Abstract
Objective
To assure the accuracy and reliability of blood pressure measurement by non-invasive blood pressure monitor using Datascope Accutorr Plus™ (Paramus, NJ, USA) against mercury manometer, among adult male participants.
Method
Eighty participants from a family physician’s office at a teaching hospital were recruited. One hundred and sixty measurements of blood pressure were performed according to BHS technique protocol.
Statistical analysis
Descriptive analysis was done according to the AAMI and BHS protocol guidelines. The limits of agreement between the device and the standard were plotted using the method of Bland and Altman plot.
Results
The mean difference ± SD between the Datascope Acutorr Plus™ and observer was 2.7 ± 5.2 mm Hg and 1.5 ± 3.26 mm Hg for systolic and diastolic blood pressure, respectively. Datascope Acutorr Plus™ obtained A/A grading for both systolic and diastolic blood pressure.
Conclusion
Datascope Accutorr Plus™ (Paramus, NJ, USA) satisfies BHS and AAMI validation protocols for both systolic and diastolic BP and may be recommended for everyday use for BP monitoring at home and in clinical use for adult population.
Keywords: AAMI, BHS, BP devices, Datascope Acutorr plus, Validation
1. Introduction
The purpose of blood pressure measurement is to detect any changes from normal values, which may indicate disease. Measurement is also performed to monitor the effectiveness of medication and other methods used to control elevated blood pressure. According to the World Health Organization report hypertension affects about 600 million people all over world and is one of the leading causes of cardiovascular morbidity and mortality (The World Health Organization, 2002) and is associated with increased risk of stroke, myocardial infarction, congestive heart failure, peripheral vascular disease and renal dysfunction (Murray and Lopez, 1997).
At present, European Society of Hypertension, European Society of Cardiology and the seventh Joint National Committee guidelines stressed home blood pressure monitoring as a supplement to the measurements in the clinic to diagnose white coat hypertension and monitor treatment effectiveness.
The selection of a blood pressure measuring device may be influenced by many factors, but a fundamental requirement must be that it gives accurate measurements. Hence, British Hypertension Society (BHS) and Association for the Advancement of Medical Instrument (AAMI) have unanimously recommended that all semi-automated and automated blood pressure devices for measuring blood pressure should be independently validated (O’Brien et al., 2002; ANSI/AAMI SP10, 2002 Protocol, 2003).
Hundreds of automated devices are available in the market for the measurement of blood pressure but still there is an increasing demand and search for accurate devices (Ng and Small, 1994). Despite widespread use of automated devices, there is limited published evidence for their reliability and accuracy due to the complex protocols. Out of many only few studies conducted on the subject were according to the validation protocols (de Greeff et al., 2007; Alpert, 2007).
Our main objective of the present study is the determination of the accuracy and performance of the Datascope Acutorr Plus™ before to replace the Mercury Sphygmometer to overcome the human error and to standardize the blood pressure measurement in adult male out patient clinics. We therefore decided to evaluate the accuracy and reliability of the Datascope Acutorr Plus™, a non-invasive oscillometric BP monitor, using the mercury manometer reference standard and AAMI (Association for the Advancement of Medical Instrumentation, SP10: 2002 protocol guidelines) as well as BHS (British Hypertension Society) validation protocol standards.
2. Method
The Datascope Accutorr Plus™ (Paramus, NJ, USA) was tested on 80 participants according to BHS and AAMI requirements. Datascope Accutorr Plus™ (Paramus, NJ, USA) is an automatic oscillometric, non-invasive BP monitor for the arm. It is light weight, portable with user configurable technology. Inflations and deflations are controlled by an automatic system. It has optional infrared or predictive temperature and recorder modules. The power is supplied by lithium ion battery.
In this cross sectional study, 80 male adult participants of above 30 years age were recruited from a family physician’s office at King Khalid University hospital in the kingdom of Saudi Arabia during January–April 2009. Both Normotensive and hypertensive but free from atrial fibrillation or any sustained arrhythmia were included in the study. Study participation was limited to one visit. Local ethics committee approval was obtained for the study and all 80 participants recruited to the study were required to give written consent.
The Datascope Accutorr Plus™ was used to measure blood pressure, which was either preceded or followed by auscultatory measurement of blood pressure. The order of the measurement was randomized so that about half of the patients had their blood pressure measured by the auscultatory method first followed by the Datascope method and the remainder of the patients had their blood pressure measured vice versa. Interval of less than 1 min was taken between the mercury sphygmomanometer and Datascope measurements. Blood pressure measurements were performed by a family physician, highly expert in BP measurement and having normal auditory as well as visual acquity. A well maintained quality stethoscope was used for auscultatory method of blood pressure measurement.
For all BP measurements, each participant was seated with the back being supported for at least 5 min before measurement of blood pressure. Prior to examination, patients were advised not to talk. Blood pressure measurements were taken from the right arm with the relaxed arm slightly flexed and placed at heart level. Cuff size was selected according to arm circumference. Technique of BP measurements by mercury sphygmomanometer was according to British Hypertension Society (BHS) protocol.
2.1. Methods of validation
The principle of validation is based on the comparison between the level of the blood pressure obtained with the reference method, which is the mercury sphygmomanometer, and the device being validated.
To meet Advancement of Medical Instrumentation Association (AAMI) criteria, the difference between the two devices has to be ⩽5 mm of mercury (mean of the values) or standard deviation (SD) must be ⩽8 mm Hg3 (O’Brien et al., 2002).
The British Hypertension Society has a different way to validate the self measurement device: The device is classified with grade A–D, from best to worse device. The BHS grading is determined by calculating the percentage of differences between the device and observer that are within 5, 10 and 15 mm Hg. To meet BHS criteria devices must achieve a grade of at least B for both systolic and diastolic measurements. Grade A denotes greatest agreement with mercury standard and D denotes least agreement (Table 1) (O’Brien et al., 2002).
Table 1.
BHS grading criteria.
| Level | Absolute difference between devices
[mmHg(%)] |
||
|---|---|---|---|
| ⩽5 | ⩽10 | ⩽15 | |
| A | 60 | 85 | 95 |
| B | 50 | 75 | 90 |
| C | 40 | 65 | 85 |
| D | <C | ||
2.2. Statistical analysis
SPSS statistical package version 16.0 was used for descriptive analysis according to BHS and AAMI protocols of validation. The limits of agreement between the device and the standard were plotted using the method of Bland and Altman plot.
3. Results
The blood pressure measurements using the Datascope Accutorr Plus™, an oscillometric monitor, was compared to measurements taken using the cuff-stethoscope-mercury sphygmomanometer technique.
Total of 160 measurements were performed on 80 participants. Participants included in the study were in blood pressure range of 88–156 mmHg for systolic and 55–90 mmHg for diastolic pressures, respectively. The systolic blood pressure in 13 (16.2%) participants was ⩽100 mmHg and 6 (7.5%) participants had measurement ⩾150 mmHg. The diastolic measurement in 14 (17.5%) participants was ⩽60 mmHg and 5 (6.25%) participants had measurement of 90 mmHg.
According to the BHS protocol the difference between Datascope Accutorr Plus™ and mercury sphygmomanometer were 66.3%, 93.8% and 100% within 5, 10 and 15 mmHg, respectively (Table 2). Diastolic blood pressure differences were 86.3%, 97.5% and 100% within 5, 10 and 15 mmHg, respectively (Table 3).
Table 2.
Discrepancy between Datascope Accutorr Plus™ (device) and mercury sphygmomanometer (observer) measurement for systolic blood pressure.
| Absolute difference between device and observer (mmHg) | Percentage of difference between the device and observer | Cumulative percent |
|---|---|---|
| ⩽5 | 66.3 | 66.3 |
| ⩽10 | 27.5 | 93.8 |
| ⩽15 | 6.3 | 100 |
Table 3.
Discrepancy between Datascope Accutorr Plus™ (device) and mercury sphygmomanometer (observer) measurement for diastolic blood pressure.
| Absolute difference between device and observer (mmHg) | Percentage of difference between the device and observer | Cumulative percent |
|---|---|---|
| ⩽5 | 86.3 | 86.3 |
| ⩽10 | 11.3 | 97.5 |
| ⩽15 | 2.5 | 100 |
Hence, according to BHS protocol guidelines, Datascope Accutorr Plus™ (Paramus, NJ, USA) obtained A/A grading for systolic as well as diastolic blood pressure.
3.1. The Bland–Altman Plot of systolic blood pressure discrepancies between Datascope Acutorr Plus™ and mercury sphygmomanometer is shown in Fig. 1
Figure 1.
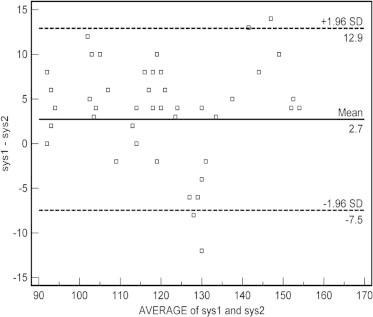
Bland–Altman scaterplot: Difference of systolic blood pressure between Datascope Accutorr Plus™ (sys1) and mercury sphygmomanometer (sys2).
Sample size (number of patients) = 80, Difference in mean systolic blood pressure values of two methods = 2.72, Standard deviation = 5.2 and 95% Confidence intervals for difference in mean = 1.56–3.88. It is expected most of the difference to lie between − 2s and + 2s, Hence the limits of agreement were obtained as:
Lower limit = −7.47; 95% CI = −9.46 to −5.48
Upper limit = +12.9; 95% CI = 10.93 to 14.90
The x-axis represents the average of the device and mercury sphygmomanometer measurement, in the range 90–170 mm Hg. The y-axis represents the difference between the device and mercury sphygmomanometer measurements. A positive value indicates that the device measurement is greater than the mercury sphygmomanometer’s measurement. The bold line shows the mean difference, and the dashed lines show the 95% limits of agreement. Ninety-five percent of discrepancies for systolic blood pressure were between −7.47 and +12.9 mm Hg (2 SD) (Fig. 1).
3.2. The Bland–Altman Plot of diastolic blood pressure discrepancies between Datascope Acutorr Plus™ and mercury sphygmomanometer is shown in Fig. 2
Figure 2.
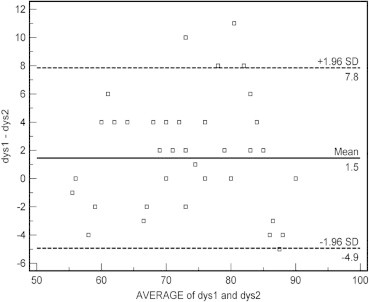
Bland–Altman scaterplot: Difference of diastolic blood pressure between Datascope Accutorr Plus™ (dys1) and mercury sphygmomanometer (dys2).
Sample size (number of patients) = 80. Difference in mean diastolic blood pressure values of two methods = 1.45. Standard deviation = 3.26 and 95% Confidence intervals for difference in mean = 0.72–2.17. It is expected most of the difference to lie between − 2s and + 2s, Hence the limits of agreement were obtained as:
Lower limit = −4.9; 95% CI = −6.2 to −3.7
Upper limit = +7.8; 95% CI = 6.6 to 9.1
The x-axis represents the average of the device and mercury sphygmomanometer measurement, in the range 50–100 mmHg. The y-axis represents the difference between the device and mercury sphygmomanometer measurements. A positive value indicates that the device measurement is greater than the mercury sphygmomanometer’s measurement. The bold line shows the mean difference, and the dashed lines show the 95% limits of agreement. Ninety-five percent of discrepancies for diastolic blood pressure were between −4.9 and +7.8 mmHg (2 SD) (Fig. 2).
To meet AAMI criteria the mean difference between the device and the mercury standard must be ⩽5 mmHg or the standard deviation must be ⩽8 mmHg.
In present study, the mean difference ± SD between the Datascope Acutorr Plus and mercury sphygmomanometer was 2.7 ± 5.2 and 1.5 ± 3.26 mmHg for systolic and diastolic blood pressure, respectively. Thus, in this study, Datascope Acutorr Plus satisfied AAMI protocol.
4. Discussion
In this study, analysis of 80 patients obtained using the Datascope oscillometric device shows that the Datascope Accutorr Plus™ fulfils the validation protocol of BHS & AAMI and could be replaced in clinical practice to overcome the human error.
Several limitations of this study may be considered in interpreting these findings. A limitation of this study is the lack of a strict adherence with BHS or AAMI protocols for validation due to the complex and extremely difficult to fulfill the conditions demanded.
In this study, patient’s distribution deviated from that expected for BHS and AAMI protocols. We have lower than expected number of participants with extreme BP values. The protocol of the AAMI needs minimum of 85 participants with 255 measurements. More than 10% of the participants were required to have a systolic blood pressure of ⩽100 mmHg and ⩾10% participants were required to have a systolic BP ⩾160 mmHg. More than 10% of the participants were required to have a diastolic BP of ⩽60 mmHg and ⩾10% participants were required to have a diastolic BP ⩾100 mmHg.
According to BHS protocol, minimum of 33 participants of both sexes with a fraction of extreme BP measurements and of three different ranges were needed and analysis was supposed to be in different phases.
Both protocols advised 3–4 observers to measure the BP for validation. In fact, it has been reported that recruiting participant with extremes of BP is difficult and some previous works about BHS grading adopted modified distributions of BP similar to this study and that work have been published and accepted by committees (Coleman et al., 2006; Braam et al., 2002; O’Brien, 2001; Cuckson and Reinders, 2002; Jones et al., 2000; Mee et al., 1998).
Findings of this study are comparable to those from other studies of simultaneous measurements using different devices, age group and clinical setups (de Greeff et al., 2007; Alpert, 2007; Zaetta et al., 2007; Wong et al., 2006; Jin et al., 2001; Myung et al., 1987; Reinders et al., 2006; Yarows, 2007). A numbers of reports contradict the findings of this study and many devices have been found to be inaccurate on formal testing (O’Brien et al., 1993; Coe and Houghton, 2002; Beaubien et al., 2002; Wattigney et al., 1996; Sun et al., 1996; O’Brien and Atkins, 1997).
In a review article, Ng and Small surveyed 423 automated devices, of which 161 were designed for self measurement (Ng and Small, 1994). O’Brien reported that only a fraction of the oscillometer devices available worldwide have been independently tested for validation according to BHS or/and AAMI protocol and only few of them passed and recommended for clinical as well as research purpose (O’Brien et al., 2001; O’Brien, 2001).
In conclusion, despite of many devices either not tested for validation or have been found to be inaccurate, datascope portable monitors are of the most popular automated devices used in clinical practice and hypertension research. It seems that purchasers and users are prepared to accept the word of manufacturers with regard to their accuracy and performance and to ignore warnings from the scientific literature as to their shortcomings. I would suggest that automated devices should be validated with particular care before to use in various studies and in clinical practice.
I also would recommend the use of Datascope Acutorr Plus™ (Paramus, NJ, USA) in clinical and research settings when accurate BP measurement is required.
5. Conclusion
According to BHS validation protocol, the Datascope Accutorr Plus™ achieved an overall A/A grade with 66.3%, 93.8% and 100% of the systolic pressures and 86.3%, 97.5% and 100% of the diastolic pressures being within 5, 10 and 15 mmHg standards. The device also met the requirements of the AAMI criteria with a mean difference of 2.72 ± SD 5.2 mmHg for systolic and mean difference of 1.45 ± SD 3.26 mmHg for diastolic BP respectively. The Bland Altman plot of BP discrepancies (95% CI) between Datascope and observer were between −7.5 and +12.9 mmHg (2 SD) for systolic and between −4.9 and + 7.8 mm Hg (2 SD) for diastolic pressure, respectively.
In summary, Datascope Accutorr Plus™ fulfilled the AAMI criteria and graded A for both systolic and diastolic blood pressure under the BHS protocol and may be recommended for every day use for BP monitoring at home and in clinical practice.
Acknowledgment
The author would like to thank Professor Zahid Shakoor for his kind advice after reviewing whole manuscript.
References
- Alpert, Bruce S. Validation of the Welch Allyn spot vital signs blood pressure device according to the ANSI/AAMI SP10: 2002. Accuracy and cost-efficiency successfully combined. Blood Pressure Monitoring. 2007;12(5):345–347. doi: 10.1097/MBP.0b013e3282c9abf7. [DOI] [PubMed] [Google Scholar]
- ANSI/AAMI SP10-2002 Protocol: Association for the Advancement of Medical Instrumentation, 2003 American National Standard. Electronic or automated sphygmomanometers ANSI/AAMI SP10-2002, 3330 Washington Boulevard, Suite 400, Arlington, VA 22201-4598, AAMI, USA.
- Beaubien E.R., Card C.M., Card S.E., Biem H.J., Wilson T.W. Accuracy of the Dinamap 1846 XT automated blood pressure monitor. Journal of Human Hypertension. 2002;16(9):647–652. doi: 10.1038/sj.jhh.1001463. [DOI] [PubMed] [Google Scholar]
- Braam R., De Maat C., Thien T. Accuracy of the Welch Allyn vital sign monitor52000 automatic blood pressure measuring device according to a modified BHS protocol. Blood Pressure Monitoring. 2002;7:185–189. doi: 10.1097/00126097-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Coe T.R., Houghton K. Comparison of the automated Dinamap blood pressure monitor with the mercury sphygmomanometer for detecting hypertension in the day case pre-assessment clinic. Ambulatory Surgery. 2002;10(15):9–15. [Google Scholar]
- Coleman A., Freeman P., Steel S., Sheenan A.H. Validation of the Omron 7051T BP monitoring device according to the BHS protocol. Blood Pressure Monitoring. 2006;11:27–32. doi: 10.1097/01.mbp.0000189788.05736.5f. [DOI] [PubMed] [Google Scholar]
- Cuckson A.C., Reinders A., Shabeeh H., Sheenan A.H. Validation of the Microlife 3BTO – a oscillometric blood pressure monitoring device according to a modified BHS protocol. Blood Pressure Monitoring. 2002;7:319–324. doi: 10.1097/00126097-200212000-00005. [DOI] [PubMed] [Google Scholar]
- de Greeff Annemarie, Reggiori, Fayrouge, Shennan, Andrew H. Clinical assessment of the Dinamap procure monitor in an adult population according to the BHS protocol. Blood Pressure Monitoring. 2007;12(1):51–55. doi: 10.1097/MBP.0b013e3280858b73. [DOI] [PubMed] [Google Scholar]
- Jin R., Donaghue K., Fairchild J., Chan A., Silink M. Comparison of Dinamap 8100 with sphygmomanometer blood pressure measurement in a prepubertal diabetes cohort. Journal of Paediatric and Child Health. 2001;37(6):545–549. doi: 10.1046/j.1440-1754.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- Jones C., Taylor K., Chowienczyk P., Poston L., Shennan Ah. A validation of the mobile O Graph (version 12) ambulatory blood pressure monitor. Blood Pressure Monitoring. 2000;5:233–238. doi: 10.1097/00126097-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Mee F., Atkin N., O’Brien E. Evaluation of the Profilomat 11 ambulatory blood pressure system according to the protocol of the BHS and AAMI. Blood Pressure Monitoring. 1998;3:353–361. [PubMed] [Google Scholar]
- Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349:1398–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Myung K., Park M.D., Shirley M., Menard R.N. Accuracy of blood pressure measurement by the Dinamap monitor in infants and children. Pediatrics. 1987;79(6):907–914. [PubMed] [Google Scholar]
- Ng K.-G., Small C.F. Survey of automated non-invasive blood pressure monitors. J Clin Eng. 1994;19:452–475. doi: 10.1097/00004669-199411000-00014. [DOI] [PubMed] [Google Scholar]
- O’Brien E. Proposal of simplifying the validation protocols of the BHS and the AAMI. Blood Pressure Monitoring. 2001;6:171–176. [Google Scholar]
- O’Brien E. State of the market in 2001 for blood pressure measuring devices. Blood Pressure Monitoring. 2001;6:171–176. doi: 10.1097/00126097-200108000-00001. [DOI] [PubMed] [Google Scholar]
- O’Brien E., Atkins N. Accuracy of the Dinamap portable monitor, model 8100: a review of the evidence for accuracy. Blood Pressure Monitoring. 1997;2:31–33. [PubMed] [Google Scholar]
- O’Brien E., Mee F., Atkins N., O’Malley K. Accuracy of the Dinamap portable monitor, model 8100 determined by the British Hypertension Society protocol. Journal of Hypertension. 1993;11:761–763. doi: 10.1097/00004872-199307000-00012. [DOI] [PubMed] [Google Scholar]
- O’Brien E., Waeber B., Parati G., Staessen J., Myers M. On behalf of the European Society of Hypertension Working Group on Blood Pressure Monitoring. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–536. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien E., Thomas Pickring, Roland Asmar, Martin Myers, Gian Fransco Parati, Jan Staessen. Working group on BP monitoring of the ESH international protocol for validation of BP measuring devices in adults. Blood Pressure Monitoring. 2002;7:3–17. doi: 10.1097/00126097-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Reinders A., Reggiori F., Shennan A.H. Validation of the DINAMAP ProCare blood pressure device according to the international protocol in an adult population. Blood Pressure Monitoring. 2006;11(5):293–296. doi: 10.1097/01.mbp.0000217998.96967.fb. [DOI] [PubMed] [Google Scholar]
- Sun M., Tien J., Jones R., Ward R. A new approach to reproducibility assessment: clinical evaluation of SpaceLabs Medical oscillometric blood pressure monitor. Biomedical Instrumentation and Technology. 1996;30:439–448. [PubMed] [Google Scholar]
- The World Health Organization The world health report: 2002, reducing risks, promoting healthy life. JAMA. 2002;288:1974. doi: 10.1001/jama.288.16.1974. [DOI] [PubMed] [Google Scholar]
- Wattigney W.A., Webber L.S., Lawrence M.D., Berenson G.S. Utility of an automatic instrument for blood pressure measurement in children. The Bogalusa heart study. American Journal of Hypertension. 1996;9:256–262. doi: 10.1016/0895-7061(95)00334-7. [DOI] [PubMed] [Google Scholar]
- Wong S.N., Tz Sung R.Y., Leung L.C. Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Pressure Monitoring. 2006;11(5):281–291. doi: 10.1097/01.mbp.0000209082.09623.b4. [DOI] [PubMed] [Google Scholar]
- Yarows S.A. Accuracy of the HoMedics BPA-300, a home blood pressure monitor using the auscultatory method. Blood Pressure Monitoring. 2007;12(5):339–343. doi: 10.1097/MBP.0b013e3282c9ac12. [DOI] [PubMed] [Google Scholar]
- Zaetta Vania, Daniele Longo, Perkovic Davor, Prattico Francesco, Barica Marlena, Perfetti Paola. Validation of the SAA-102 home blood pressure monitor according to the protocols of the European Society of Hypertensión, AAMI and the BHS. Blood Pressure Monitoring. 2007;12(6):363–368. doi: 10.1097/MBP.0b013e3282c9ad53. [DOI] [PubMed] [Google Scholar]


