Abstract
Varicella zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus. Primary infection causes varicella (chickenpox), after which the virus becomes latent in ganglionic neurons along the entire neuraxis. With advancing age or immunosuppression, cell-mediated immunity to VZV declines, and the virus reactivates to cause zoster (shingles), dermatomal distribution, pain, and rash. Zoster is often followed by chronic pain (postherpetic neuralgia), cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis, and multiple ocular disorders. This review covers clinical, laboratory, and pathological features of neurological complications of VZV reactivation, including diagnostic testing to verify active VZV infection in the nervous system. Additional perspectives are provided by discussions of VZV latency, animal models to study varicella pathogenesis and immunity, and of the value of vaccination of elderly individuals to boost cell-mediated immunity to VZV and prevent VZV reactivation.
Keywords: Varicella zoster virus, Nervous system, Latency, Pathogenesis, Immunity, Immunization, Animal model, Variegate neurological manifestations
Introduction
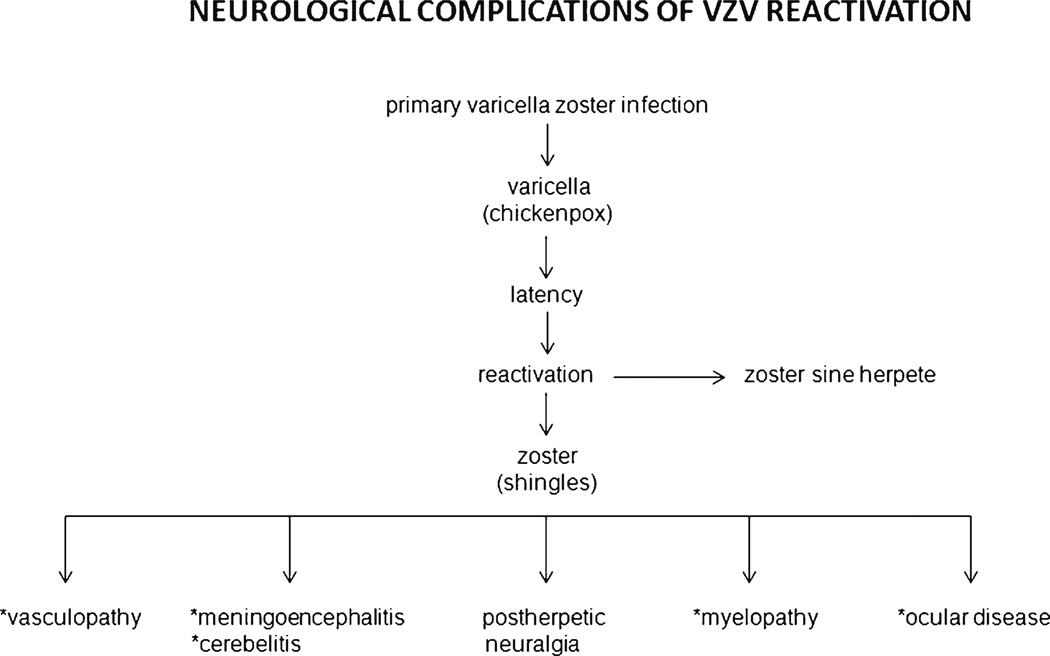
Varicella zoster virus (VZV), an exclusively human neurotropic alphaherpesvirus, causes varicella (chickenpox). The virus then becomes latent in ganglionic neurons along the entire neuraxis. With advancing age or immunosuppression, cell-mediated immunity to VZV declines and the virus reactivates to cause herpes zoster (shingles), which is often complicated by chronic pain [postherpetic neuralgia (PHN)], cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis and cerebellitis, myelopathy and multiple ocular disorders (Fig. 1). VZV reactivation also produces chronic radicular pain without rash (zoster sine herpete).
Fig. 1.
Neurological complications of varicella zoster virus VZV reactivation. (With permission from Gilden D, Mahalingam R, Nagel MA, Pugazhenthi S, Cohrs RJ. The neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441– 63) [52]
Neurological Complications of VZV Reactivation
Zoster
VZV reactivation is usually manifest by a painful dermatomal distribution vesicular eruption on an erythematous base, as well as unpleasant sensations (dysesthesias) produced by touch (allodynia). Rash and pain usually develop within a few days of each other, although pain can precede rash by weeks to months [1] Because VZV becomes latent in cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia, zoster can develop anywhere on the body. Zoster most frequently occurs in the elderly [2] as cell-mediated immunity to VZV declines. Other groups at risk for zoster are organ transplant recipients and immunocompromised patients with cancer or AIDS [3]. Zoster in an otherwise healthy young person may be the first manifestation of HIV infection [4]. Varicella in infancy also predisposes to zoster in early adulthood.
Zoster is associated with optic neuritis and ophthalmoplegia. Involvement of the maxillary and mandibular distribution of the trigeminal nerve can produce osteonecrosis and spontaneous tooth exfoliation. Geniculate zoster causes weakness or paralysis of ipsilateral facial muscles. Facial palsy and vesicles in the external auditory canal or on the tympanic membrane (zoster oticus), or on the ipsilateral anterior two-thirds of the tongue or hard palate constitutes Ramsay Hunt syndrome; Ramsay Hunt syndrome is frequently associated with tinnitus, hearing loss, nausea, vomiting, vertigo, and nystagmus, indicating involvement of cranial nerve VIII within the bony facial canal. Zoster is also followed by involvement of cranial nerves IX, X, XI, and XII.
Cranial neuropathies occur days to weeks after zoster and multiple cranial nerves are frequently affected. This is best explained by the likelihood that virus particles spread transaxonally along trigeminal and other ganglionic afferent fibers to cause occlusion of small vessels supplying cranial nerves in the same manner that produces VZV vasculopathy in larger arteries (see ‘VZV vasculopathy’ section). Because cranial neuropathies frequently occur weeks after zoster, disease may be due to micro-infarction of cranial nerves. The blood supply of cranial nerves III, IV, V1 and VI comes from the internal carotid circulation, while V2, V3 and VII, IX, X, XI and XII are supplied by the external carotid circulation [5]. Trigeminal- [6••] and facial-distribution zoster, as well as polyneuritis cranialis due to VZV, may occur in the absence of rash.
Zoster paresis (weakness) may present with arm weakness or diaphragmatic paralysis after cervical distribution rash; leg weakness after lumbar or sacral distribution rash; or urinary retention after sacral distribution zoster. Magnetic resonance imaging (MRI) of patients with zoster paresis reveals involvement of both anterior and posterior roots at spinal levels, corresponding to clinical deficit. Rarely, clinical deficit in cervical zoster paresis extends to the brachial plexus, confirmed by both electrodiagnostic testing and MRI. Thoracic zoster has been associated with abdominal muscle weakness, resulting in abdominal hernia.
The cardinal pathological features of zoster are characterized by inflammation and hemorrhagic necrosis with associated neuritis, localized leptomeningitis, unilateral segmental poliomyelitis, and degeneration of related motor and sensory roots [7••]. Demyelination is seen in areas with mononuclear cell (MNC) infiltration and microglial proliferation. Intranuclear inclusions, viral antigen, and herpesvirus particles are found in acutely infected ganglia. Antiviral drugs speed healing of rash and shorten the duration of acute pain. Immunocompromised patients should be treated with valacyclovir (1 g three times daily for 7 days); if rash does not resolve within a few days, these patients may need intravenous acyclovir (5–10 mg/kg three times daily for 5–7 days).
PHN
Dermatomal distribution pain persisting for more than 3 months after zoster constitutes PHN. Age is the most important factor in predicting the development of PHN. More than 40 % of zoster patients >60 years of age experience chronic pain. Analysis of ganglia from an early case of PHN of 2.5 months’ duration revealed diffuse and focal infiltration by chronic inflammatory cells—an observation confirmed by Watson et al. [8•], who found prominent collections of lymphocytes in ganglia from a patient with PHN of 2 years’ duration. The inflammatory response in ganglia raised the possibility of prolonged viral infection. Further evidence that PHN may be produced by low-level ganglionitis has come from the detection of VZV DNA and proteins in blood MNCs of many patients with PHN and from the favorable response of some PHN patients to antiviral treatment.
PHN is difficult to manage, and no universal treatment exists. First-line therapies include tricyclic antidepressants, gabapentin and pregabalin, and topical lidocaine patches. Opioids, tramadol, capsaicin cream, and the capsaicin 8 % patch are second- or third-line therapies. A newer, potentially promising treatment for PHN is percutaneous peripheral nerve field stimulation. Rare reports indicate its effectiveness for refractory PHN. Patients became pain-free with minimalto-no medication needed after ophthalmic, cervical, and thoracic distribution PHN.
VZV Meningitis, Meningoencephalitis, Meningoradiculitis, and Cerebellitis
All these neurological complications of VZV reactivation can occur after zoster. Like VZV vasculopathy, they also occur in the absence of zoster rash, as demonstrated by reports of VZV meningitis [9], meningoradiculitis [10], and cerebellitis [11], in which diagnosis was confirmed by the detection of VZV DNA or anti-VZV antibody or both in CSF.
VZV myelopathy
VZV myelopathy can present as a self-limiting, monophasic spastic paraparesis, with or without sensory features and sphincter problems. This so-called post-infectious myelitis usually occurs in immunocompetent patients, days to weeks after acute varicella or zoster. Its pathogenesis is unknown. The CSF usually contains a mild mononuclear pleocytosis, with a normal or slightly elevated protein. Steroids are used to treat these patients, although some improve spontaneously. VZV can also invade the spinal cord. In such instances, VZV myelopathy presents as an insidious, progressive, and sometimes fatal myelitis, mostly in immunocompromised individuals, such as patients with AIDS. MRI reveals longitudinal serpiginous enhancing lesions. Diagnosis is confirmed by the presence of VZV DNA or anti-VZV IgG, or both in cerebrospinal fluid (CSF) [12]. Pathological and virological analyses of the spinal cord from fatal cases have revealed frank invasion of VZV in the parenchyma [13] and, in some instances, spread of virus to adjacent nerve roots. Early diagnosis and aggressive treatment with intravenous acyclovir have been helpful, even in immunocompromised patients [14]. Rarely, VZV myelitis recurs, even in immunocompetent patients [12]. VZV myelitis also occurs in the absence of zoster rash. VZV can also produce spinal cord infarction identified by diffusion-weighted MRI and confirmed virologically [15]. Thus, VZV vasculopathy can cause stroke in the spinal cord, as well as in the brain.
VZV Vasculopathy
VZV vasculopathy is caused by productive infection of cerebral arteries resulting in transient ischemic attacks, and ischemic and hemorrhagic stroke. While the exact incidence is unknown, it is most likely more common than previously believed given recent studies which showed that patients with herpes zoster have a 30 % increased risk of stroke within the following year [16], and a 4.5-fold increased risk with ophthalmic-distribution zoster [17].
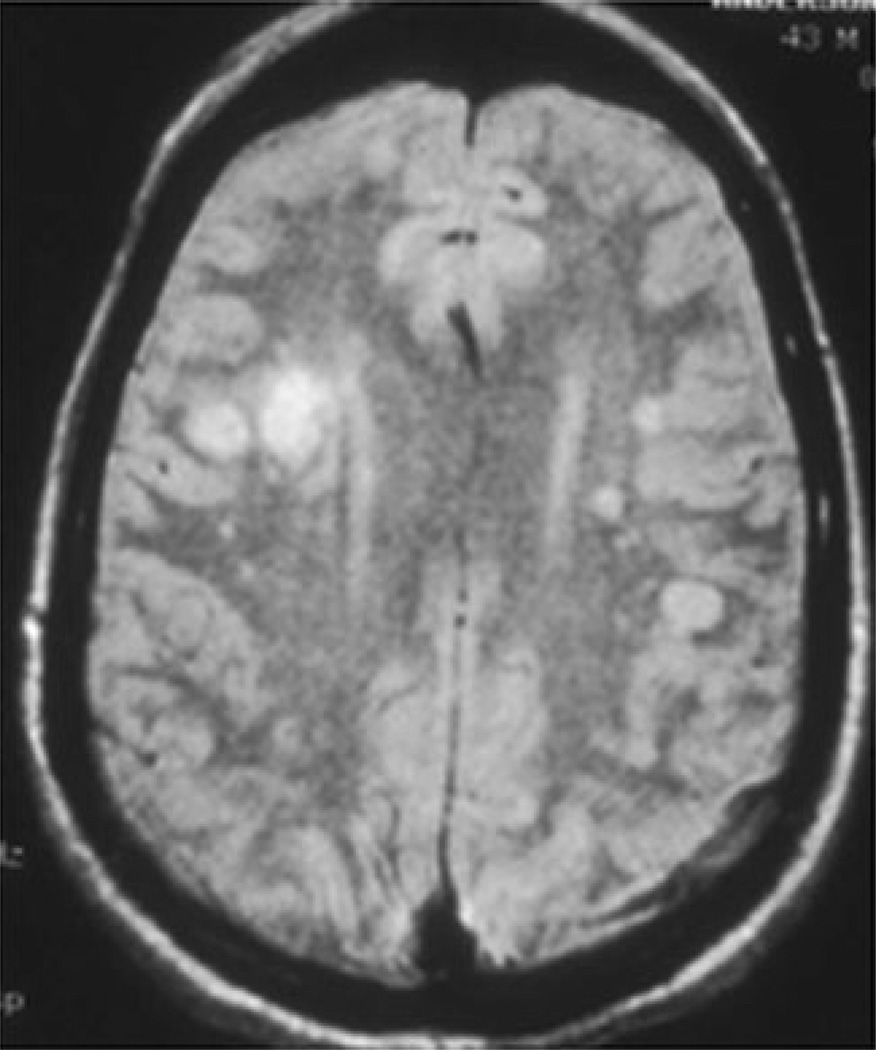
A study of 30 patients with virologically confirmed VZV vasculopathy [18••] revealed that rash was present in 63 %, CSF pleocytosis in 67 %, and imaging abnormalities in 97 % of the 30 patients (Fig. 2) Angiography revealed abnormalities in 70 % of patients. Large and small arteries were involved in 50 %, small arteries in 37 %, and large arteries in only 13 % of 30 patients. The average time from rash to neurological symptoms and signs was 4.1 months [18••]. The CSF of 30 % of patients contained VZV DNA; in contrast, 93 % had anti-VZV IgG antibody in CSF with a reduced serum/CSF ratio of anti-VZV IgG that confirmed intrathecal synthesis of anti-VZV IgG. Thus, detection of anti-VZV IgG antibody in CSF is the best test to diagnose VZV vasculopathy [19]. VZV vasculopathy is treated with intravenous acyclovir. Importantly, diagnosis of this treatable cause of stroke is often missed because there is no history of zoster rash in one third of patients, the CSF is normal in one third of subjects, there is an average 4.2-month delay from zoster to neurological symptoms and signs, and VZV DNA is often not present in CSF.
Fig. 2.
Brain magnetic resonance image (MRI) of a patient with varicella zoster virus multifocal vasculopathy. Proton-density brain MRI scan shows multiple areas of infarction, particularly involving gray–white matter junctions, in both hemispheres.(With permission from Gilden DH, Mahalingam R, Cohrs RJ, Kleinschmidt-DeMasters BK, Forghani B. The protean manifestations of varicella-zoster vasculopathy. J Neurovirol 2002;8(Suppl. 2):75–9) [53]
When VZV reactivates from cranial nerve ganglia, it most likely spreads transaxonally to the outermost layer of the artery wall (adventitia, adventitia-media region), a notion supported by detection of VZV infection in adventitia in early VZV vasculopathy [20•]. VZV infection of cerebral arteries is associated with a thickened intima composed of myofibroblasts, which can potentially lead to ischemia, a disrupted internal elastic lamina, and a paucity of smooth muscle cells, which can result in aneurysm formation and hemorrhage [20•]. Furthermore, inflammatory cells (primarily CD4 and CD8 T-cells, and CD68 macrophages) are present predominantly in the adventitia and, to a lesser degree, in the luminal surface of the thickened intima [21•]. In early VZV vasculopathy, there is a striking number of neutrophils in the adventitia. A remarkable finding in a VZV-infected artery was the association of inflammation with a thickened intima, supporting findings in the cardiovascular and pulmonary vascular fields that inflammation is intimately involved in vascular remodeling.
Multifocal VZV Vasculopathy with Temporal Artery Infection
Recently, we encountered three patients with a novel variant of multifocal VZV vasculopathy with temporal artery infection. All three patients presented with ischemic optic neuropathy, followed by, in one instance, acute retinal necrosis. VZV infection of the ipsilateral temporal artery was found in all three patients. Importantly, these patients experienced symptoms, signs, and laboratory abnormalities characteristic of giant cell arteritis (GCA), a vasculitis of unclear etiology that is treated with corticosteroids; histopathological examination of their temporal arteries was negative for GCA. These cases illustrate that patients with suspected GCA, but whose arteries are pathologically negative for GCA, may have multifocal VZV vasculopathy with temporal artery infection. It is essential to differentiate GCA from multifocal VZV vasculopathy because treatment with corticosteroids for presumed GCA may potentiate VZV infection and lead to loss of vision. In contrast, patients with multifocal VZV vasculopathy require immediate antiviral treatment.
To further address the incidence of VZV infection in GCA biopsy-negative patients, 24 temporal arteries from patients with clinically suspected GCA, but whose biopsies were negative, were examined by immunohistochemistry for the presence of VZV antigen. Remarkably, five (21 %) were found to contain VZV [22••]. All five patients whose temporal arteries contained VZV antigen presented with clinical and laboratory features of GCA and early visual disturbances. Thirteen normal temporal arteries did not contain VZV antigen. Overall, multifocal VZV vasculopathy can present with the full spectrum of clinical features and laboratory abnormalities characteristically seen in GCA.
Zoster Sine Herpete (Radicular Pain in the Absence of Rash)
Zoster sine herpete was first described in a report of multiple patients with dermatomal distribution radicular pain in areas distinct from pain with rash in zoster [23]. The first two virologically confirmed cases of zoster sine herpete were verified by detection of VZV DNA in CSF [24•]. A third case of thoracic-distribution zoster sine herpete, in which electromyography of paraspinal muscles demonstrated frequent fibrillation potentials restricted to chronically painful thoracic root segments was confirmed by detection of VZV DNA in blood MNCs and anti-VZV IgG antibody in CSF [25]. Blumenthal et al. [26] recently described a patient with zoster sine herpete whose CSF did not contain amplifiable VZV DNA but did contain anti-VZV IgG with reduced serum/CSF ratios of anti-VZV IgG indicative of intrathecal synthesis. Perhaps the most compelling evidence that persistent radicular pain without rash can be caused by chronic active VZV ganglionitis came from analysis of a trigeminal ganglionic mass removed from an immunocompetent adult who had experienced relentless trigeminal-distribution pain for more than a year; pathological and virological analyses of the ganglionic mass revealed active VZV ganglionitis [6••].
Ocular Disease
VZV infection produces acute retinal necrosis (ARN) or progressive outer retinal necrosis (PORN). VZV is the most common cause of PORN, although herpes simplex virus (HSV) and cytomegalovirus can also cause this disease. Most cases are seen in AIDS patients with CD4+ T-cell counts <10 cells/mm3 of blood, as well as in other immunosuppressed individuals. PORN may be preceded by retrobulbar optic neuritis and aseptic meningitis [27], central retinal artery occlusion or ophthalmic-distribution zoster [28], and may occur together with multifocal vasculopathy or myelitis. PORN patients treated with ganciclovir alone or in combination with foscarnet had a better final visual acuity than those treated with acyclovir or foscarnet. The best treatment for PORN in AIDS patients may be prevention with highly active antiretroviral therapy. Like all neurological disorders caused by VZV, ocular disease caused by VZV can also occur in the absence of rash.
Diagnostic Tests
Examination of CSF and serum is necessary in patients with neurological disease caused by VZV, in the absence of rash. Routine cell count can be helpful, as a mild lymphocytic pleocytosis is characteristically found in VZV vasculopathy, myelitis and meningoencephalitis. Furthermore, increased red blood cells and neutrophils may also be seen when VZV infects the nervous system. In the absence of rash, CSF should be examined for VZV DNA by polymerase chain reaction (PCR), and for anti-VZV IgG and IgM. Importantly, many cases of VZV vasculopathy are protracted, and VZV DNA is only found ~30 % of the time [18••]. The detection of anti-VZV IgG antibody in CSF with intrathecal synthesis is superior to detection of VZV DNA in CSF to diagnose VZV vasculopathy [19], recurrent myelopathy, and brainstem encephalitis produced by VZV [29].
VZV Pathogenesis
VZV is highly contagious. While VZV DNA can be found on surfaces such as door knobs, toys, air-conditioning filters, and tables in rooms where children or adults have active varicella or zoster, enveloped VZV is highly sensitive to desiccation such that infection is typically acquired through inhalation of aerosolized virus. VZV is restricted to humans. After exposure, VZV infects Langerhans and plasmacytoid dendritic cells of the upper respiratory mucosa and nasopharyngeal region, with subsequent transfer of virus by these antigen-presenting cells to CD4 T-cells, which, in turn, transmit virus to dermal endothelial cells. During primary infection, VZVinfects ganglionic neurons, most likely hematogenously, as supported by the finding that ganglionic infection with simian varicella virus, a closely related neurotropic alphaherpesvirus, precedes rash [30•].
VZV Latency
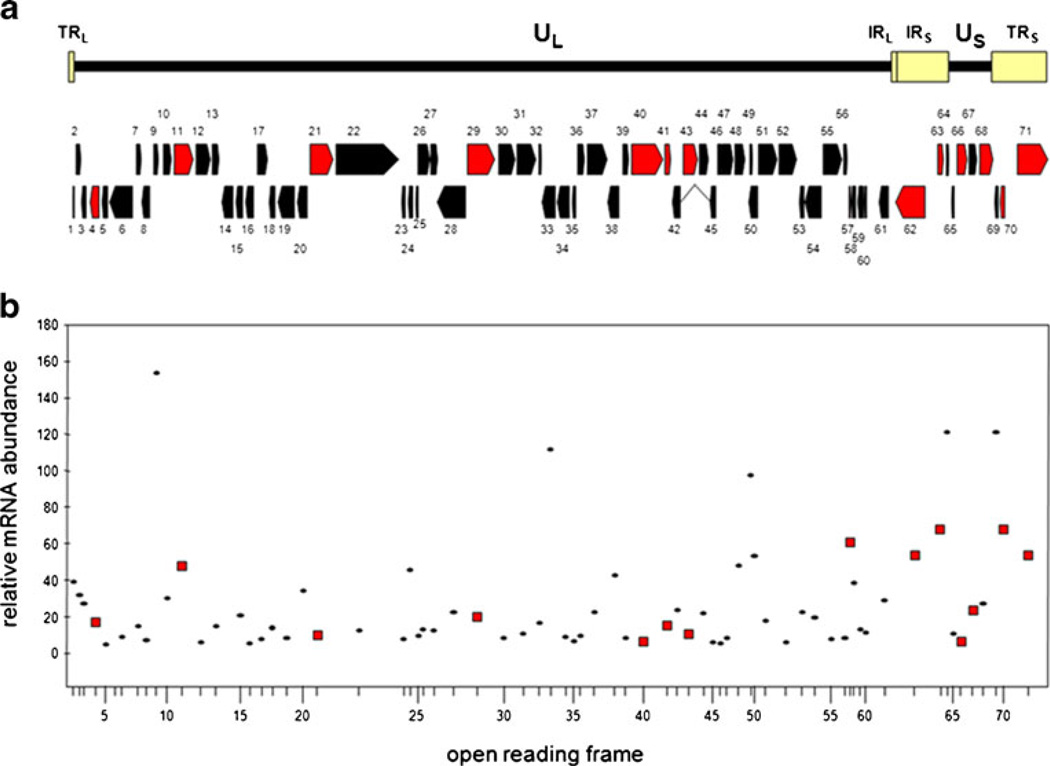
The linear double-stranded VZV DNA genome contains ~125,000 base pairs and consists of two unique regions each bound by invert repeats. Sequence analysis reveals 71 annotated open reading frames (ORFs) encoding 68 unique genes [31] (Fig. 3). All 68 VZV genes are transcribed during productive virus infection in tissue culture cells [32] where virus spreads from cell to cell, resulting in extensive cytopathologic effects and apoptotic cell death in 3–5 days [33]. However, during latency VZV transcription is limited, no cytopathologic effects are evident, and production of infectious virions is inhibited. Reactivation of latent VZV results in extensive virus gene expression, assembly, and release of infectious virus, and causes disease far more serious than primary infection. A full understanding of virus latency will help in design of therapies to mitigate disease associated with VZV reactivation.
Fig. 3.
The varicella zoster virus (VZV) genome, open reading frames (ORFs), and transcriptome in productively infected cells. The 125-kbp VZV genome is compose of long and short unique regions (UL and US), each containing terminally and internally located invert repeats (TRL, IRL and TRS, IRS). DNA analysis identifies 71 ORFs numbered consecutively from the left end of the virus genome. Relative location and direction of transcription is indicated by arrows, and ORFs transcribed in latently infected human ganglia are identified in red (A). ORFs 62–64 are located within TRS and are also present in IRS as ORFs 69, 70, and 71. The abundance of each ORF (except ORF 14) was determined and compared with the abundance of housekeeping genes actin and GAPdH (B). VZV ORFs transcribed in latently infected human ganglia are identified by red squares
VZV becomes latent in geniculate, vestibular, trigeminal, cervical, thoracic, and sacral ganglia [34, 35••, 36, 37]. During latency, the virus DNA termini join to form an “endless” molecule [38] with 35–3,500 copies present per 100 ng ganglionic DNA [39, 40•]. Owing to the paucity of virus mRNA in human ganglia, identification of the full extent of VZV gene transcription during latency required development of multiplex PCR analysis capable of detecting all 68 unique virus gene transcripts in five reactions [41]. Application of this novel technology to human trigeminal ganglia shows that VZV transcription during latency is unique among alphaherpesviruses. While all other neurotropic alphaherpesviruses, i.e., HSV types 1 and 2, bovine herpesvirus, equine herpesvirus, and pseudorabies virus, encode similarly located latency associated transcripts, at least 12 VZV genes located throughout the genome are transcribed during latency (Fig. 3) [20•]. This genome-wide search of VZV transcripts confirmed our earlier studies, which showed a unique pattern of latent virus gene transcription, of which ORF 63 is the VZV gene most abundantly and prevalently transcribed [42].
Transcription of VZV genes requires assembly of a functional transcription complex on the virus promoter. The first virus genes transcribed during productive infection belong to the immediate-early (IE) class and have promoters with weak basal activity. This low abundant IE gene transcription is enhanced by IE62, the major VZV transcription cofactor located within the virion. IE62 binds to host transcription factors and enhances VZV gene transcription. However, during herpesvirus latency, incoming virus is not present to supply essential IE transcription factors. Additionally, during latency, herpesvirus DNA contains multiple tightly-bound histone complexes that limit transcription complex access to virus promoters. Thus, reactivation is initiated when the latent virus genome undergoes transformation from transcriptionally silent (heterochromatic) to transcriptionally active (euchromatin).
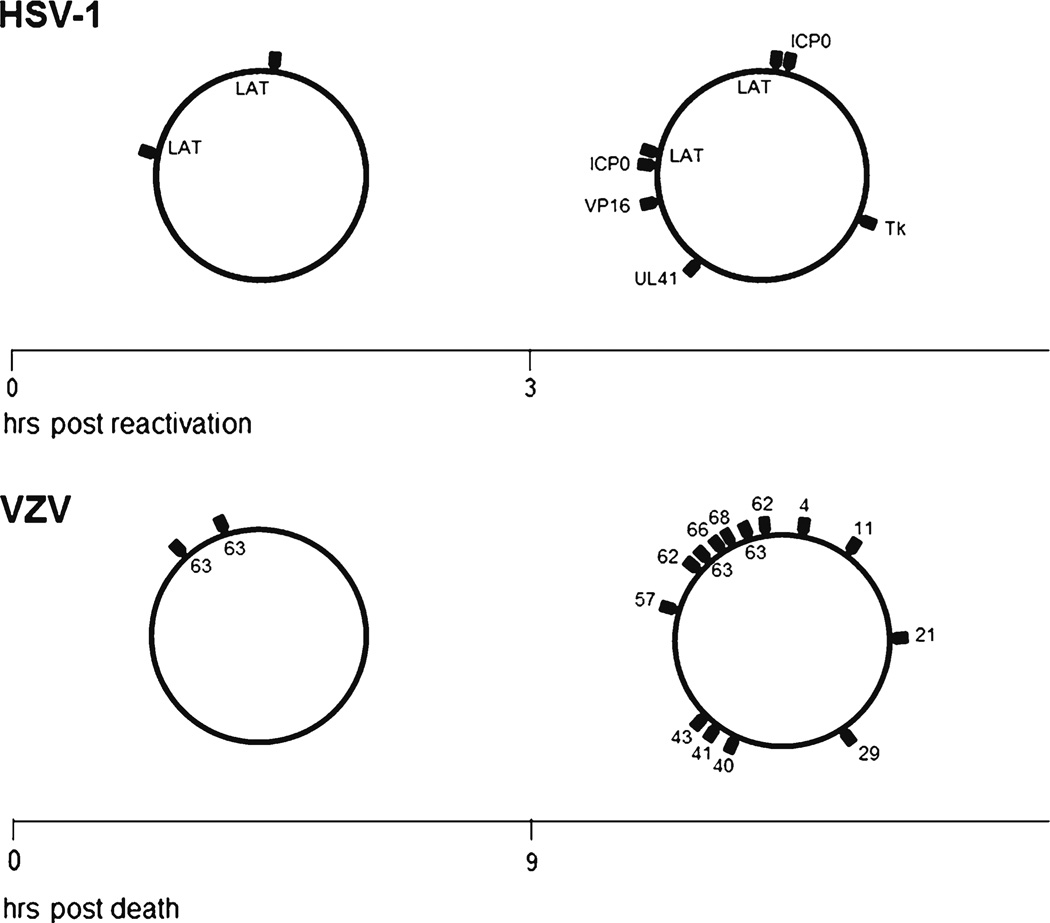
HSV-1, the prototype human neurotropic alphaherpesvirus, has been studied extensively. During latency, both HSV-1 DNA and VZV DNA are episomal, and most virus genes are not transcribed. The mechanism by which transcription of alphaherpesvirus genes is controlled during latency is under investigation. Current evidence indicates that virus genes transcribed during latency are associated with histones that contain euchromatic post-translation modifications [43]. Promoters for VZV and HSV-1 genes not transcribed during latency lack histones with euchromatic post-translational modification, and, in the case of HSV-1, contain histones with heterochromatic post-translational modifications. During HSV-1 reactivation, heterochromatic histone modifications are lost and euchromatic histone modifications are gained with increased virus transcription. The transition from heterochromatin to euchromatin is not localized to a specific region of the HSV-1 genome, but results in generalized deregulation of latent virus gene transcriptional control and transcription of multiple virus genes located throughout the latent virus episome. The general loss of HSV-1 transcription regulation observed early during virus reactivation is also seen in human ganglia latently infected with VZV (Fig. 4). VZV transcription in human ganglia removed less than 9 h after death is limited to ORF 63, whereas after 9 h multiple VZV ORFs are transcribed [44]. Consequently, generalized virus deregulation of gene transcription seen in human ganglia latently infected with VZV is similar to generalized virus deregulation of gene transcription seen in mouse ganglia latently infected with HSV-1. This generalized deregulation of virus gene transcription, named animation, is the first stage of virus reactivation and can initiate events leading to release of infectious virus or re-establishment of virus gene regulation and a return to latency. In either instance, regulation of latent VZV (and HSV-1) gene transcription is key to virus reactivation, and novel “epigenetic therapies” could potentially mitigate disease associated with virus reactivation.
Fig. 4.
Herpes simplex virus-1 (HSV-1) and varicella zoster virus (VZV) transcription after death. Mouse trigeminal ganglia latently infected with HSV-1 were explanted and virus transcripts detected by reverse-transcription quantitative polymerase chain reaction (PCR). Before 3 h post-explantation, latency associated transcripts (LAT) are transcribed from the episomal genome. Three h post-explantation, multiple transcripts are detected. Human trigeminal ganglia were removed at various times post-mortem and VZV transcripts detected by reverse transcription multiplex PCR [54••]. Before 9 h after death, transcripts for only VZV ORF 63 were detected from the virus episome. Nine h post-mortem, multiple VZV transcripts were detected
VZV-Specific Immunity
Primary VZV infection is followed by production of VZV-specific antibody and VZV-specific T-cell-mediated immunity. Antibodies to VZV glycoproteins I–IV and to three nonglycosylated proteins remain throughout life. VZV-specific antibodies are also present in some adults with no history of varicella or zoster, indicative of subclinical infection [45]. T-cell immunity to VZV is more important than the antibody response. For example, agammaglobulinemic humans who are unable to produce VZV-specific antibodies are protected against second episodes of varicella because of their ability to mount a VZV-specific T-cell-mediated immune response. Furthermore, individuals with T-cell immune deficiency disorders have more severe disease than normal hosts, and in stem cell transplant recipients who received inactivated VZV vaccine, protection was correlated with VZV-specific T-cell immunity, not with anti-VZV antibody.
VZV-specific T-cell-mediated immunity maintains VZV latency in ganglia. The immune response is also boosted by subclinical reactivation of latent virus or environmental exposure to virus. Importantly, the incidence of zoster increases with age as VZV-specific T-cell-mediated immunity declines. The frequency of VZV-specific memory CD4 T-cells is significantly influenced by age. CD4 T-cells decrease during the first 3 years after varicella. A comparison of the cell-mediated immune response to VZV antigen in vitro in young adults and individuals >60 years of age revealed fivefold fewer CD4 cells producing interferon-gamma or interleukins 4 and 5, as well as fewer CD4 early effectors and CD8 effector memory cells in the older group.
While reduced cell-mediated immunity to VZV with age or after exposure to immunosuppressive regimens in cancer patients or bone marrow transplant recipients often results in VZV reactivation, virus-specific T-cells are rarely seen in human ganglia latently infected with VZV. Analysis of human ganglia from donors who had zoster 1–5 months before death revealed VZV glycoprotein E in neurons and infiltration of non-cytolytic CD8+ T cells, but neurons that were positive for VZV glycoprotein E were neither positive for major histocompatibility complex class I antigens nor surrounded by T-cells, suggesting that immune control of virus reactivation may not depend on direct contact with Tcells. As VZV has been shown to downregulate major histocompatibility complex class I surface expression, virus latency is probably regulated by an innate immune response involving cytokines or chemokines. C-X-C motif chemokine 10 has been proposed as a potential driver of T-cell recruitment, based on its detection and that of its receptor (C-X-C motif receptor 3) in human ganglia from zoster patients. Recognition of the essential role of cell-mediated immunity to VZV for protection against and recovery from varicella and zoster has led to studies designed to boost the cell-mediated immune response to VZV by immunization of elderly adults (see below).
Prevention of VZV Reactivation
Zostavax (highly potent attenuated VZV) is indicated for prevention of zoster in individuals aged 60 years and older. Zoster vaccine resulted in increased numbers of CD4 and CD8 cells, CD4 and CD8 effector memory T-cells, and CD8 early-effector T-cells; the half-life of the boost in T-cell immunity to VZV is at least 5 years. Zoster vaccine also boosts VZV-specific immunity in adults with a history of zoster before vaccination or with chronic illness.
The Shingles Prevention Study (SPS) of the licensed zoster vaccine was a placebo-controlled, double-blind study of more than 38,000 adults over the age of 60 years, and randomized to receive either zoster vaccine or placebo. All participants were monitored for zoster. Endpoints included the burden of illness due to zoster and zoster-associated pain, as well as the incidence of clinically significant PHN. Participants received a single dose of Zostavax (n=19,270) or placebo (n=19,276). Racial distribution across both vaccination groups was similar: Caucasian (95 %), African-American (2 %), Hispanic (1 %), and other (1 %). The gender distribution was 59 % men and 41 % women in both groups. The most common side effects reported by participants after zoster vaccination were redness, pain, itching, swelling, warmth, or bruising at the injection site, and sometimes headache. Varicella-like rashes at the injection site were more common in zoster vaccine than in placebo recipients (0.1 % versus 6.4 %; p <0.05).
After a mean follow-up of 3 years, the SPS found that Zostavax vaccine reduced the incidence of zoster by 51 %. Participants in the immunization group who developed zoster reported significantly less pain and discomfort than those in the placebo group, and PHN was less frequent (an overall 61 % lower burden of disease). While the vaccine group had a significantly greater risk of a serious adverse event (1.9 % versus 1.3 %) and experienced more adverse events at the injection site (48.3 % versus 16.6 %) than the placebo group during the first 42 days after vaccination, no significant differences were seen between the groups in the incidence of serious adverse vaccine-related events (both <0.1 %).
In the USA, the Center for Disease Control and Prevention Advisory Committee on Immunization Practices recommended zoster vaccine for all persons over the age of 60 years. By 2008, 3 years after zoster vaccine was licensed and recommended by the Advisory Committee on Immunization Practices for persons aged 60 years and older, less than 7 % of the age group in the USA was vaccinated [46]. This was owing to a combination of lack of patient awareness regarding the availability of a vaccine, physicians’ uncertainty about the duration of protection, and different cost-sharing plans for immunization. This is disappointing. The zoster vaccine should be universally administered to all individuals over the age of 50 years.
Efforts to Produce VZV Infection in Animals
VZV is an exclusively human alphaherpesvirus. Over the last two decades, several laboratories have attempted to develop experimental animal models that recapitulate the pathogenesis of VZV in humans. A satisfactory model of VZV latency must fulfill the following criteria: (i) presence of virus exclusively in ganglia; (ii) presence of virus only in neurons; (iii) ability to reactivate latent virus; and (iii) limited virus transcription. None of the small animal models fulfill these criteria. For example, experimental VZV infection of mice, rats, and rabbits results only in seroconversion in the absence of varicella. Furthermore, VZV DNA is found in both neurons and non-neuronal cells in ganglia, as well as in nonganglionic tissues 1 month after corneal inoculation of VZV in mice. Similarly, VZV DNA is found in both neurons and non-neuronal cells in ganglia of rats 1–3 months after footpad inoculation. While VZV infects human ganglia implanted under the kidney capsule of severe combined immunodeficient mice, the absence of an adaptive immune response in this model makes it difficult to study pathogenesis. Finally, VZV reactivation has not been demonstrated in any of these models.
Simian Varicella Virus Infection in Nonhuman Primates
Clinical, pathological, immunological, and virological features of simian varicella virus (SVV) infection of monkeys closely resemble human VZV infection. Like VZV in humans, primary SVV infection of monkeys produces varicella, followed by virus latency and spontaneous reactivation [47]. Experimental infection of monkeys with SVV has been used to demonstrate hematogenous spread of SVV to ganglia confirmed by the presence of virus DNA in ganglia in the absence of varicella rash [30•, 44]. Intrabronchial inoculation of rhesus macaques results in primary infection followed by establishment of latency, the molecular and immunological features of which closely mimic VZV infection in humans, including limited SVV transcription 3 months postinfection [48]. Experimental immunosuppression and stress result in clinical and subclinical reactivation of SVV in latently-infected African green monkeys and cynomolgus macaques [47, 49••]. Subclinical reactivation is also seen in irradiated, latently-infected rhesus macaques [50]. Recently, transient T-cell infiltration was found in ganglia with reactivated SVV in cynomolgus macaques and correlated with expression of CXCL10, a chemokine that recruits activated T-cells and natural killer cells [51].
Conclusion
VZV causes varicella (chickenpox). The virus then becomes latent in ganglionic neurons along the entire neuraxis. With advancing age or immunosuppression, cell-mediated immunity to VZV declines and the virus reactivates to cause herpes zoster (shingles), which is often complicated by chronic pain (PHN), cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis and cerebellitis, myelopathy, and multiple ocular disorders [52].
VZV reactivation also produces chronic radicular pain without rash (zoster sine herpete). In fact, all the neurologic and ocular complications of VZV reactivation may occur without rash. Most recently, multifocal VZV vasculopathy with temporal artery infection has been found in patients with symptoms, signs, and laboratory abnormalities characteristic of GCA, but whose arteries are pathologically negative for GCA. It is essential to differentiate GCA from multifocal VZV vasculopathy because treatment with corticosteroids for presumed GCA may potentiate VZV infection and lead to loss of vision. In contrast, patients with multifocal VZV vasculopathy require immediate antiviral treatment.
Examination of CSF and serum is necessary in patients with neurological disease caused by VZV. A CSF lymphocytic pleocytosis is characteristically found in VZV vasculopathy, myelitis, and meningoencephalitis. Furthermore, increased red blood cells and neutrophils may also be seen when VZV infects the nervous system. In the absence of rash, CSF should be examined for VZV DNA by PCR, and for anti-VZV IgG and IgM. Importantly, many cases of VZV vasculopathy are protracted and VZV DNA is only found ~30%of the time. The detection of anti-VZV IgG antibody in CSF with intrathecal synthesis is superior to detection of VZV DNA in CSF to diagnose VZV vasculopathy, recurrent myelopathy, and brainstem encephalitis produced by VZV.
T-cell immunity to VZV is more important than the antibody response. Agammaglobulinemic humans unable to produce VZV-specific antibodies are protected against second episodes of varicella because of their ability to mount a VZV-specific T-cell-mediated immune response, and individuals with T-cell immune deficiency disorders have more severe disease than normal hosts. In stem cell transplant recipients who received inactivated VZV vaccine, protection was correlated with VZV-specific T-cell immunity, not with anti-VZV antibody.
Zostavax (highly potent attenuated VZV) is indicated for prevention of zoster in individuals aged 60 years and older. The SPS found that the Zostavax vaccine reduced the incidence of zoster by 51 %. Serious adverse vaccine-related events were <0.1 %. Zoster vaccine should be universally administered to all individuals over the age of 50 years.
In latently-infected human ganglia, VZV transcription is limited and VZV ORF 63 is the most abundant and prevalent VZV gene transcribed. Generalized deregulation of virus gene transcription is the first stage of virus reactivation and can initiate events leading to release of infectious virus or re-establishment of virus gene regulation, and a return to latency. Regulation of latent VZV gene transcription is key to virus reactivation, and novel epigenetic therapies could potentially mitigate disease associated with virus reactivation.
SVV infection of monkeys is the best animal model of human VZV infection. Like VZV in humans, primary SVV infection of monkeys produces varicella followed by virus latency and spontaneous reactivation. Intrabronchial inoculation of rhesus macaques results in primary infection followed by establishment of latency, the molecular and immunological features of which closely mimic VZV infection in humans, including limited SVV transcription 3 months later. Experimental immunosuppression and stress result in clinical and subclinical reactivation of SVV in latently-infected African green monkeys and cynomolgus macaques.
Acknowledgments
This work was supported by NIH grants AG006127 to DG, AG032958 to DG, RJC and RM, NS082228 to RJC, and NS067070 to MAN.
We thank Marina Hoffman for editorial assistance and Lori DePriest for help with the manuscript preparation.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest All authors report no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Don Gilden, Departments of Neurology and Microbiology, University of Colorado School of Medicine, 12700 E. 19th Avenue, Box B182, Aurora, CO 80045, USA don.gilden@ucdenver.edu.
Maria A. Nagel, Department of Neurology, University of Colorado School of Medicine, Aurora, CO, USA
Randall J. Cohrs, Department of Neurology, University of Colorado School of Medicine, Aurora, CO, USA
Ravi Mahalingam, Department of Neurology, University of Colorado School of Medicine, Aurora, CO, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Gilden DH, Dueland AN, Cohrs R, et al. Preherpetic neuralgia. Neurology. 1991;41:1215–1218. doi: 10.1212/wnl.41.8.1215. [DOI] [PubMed] [Google Scholar]
- 2.Harnisch JP. Zoster in the elderly: clinical, immunologic and therapeutic considerations. J Am Geriatr Soc. 1984;32:789–793. doi: 10.1111/j.1532-5415.1984.tb06298.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilden DH, Cohrs RJ, Mahalingam R. Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol. 2003;16:243–258. doi: 10.1089/088282403322396073. [DOI] [PubMed] [Google Scholar]
- 4.Tyndall MW, Nasio J, Agoki E, et al. Herpes zoster as the initial presentation of human immunodeficiency virus type 1 infection in Kenya. Clin Infect Dis. 1995;21:1035–1037. doi: 10.1093/clinids/21.4.1035. [DOI] [PubMed] [Google Scholar]
- 5.Lapresle J, Lasjaunias P. Cranial nerve ischaemic arterial syndromes. Brain. 1986;109:207–15. doi: 10.1093/brain/109.1.207. [DOI] [PubMed] [Google Scholar]
- 6. Hevner R, Vilela M, Rostomily R, et al. An unusual cause of trigeminal-distribution pain and tumor. Lancet Neurol. 2003;2:567–572. doi: 10.1016/s1474-4422(03)00506-4. A remarkable case of an inflammatory mass that produced prolonged dermatomal- distribution pain caused by VZV in the absence of rash.
- 7. Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. A classic paper that lead to identification of dermatomes served by specific ganglia.
- 8. Watson CP, Deck JH, Morshead C, Van der Kooy D, Evans RJ. Postherpetic neuralgia: further post-mortem studies of cases with and without pain. Pain. 1991;44:105–117. doi: 10.1016/0304-3959(91)90124-G. Correlation of PHN with pathological changes in ganglia.
- 9.Habib AA, Gilden D, Schmid DS, Safdieh JE. Varicella zoster virus meningitis with hypoglycorrhachia in the absence of rash and in an immunocompetent woman. J Neurovirol. 2009;15:206–208. doi: 10.1080/13550280902725550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunson RN, Aitken C, Gilden D. A woman with acute headache and sacral dermatomal numbness. J Clin Virol. 2011;50:191–193. doi: 10.1016/j.jcv.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moses H, Nagel MA, Gilden DH. Acute cerebellar ataxia in a 41 year old woman. Lancet Neurol. 2006;5:984–988. doi: 10.1016/S1474-4422(06)70601-9. [DOI] [PubMed] [Google Scholar]
- 12.Gilden DH, Beinlich BR, Rubinstien EM, et al. Varicella zoster virus myelitis: an expanding spectrum. Neurology. 1994;44:1818–1823. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- 13.Kleinschmidt-DeMasters BK, Gilden DH. Varicella zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–780. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 14.de Silva SM, Mark AS, Gilden DH, et al. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–931. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- 15.Orme HT, Smith AG, Nagel MA, et al. VZV spinal cord infarction identified by diffusion-weighted magnetic resonance imaging (DWI) Neurology. 2007;69:398–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- 16.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2010;40:3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 17.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–797. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 18. Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging and virological features. Neurology. 2008;70:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. Comprehensive study of the VZV vasculopathies.
- 19.Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–1073. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- 20. Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77:364–370. doi: 10.1212/WNL.0b013e3182267bfa. Correlative analysis of viral and muscle cell markers in cerebral arteries from patients with VZV vasculopathy.
- 21. Nagel MA, Traktinskiy I, Stenmark KR, et al. Varicella zoster virus vasculopathy: Immune characteristics of virus-infected arteries. Neurology. 2013;80:62–68. doi: 10.1212/WNL.0b013e31827b1ab9. Analysis of the immune repertoire in arteries of patients with VZV vasculopathy.
- 22. Nagel MA, Bennett JL, Khmeleva N, et al. Multifocal VZV vasculopathy with temporal artery infection mimics giant cell arteritis. Neurology. 2013;80:17–21. doi: 10.1212/WNL.0b013e318294b477. Exciting new study indicating that the clinical features of multifocal VZV vasculopathy with temporal artery infection can be the same as seen in classic GCA.
- 23.Lewis GW. Zoster sine herpete. Br Med J. 1958;2:418–421. doi: 10.1136/bmj.2.5093.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilden DH,Wright RR, Schneck SA, Gwaltney JM, Jr, Mahalingam R. Zoster sine herpete, a clinical variant. Ann Neurol. 1994;35:530–533. doi: 10.1002/ana.410350505. Correlative clinical-virologic analysis proving that zoster sine herpete is produced by VZV.
- 25.Amlie-Lefond C, Mackin GA, Ferguson M, et al. Another case of virologically confirmed zoster sine herpete with electrophysiologic correlation. J Neurovirol. 1996;2:136–138. doi: 10.3109/13550289609146547. [DOI] [PubMed] [Google Scholar]
- 26.Blumenthal DT, Shacham-Shmueli E, Bokstein F, et al. Zoster sine herpete: virological verification by detection of anti-VZV IgG antibody in CSF. Neurology. 2011;76:484–485. doi: 10.1212/WNL.0b013e31820a0d28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco-Paredes C, Bellehemeur T, Merchant A, et al. Aseptic meningitis and optic neuritis preceding varicella zoster progressive outer retinal necrosis in a patient with AIDS. AIDS. 2002;16:1045–1049. doi: 10.1097/00002030-200205030-00011. [DOI] [PubMed] [Google Scholar]
- 28.Menerath JM, Gerard M, Laurichesse H, et al. Bilateral acute retinal necrosis in a patient with acquired immunodeficiency syndrome. J Fr Ophtalmol. 1995;18:625–633. [PubMed] [Google Scholar]
- 29.Haug A, Mahalingam R, Cohrs RJ, et al. Recurrent polymorphonuclear pleocytosis with increased red blood cells caused by varicella zoster virus infection of the central nervous system. J Neurol Sci. 2010;292:85–88. doi: 10.1016/j.jns.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahalingam R, Wellish M, Soike K, et al. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. The first study to show that during primary varicella infection, ganglia are infected hematogenously.
- 31.Davison AJ, Scott JE. The complete DNA sequence of varicella zoster virus. J Gen Virol. 1986;66:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 32.Cohrs RJ, Hurley MP, Gilden DH. Array analysis of viral gene transcription during lytic infection of cells in tissue culture with Varicella zoster virus. J Virol. 2003;77:11718–11732. doi: 10.1128/JVI.77.21.11718-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brazeau E, Mahalingam R, Gilden D, et al. Varicella zoster virus–induced apoptosis in MeWo cells is accompanied by down-regulation of Bcl-2 expression. J Neurovirol. 2010;16:133–140. doi: 10.3109/13550281003682547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furuta Y, Takasu T, Fukuda S, et al. Detection of varicella zoster virus DNA in human geniculate ganglia by polymerase chain reaction. J Infect Dis. 1992;166:1157–1159. doi: 10.1093/infdis/166.5.1157. [DOI] [PubMed] [Google Scholar]
- 35. Gilden DH, Vafai A, Shtram Y, et al. Varicella zoster virus DNA in human sensory ganglia. Nature. 1983;306:478–480. doi: 10.1038/306478a0. A classic paper that first proved that VZV DNA is latent in human ganglia.
- 36.Gilden DH, Gesser R, Smith J, et al. Presence of VZVand HSV-1 DNA in human nodose and celiac ganglia. Virus Genes. 2001;23:145–147. doi: 10.1023/a:1011883919058. [DOI] [PubMed] [Google Scholar]
- 37.Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden DH. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol. 1992;31:444–448. doi: 10.1002/ana.410310417. [DOI] [PubMed] [Google Scholar]
- 38.Clarke P, Beer T, Cohrs R, Gilden DH. Configuration of latent varicella zoster virus DNA. J Virol. 1995;69:8151–8154. doi: 10.1128/jvi.69.12.8151-8154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahalingam R, Wellish M, Lederer D, et al. Quantitation of latent varicella zoster virus DNA in human trigeminal ganglia by polymerase chain reaction. J Virol. 1993;67:2381–2384. doi: 10.1128/jvi.67.4.2381-2384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. First real-time PCR analysis of alphaherpesvirus transcription in human ganglia.
- 41.Nagel MA, Gilden D, Shade T, Gao B, Cohrs RJ. Rapid and sensitive detection of 68 unique varicella zoster virus gene transcripts in five multiplex reverse transcription-polymerase chain reactions. J Virol Methods. 2009;157:62–68. doi: 10.1016/j.jviromet.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella zoster virus genes in human ganglia. J Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gary L, Gilden DH, Cohrs RJ. Epigenetic regulation of varicella zoster virus open reading frames 62 and 63 in latently infected human trigeminal ganglia. J Virol. 2006;80:4921–4926. doi: 10.1128/JVI.80.10.4921-4926.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouwendijk W, Mahalingam R, Traina-Dorge V, et al. Simian varicella virus infection of Chinese rhesus macaques produces ganglionic infection in the absence of rash. J Neurovirol. 2012;18:91–99. doi: 10.1007/s13365-012-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vafai A, Wellish M, Gilden DH. Expression of varicella zoster virus in blood mononuclear cells of patients with postherpetic neuralgia. Proc Natl Acad Sci USA. 1988;85:2767–2770. doi: 10.1073/pnas.85.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu PJ, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years and older in the US 2008. Am J Prev Med. 2011;40:e1–e6. doi: 10.1016/j.amepre.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Mahalingam R, Traina-Dorge V, Wellish M, et al. Simian varicella virus reactivation in cynomolgus monkeys. Virology. 2007;368:50–59. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 48.Messaoudi I, Barron A, Wellish M, et al. Simian varicella virus infection of rhesusmacaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5:e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mahalingam R, Traina-Dorge V, Wellish M, et al. Latent simian varicella virus reactivates in monkeys treated with tacrolimus with or without exposure to irradiation. J Neurovirol. 2010;16:342–354. doi: 10.3109/13550284.2010.513031. First study revealing that virus reactivates in experimentally immunosuppressed primates latently-infected with SVV.
- 50.Kolappaswamy K, Mahalingam R, Traina-Dorge V, et al. Disseminated simian varicella virus infection in an irradiated rhesus macaque (Macaca mulatta) J Virol. 2007;81:411–415. doi: 10.1128/JVI.01825-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouwendijk WJD, Abendroth A, Traina-Dorge V, et al. T-cell infiltration correlates with CXCL10 expression in ganglia of cynomolgus macaques with reactivated simian varicella virus. J Virol. 2013;87:2979–2982. doi: 10.1128/JVI.03181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilden D, Mahalingam R, Nagel MA, Pugazhenthi S, Cohrs RJ. The neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441–463. doi: 10.1111/j.1365-2990.2011.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilden DH, Mahalingam R, Cohrs RJ, Kleinschmidt-DeMasters BK, Forghani B. The protean manifestations of varicella-zoster vasculopathy. J Neurovirol. 2002;8(Suppl 2):75–79. doi: 10.1080/13550280290167902. [DOI] [PubMed] [Google Scholar]
- 54. Nagel MA, Choe A, Traktinskiy I, et al. Varicella zoster virus transcriptome in latently infected human ganglia. J Virol. 2011;85:2276–2287. doi: 10.1128/JVI.01862-10. State-of-the-art analysis of the VZV transcriptome in latently infected human ganglia.