Abstract
Purpose
To evaluate the efficacy of adjuvant chemoradiation therapy (CRT) for pancreatic adenocarcinoma patients ≥75 years of age.
Methods
The study group of 655 patients underwent pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma at the Johns Hopkins Hospital over a 12-year period (8/30/1993 to 2/28/2005). Demographic characteristics, comorbidities, intraoperative data, pathology data, and patient outcomes were collected and analyzed by adjuvant treatment status and age ≥75 years. Cox proportional hazards analysis determined clinical predictors of mortality and morbidity.
Results
We identified 166 of 655 (25.3 %) patients were ≥75 years of age and 489 of 655 patients (74.7 %) were <75 years of age. Forty-nine patients in the elderly group (29.5%) received adjuvant CRT. For elderly patients, node-positive metastases (p = 0.008), poor/anaplastic differentiation (p = 0.012), and undergoing a total pancreatectomy (p = 0.010) predicted poor survival. The 2-year survival for elderly patients receiving adjuvant therapy was improved compared with surgery alone (49.0% vs. 31.6%, p = 0.013); however, 5-year survival was similar (11.7% vs. 19.8%, respectively, p= 0.310). After adjusting for major confounders, adjuvant therapy in elderly patients had a protective effect with respect to 2-year survival (relative risk [RR] 0.58, p = 0.044), but not 5-year survival (RR 0.80, p = 0.258). Among the nonelderly, CRT was significantly associated with 2-year survival (RR 0.60, p < 0.001) and 5-year survival (RR 0.69, p < 0.001), after adjusting for confounders.
Conclusions
Adjuvant therapy after PD is significantly associated with increased 2-year but not 5-year survival in elderly patients. Additional studies are needed to select which elderly patients are likely to benefit from adjuvant CRT.
Keywords: Chemoradiation, Adjuvant therapy, Pancreatic cancer, Aging, Elderly
INTRODUCTION
The expanding elderly population of the United States is creating new demands on the medical system to serve the elderly. Current estimates place the number of individuals in United States aged 65 or older at nearly 35 million, with approximately 47% of these individuals aged 75 or older (1). The elderly population is expected to grow by more than 50% by 2050. It has been estimated that the increase in the elderly population will account for up to a 51% increase in the number of patients undergoing oncologic procedures by 2020 (2).
Pancreatic adenocarcinoma is a common cancer, with an estimated 42,470 cases diagnosed in the United States in 2009 (3). The disease is highly lethal, with approximately 95% patients dying within 5 years of diagnosis (4). Although the incidence of pancreatic adenocarcinoma is stable overall, the incidence of disease increases dramatically with age (5). Given the aging population of the country, the prevalence of pancreatic cancer can be expected to increase over the next half century. In fact, according to Surveillance, Epidemiology, and End Results data, 32% of patients who are diagnosed with pancreatic adenocarcinoma are at least 75 years old (6).
Research into the management of pancreatic adenocarcinoma has shown that age itself is not a contraindication to surgery, with no differences in morbidity, mortality, or reoperation found between elderly and nonelderly patients (7). Among the oldest patients treated with pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma, age has been found to contribute less than 1% to the outcome of death or a complication (8).
Most studies evaluating the tolerability of chemotherapy and radiation in elderly patients have been based on conventional outdated radiation delivery methods (9). More recent research suggests that radiation can be safely delivered to elderly patients and result in improved survival and quality of life (10, 11). We have found that adjuvant 5-fluorouracil (5-FU)-based chemoradiation therapy (CRT) results in improved survival (12). Whether this same benefit is maintained in elderly patients is unknown. Therefore, the purpose of this study was to evaluate the efficacy of adjuvant 5-FU-based CRT in elderly patients who underwent a curative resection for pancreatic cancer at the Johns Hopkins Hospital (JHH).
PATIENTS AND METHODS
Patient selection
Approval for this study was granted by the Johns Hopkins Hospital Institutional Review Board before data collection. Between August 30, 1993 and February 28, 2005, data were prospectively collected on all patients undergoing elective pancreaticoduodenectomy or total pancreatectomy at JHH. Distal pancreatectomy alone, duodenal, ampullary, bile duct adenocarcinomas, cystic neoplasms, and neuroendocrine tumors were excluded. A single pathologist reviewed all pathology specimens to ensure consistent interpretation of ductal adenocarcinoma histology, tumor margin, and nodal status (12).
Patients underwent a pylorus-preserving, classic, or total pancreaticoduodenectomy (PD). Lymph nodes were considered positive if any lymph node in the resection specimen contained tumor, whether it was involved by direct extension or was not contiguous with the primary tumor. Resection margins were considered positive if the tumor was present at the final pancreatic neck, uncinate process, bile duct, duodenal, or retroperitoneal soft tissue margin. Close margins were considered negative.
Nine hundred and eight patients underwent surgical resection for ductal adenocarcinoma at JHH. Patients were excluded from the final analysis if they were found to have T4 or M1 disease at the time of surgery (n = 16) or if death occurred ≤60 days after PD (n = 43). Patients were excluded if they received preoperative therapy (n = 19), if metastatic disease was identified before CRT (n = 17), if it was unknown whether they had received CRT (n = 44), or they received adjuvant chemotherapy or radiation alone (n = 73). Those missing data on age, race, sex, tumor size, node status, margin status, or histologic grade were also excluded (n= 41). The final study cohort includes 655 patients; 342 (52.2%) received no adjuvant CRT, 184 (28.1%) received 5-FU-based CRT at JHH, and 129 (19.7%) received 5-FU-based CRT at an outside hospital or clinic. Patient follow-up information was obtained from physical and electronic hospital charts. Survival was determined and cross-checked by review of clinical follow-up information, cancer center abstracting services, and the Social Security Death Index.
Patients were defined as elderly if the were aged 75 or more years at the time of surgery. Patients less than 75 years of age at the time of surgery were defined as. nonelderly.
Presurgical, intraoperative, and postoperative variables
Preoperative information such as weight loss, pain, jaundice, and comorbid conditions were collected. Comorbid conditions included coronary artery disease, chronic obstructive pulmonary disorder, cerebrovascular accidents, diabetes mellitus before surgical resection, and hypertension. Data describing postoperative complications were collected and described elsewhere (13).
Adjuvant therapy
Postoperatively, all patients were seen by a medical and radiation oncologist and offered JHH standard therapy, which consisted of continuous infusion or oral (capecitabine) 5-FU (86%) with radiation therapy followed by maintenance 5-FU (83%) for an additional 2–6 months. All patients who had a satisfactory recovery from the PD by postoperative Day 60 were encouraged to accept either standard or protocol therapy. Patients treated elsewhere were given the same recommendations before discharge as those patients treated at JHH. These recommendations were often communicated in a dictated consultation. Most patients who elected to receive no therapy did so after being informed fully about the potential risks and benefits of such therapy.
For those patients treated at JHH, the tumor bed and adjacent draining lymph nodes plus a 1.5- to 2-cm margin were used to cover possible microscopic extension and daily treatment setup error. The median daily fraction size and total dose were 180 cGy and 5,000 cGy, respectively. The majority of patients treated at JHH received a continuous course of radiation therapy without a planned break (79%). The details of therapy and toxicities could not be fully assessed for patients treated elsewhere.
Statistical analysis
Statistical analyses were performed using STATA, version 9.0 (Stata, College Station, TX). Tests of differences in patient characteristics by treatment group were performed using t tests and chi-square tests. For characteristics with individuals who were missing data, chi-square tests were performed including only those with known status, as indicated. The primary outcome variable was overall survival (OS), defined as the time from surgical resection for pancreatic adenocarcinoma to death. Patients were censored at March 29, 2007. Survival curves were estimated using the Kaplan-Meier method (14). Comparisons of overall survival between groups were made using the log-rank test. Median overall survival (in months) with 95% confidence intervals (CIs) was estimated for each treatment group stratified by risk group. The proportion of individuals surviving up to 2 and 5 years was calculated using life tables, with comparison by adjuvant treatment performed using the log-rank test with survival time censored at 2 and 5 years, respectively.
Proportional hazards models were used to examine the association with mortality of adjuvant treatment and other patient characteristics (15). To examine Cox proportional hazards analyses for 2-year survival, we censored follow-up at 24 months. For overall Cox proportional hazards survival analysis, follow-up was complete until date of death or censored at March 29, 2007. To examine the independent association of adjuvant therapy and overall survival after surgical resection, multivariate analyses were performed adjusting for confounders.
RESULTS
Patient demographics and tumor pathologic features
Patient demographic and tumor pathologic features are displayed in Table 1 The median age. of nonelderly patients was 62.0 years (range, 34–74), and the median age of elderly patients was 79.0 years (range, 75–90; p < 0.001). Among nonelderly patients, 56.0% had comorbid diseases (hypertension, diabetes mellitus, chronic obstructive pulmonary disorder, or cardiovascular disease); 66.3% of elderly patients had these same comorbidities (p = 0.021). The presence of positive lymph nodes varied significantly between age groups. Positive nodes were found in 82.6% of nonelderly and 75.3% of elderly patients (p = 0.039). Nonelderly patients had a significantly higher percentage of poorly differentiated, anaplastic, or undifferentiated tumors than elderly patients (45.4% vs. 34.9%, p = 0.019).
Table 1.
Patient demographic and pathologic features
| Nonelderly (%) | Elderly (%) | p value | |
|---|---|---|---|
| N | 489 | 166 | |
| Average age (range) | 62.0 (34–74) | 79.0 (75–90) | <0.001 |
| Female | 221 (45.2) | 92 (55.4) | 0.023 |
| Non-white | 46 (9.4) | 12 (7.2) | 0.393 |
| Tumor size | |||
| ≤3 cm | 297 (60.7) | 110(66.3) | 0.204 |
| >3 cm | 192 (39.3) | 56 (33.7) | |
| Number of positive nodes | |||
| 0 | 85 (17.4) | 41 (24.7) | 0.039 |
| 1+ | 404 (82.6) | 125 (75.3) | |
| Surgery type | |||
| Classic PD | 128 (26.2) | 42 (25.3) | 0.976 |
| Pylorus-preserving PD | 332 (67.9) | 114(68.7) | |
| Pylorus-preserving/total | 29 (5.9) | 10 (6.0) | |
| pancreatectomy | |||
| Positive margins | 222(45.4) | 81 (48.8) | 0.448 |
| Differentiation | |||
| Well/moderate | 267 (54.6) | 108 (65.1) | 0.019 |
| Poor/anaplastic/undifferentiated | 222 (45.4) | 58 (34.9) | |
| Perioperative complication | 169 (34.6) | 68 (41.0) | 0.138 |
| HTN, DM, COPD, or CVD | 274 (56.0) | 110 (66.3) | 0.021 |
| Received adjuvant therapy | 264 (54.0) | 49 (29.5) | <0.001 |
Abbreviations: COPD = chronic obstructive pulmonary disorder; CVD = cardiovascular disease; HTN = hypertension; PD = pancreaticoduodenectomy.
p value for patient age at time of surgery was determined using Bartlett’s test for equal variances. Other p values were determined using the Pearson chi-square test, p values ≤0.05 are in bold.
There was a significant difference between the two groups in the percentage of patients who received adjuvant chemoradiation therapy: 54.0% of nonelderly patients received adjuvant therapy, whereas only 29.5% of elderly patients received adjuvant therapy (p < 0.001).
Additional demographic and pathologic data for elderly patients who received either surgery alone or adjuvant CRT (Johns Hopkins Hospital [JHH] and non-JHH facility), are displayed in Table 2. Patients receiving surgery alone had an average age of 79.4 years, whereas those who also received adjuvant therapy had an average age of 78.2 (JHH = 78.8, non-JHH = 77.5) years (p = 0.023). Demographic data such as race and sex did not differ significantly between those receiving surgery alone and those who received adjuvant CRT. Also, tumor features and rate of perioperative complication were similar between the two groups.
Table 2.
Demographic and pathologic features for patients aged ≥75 years, by adjuvant CRT status and location (JHH and non-JHH facility)
| Surgery only (%) | Surgery + adjuvant CRT JHH (%) | Surgery + adjuvant CRT non-JHH (%) | p value | |
|---|---|---|---|---|
| N | 117 | 27 | 22 | |
| Average age (range) | 79.4 (75–90) | 78.8 (75–84) | 77.5 (75–84) | 0.023 |
| Female | 63 (53.9) | 12 (44.4) | 17 (77.3) | 0.058 |
| Non-white | 10 (8.6) | 1 (3.7) | 1 (4.6) | 0.595 |
| Tumor size | ||||
| ≤3 cm | 74 (63.3) | 22 (81.5) | 14 (63.6) | 0.188 |
| >3 cm | 43 (36.8) | 5 (18.5) | 8 (36.4) | |
| Number of positive nodes | ||||
| 0 | 28 (23.9) | 7 (25.9) | 3 (27.3) | 0.934 |
| 1+ | 89 (76.1) | 20(74.1) | 16 (72.7) | |
| Surgery type | ||||
| Classic PD | 31 (26.5) | 2 (7.4) | 9 (40.9) | 0.112 |
| Pylorus-preserving PD | 79 (67.5) | 23 (85.2) | 12 (54.6) | |
| Total pancreatectomy | 7 (6.0) | 2 (7.4) | 1 (4.6) | |
| Positive margins | 57 (48.7) | 13 (48.2) | 11 (50.0) | 0.991 |
| Differentiation | ||||
| Well/moderate | 73 (62.4) | 20(74.1) | 15 (68.2) | 0.490 |
| Poor/anaplastic/undifferentiated | 44 (37.6) | 7 (25.9) | 7(31.8) | |
| Perioperative complication | 51 (43.6) | 7 (25.9) | 10 (45.5) | 0.219 |
| HTN, DM, COPD, or CVD | 81 (69.2) | 15 (55.6) | 14 (63.6) | 0.384 |
Abbreviations: COPD = chronic obstructive pulmonary disorder; CRT = adjuvant chemoradiation therapy; CVD = cardiovascular disease; HTN = hypertension; JHH = Johns hopkins hospital; PD = pancreaticoduodenectomy.
p value for patient age at time of surgery was determined using Bartlett’s test for equal variances. Other p values were determined using the Pearson chi-square test. P values ≤ 0.05 are in bold.
Survival analysis
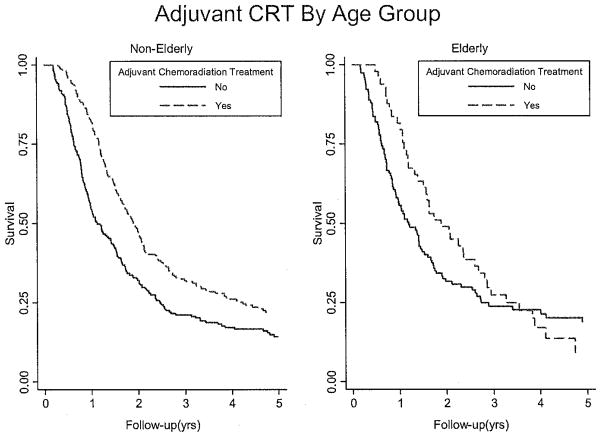
Nonelderly patients (aged <75) who received adjuvant therapy had significantly greater survival than those who did not receive adjuvant therapy (Fig. 1, p < 0.001). In the <75 cohort, the median survival was 22.7 months (95% CI, 20.0–24.9) for patients who received adjuvant therapy and 13.5 months (95% CI, 11.5–17.0) for those who underwent surgery alone (p < 0.001; Table 3). Two-year survival rates were 46.8% and 32.0% for those receiving vs. not receiving adjuvant therapy, respectively (p < 0.001). Five-year survival rates in the <75 cohort were 21.8% and 13.9% for those receiving vs. not receiving adjuvant therapy, respectively (p < 0.001).
Fig 1.
Kaplan-Meier curve of survival based on age group. Among nonelderly patients (aged <75 years), compared with patients who received surgery only, those who received adjuvant therapy had improved survival (p < 0.001). Among elderly patients (aged ≥75 years), survival during follow-up after PD was not statistically significantly different between those who did and did not receive adjuvant chemoradiation therapy (CRT; p = 0.273). However, survival censored at 2 years was significant among both nonelderly (p < 0.001) and elderly patients (p = 0.013).
Table 3.
Two- and five-year survival for elderly and nonelderly patients, stratified by adjuvant therapy
| Adjuvant therapy | Median survival, months (95% CI) | p value | Two-year survival, % | p value | Five-year survival, % | p value | |
|---|---|---|---|---|---|---|---|
| All patients (N = 655) | All | 18.4(16.9–20.0) | 39.1 | 17.9 | |||
| Nonelderly | Yes (n = 264) | 22.7 (20.0–24.9) | <0.001 | 46.8 | <0.001 | 21.8 | <0.001 |
| No (n = 225) | 13.5(11.5–17.0) | 32.0 | 13.9 | ||||
| Elderly | Yes (n = 49) | 22.6 (16.6–31.1) | 0.273 | 49.0 | 0.013 | 11.7 | 0.310 |
| No (n = 117) | 14.3 (11.0–17.7) | 31.6 | 19.8 |
Abbreviation: CI = confidence interval
Among elderly patients (aged ≥75 years), median survival was 22.6 months (95% CI, 16.6–31.1) for those who received adjuvant therapy and 14.3 months (95% CI, 11.0–17.7) for those who received surgery alone (p = 0.273; Figure 1). In the ≥75 cohort, 2-year (49.0% and 31.6%, p = 0.013) survival was significantly improved for patients receiving CRT vs. surgery alone. However, this benefit was no longer seen at 5-years (11.7% and 19.8%, p = 0.310)
Multivariate analysis
A multivariate analysis performed for elderly and nonelderly patients is displayed in Table 4. Demographic factors such as patient sex and race were not significantly associated with 2-year or overall survival for either elderly or nonelderly patients.
Table 4.
Multivariate analysis stratified for nonelderly (aged <75 years) and elderly (aged ≥75 years) patients, performed by Cox proportional hazard analysis
| Characteristic | Two-year survival
|
Overall survival
|
||
|---|---|---|---|---|
| Nonelderly RR (95% CI), p value | Elderly RR (95% CI), p value | Nonelderly RR (95% CI),. p value | Elderly RR (95% CI), p value | |
| Adjuvant therapy | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 0.60 (0.47–0.76), <0.001 | 0.58 (0.36–0.92), 0.02 | 0.69 (0.56–0.84), <0.001 | 0.80 (0.55–1.17), 0.258 |
| Sex | ||||
| Female | 1 | 1 | 1 | 1 |
| Male | 0.79 (0.62–0.99), 0.044 | 0.98 (0.65–1.47), 0.919 | 0.82(0.67–1.01), 0.059 | 0.86(0.60–1.22), 0.390 |
| Race | ||||
| White | 1 | 1 | 1 | 1 |
| Non-white | 1 (0.67–1.49), 0.989 | 0.51 (0.21–1.24), 0.138 | 0.83(0.58–1.19), 0.321 | 0.64(0.31–1.31), 0.223 |
| Tumor diameter | ||||
| ≤3 cm | 1 | 1 | 1 | 1 |
| >3 cm | 1.26(0.99–1.60), 0.059 | 1.18 (0.76–1.82), 0.462 | 1.28(1.04–1.57), 0.020 | 1.18(0.80–1.74), 0.396 |
| Positive nodes | ||||
| 0 | 1 | 1 | 1 | 1 |
| 1+ | 0.97(0.71–1.33), 0.852 | 2.04(1.18–3.52), 0.01 | 0.99(0.76–1.30), 0.961 | 1.81 (1.17–2.81), 0.008 |
| Positive margins | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.52 (1.20–1.60), 0.001 | 1.23 (0.81–1.87), 0.323 | 1.31 (1.07–1.61), 0.009 | 1.32 (0.91–1.89), 0.141 |
| Differentiation | ||||
| Well/moderate | 1 | 1 | 1 | 1 |
| Poor/anaplastic | 1.76 (1.39–2.22), 0.001 | 1.81 (1.21–2.71), 0.004 | 1.63(1.33–1.99), 0.001 | 1.60(1.11–2.30), 0.012 |
| Surgery type | ||||
| Classic PD | 1 | 1 | 1 | 1 |
| Pylorus preserving PD | 1.01 (0.77–1.32), 0.946; | 0.95 (0.60–1.50), 0.821; | 1.20(0.95–1.52), 0.133; | 1.07 (0.72–1.60), 0.724; |
| Total pancreatectomy | 1.05(0.64–1.74), 0.839 | 2.79 (1.16–6.71), 0.022 | 1.14(0.72–1.79), 0.574 | 2.89 (1.28–6.50), 0.010 |
| Complication | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.13 (0.88–1.44), 0.342 | 1.25 (0.83–1.86), 0.286 | 1.12(0.91–1.39), 0.281 | 1.22 (0.85–1.76), 0.273 |
| HTN, DM, COPD, or CVD | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.23(0.97–1.57), 0.087 | 1.11 (0.71–1.72), 0.655 | 1.29(1.05–1.59), 0.016 | 1.23 (0.84–1.79), 0.279 |
Abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disorder; CRT = adjuvant chemoradiation therapy; CVD = cardiovascular disease; HTN = hypertension; PD = pancreaticoduodenectomy; RR = relative risk.
For 2-year survival, follow-up time was censored at 24 months. For overall survival, analysis was performed using complete follow-up time. p values ≤0.05 are in bold.
Among the elderly, patients who underwent a total pancreatectomy were at an increased risk of death at both 2 years (relative risk [RR] 2.79, 95% CI, 1.18–6.71; p = 0.022) and overall (RR 2.89,95% CI, 1.28–6.50; p = 0.010) than elderly patients who underwent classic or pylorus-preserving PD. Among nonelderly patients, surgery type was not significantly associated with a change in mortality. The presence of intra- or postoperative complications were not associated with a change in mortality for either group.
Tumor diameter >3 cm was significantly associated with an increased risk of death in the nonelderly at 2 years (RR 1.28, 95% CI, 1.01–1.62; p = 0.045) and overall (RR 1.81, 95% CI, 1.17–2.81; p = 0.008). Tumor diameter was not significantly associated with an increase in mortality in the elderly at 2 years or overall (RR 1.16, 95% CI, 0.75–1.80, p = 0.500 and RR 1.18, 95% CI, 0.80–1.74, p = 0.396, respectively). At 2 years, the presence of positive lymph nodes was associated with an increased risk of death in nonelderly (RR 2.04, 95% CI, 1.18–3.52; p = 0.01) and elderly patients (RR 1.81, 95% CI, 1.17–2.81; p = 0.008). Positive margins were associated with a significantly increased overall relative risk of death in the nonelderly (RR 1.31, 95% CI, 1.07–1.61; p = 0.009) but not in the elderly (RR 1.32, 95% CI, 0.91–1.89; p = 0.141). Both nonelderly (RR 1.63, 95% CI, 1.33–1.99; p < 0.001) and elderly (RR 1.60, 95% CI, 1.11–2.30; p = 0.012) patients with poorly differentiated, anaplastic, or undifferentiated tumors had significantly increased overall risks of death compared with those with well or moderately differentiated tumors.
Nonelderly patients with comorbidities were at an increased risk of death overall (RR 1.29, 95% CI, 1.05–1.59; p = 0.016) but not at 2 years (RR 1.23, 95% CI, 0.97–1.57; p = 0.087). Elderly patients with comorbid conditions did not have a significant change in risk of mortality at 2 years (RR 1.11, 95% CI, 0.71–1.72; p = 0.655) or overall (RR 1.23, 95% CI, 0.84–1.79; p = 0.279).
For survival up to 2 years, compared with surgery alone, adjuvant CRT was protective among both nonelderly (RR 0.60, 95% CI, 0.47–0.76; p < 0.001) and elderly patients (RR 0.58, 95% CI, 0.36–0.92; p = 0.02). With respect to overall survival, adjuvant CRT was only significantly protective for patients <75 years (RR 0.69, 95% CI, 0.56–0.84; p< 0.001) and not among those ≥75 years (RR 0.80, 95% CI, 0.55–1.17; p = 0.258).
DISCUSSION
The appropriate use of adjuvant CRT in patients with resectable pancreatic cancer is controversial. The findings of the Gastrointestinal Tumor Study Group (GITSG) support the use of adjuvant chemoradiation (16). Two European Study Group for Pancreatic Cancer (ESPAC) trials have suggested a possible detrimental effect with adjuvant C)RT (17, 18), although these have been criticized for lack of quality control in the delivery of radiation. Our institution previously reported a benefit in median, 2-, and 5-year survival with adjuvant CRT (12). A retrospective study from another institution demonstrated similar survival with chemoradiation as seen in the GITSG study (19). Furthermore, a combined analysis from Johns Hopkins Hospital and the Mayo Clinic showed a significant improvement in survival with adjuvant CRT; CRT in this study was not associated with decreased survival in any risk group (20).
This study aimed to determine whether adjuvant therapy is efficacious in the elderly. In our analysis, patients aged less than 75 years clearly benefit from the addition of adjuvant CRT, whereas those aged 75 years or more did not significantly benefit from adjuvant therapy. Elderly and nonelderly patients, however, had similar 2-year survival benefits after receiving adjuvant therapy. After approximately 3 years, by Kaplan-Meier analysis, elderly patients who received adjuvant therapy had worse overall survival than those who did not receive adjuvant therapy. At 5 years, elderly patients who received adjuvant therapy continued to have worse survival than those who received only surgery, although this was not statistically significant. It is possible that other competing causes of mortality may explain the decreased survival in the elderly group. Unfortunately, the number of patients alive 5 years after surgery is small: 17 in the elderly group. It is difficult to discern true effects from random noise in a group this small. Based on our data, we would suggest that CRT appears to have a benefit over 2–3 years but that there is insufficient long-term data to indicate whether there is truly a benefit to adjuvant chemoradiation or whether there is the potential for harm.
It is important to note differences between the elderly and nonelderly patients that may provide insight into the lack of benefit of adjuvant CRT in the elderly. The female predominance of the elderly group is not surprising, given the average longer life expectancy of females in the United States. Elderly patients were more likely to have well-differentiated tumors than their nonelderly counterparts. Additionally, elderly patients were less likely than the nonelderly to have node-positive disease at the time of surgery. Both node-positivity and poorly differentiated or anaplastic tumor biology were found to increase the relative risk of death. Perhaps the biology of tumors in the elderly may differ from younger patients. For example, tumors in the elderly may represent the result of transformation from precursor intraductal papillary mucinous neoplasms, which have more favorable survival characteristics that may confound our analysis (21). Also, elderly patients were less likely than their nonelderly counterparts to receive adjuvant therapy. Numerous studies have previously found that elderly patients are less likely than the nonelderly to receive adjuvant therapy for a variety of cancers, and elderly patients are often less likely to receive multimodality therapy (chemotherapy and radiation) (22–24). This may reflect a selection bias that could confound our analysis.
Among elderly patients, those who received surgery alone had similar tumor characteristics as elderly patients who received adjuvant CRT. Elderly patients undergoing surgery alone only differed from their elderly counterparts who also received adjuvant therapy in their age. For example, those elderly patients undergoing surgery alone were older than those who received adjuvant therapy. This age difference, although statistically significant, represents only 1.2 years, which is unlikely to be clinically relevant.
Limitations of our study include that it is a retrospective study of adjuvant CRT, although the patients were identified from a prospectively collected database, and is subject to all forms of bias characteristic to retrospective studies. Additionally, this is a nonrandomized study, and thus patient selection may have influenced survival outcomes. Performance status data were not available for sufficient patients to be included in this analysis, nor was it possible to account for comorbidities using tools such as the Charlson Score of the Adult Comorbidity Evaluation—27. Co-morbid conditions and performance status likely play important roles in the tolerance of patients to therapy and are valuable factors in selecting patients for aggressive therapies; this should be investigated in future studies of pancreatic cancer in the elderly. We conclude that although adjuvant therapy for pancreatic cancer may not demonstrate long-term survival benefits for all elderly patients, adjuvant CRT may be effective in select elderly patients. Moreover, this study further supports the use of adjuvant CRT in patients with resectable pancreatic adenocarcinoma aged less than 75 years. Among these older patients, it should be investigated whether they might benefit from neoadjuvant therapy, which uses smaller radiation fields and may result in less toxicity. The Comprehensive Geriatric Assessment is a tool that has been used to predict tolerance to treatment and survival for other geriatric cancer patients and should be investigated for elderly patients with pancreatic adenocarcinoma to identify those patients likely to benefit from adjuvant therapy (25, 26). Further study is needed to determine which elderly patients are likely to benefit from adjuvant CRT vs. chemotherapy alone.
Footnotes
Presented at the 49th Annual Meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO), Los Angeles, California, 2007.
Conflict of interest: none.
References
- 1.U.S. Census Bureau. Projections of the total resident population by 5-year age groups and sex with special age categories: Middle series 2025 to 2045, 2050 to 2070. Bethesda, MD: Population Projection Program, U.S. Census Bureau; 2000. [Google Scholar]
- 2.Etzioni DA, Liu JH, Maggard MA, et al. Workload projections for surgical oncology: Will we need more surgeons? Ann Surg Oncol. 2003;10:1112–1117. doi: 10.1245/aso.2003.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 5.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: Changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 6.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: 2007. [Accessed 4/2/08]. http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site. [Google Scholar]
- 7.Brozzetti S, Mazzoni G, Miccini M, et al. Surgical treatment of pancreatic head carcinoma in elderly patients. Arch Surg. 2006;141:137–142. doi: 10.1001/archsurg.141.2.137. [DOI] [PubMed] [Google Scholar]
- 8.Makary M, Winter JM, Cameron JL, et al. Pancreaticoduodenectomy in the very elderly. / Gastrointest Surg. 2006;10:347–355. doi: 10.1016/j.gassur.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Horiot JC. Radiation therapy and the geriatric oncology patient. J Clin Oncol. 2007;25:1930–1935. doi: 10.1200/JCO.2006.10.5312. [DOI] [PubMed] [Google Scholar]
- 10.Wasil T, Lichtman S, Gupta V, Rush S. Radiation therapy in cancer patients 80 years of age and older. Am J Clin Onc. 2000;23:526–530. doi: 10.1097/00000421-200010000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 12.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: Results of a large prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588. doi: 10.1097/00000658-199510000-00014. discussion 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein JP, Moeschberger ML, editors. Survival analysis: Techniques for censored and truncated data. 2. New York: Springer-Verlag; 2003. [Google Scholar]
- 15.Cox DR, Oakes D, editors. Analysis of Survival Data. London: Chapman and Hall; 1984. [Google Scholar]
- 16.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 17.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 18.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 19.Hattangadi J, Hong T, Yeap B, Harvey M. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115:2640–2650. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu C, Herman J, Corsini M, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: The Johns Hopkins Hospital— Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hruban R, Maitra A, Kern S, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831–849. doi: 10.1016/j.gtc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Rijke JM, Schouten LJ, Schouten HC, et al. Age-specific differences in the diagnostics and treatment of cancer patients aged 50 years and older in the province of Limburg, The Netherlands. Ann Oncol. 1996;7:677–685. doi: 10.1093/oxfordjournals.annonc.a010716. [DOI] [PubMed] [Google Scholar]
- 23.Giordano SH, Hortobagyi GN, Kau SW, et al. Breast cancer treatment guidelines in older women. J Clin Oncol. 2005;23:783–791. doi: 10.1200/JCO.2005.04.175. [DOI] [PubMed] [Google Scholar]
- 24.Popescu RA, Norman A, Ross PJ, et al. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol. 1999;17:2412–2418. doi: 10.1200/JCO.1999.17.8.2412. [DOI] [PubMed] [Google Scholar]
- 25.Freyer G, Geay J-F, Touset S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann Oncol. 2005;16:1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 26.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]