eye development index were both significant in Atlantic sturgeon, p = 0.011 and p = 0.009, respectively (Fig. 5B).
Larval life span
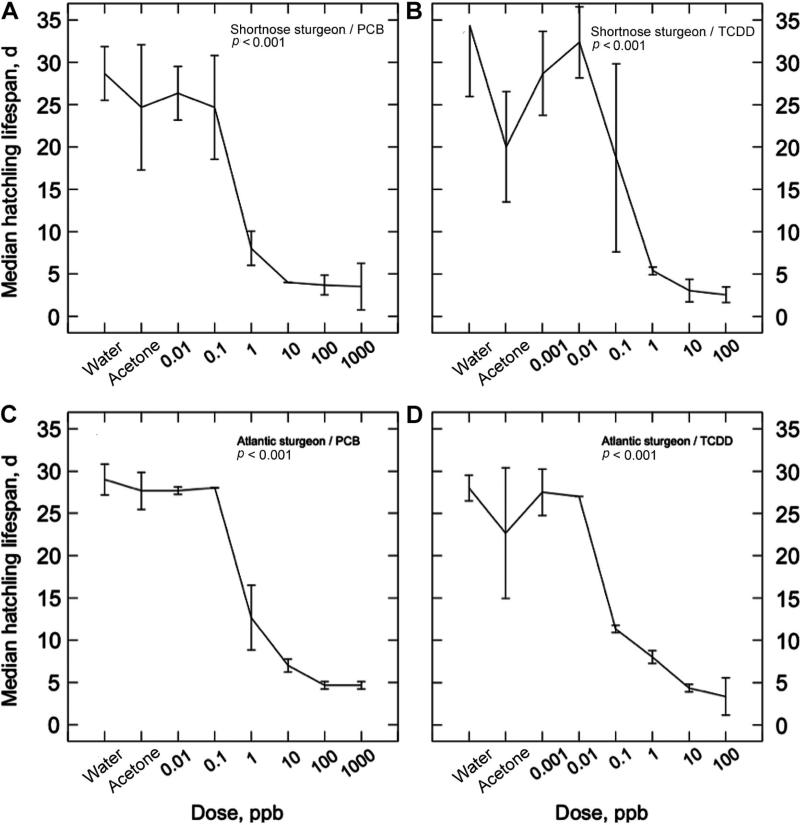
Larvae of both species are large and robust at hatching compared with most riverine and estuarine finfish. Shortnose sturgeon are larger at hatching than Atlantic sturgeon (11.2 vs 8.7 mm SL, respectively), but the species were comparably resilient to starvation in our analyses, and individuals from control or lower concentrations of contaminants could persist for over 1 month at our 15° test temperature (life span of unfed larvae from control treatments averaged 27 d for both shortnose and Atlantic sturgeon). The dose of PCB126 had a highly significant effect on larval life span of shortnose sturgeon (r2 = 0.86, p < 0.001). Larval life span was little affected by PCB126 at doses of ≤0.1 ppb but decreased dramatically at concentrations of >0.1 ppb. At concentrations of 10 ppb and above, shortnose larval life span averaged under 5 d (Fig. 6A). The response of shortnose sturgeon larval life span to TCDD was also highly significant (r2 = 0.78, p < 0.001) and similar in pattern to that elicited by PCB126, except that the effect was evident at approximately an order of magnitude lower concentrations (i.e., between 0.01 and 0.1 ppb TCDD). At concentrations of 1 ppb and above, shortnose sturgeon larval life span averaged ≤5 d and larval life spans of replicate populations at 0.1 ppb were highly variable (Fig 6B).
Fig. 6.
Life span of unfed hatched larvae of shortnose sturgeon (A,B) and Atlantic sturgeon (C,D) in response to graded nominal water doses of polychlorinated biphenyl 126 (PCB126) (A,C) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (B,D) administered during the midembryonic period. See Table 3 for relationship between nominal water doses and approximate body burdens of PCB126. Each panel displays the median age at death (mean ±SD) of unfed larvae of two or three replicate treatments of each species. Statistics are from one-way univariate analysis of variance (ANOVAs) with dose as the treatment. Water and acetone (solvent) were used as controls.
The larval life span of Atlantic sturgeon in response to the two toxicants mirrored that exhibited by shortnose sturgeon. The PCB126 had a highly significant effect on larval life span of Atlantic sturgeon (r2 = 0.97, p < 0.001). Larval life span was little affected by PCB126 at doses ≤0.1 ppb, decreased dramatically at concentrations of PCB126 >0.1 ppb, and at concentrations of 10 ppb and above averaged 7 d (Fig. 6C). The response of Atlantic sturgeon larval life span to TCDD was also highly significant (r2 = 0.91, p < 0.001) and similar in pattern to that elicited by PCB126, except that the effect was evident at approximately an order of magnitude lower concentration (i.e., by 0.1 ppb TCDD; Fig 6D). At concentrations of 1 ppb and above Atlantic sturgeon larval life span averaged ≤10 d.
DISCUSSION
Adult and early-life-stage shortnose sturgeon and Atlantic sturgeon are likely exposed to high levels of PCBs and PCDD/Fs in contaminated natal estuaries such as the Hudson and Delaware Rivers. Limited reports indicate that both species bioaccumulate these pollutants at these locales [29] (http://response.restoration.noaa.gov/querrymanager); however, their exposure on a TCDD TEQ basis has yet to be determined within these or other impacted estuaries. The sensitivities of these and other sturgeon to these contaminants have not been previously reported, so our objective was to determine whether these contaminants could induce early-life-stage toxic effects in sturgeon at environmentally relevant concentrations. We have demonstrated that both sturgeon are sensitive at a variety of endpoints to environmentally relevant levels of PCB126 and TCDD-induced early-life-stage toxicities.
We have previously shown that CYP1A mRNA in both sturgeon is inducible and dose responsive to PCB126 and TCDD at environmentally relevant embryo burdens (minimal nominal water concentrations of 0.010 ppb for PCB126 and 0.001 ppb for TCDD) [30]. These results are indicative of activation of the AHR pathway and suggest that some early-life-stage toxicities mediated by AHR are possibly cooccurring. We focused on toxicities to these early life stages because many fish are known to be particularly vulnerable as embryos and larvae to toxicities of coplanar PCBs and TCDD [5,7,10], with recruitment failure in wild populations as a possible consequence [6]. Our study is the first to demonstrate that early life stages of both sturgeon are sensitive in a variety of AHR-mediated biological responses to environmentally relevant embryo burdens of PCB126 and TCDD. Many of these responses, including reduced hatching success, morphometric alterations, compromised eye development, and shortened life span of unfed larvae, would lessen the likelihood of successful recruitment of larval sturgeon to the adult population and thus contribute to their failure to rebuild and the eventual demise of impacted populations.
Evaluation and optimization of the culture and maintenance conditions of early life stages needed to conduct these experiments have not been reported for sturgeon. This is a necessary step in identifying protocols that are efficient, reproducible, and cost-effective for rearing early life stages for toxicological studies. Traditionally, MacDonald-type jars have been used to maintain sturgeon eggs in hatchery programs that seek to maximize outputs for commercial production or to supplement natural populations. For purposes of toxicological experiments, however, we found that sturgeon eggs to which de-adhesion methods have been applied can be maintained well in small volumes of water (≤250 ml) in glass or plastic containers without aeration and with twice daily water changes. This protocol provides a simple, cost-effective, and efficient means to successfully maintain large numbers (over 100) of small groups (N ~30) of embryos and prefeeding larvae as needed for multifactorial and replicated experimental designs appropriate for toxicological analyses.
Our experiments in which embryos of both species were exposed to 3H-PCB126 for various durations allowed us to estimate actual uptake of PCB126 and to calibrate toxic responses that we observed to estimated embryo burdens of PCB126. We found significant reductions in viability of larvae (decreased hatching success) and various sublethal effects (shortened body length, reduction in yolk, and delayed eye development) at embryo burdens of approximately 1 ng PCB126/g in shortnose sturgeon and 4 ng PCB126/g in Atlantic sturgeon. Applying the toxic equivalency factor (TEF) of 0.05 derived for PCB126 in fish to our data, we find that a minimum TCDD TEQ of 50 pg/g induces significant toxicities in short-nose sturgeon and 200 pg/g TCDD TEQ in Atlantic sturgeon. It should be noted that we might have underestimated the threshold concentrations of PCB126 and TCDD that actually induce toxicity in natural populations, because embryos in the wild would be exposed at fertilization by maternal transfer and immediately thereafter when they settle to contaminated sediments. Walker et al. [34] determined that the median lethal concentration (LC50) in lake trout embryos exposed to TCDD for 24 h was 65 pg/g. Elonen et al. [10] estimated LC50 values for TCDD among seven species of freshwater fish and found that they varied between 540 and 2,600 pg/g TCDD. Although we do not report LC50s here (survival to hatching exceeded 50% for all but shortnose sturgeon exposed to PCB126), our results from a variety of sublethal responses indicate that both sturgeon species are at the more sensitive end of the sensitivity spectrum of fish to TCDD-like toxicants. Because we did not quantify the uptake of TCDD in our study, we can calibrate toxic responses in sturgeon to that chemical only to the nominal water concentrations used. That calculation shows that the level of 1 ng/g TCDD that initially elicited significant toxicity in our study was also in the range of the more sensitive fish previously investigated. Thus, both Atlantic and shortnose sturgeon are among the more sensitive fish to coplanar PCB- and TCDD-induced toxicities reported to date.
The only previous controlled laboratory study of toxicity in sturgeon investigated the sensitivity of early life stages of shovelnose sturgeon, Scaphirhynchus platorynchus, and U.S. federally endangered pallid sturgeon, Scaphirhynchus albus, to PCB126 [35] and the responses of offspring of environmentally exposed shovelnose sturgeon females from the middle Mississippi River [36]. The investigators found that embryo burdens of 213 ng PCB126/g caused craniofacial malformations and shortened snouts in shovelnose sturgeon (pathologies not quantified in pallid sturgeon). They also found that percentage of hatching success and survival of larvae at 2 to 5 d post-fertilization were inversely correlated to persistent organic pollutants (PCBs, polybrominated diphenyl ether, and organo-chlorine pesticide) content in offspring of environmentally exposed shovelnose sturgeon. They reported PCB126 LD50s of 186 ng/g egg and 224 ng/g egg for shovelnose and pallid sturgeon, respectively. The LD50s for the two sturgeon species expressed as TCDD TEQs were approximately twice the LD50 of Japanese medaka, Orzyias latipes [10], and an order of magnitude higher than of lake trout [34].
To our knowledge, no study has quantified burdens of PCDD/Fs or PCBs on a congener-specific basis in tissues of environmentally exposed individuals of Atlantic or shortnose sturgeon, precluding a comparison with concentrations eliciting toxic responses in our study. We can compare toxicity thresholds in our experiments with tissue burdens of TCDD TEQs in Atlantic tomcod, Microgadus tomcod, another bottom-dwelling species that is largely sympatric with both sturgeon in the Hudson River [24]. We did this by comparing nominal water doses in our toxicity experiments with both sturgeons to embryo burdens of PCB126 estimated from our radiolabeled PCB126 uptake experiment (using a TEF for PCB126 of 0.05). For tomcod, we reported mean hepatic TCDD TEQs (N = 10 individuals) of 736 pg/g and 1,044 pg/g, largely dominated by TCDD, in 6-month-old juvenile tomcod from Newark Bay and the Hackensack River, respectively, in the western Hudson River Estuary [26]. Furthermore, mean hepatic TCDD TEQs (N = 10 individuals) ranged between 30 pg/g and 79 pg/g with a mean of 56.2 pg/g at five sites in the main-stem lower Hudson River at 0, 8, 10, 17, and 39 miles upriver from the southern tip of Manhattan. For one of the most sensitive endpoints reported here, life span of unfed larvae, we found an initial significant decrease in both sturgeon species at 5 to 10 pg/g TCDD TEQs. For the eye development index, signifi-cant impairment began in our experiments at 50 to 100 pg/g TCDD TEQs. Thus, hepatic burdens of dioxin-like chemicals in environmentally exposed tomcod from throughout the lower Hudson River Estuary approached and usually exceeded those that elicited sensitive toxicities in sturgeon in our experiments. However, we should caution that we have compared hepatic levels of these contaminants in environmentally exposed tomcod, which may be substantially greater than egg burdens in environmentally exposed sturgeon from the lower Hudson River.
We found that the degree of eye pigmentation of recently hatched larvae was one of the most sensitive and possibly ecologically important indicators of effect of exposure to these compounds. Furthermore, the degree of eye pigmentation, unlike survival, life span, or behavioral measures, is based on a snapshot-sample method, which makes it an efficient variable for observing and scoring as well as a likely proxy for fitness. Although many studies have investigated the toxic effects of AHR agonists on the early life stages of fish, few have evaluated mechanisms or potential ecological impacts of these exposures on eye development. Studies in mammals have demonstrated that AHR agonists can induce neurological damage perhaps through reduced blood flow to the dorsal midbrain, apoptosis, or increased albumin permeability. Visual pigments, the opsins located within the outer segments of retinal cones of developing larvae, provide light sensitivity, visual acuity, and light contrast. Furthermore, studies in fish have revealed a strong relationship between retinal structure and behavioral capabilities such as foraging success and predator avoidance. Carvalho and Tillitt [37] injected TCDD (113 or 300 pg TCDD/g) into rainbow trout embryos and found a significant reduction at both doses in the densities of retinal ganglion cells and corresponding deficits in detail discrimination, motion detection, and low-light sensitivity in hatchlings. Larvae at the higher dose of TCDD exhibited a decrease in their rate of capture of daphnia, Ceriodaphnia dubia, prey. Similarly, treatment of killifish, Fundulus heteroclitus, embryos with environmentally relevant concentrations of PCB126 caused a significant decrease in the ability of treated larvae to capture brine shrimp nauplii, Artemia franciscana, prey [38]. Histopathological alterations were also reported for the eyes of fathead minnows, Pimephales promelas, and white suckers, Catostomus commersonii, exposed as embryos to oil-sands-contaminated (PAHs) sediments [39]. In this case, retinas of treated fish were poorly differentiated, focally necrotic, and inflamed. Although we have demonstrated a significant decrease in eye pigmentation of young sturgeon larvae by both PCB126 and TCDD at environmentally relevant concentrations, we have yet to determine its mechanistic basis or its ecological significance in experiments evaluating the prey-capture ability or predator avoidance of treated larvae.
Several caveats are warranted regarding the applicability of our results to the likely effects of coplanar PCB- and TCDD-induced toxicities to sturgeon in nature. First, our treatment of embryos was through a water-borne route of exposure with the use of a solvent vehicle at 3 or 4 d postfertilization. It is far more likely that embryos in chemically impacted natural populations are exposed to these aromatic hydrocarbon contaminants through maternal transfer. Thus, unfertilized eggs in natural populations will likely be carrying high levels of these contaminants, and this will occur throughout their gerμline development and even prior to fertilization. The experimental subjects for the toxicity experiment reported here were from the Saint John River and were unlikely to have high maternal burdens of these contaminants. In impacted locales, however, the exposure of embryos in natural populations begins at an earlier stage of embryonic development than we used in our experimental protocol. This difference could result in different, and likely higher-biological-level toxic responses than we observed. Second, in our study, embryos were treated with single PCDD or PCB congeners, unlike the exposures to mixtures of PCDD/Fs, PCBs, and other exogenous compounds that are likely to occur in contaminated natural systems. Several studies have demonstrated that the binding of agonists to AHR is impacted by the presence of noncoplanar congeners and other contaminants. Thus, exposures to mixtures containing AHR agonists may elicit antagonistic or synergistic toxic responses compared with responses that we observed for single compounds in our experiments. Finally, empirical studies are needed to measure actual tissue and egg burdens of AHR agonists in impacted sturgeon populations.
In summary, we found that shortnose and Atlantic sturgeon early life stages can be cultured under conditions conducive to replicated toxicology experimental designs. Both species exhibited significant toxic responses in body size, shape, and development to environmentally relevant exposure concentrations of PCB126 and TCDD, with TCDD being approximately tenfold more toxic. Furthermore, we found that the eye development index was among the most sensitive attributes that we examined. We would expect fish attributes such as behavioral traits that in nature are at a fine balance between the organism and its environment to be most sensitive to these toxicants. Finally, the condition of a fish larva and the capability of larvae to persist in a food-free environment showed a dramatic dose response to both contaminants, with over a fivefold reduction in life span compared with baseline longevity. The dose responses that we observed indicate that both shortnose and Atlantic sturgeon exhibit toxic responses to PCB126 and TCDD and at concentrations within the range that could be bioaccumulated in contaminated estuaries such as the Hudson River.
Acknowledgement
Funding was provided by the Hudson River Natural Resource Trustees as part of the ongoing Hudson River Natural Resource Damage Assessment. We acknowledge the support of the Life History and RecruitmentGroupat the NOAANortheast FisheriesScience CenterJames J. Howard Marine Sciences Laboratory and Molecular Facilities Cores of NYU NIEHS Center grant ES00260 and NYU SRP grant ES10344. The conclusions and opinions presented in these presentations are those of the authors and do not represent the official position of NOAA, the Hudson River Trustees, or the U.S. Government.
REFERENCES
- 1.Bemis WE, Kynard B. Sturgeon rivers: An introduction of acipenseriform biogeography and life history. Environ Biol Fishes. 1997;48:167–183. [Google Scholar]
- 2.Birstein VJ, Bemis WE, Waldman JR. The threatened status of acipenseriform fishes: A summary. Environ Biol Fishes. 1997;48:427–435. [Google Scholar]
- 3.Boreman J. Sensitivity of North American sturgeons and paddlefish to fishing mortality. Environ Biol Fishes. 1997;48:399–405. [Google Scholar]
- 4.Feist GW, Webb MAH, Gundersen DT, Foster EP, Schreck CB, Maule AG, Fitzpatrick MS. Evidence of detrimental effects of environmental contaminants on growth and reproductive physiology of white sturgeon in impounded areas of the Columbia River. Environ Health Perspect. 2005;113:1675–1682. doi: 10.1289/ehp.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spitsbergen JM, Walker MK, Olson JR, Peterson RE. Pathological alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat Toxicol. 1991;19:41–71. [Google Scholar]
- 6.Tillitt DE, Cook PM, Giesy JP, Heideman W, Peterson RE. Reproductive impairment of Great Lakes lake trout by dioxin-like chemicals. In: Di Giulio RT, Hinton DE, editors. The Toxicology of Fishes. CRC; Boca Raton, FL, USA: 2008. pp. 819–875. [Google Scholar]
- 7.Hornug MW, Spitsbergen JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters cardiovascular and craniofacial development and function in sac fry of rainbow trout (Oncorhynchus mykiss). Toxicol Sci. 1999;47:40–451. doi: 10.1093/toxsci/47.1.40. [DOI] [PubMed] [Google Scholar]
- 8.Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- 9.Kopf PG, Walker MK. Overview of developmental heart defects by dioxins, PCBs, and pesticides. J Environ Sci Health C. 2009;27:276–285. doi: 10.1080/10590500903310195. [DOI] [PubMed] [Google Scholar]
- 10.Elonen GE, Spehar RL, Holcombe GW, Johnson RD, Fernandez JD, Erickson RJ, Tietge JE, Cook PM. Comparative toxicity of 2,3,7,8 tetrachlorodibenzo-p-dioxin to seven freshwater fish species during early life-stage development. Environ Toxicol Chem. 1998;17:472–483. [Google Scholar]
- 11.Hahn ME, Hestermann EV. Receptor-mediated mechanisms of toxicity. In: Di Giulio RT, Hinton DE, editors. The Toxicology of Fishes. CRC; Boca Raton, FL, USA: 2008. pp. 235–272. [Google Scholar]
- 12.Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc Natl Acad Sci U S A. 1997;94:13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebra-fish. Toxicol Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- 14.Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- 15.Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus). Aquat Toxicol. 2010;99:232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshioka W, Peterson RE, Tohyama C. Molecular targets that link dioxin exposure to toxicity phenotypes. J Steroid Biochem. 2011;127:96–101. doi: 10.1016/j.jsbmb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepin P. Effect of temperature and size on development, mortality, and survival rates of the pelagic early life-history stages of marine fish. Can J Fish Aquat Sci. 1991;48:503–518. [Google Scholar]
- 18.Wirgin I, Grunwald C, Stabile J, Waldman JR. Delineation of discrete population segments of shortnose sturgeon Acipenser brevirostrum based on mitochondrial DNA control region sequence analysis. Conserv Genet. 2010;11:689–708. doi: 10.1046/j.1365-294x.2002.01575.x. [DOI] [PubMed] [Google Scholar]
- 19.Dadswell MJ, Taubert BD, Squires TS, Marchette D, Buckley J. NOAA OPR. Silver Spring; MD, USA: 1984. Synopsis of biological data on shortnose sturgeon, Acipenser brevirostrum LeSueur 1818. p. 45. [Google Scholar]
- 20.VanEenennaam JP, Doroshov SI, Moberg GP, Watson JG, Moore DS, Linares J. Reproductive conditions of the Atlantic sturgeon (Acipenser oxyrinchus) in the Hudson River. Estuaries. 1996;19:769–777. [Google Scholar]
- 21.Wirgin I, Maceda L, Waldman JR, Wehrell S, Dadswell M, King T. Stock origin of migratory Atlantic sturgeon in the Minas Basin, Inner Bay of Fundy, Canada, determined by microsatellite and mitochondrial DNA analyses. Trans Am Fish Soc. 2012 DOI: 10.1080/00028487.2012.700899. [Google Scholar]
- 22.Smith TIJ. The fishery, biology, and management of Atlantic sturgeon, Acipenser oxyrhynchus, in North America. Environ Biol Fishes. 1985;14:61–72. [Google Scholar]
- 23.TAMS/Gradient . Further site characterization and analysis database report. Phase 2 report, review copy. U.S. Environmental Protection Agency; Region II, New York, NY.: 1995. [Google Scholar]
- 24.Wirgin I, Weis JS, McElroy AE. Physiological and genetic aspects of toxicity in Hudson River species. In: Levinton J, Waldman JR, editors. The Hudson River Ecosystem. Cambridge University Press; New York, NY, USA: 2006. pp. 441–464. [Google Scholar]
- 25.Sloan RJ, Kane MW, Skinner LC. 2005.Of time, PCBs,andthe fish of the Hudson River. Bureau of Habitat, Division of Fish, Wildlife and Marine Resources, NewYork State Department of Environmental Conservation; Albany, New York, USA: [Google Scholar]
- 26.Fernandez M, Ikonomou M, Courtenay S, Wirgin II. Spatial variation and source prediction of PCBs and PCDD/Fs among young-ofthe-year and adult tomcod (Microgadus tomcod) in the Hudson River Estuary. Environ Sci Technol. 2004;38:976–983. doi: 10.1021/es034177f. [DOI] [PubMed] [Google Scholar]
- 27.Kreeger D, Tudor R, Sharp J, Kilham S, Soeder D, Maxwell-Doyle M, Kraeuter J, Frizzera D, Hameedi J, Collier C. Partnership for the Delaware Estuary. Wilmington, DE, USA: 2006. White paper on the status and needs of science in the Delaware Estuary. p. 72. [Google Scholar]
- 28.Wirgin I, Grunwald C, Stabile J, Waldman J. Genetic evidence for relict Atlantic sturgeon stocks along the mid-Atlantic coast of the USA. North Am J Fish Manag. 2007;27:1214–1229. [Google Scholar]
- 29.Environmental Research and Consulting . Contaminant analysis of tissues from two shortnose sturgeon (Acipenser brevirostrum) collected in the Delaware River. NMF Service; Gloucester, MA, USA: 2002. pp. 1–13. [Google Scholar]
- 30.Roy NK, Walker N, Chambers RC, Wirgin I. Characterization and expression of cytochrome P4501A in Atlantic sturgeon and shortnose sturgeon experimentally exposed to coplanar PCB 126 and TCDD. Aquat Toxicol. 2011;104:23–31. doi: 10.1016/j.aquatox.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courtenay S, Grunwald C, Kreamer G-L, Fairchild WL, Arsenault JT, Ikonomou M, Wirgin I. A comparison of the dose and time response of CYP1A1 mRNA induction in chemically treated Atlantic tomcod from two populations. Aquat Toxicol. 1999;47:43–69. [Google Scholar]
- 32.Mohler JW. Culture manual for the Atlantic sturgeon. U.S. Fish and Wildlife Service; Hadley, MA: 2003. p. 66. [Google Scholar]
- 33.Conte FS, Doroshov SI, Lutes PB, Strange EM. BT Hatchery Manual for the White Sturgeon (Acipenser transmontanus Richardson) With Application to Other North American Acipenseridae. Division of Agriculture and Natural Resources, University of California; Oakland, CA, USA: 1988. p. 102. Publication 3322. [Google Scholar]
- 34.Walker MK, Spitsbergen JJ, Olson JR, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) toxicity during early life stage development of lake trout (Salvelinus namaycush). Can J Fish Aquat Sci. 1991;48:875–883. [Google Scholar]
- 35.Buckler J, Candrl J, Mckee M, Papoulias D, Tillitt D. Abstract, Society of Environmental Toxicology and Chemistry National Meeting. New Orleans, LA, USA: 2009. Effects of contaminants on the shovelnose sturgeon as a surrogate for the endangered pallid sturgeon. p. 283. [Google Scholar]
- 36.Buckler J, Candrl J, Mckee M, Tillitt DE, Galat D. Abstract, Society of Environmental Toxicology and Chemistry National Meeting. Boston, MA, USA: 2011. Persistent organic pollutant (POP) effects on shovelnose sturgeon (Scaphirynchus platorynchus) reproduction and early life stages; p. 311. [Google Scholar]
- 37.Carvalho PSM, Tillitt DE. 2,3,7,8-TCDD effects on visual structure and function in swim-up rainbow trout. Environ Sci Technol. 2004;38:6300–6306. doi: 10.1021/es034857i. [DOI] [PubMed] [Google Scholar]
- 38.Couillard CM, Legare B, Bernier A, Dionne Z. Embryonic exposure to environmentally relevant concentrations of PCB126 affect prey capture ability of Fundulus heteroclitus larvae. Mar Environ Res. 2011;71:257–265. doi: 10.1016/j.marenvres.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Colavecchia MV, Hodson PV, Parrott JL. The relationships among CYP1A induction, toxicity, and eye pathology in early life stages of fish exposed to oil sands. J Toxicol Environ Health A. 2007;70:1542–1555. doi: 10.1080/15287390701384726. [DOI] [PubMed] [Google Scholar]