Abstract
Background
Adult-onset stressors exert opposing effects on hippocampal neurogenesis and cognition, with enhancement observed following mild stress and dysfunction following severe chronic stress. While early life stress evokes persistent changes in anxiety, it is unknown whether early stress differentially regulates hippocampal neurogenesis, trophic factor expression, and cognition across the life span.
Methods
Hippocampal-dependent cognitive behavior, neurogenesis, and epigenetic regulation of brain-derived neurotrophic factor (Bdnf) expression was examined at distinct time points across the life span in rats subjected to the early stress of maternal separation (ES) and control groups. We also examined the influence of chronic antidepressant treatment on the neurogenic, neurotrophic, and cognitive changes in middle-aged ES animals.
Results
Animals subjected to early stress of maternal separation examined during postnatal life and young adulthood exhibited enhanced hippocampal neurogenesis, decreased repressive histone methylation at the Bdnf IV promoter along with enhanced Bdnf levels, and improved performance on the stress-associated Morris water maze. Strikingly, opposing changes in hippocampal neurogenesis and epigenetic regulation of Bdnf IV expression, concomitant with impairments on hippocampal-dependent cognitive tasks, were observed in middle-aged ES animals. Chronic antidepressant treatment with amitriptyline attenuated the maladaptive neurogenic, epigenetic, transcriptional, and cognitive effects in middle-aged ES animals.
Conclusions
Our study provides novel insights into the short- and long-term consequences of ES, demonstrating both biphasic and unique, age-dependent changes at the molecular, epigenetic, neurogenic, and behavioral levels. These results indicate that early stress may transiently endow animals with a potential adaptive advantage in stressful environments but across a life span is associated with long-term deleterious effects.
Keywords: Amitriptyline, cognition, histone modification, maternal separation, Morris water maze, novel object recognition
Stress has manifold effects on neurocircuitry ranging from adaptive modifications that facilitate future stress responses to maladaptive states that disrupt homeostasis and impair stress responses (1,2). Stress responses are shaped by diverse factors including intensity, duration, predictability, and controllability of the stressor. Adult-onset, chronic, unpredictable stress induces a decline in adult hippocampal neurogenesis (3,4); enhances anxiety and depressive behaviors (5); and impairs hippocampal-dependent cognition (4,6). In contrast, mild, predictable adult stressors increase hippocampal neurogenesis (7,8), attenuate anxiety, and improve cognition (7,8). It is noteworthy that stress effects are often critically dependent on the age of exposure to the stressor (9,10). While adult-onset stressors evoke predominantly transient effects (11,12), in contrast, early life stress results in relatively persistent changes in anxiety behavior (13–15).
The early stress of maternal separation (ES) has served as a key model to examine the effects of early adverse experience (16,17). While most studies have focused on the deleterious consequences of ES (14,15), relatively little is known about its potentially adaptive consequences (18–20). We examined whether ES alters hippocampal-dependent cognitive behavior, as well as molecular and cellular correlates strongly associated with cognition, across the life span. We found that ES history is associated with distinct consequences on hippocampal neurogenesis, epigenetic regulation of Bdnf expression, and hippocampal-dependent cognitive function that manifests in a temporally regulated manner. Our results demonstrate a continuum of ES-evoked responses with transiently adaptive changes soon after ES in postnatal life and young adulthood and relatively slow-emerging maladaptive effects in middle-aged life. Chronic antidepressant treatment ameliorated the molecular, neurogenic, and behavioral changes observed in middle-aged ES animals. Our findings suggest that ES-evoked changes may transiently enhance an individual's fitness in stress coping, but across the life span, increase susceptibility for aging-associated cognitive decline and hippocampal damage.
Methods and Materials
Animal Paradigms
Male Sprague-Dawley rats bred in the Tata Institute of Fundamental Research animal facility were group housed and maintained on a 12-hour light/dark cycle with access to food and water ad libitum. Animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Tata Institute of Fundamental Research Animal Ethics Committee. Animals were subjected to ES from postnatal day (P)2 to P14 for 3 hours daily and examined at P21, 2 months (young adult), and 15 months (middle-aged) of age.
Drug Treatments
Animals received the mitotic marker 5 bromo-2′-deoxyuridine (BrdU) (Sigma, St. Louis, Missouri) (P21: 100 mg/kg, young adult and middle-aged animals: 200 mg/kg, via intraperitoneal injection) and sacrificed 2 hours late n = 4–6/group/age). To examine the effects of chronic antidepressant treatment, ES animals (10– month–old) received treatment with amitriptyline (ES Ami) (10 mg/kg of amitriptyline per animal per day) (Sigma) in drinking water for 5 months. Control animals and ES groups received drinking water (n = 5–11/group for chromatin immunoprecipitation [ChIP], quantitative polymerase chain reaction [PCR], in situ, and behavior analysis, and n = 12–21/group for immature neuron analysis). Animal weights, water consumption, and drug intake were monitored twice a month.
Behavior Paradigms
Morris Water Maze
Control and ES groups were tested using the Morris water maze (MWM) task (n = 7–11/group/age). Animals were trained to locate a hidden platform in a tank (150 cm diameter) over 4 days of training, 4 trials per day (trial duration: 120 sec, intertrial interval: 60 sec). Morris water maze training performance was determined by measuring escape latency. Short- and long-term spatial memory retention was assessed through probe tests (90 sec) performed 24 hours and 10 days posttraining. In addition, middle-aged control and ES animals treated with BrdU 35 days before the MWM task were tested for long-term retention of spatial memory 6 days post-training (n = 7–8/group). We used an earlier time point for the long-term retention test, as the hippocampus demonstrates a progressively reduced role in spatial memory recall over time (21,22). Body weight and swim speed were noted for all MWM experiments (Table S1 in Supplement 1). Behavior in the MWM was scored using an automated tracking system (Noldus Ethovision 3.1, Noldus Information Technology, Wageningen, The Netherlands).
Novel Object Recognition
Object memory was tested using the novel object recognition (NOR) paradigm (23) (2 month: n 17–20/group; 15 month: n = 9–20/group). Control and ES animals were acclimatized to a novel arena (40 cm × 60 cm × 29 cm) over 3 days (30 min daily). On day 1, animals explored two identical objects in the arena (15 min). Object memory was tested (day 2) by replacing one of the identical objects with a novel object of similar dimensions. On day 7, object memory was tested by replacing the first novel object with a second novel object. Discrimination index {[(time spent exploring the novel object – time spent exploring the familiar object) / total object exploration time] × 100} was determined to ascertain object memory over a 5-minute exploration period. Object selection was based on pilot experiments with a separate cohort of animals to ensure no inherent object bias.
Immunohistochemistry and Immunofluorescence
Hippocampal proliferation and immature neuron numbers were assessed using BrdU and doublecortin (DCX) immunohistochemistry as previously described (24) (n = 4–6/group/age). For BrdU and DCX immunohistochemistry, hippocampal sections were incubated sequentially with primary antibody and biotinylated secondary antibodies. Signal amplification was performed using an avidinbiotin Elite ABC system (Vector Laboratories, Burlingame, California) and visualized using diaminobenzidine (Sigma). For DCX immunofluorescence, following primary and biotinylated secondary antibody exposure, sections were exposed to Alexa 488-conjugated streptavidin (Molecular Probes, Grand Island, New York). Immunofluorescence experiments were visualized by confocal microscopy (LSM5 Exciter, Zeiss, Oberkochen, Germany).
Cell Counting Analysis
Quantitation of BrdU-positive cell numbers in tissue sections spanning the rostrocaudal extent of the hippocampus was performed using a modified, unbiased stereology protocol (25). The number of BrdU-positive cells per subgranular zone (SGZ)/ granule cell layer was estimated by multiplying the total number of marker-positive cells counted by the section periodicity. To determine DCX-positive cell number, DCX-positive cells per section were counted on a Zeiss Axioskop camera at a magnification of 400× . To quantitate the number of DCX-positive cells in control and ES animals at P21, the immunopositive cells were counted in three SGZ subfields of specified area across the span of the dentate gyrus (DG) granule cell layer blade on an LSM5 exciter confocal microscope. The number of DCX-positive cells per section were then estimated based on the average of the DCX-positive cells counted per subfield and the total area of the SGZ in the section.
In Situ Hybridization and Densitometric Analysis
Hippocampal coronal sections were subjected to in situ hybridization for Bdnf exon IV and Bdnf exon IX (total Bdnf) transcripts as described previously (26) (n = 4–7/group/age). In brief, slides were incubated with 35S-uridine triphosphate labeled rat exon-specific Bdnf riboprobes (1 × 106 cpm/slide) generated from plasmids kindly provided by Dr. Julie Lauterborn (University of California, Irvine, California) and then subjected to stringent washes. Slides were exposed to Hyperfilm β-max (GE Healthcare, Buckinghamshire, United Kingdom) and densitometric measurements of messenger RNA (mRNA) levels in hippocampal subfields were generated with the Scion Image software (Scion Corporation, Houston, Texas).
ChIP Assay
Chromatin immunoprecipitation assays were performed on hippocampal tissue as previously described (27,28) (n = 7–9/ group/age). Tissue was cross-linked, homogenized, and sonicated to generate 400 base pair (bp) to 800 bp chromatin fragments. Chromatin lysate (50 mg) was immunoprecipitated with 1 mg of antibody directed against histone 3-lysine 9 dimethylation (H3K9me2) (Millipore Bioscience Research Reagents, Billerica, Massachusetts). The histone modification-associated chromatin was extracted with phenol/chloroform/isoamyl alcohol before quantitative polymerase chain reaction (qPCR) analysis.
Quantitative PCR
To estimate chromatin pulled down by ChIP assays and to assess levels of Bdnf exon specific transcripts (Bdnf exon I, II, IV, VI, and IX), qPCR was carried out (n = 7–11/group/age). RNA was extracted using Trizol reagent (Sigma) and reverse transcribed. The synthesized complementary DNA was subjected to qPCR (Applied Biosystems, Foster City, California) with exon-specific Bdnf primers (Table S2 in Supplement 1). Quantitative PCR data analysis was performed using the ΔΔCt method (27,29). Gene expression analysis data were normalized to the endogenous housekeeping gene, Hprt1. A region 3000 bp upstream of the Bdnf IV promoter was used as a normalizing control region for ChIP analysis.
Western Blot Analysis
Hippocampal tissue (n = 4–6/group/age) was lysed and separated by sodium dodecyl sulfate polyacrylamide gel (12.5%) electrophoresis and then transferred to polyvinylidene difluoride (GE Healthcare) membranes. Following blocking, membranes were incubated with rabbit anti-brain-derived neurotrophic factor (Bdnf) (1:500) (Santa Cruz Biotechnology, Santa Cruz, California) or rabbit anti-beta-III-tubulin (1:5000) (Covance, Princeton, New Jersey). Membranes were incubated with a 1:5000 dilution of horseradish peroxidase-conjugated donkey anti-rabbit (GE Healthcare) secondary antibody. Protein-antibody complexes were detected with an enhanced chemiluminescent substrate (GE Healthcare). Densitometric analysis was performed using Scion image software.
Statistical Analysis
Experiments with two and three groups were analyzed using unpaired Student t test and one-way analysis of variance (ANOVA), followed by Tukey-Kramer post hoc tests, respectively (Instat, Graphpad Software Inc., San Diego, California). Repeated measures ANOVA with paired Student t test for group-wise post hoc comparisons was used for analysis of MWM escape latencies (SPSS output; IBM Corporation, Armonk, New York). Target quadrant preference above chance in the MWM was assessed using one sample t test. Correlation between newborn neuron number and long-term spatial memory was assessed using the Pearson correlation test. Significance level was determined at p < .05 for all tests.
Detailed methodological procedures are provided in Supplemental Methods in Supplement 1.
Results
ES Evokes Enhanced Spatial Learning in Young Adulthood and a Decline in Spatial Memory in Middle-Aged Life
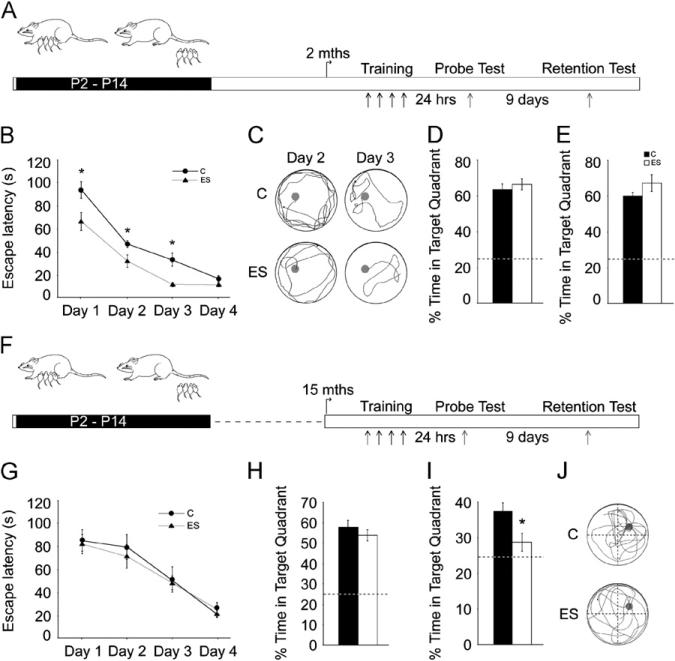
Young adult and middle-aged ES animals and control animals were tested on the MWM task (30), a spatial learning task involving stressful components such as forced swim (31). Young adult ES animals showed accelerated spatial learning with significantly lower escape latencies (Figure 1B,C) [effect of group:F(1,19) = 18.48, p < .001]. Decreased escape latencies were noted on the first MWM training day in ES animals (Figure S1 in Supplement 1) [effect of group:F(1,19) 7.44, p = .01]. Probe test performance, 24-hour and 10-day (Figure = 1D,E), did not differ between young adult control and ES animals.
Figure 1.
Age-dependent biphasic changes evoked by early stress in spatial learning and memory. (A, F) Shown are schematic representations of the experimental design. Animals subjected to the early stress of maternal separation (ES) and their age-matched control animals (C) were trained at 2 months (mths) (A) or 15 months (F) of age on the Morris water maze (MWM) task and tested using probe tests 24 hours and 10 days after training. (B) Young adult ES animals exhibited a significant reduction in escape latency across days of training as compared with age-matched control animals (n = 10–11/group; *p < .05, repeated measures analysis = of variance). (C) Shown are representative tracks for 2-month-old control and ES animals on day 2 and day 3 of MWM training. (D, E) Recollection of the hidden platform location assessed using the probe tests 24 hours (D) and 10 days (E) following the end of training did not differ between the 2-month-old control and ES groups. (G) Middle-aged ES animals did not differ from control animals in their escape latency during MWM training. (H) Middle-aged ES animals did not differ from control animals in performance on the 24-hour probe test. (I) Middle-aged ES animals exhibited a significant decline in the percent time spent in the target quadrant as compared with age-matched control animals on the 10-day probe test (n = 7–8/group; *p< .05; unpaired Student t test). (J) Shown are representative tracks for middle-aged control and ES animals from the 10-day probe test. The dashed lines in the bar graphs represent the chance percentage time (25%) spent in the target quadrant. Results are expressed as the mean ± SEM and represent the time in seconds for escape latency during MWM training and the percent time in target quadrant for the probe tests performed 24 hours and 10 days after MWM training. P, postnatal day.
Middle-aged control and ES animals did not exhibit significant differences in MWM training or the 24-hour probe test (Figure 1G,H). In the 10-day probe test, middle-aged ES animals showed no preference over chance for the target quadrant (p= .16) and were significantly different from their age-matched control animals (p= .04) (Figure 1I,J). These results indicate that middle-aged ES animals show impaired long-term spatial memory on the MWM. Our results demonstrate that ES evokes temporally specific changes in hippocampal dependent cognition, with enhanced spatial learning in young adulthood and a decline in long-term spatial memory in middle-aged life.
ES Animals Exhibit an Age-Dependent Decline in Object Memory
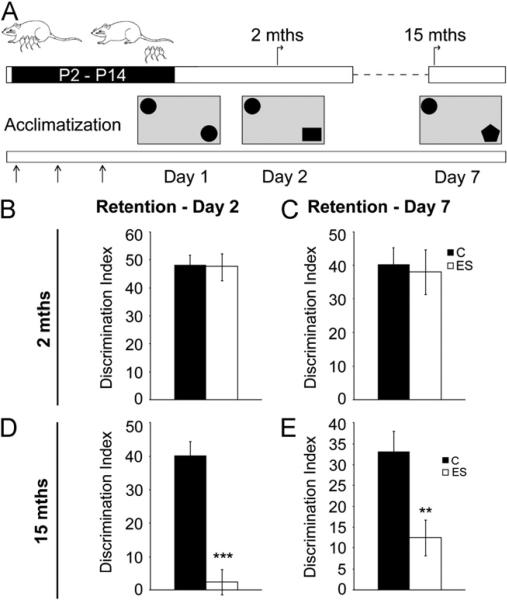
Object memory was examined using the NOR task, a non-spatial, emotionally neutral test (32). Young adult control and ES animals exhibited no significant difference in short- or long-term object memory (Figure 2B,C; Table S2 in Supplement 1). In contrast, middle-aged ES animals exhibited a significant decline in object memory (day 2 test: p < .001; day 7 test: p < .01) (Figure 2D,E; Table S2 in Supplement 1). The total object exploration time did not differ between the groups at the two ages examined (data not shown). Our results indicate that ES animals show an age-dependent decline in object memory in middle-aged life.
Figure 2.
Early stress history influences performance on the novel object recognition test selectively in middle-aged life. (A) Shown is a schematic representation of the experimental design. Animals subjected to early stress (ES) and their age-matched control animals (C) were trained at 2 and 15 months (mths) of age on an emotionally neutral task of learning, the novel object recognition test. Post 3 days of arena acclimatization (A) (arrows), control and ES animals were exposed to two identical objects in the arena (day 1). Object memory was tested 24 hours later (day 2). Animals were retested for object memory using a second novel object on day 7. (B, C) Object memory on day 2 (B) or day 7 (C) was not different between young adult control and ES animals. (D) Middle-aged ES animals exhibited a significant impairment in object memory on day 2. (E) Middle-aged ES animals showed a significant impairment in object memory when retested on day 7. Results are the mean ± SEM of the discrimination index (2 months: n = 17–20/group; 15 months: control animals n = 9, ES n = 20; *p < .05, **p < .01, ***p < .001, unpaired Student t test). P, postnatal day.
ES Induces Temporally Dependent, Biphasic Effects on Adult Hippocampal Neurogenesis
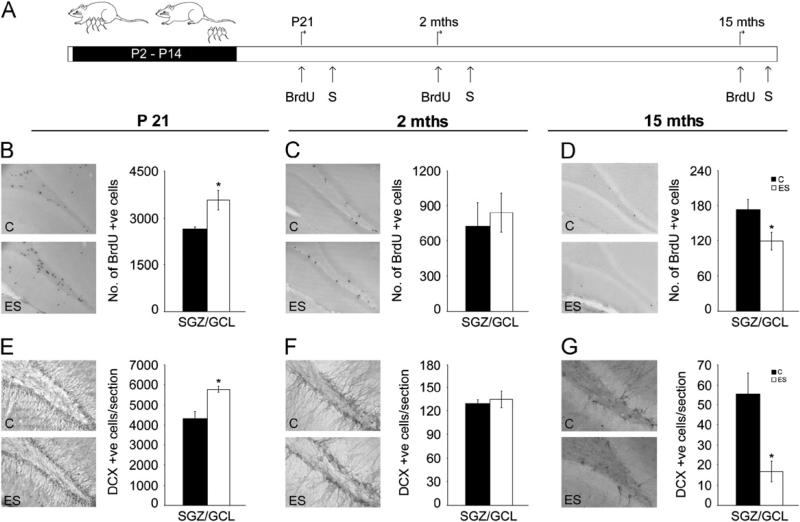
We next examined if ES animals across the life span exhibit alterations in neurogenesis, a form of hippocampal plasticity modulated by stress (4,33) and strongly correlated with cognition (34,35). Hippocampal neurogenesis was assessed using the proliferation marker BrdU and the immature neuronal marker DCX. As we have reported previously (26), P21 ES animals exhibit a significant increase in progenitor proliferation (p = .03) (Figure 3B). Enhanced proliferation was accompanied by a significant increase in the hippocampal DCX-immunopositive immature neurons in P21 ES animals (p.01) (Figure 3E). Young adult ES animals exhibited no significant=difference in BrdU- and DCX-positive cell numbers (Figure 3C,F). Strikingly, middle-aged ES animals exhibited significantly decreased hippocampal BrdU-positive cells (p = .04) and DCX-positive immature neurons (p = .02) (Figure 3D,G). While proliferating progenitors were reduced in middle-aged ES animals, Sox-2/glial fibrillary acidic protein double-positive quiescent stem cells were unaltered at this age (p = .44) (Figure S2 in Supplement 1). Further, progenitor differentiation was unaltered at 2 and 15 months of age in ES animals (Figure S3 in Supplement 1).
Figure 3.
Early stress evokes bidirectional changes in hippocampal neurogenesis across the life span. Hippocampal neurogenesis in animals with a history of early stress (ES) was studied using the exogenous mitotic marker 5-bromo-2-deoxyuridine (BrdU) to assess hippocampal progenitor proliferation and the endogenous marker doublecortin (DCX) to determine immature neuron number. (A) Shown is a schematic of the experimental design. Early stress and control (C) animals received treatment with BrdU in postnatal life (postnatal day [P]21), young adulthood (2 months [mths]), and in middle-aged life (15 months) and were killed (S) 2 hours post BrdU administration. Shown are representative images from control and ES animals, of BrdU- and DCX-positive (+ve) cells within the subgranular zone (SGZ)/granule cell layer (GCL) at P21 (B, E), 2 months (C, F), and 15 months (D, G). (B) Stereological analysis revealed a significant increase in BrdU-positive cell numbers at P21. (C) In young adulthood, no change was observed in BrdU-positive cell numbers within the SGZ/GCL. (D) In striking contrast, middle-aged ES animals exhibited a significant decline in BrdU-positive cell numbers. (G) Quantitative analysis revealed a bidirectional change in immature neuron number with an increase at P21 (E) and a steep decline at 15 months of age (G). (F) In young adulthood, no change was observed in DCX-positive cell numbers within the SGZ/GCL. Results are expressed as mean ± SEM of BrdU-positive cells in the SGZ/GCL and mean ± SEM of DCX-positive cells/section (n = 4–6/group/age; *p < .05, unpaired Student t test).
Compelling evidence indicates that 4- to 8-week-old neurons are preferentially recruited into hippocampal spatial memory networks (36). We examined the relationship between the extent of neurogenesis and spatial memory recall in middle-aged animals. Control and ES groups, treated with BrdU before MWM, showed no difference in performance during training and the 24-hour probe test (Figure S4B,C in Supplement 1). Animals with early stress of maternal separation showed a significant deficit in platform recall in the 6-day probe test (p = .01) (Figure S4D in Supplement 1) accompanied by decreased BrdU/ NeuN double-positive neuron number (p = .03) (Figure S4E in Supplement 1). A strong positive correlation (r = .90, p = .0003) was found between the percent time in target quadrant and BrdU/NeuN double-positive cell number (Figure S4F in Supplement 1). These results suggest that the decreased hippocampal neurogenesis in middle-aged ES animals may underlie the MWM memory deficit.
Our results indicate that ES animals show a transient increase in hippocampal neurogenesis in postnatal life and a steep decline in hippocampal neurogenesis at 15 months of age coincident with impairments in hippocampal-dependent cognitive tasks.
ES Results in Age-Dependent Bidirectional Alterations in Epigenetic Regulation of Hippocampal Bdnf Expression
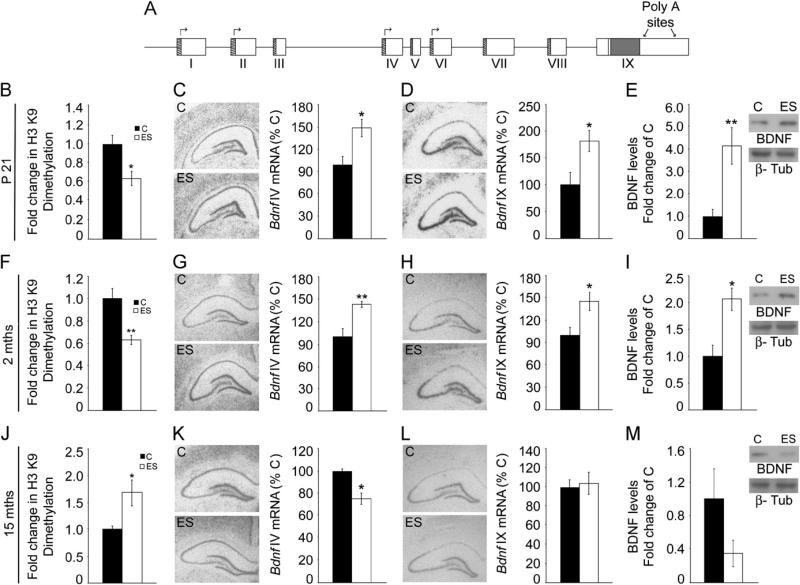
Given that ES alters hippocampal neurogenesis and cognitive function, we hypothesized that ES may influence levels of Bdnf, a key modulator of neurogenesis (37,38) and hippocampal-dependent cognition (39,40). Bdnf expression is under the transcriptional control of eight distinct promoters, which drive the expression of unique noncoding 5’ exons (I–VIII) alternatively spliced to a common 3’ coding exon (IX), giving rise to exonspecific Bdnf transcripts (41) (Figure 4A). We examined epigenetic histone modifications at Bdnf promoters (I, II, IV, VI) that regulate the expression of Bdnf transcript variants at P21, 2 months, and 15 months in control and ES animals.
Figure 4.
Age-dependent biphasic alterations in the epigenetic regulation of brain derived neurotrophic factor (Bdnf) expression following early stress exposure. (A) Shown is a schematic representation of the Bdnf gene composed of eight 5′ noncoding exons (I–VIII) that are alternatively spliced to the common 3′ coding exon IX, with the expression of each 5′ noncoding transcript regulated by its own unique promoter. The arrows show the Bdnf promoters profiled for histone modifications. Chromatin immunoprecipitation (ChIP) analysis for the repressive histone 3 lysine 9 (H3 K9) dimethylation histone modification at the Bdnf IV promoter, expression of Bdnf IV and Bdnf IX messenger RNA (mRNA) levels, and hippocampal mature Bdnf protein levels were analyzed in animals with early stress exposure (ES) and control animals (C) at postnatal day (P)21, 2 months (mths), and 15 months. The Bdnf IV promoter revealed a significant downregulation in the repressive H3 K9 dimethylation modification at P21 (B) and in 2-month-old ES animals (F), and in contrast, an enhancement of this repressive modification was observed in 15-month-old ES animals (J). A significant increase in Bdnf IV mRNA expression in the hippocampal dentate gyrus subfield of ES animals was observed at P21 (C) and 2 months (G) with a significant decline in expression of this Bdnf variant at 15 months (K). A significant increase in Bdnf IX mRNA expression in the hippocampal dentate gyrus subfield of ES animals was noted at P21 (D) and 2 months (H). No change in Bdnf IX mRNA expression was observed in ES animals at 15 months of age (L). Shown are the representative autoradiographs of Bdnf IV and Bdnf IX containing transcripts for all the three ages examined. (E, I) Increased levels of hippocampal Bdnf protein were observed in ES animals at P21 (E) and at 2 months of age (I). (M) Brain derived neurotrophic factor protein was observed to be unchanged in 15-month-old ES animals as compared with their control animals. Shown are representative western blots for Bdnf and beta-III-tubulin (b-Tub). Results are expressed as mean ± SEM percentage of control for mRNA expression, mean ± SEM fold change of control for ChIP and western blot analysis (in situ hybridization: n = 4–7/group/age; ChIP: n = 7–9/group/age; western blot analysis: n = 4–6/group/age, *p < .05, **p < .01, unpaired Student t test).
Animals with early stress of maternal separation showed a significant induction in Bdnf IV mRNA at P21 (p = .02) and at 2 months (p = .007) (Figure 4C,G) in the hippocampal DG subfield, accompanied by decreased repressive H3K9me2 modification at this promoter at both ages (P21: p = .03; 2 months: p = .007) (Figure 4B,F; Figure S5B,C in Supplement 1). No changes were observed in other Bdnf transcript variants examined (Figure S5B,C in Supplement 1). Enhanced Bdnf IV expression also resulted in increased Bdnf IX (total Bdnf) mRNA levels in the DG of postnatal (p = .04) and young adult ES animals (p = .04) (Figure 4D,H). Enhanced Bdnf IV and IX expression at P21 and 2 months was confirmed using qPCR (P21 Bdnf IV: control = 1.00 ± .04, ES = 1.15 ± .04, p = .02; Bdnf IX: control = 1.00 ± .06, ES = 1.18 ± .04, p = .04; 2-month Bdnf IV: control = 1.00 ± .14, ES = 1.60 ± .26, p = .04; Bdnf IX: control = 1.00 ± .04, ES = 1.26 ± .06, p = .001, unpaired Student t test; results are the mean ± SEM fold change of control). A robust enhancement of hippocampal mature Bdnf protein levels in ES animals was also noted at both P21 (p= .007) and in young adulthood (p = .02) (Figure 4E,I).
In striking contrast, middle-aged ES animals demonstrated a significant decline in Bdnf IV mRNA in the DG (p = .01), accompanied by increased repressive H3K9me2 modification at this promoter (p = .01) (Figure 4K,J; Figure S5D in Supplement 1). Other Bdnf transcript variants showed no change in expression (Figure S5D in Supplement 1). Middle-aged ES animals demonstrated no change in Bdnf IX mRNA levels in the DG (Figure 4L). These findings were confirmed by qPCR analysis, which revealed decreased hippocampal Bdnf IV but not IX expression in middle-aged ES animals (Bdnf IV: control = 1.00 ± .09, ES = .61 ± .06, p = .004; Bdnf IX: control = 1.00 ± .14, ES = .99 ± .10, p = .99; unpaired Student t test; results are the mean ± SEM fold change of control). Hippocampal Bdnf protein levels were unaltered in middle-aged ES animals (p = .11) (Figure 4M). It is noteworthy that ES animals at younger ages exhibit reduced H3K9me2 at the Bdnf IV promoter along with enhanced Bdnf IV expression, whereas middle-aged ES animals show enhanced repressive H3K9me2 at the Bdnf IV promoter concomitant with decreased Bdnf IV expression, indicating bidirectional effects of ES on the epigenetic regulation of hippocampal Bdnf IV expression.
Antidepressant-Mediated Amelioration of ES Evoked Changes in Middle-Aged Life
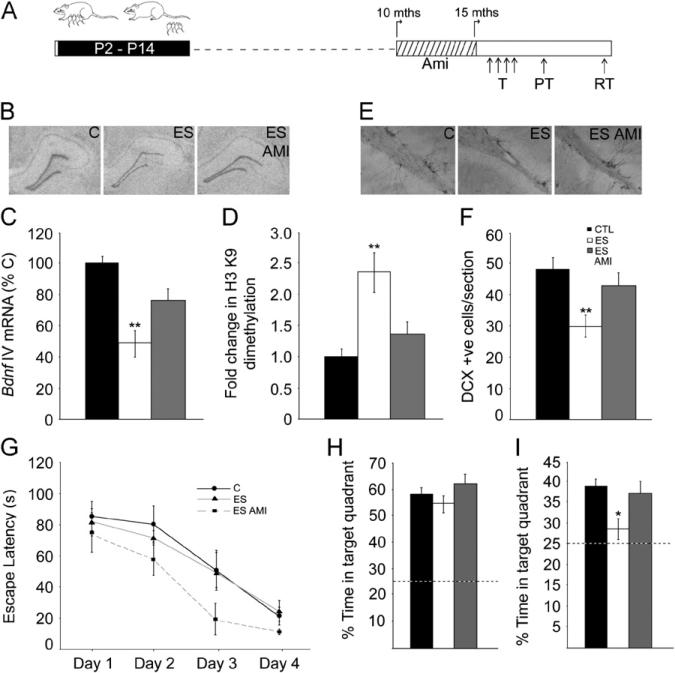
We next addressed whether chronic antidepressant treatment could alleviate the consequences observed in middle-aged ES animals. Amitriptyline treatment prevented the decreased Bdnf IV expression within the DG (Figure 5B,C) [F(2,14) = 8.87, p = .003] and also attenuated the increased repressive H3K9me2 modification at the Bdnf IV promoter in the hippocampi of middle-aged ES animals (Figure 5D) [F(2,21) = 8.01, p = .003]. Amitriptyline treatment did not alter Bdnf IX expression inES animals (control 100 ± 7.97, ES = 103.05 ± 10.17, ES Ami = 106 ± 8.46, p = .90, one-way ANOVA; results are the mean ± SEM percentage of controls). Amitriptyline treatment rescued the DCX-positive neuron number decline in middle-aged ES animals (Figure 5E,F) [F(2,46) = 6.03, p = .005]. No significant differences were noted across groups on the MWM task during training and the 24-hour probe test (Figure 5G,H). In the 10-day probe test, middle-aged ES animals exhibited no greater than chance preference for the target quadrant (p = .14), whereas both the control (p = .002) and ES Ami (p = .005) groups showed significant preference over chance for the target quadrant. One-way ANOVA analysis revealed an amitriptyline-mediated attenuation of the ES-induced cognitive decline (Figure 5I) [F(2,22) = 5.29, p = .01]. These results indicate that chronic amitriptyline treatment attenuates the maladaptive neurogenic, neurotrophic, and cognitive changes observed in middle-aged ES animals.
Figure 5.
Antidepressant treatment ameliorates the epigenetic repression of Bdnf IV expression and the neurogenic and cognitive decline in middle-aged animals with early stress history. (A) Shown is a schematic representation of the experimental design. A cohort of animals with early stress history (ES) received treatment with the antidepressant amitriptyline (Ami) for a period of 5 months before assessing histone 3 lysine 9 (H3 K9) dimethylation histone modification at the Bdnf IV promoter, Bdnf IV expression, hippocampal neurogenesis, and Morris water maze (MWM) performance at 15 months (mths) of age. Middle-aged animals from all groups, namely control (C), ES, and ES Amitriptyline (ES AMI), were trained on the MWM and subjected to 24-hour and 10-day probe tests. (B, C) Chronic amitriptyline treatment attenuated the significant decline in Bdnf IV messenger RNA (mRNA) levels within the hippocampal dentate gyrus subfield observed in middle-aged ES animals. (B) Shown are representative autoradiographs of hippocampal Bdnf IV expression for middle-aged control, ES, and ES AMI animals. (D) Chronic amitriptyline treatment ameliorated the enhanced repressive H3 K9 dimethylation modification at the Bdnf IV promoter in middle-aged ES animals as assessed using chromatin immunoprecipitation (ChIP) analysis. (E, F) The significant decline in hippocampal doublecortin (DCX)-immunopositive cells observed in middle-aged ES animals was also prevented by chronic amitriptyline treatment. (E) Shown are representative images of DCX-positive (+ve) cells from middle-aged control, ES, and ES AMI animals. Values are expressed as mean ± SEM percentage of control for mRNA expression, mean ± SEM fold change of control for ChIP analysis (n = 5–9/group, **p < .01 as compared with control, one-way analysis of variance [ANOVA], Tukey-Kramer post hoc test) and mean ± SEM of DCX-positive cells/section (n = 12–21/ group, **p < .01 as compared with control, one-way ANOVA, Tukey-Kramer post hoc test). (G) Middle-aged animals from all groups did not exhibit any difference in escape latencies across MWM training (T) (n = 8–9/group, p > .05, repeated measures ANOVA). (H) In the 24-hour probe test (PT), animals of all three groups did not differ in the percent time in the target quadrant. (I) Chronic amitriptyline treatment prevented the significant decline in percent time spent in the target quadrant on the 10-day probe test (RT) in ES animals (*p < .05 as compared with control, one-way ANOVA, Tukey-Kramer post hoc test). The dashed line in the bar graphs represents the chance percentage time (25%) spent in the target quadrant. All values are expressed as mean ± SEM of the time taken to reach the hidden platform during MWM training and mean ± SEM of the percent time spent in the target quadrant for the probe tests. P, postnatal day.
Discussion
Our results reveal that ES animals at younger ages exhibit enhanced hippocampal neurogenesis, epigenetic modifications associated with enhanced Bdnf expression, and improved cognitive performance selectively on stress–associated, hippocampal-dependent learning tasks. In contrast, middle-aged ES animals show reduced neurogenesis and epigenetic modifications associated with decreased Bdnf IV expression. Concomitantly, significantly impaired performance was noted on both stress-associated and none-motive cognitive tasks in middle-aged ES animals. Chronic antidepressant treatment attenuated the repressive epigenetic modification at the Bdnf IV promoter, the decreased Bdnf IV expression, the neurogenic decline, and the memory deficit in middle-aged ES animals.
Early stress of maternal separation has predominantly been associated with negative consequences, including perturbed stress-response neurocircuitry and enhanced emotionality (14,15). However, ES may also facilitate adaptive changes that calibrate responses to hostile future environments. Here, we provide novel evidence of cognitive, molecular, and neurogenic changes observed at younger ages in ES animals, suggestive of adaptive outcomes. It is striking to note the improved spatial learning in the stress-associated MWM task in young adult ES animals. This improved performance may involve differential behavioral reactivity and attention to cues. Our results are in agreement with evidence linking models of differential maternal care, neonatal novelty, and juvenile stress to improvements on cognitive tasks (18–20). Our results are in concert with the tuning hypothesis (42), which suggests that adverse early experience fine-tunes neurocircuitry to provide a survival advantage in stressful environments.
Postnatal ES animals exhibit enhanced hippocampal neuro-genesis and increased Bdnf levels, suggesting a role for increased trophic signaling in the enhanced newborn neuron availability (43). However, hippocampal neurogenesis is only transiently enhanced during postnatal life, whereas enhanced Bdnf levels are sustained into adulthood, suggesting an apparent dissociation between the neurogenic and neurotrophic changes evoked by ES. Increased Bdnf expression is driven via the activity-responsive Bdnf IV promoter (44), with a significant decline in the same repressive H3K9me2 mark at both ages. Several studies with diverse adult-onset stress models have observed enhanced repressive histone modifications at the Bdnf IV promoter (27,45), in stark contrast to our results of decreased repressive histone marks at this Bdnf promoter in postnatal and young adult ES animals. Enhanced Bdnf has been implicated in structural plasticity (37,38) and hippocampal-dependent learning (39,40). Despite enhanced Bdnf levels, ES animals exhibit selective improvement on stress-associated, but not emotionally neutral, learning tasks. While enhanced Bdnf may provide a suitable substrate, the motivational and arousal cues involved in stressful learning tasks may be important to interact with this substrate to manifest as improved cognitive performance. Taken together, the molecular and cellular changes, along with the enhanced hippocampal-dependent spatial learning, observed soon after ES likely represent adaptive changes that facilitate stress coping.
Most studies have examined ES effects only at specific time points, failing to provide insights into consequences across a life span. We find that ES-evoked changes differ substantially across life, with potentially adaptive outcomes shifting to deleterious consequences on neurogenesis and trophic factor expression, concomitant with memory impairments in middle-aged ES animals. Molecular and cellular hippocampal changes in middle-aged ES animals may contribute to the cognitive decline observed in the MWM and NOR. However, given the steep decline in middle-aged ES animals in object memory, which involves a strong nonhippocampal component, we cannot preclude the role of other brain regions in the ES-evoked cognitive deficits (46,47). Our results agree with previous studies that demonstrate that models of inadequate maternal care lead to cognitive impairments in middle-aged life (48,49). A key structural correlate of the cognitive impairment in middle-aged ES animals is the hippocampal neurogenic decline. We observed a strong positive correlation between hippocampal neurogenesis and long-term MWM memory in middle-aged animals. This agrees with prior reports of positive correlations between individual MWM performance in aged animals and rates of neurogenesis (50,51). In contrast to younger ES animals, middle-aged ES animals showed an increased repressive H3K9me2 modification at the Bdnf IV promoter with reduced levels of this Bdnf transcript, which did not translate into reduced total Bdnf IX mRNA. However, given that the Bdnf IV promoter is regulated by learning paradigms (52), it is possible to speculate that the responsivity of this promoter may be compromised in middle-aged ES animals. Our results indicate biphasic age-dependent changes in Bdnf expression, hippocampal neurogenesis, and cognition in ES animals. Previous reports have examined neurogenic changes evoked by ES predominantly in adulthood (2–7 months of age). While our results are in agreement with a prior report of no neurogenic change in young adult ES animals (53), they differ from prior reports demonstrating decreased hippocampal neurogenesis in young adult animals with a history of ES (54–56). The discrepancy of these results could arise as a consequence of differences in the paradigm of maternal separation, time points examined, or animal strains.
The antidepressant-mediated amelioration of epigenetic and gene expression changes at the Bdnf IV promoter and the attenuation of the neurogenic and cognitive decline in middle-aged ES animals provide support for the hypothesis that impaired neurotrophic signaling and hippocampal neurogenesis may contribute to the long-term memory decline in middle-aged ES animals. These findings suggest that antidepressant treatment long after the cessation of ES, through epigenetic modulation of critical molecular factors, may serve to reverse late-onset cytoarchitectural and behavioral consequences associated with ES history.
In conclusion, our study provides novel insights into the diverse consequences of ES across a life span, highlighting the emergence of distinct and sometimes bidirectional, temporally specific changes at the molecular, epigenetic, neurogenic, and behavioral levels. Our results suggest transient and early adaptive changes that eventually tip into maladaptive consequences at the molecular, structural, and functional levels in the hippocampus later in life. These results compel a reappraisal of the traditional notion that early stress is deterministic for future negative outcomes. Rather, our results indicate that when viewed across a life span, ES-evoked changes are temporally quite distinct and span a wide spectrum, raising the intriguing possibility that while early adaptive outcomes may enhance cognitive function under stressful conditions, such enhanced fitness may eventually extract a high cost.
Supplementary Material
Acknowledgments
This research was supported by a Wellcome Trust Senior Overseas Fellowship (VAV) (0408200314133), a Tata Institute of Fundamental Research Intramural Grant (VAV), and support from the Department of Biotechnology, Centre of Excellence in Epigenetics, Indian Institute of Science Education and Research (SG and VAV) (BT/01/COE/09/07).
We acknowledge Ranjith Vijayakumar, Brigitte Pinheiro, and Kamalvishnu Prasad Gottimukkala for technical assistance and Professor John McGrath and Dr. Thomas Burne, University of Queensland, for input on statistical analysis.
Footnotes
These data were published as an abstract and presented as a poster at the Society for Neuroscience conference; Chicago, Illinois; November 2009.
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 2.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 3.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippo-campal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: From brain homeostasis to behavior. Life Sci. 2008;82:934–942. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Mineur YS, Belzung C, Crusio WE. Effect of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ, Lee HJ, Welday AC, Song E, Cho J, Sharp PE, et al. Stress-induced alterations in hippocampal plasticity, place cells, and spatial memory. Proc Natl Acad Sci U S A. 2007;104:18297–18302. doi: 10.1073/pnas.0708644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyons DM, Buckmaster PS, Lee AG, Wu C, Mitra R, Duffey LM, et al. Stress coping stimulates hippocampal neurogenesis in adult monkeys. Proc Natl Acad Sci U S A. 2010;107:14823–14827. doi: 10.1073/pnas.0914568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neuro-genesis and memory. Mol Psychiatry. 2011;16:171–183. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenig JI, Walker CD, Romeo RD, Lupien SJ. Effects of stress across the lifespan. Stress. 2011;14:475–480. doi: 10.3109/10253890.2011.604879. [DOI] [PubMed] [Google Scholar]
- 10.Ricon T, Toth E, Leshem M, Braun K, Richter-Levin G. Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress. 2012;15:11–20. doi: 10.3109/10253890.2011.572207. [DOI] [PubMed] [Google Scholar]
- 11.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y, Westenbroek C, Bakker P, Termeer J, Liu A, Li X, Ter Horst GJ. Effects of long-term stress and recovery on the prefrontal cortex and dentate gyrus in male and female rats. Cereb Cortex. 2008;18:2762–2774. doi: 10.1093/cercor/bhn035. [DOI] [PubMed] [Google Scholar]
- 13.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 1999;64:705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 15.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 16.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 17.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- 19.Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci U S A. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, et al. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- 22.Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, et al. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res. 2009;202:114–121. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Yanpallewar SU, Fernandes K, Marathe SV, Vadodaria KC, Jhaveri D, Rommelfanger K, et al. Alpha2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci. 2010;30:1096–1109. doi: 10.1523/JNEUROSCI.2309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic anti-depressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, Vaidya VA. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32:1504–1519. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- 27.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 28.Jayani RS, Ramanujam PL, Galande S. Studying histone modifications and their genomic functions by employing chromatin immuno-precipitation and immunoblotting. Methods Cell Biol. 2010;98:35–56. doi: 10.1016/S0091-679X(10)98002-3. [DOI] [PubMed] [Google Scholar]
- 29.Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Yu J, Mao-Ying QL, Mi WL, Li B, Wang YQ, et al. Repeated clomipramine treatment reversed the inhibition of cell proliferation in adult hippocampus induced by chronic unpredictable stress. Pharmacogenomics J. 2008;8:375–383. doi: 10.1038/sj.tpj.6500485. [DOI] [PubMed] [Google Scholar]
- 34.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 35.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 38.Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampusspecific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beery AK, Francis DD. Adaptive significance of natural variations in maternal care in rats: A translational perspective. Neurosci Biobehav Rev. 2011;35:1552–1561. doi: 10.1016/j.neubiorev.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39:372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 45.Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Good MA, Barnes P, Staal V, McGregor A, Honey RC. Context-but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci. 2007;121:218–223. doi: 10.1037/0735-7044.121.1.218. [DOI] [PubMed] [Google Scholar]
- 47.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2009;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greisen MH, Altar CA, Bolwig TG, Whitehead R, Wörtwein G. Increased adult hippocampal brain-derived neurotrophic factor and normal levels of neurogenesis in maternal separation rats. J Neurosci Res. 2005;79:772–778. doi: 10.1002/jnr.20418. [DOI] [PubMed] [Google Scholar]
- 54.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 55.Aisa B, Elizalde N, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: Implications for spatial memory. Hippocampus. 2009;19:1222–1231. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- 56.Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, et al. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.