Abstract
Mounting evidence suggests that immunological responses may be altered in endometriosis. The baboon (Papio anubis) is generally considered the best model of endometriosis pathogenesis. The objective of the current study was to investigate for the first time immunological changes within uterine and peritoneal draining lymph nodes in a nonhuman primate baboon model of endometriosis. Paraffin-embedded femoral lymph nodes were obtained from 22 normally cycling female baboons (induced endometriosis n = 11; control n = 11). Immunohistochemical staining was performed with antibodies for endometrial stromal cells, T cells, immature and mature dendritic cells, and B cells. Lymph nodes were evaluated using an automated cellular imaging system. Endometrial stromal cells were significantly increased in lymph nodes from animals with induced endometriosis, compared to control animals (P = .033). In animals with induced endometriosis, some lymph node immune cell populations including T cells, dendritic cells and B cells were increased, suggesting an efficient early response or peritoneal drainage.
Keywords: lymph nodes, baboon, endometriosis, immune response, endometrial stromal cells
Introduction
Endometriosis is a benign gynecological condition which affects up to 10% to 15% of reproductive-aged women. The disease is characterized by the growth of lesions resembling the endometrium in sites outside the uterus. It is frequently associated with pelvic pain and infertility.1 Despite extensive investigation, the pathophysiology of endometriosis is unclear, however, abnormal implantation of viable endometrial fragments shed at menstruation is the most widely accepted theory. It is hypothesized that sloughed endometrial fragments, disseminated via retrograde menstruation and the lymphatic and blood circulation, can implant and establish as endometriotic lesions at distant sites.2–4
Endometriosis is an inflammatory condition, and mounting evidence suggests that dysregulated immune and other responses within the eutopic endometrium are likely to precede well-documented changes at ectopic sites.5–11 Investigating immunological responses within the uterine- and lesion-draining lymph nodes (LNs) should be important in terms of trying to understand endometriosis pathophysiology. Endometriosis has invasive characteristics as endometrial tissue must attach to and invade the peritoneum, establish a blood supply, and continue to proliferate to form an ectopic lesion.12–15 Understanding lymphatic drainage is considered crucial in diagnosis, prognosis, and treatment of invasive diseases. Also, lymphatic spread is hypothesized to play a role in pathogenesis of endometriosis and is thought to account for displacement of endometrial cells at unusual locations, other than those that would otherwise be accessible to retrograde menstrual flow.2,4,16–22
We have recently reported a novel preliminary study of immune cell populations and CD10+ (endometrial stromal) cells in the sentinel uterine-draining obturator LNs through the menstrual cycle in women with endometriosis.23 We observed a significant increase in the numbers of CD10+ endometrial stromal cells and decreases in T cell, B cell, and dendritic cell (DC) populations in LNs from women with endometriosis compared to control participants. These findings are thought to reflect an increased dissemination of endometrial cells via the lymphatic circulation and a decreased ability of the immune system to effectively target endometrial tissue in women with endometriosis. These findings probably reflect the situation in well-established peritoneal disease.
Animal models of endometriosis are important for the investigation of disease pathogenesis and efficacy of therapeutic intervention strategies. The Olive baboon (Papio anubis) is generally considered to be the best model for study of endometriosis pathogenesis. Their reproductive anatomy and menstrual cycle are similar to that of humans, and baboons can develop spontaneous endometriosis. Endometriosis can be induced in baboons by inoculation of menstrual endometrium into the peritoneal cavity, a process which mimics Sampson’s theory of retrograde menstruation. Baboons with induced disease show a range of functional molecular changes of the eutopic endometrium and ectopic lesions, which are similar to women with endometriosis.24–26
Femoral LNs were selected for study due to a previous observation of the presence of cytotrophoblast within these nodes in normal cycling and pregnant animals. Cytotrophoblast was not seen in internal pelvic LNs, including obturator nodes (Fazleabas et al., unpublished data). In the human, some efferent lymphatic vessels extend from the uterus to the femoral nodes.27 Furthermore, femoral LNs also drain the abdominal wall and peritoneal cavity.28 There are currently no published reports of leukocyte subsets in LNs from the baboon model of endometriosis.
This study has aimed to investigate endometrial stromal cells and immune cell populations within lymph nodes in a nonhuman primate model of endometriosis. These specific cell populations (CD10, CD3, CD20, DC-Lamp, and DC-Sign) were recently found to be altered in LNs from women with endometriosis, likely to reflect the situation in well-established peritoneal disease.23 However, the baboon model allows us to investigate early disease establishment, and to gain insights into how therapeutic approaches (such as lesion excision) may affect the immune response and modulate disease progression. Understanding initial responses is crucial for developing more effective early intervention strategies.
Materials and Methods
Tissue Collection
Femoral LNs were obtained from 22 female baboons (Papio anubis). All animal studies were approved by the Animal Care Committee at the University of Illinois at Chicago. The LNs were collected from normally cycling animals, aged 7 to 12 years and 12 to 18 kg in weight. They were housed in individual cages in the Biological Research Laboratories of the University of Illinois. No spontaneous endometriosis was observed in these animals. Endometriosis was experimentally induced in 11 animals by intraperitoneal inoculation of menstrual endometrium in 2 consecutive menstrual cycles. Details of the inoculation procedure have been described previously.29 Of the 11 animals with induced endometriosis, 6 had laparoscopic evaluation post inoculation without excision of endometriotic lesions (endometriosis without lesion excision = EWE), and 5 had laparoscopic excision of endometriosis (endometriosis with lesion excision = ELE). Control lymph node tissues were harvested from 5 normally cycling baboons during the secretory phase of the menstrual cycle and 6 normally cycling animals who had undergone evaluative laparoscopic procedures at the same time points as the diseased animals (time of disease induction and 1 and 6 months post induction; surgical controls) but with no induction of disease. Lymph nodes were collected 15 months after the initiation of experimental protocols. Tissues from all groups were collected between days 9 and 11 postovulation, which coincides with the window of uterine receptivity in the baboon.
Tissue samples were fixed in 10% buffered formalin and processed through a series of alcohols to paraffin wax according to a standardized protocol. Paraffin-embedded tissue blocks were cut at 4 µm and mounted onto glass slides. Dried slides were deparaffinised through xylene and a series of alcohols into water. Rehydrated slides were treated in alcohol-ammonia solution for 1 hour to remove formalin pigment.
Immunohistochemistry
Deparaffinized slides for immunohistochemical staining underwent antigen retrieval for 20 minutes at 95 to 99°C in preheated pH9 Target Retrieval Solution (Dako, Glostrup, Denmark). Dual Endogenous Enzyme Block (Dako, Glostrup, Denmark) was applied for 10 minutes followed by primary antibodies for 30 minutes as detailed in Table 1 .
Table 1.
Primary Antibodies Used in Baboon Lymph Node Immunostaining
| Antibody | Identifies | Dilution | Species | Supplier |
|---|---|---|---|---|
| CD10 | Endometrial stromal cells | Ready-to-use product | Monoclonal mouse (clone 56C6) | Dako, Glostrup, Denmark |
| CD3 | All T cells | 1:600 | Polyclonal rabbit | Dako, Glostrup, Denmark |
| DC-SIGN | Immature DCs | 1:33 | Monoclonal mouse (clone 102E11.06) | Dendritics, Lyon, France |
| DC-LAMP | Mature DCs | 1:50 | Monoclonal mouse (clone 1010E1.01) | Dendritics, Lyon, France |
| CD20 | All B cells | 1:400 | Monoclonal mouse (clone L26) | Dako, Glostrup, Denmark |
EnVision+ Dual Link System-horseradish peroxidise (HRP) detection system was applied for 30 minutes followed with Liquid Diaminobenzidine+ (DAB+) Substrate Chromagen System (Dako, Glostrup, Denmark) for 10 minutes. With the enzyme-substrate reaction, DAB+ produced a brown end product at the site of the target antigen. The Dako Autostainer Plus Model S3400 (Dako, Glostrup, Denmark) was used to perform all immunostaining. Following immunostaining, slides were counterstained using Mayer’s haematoxylin solution, dried, and cover-slipped using ultramount.
As a part of the optimization process, staining with isotype controls matched to the host species and working concentration of the primary antibody (μg/mL) was performed. All staining with isotype controls was negative.
Quantification
Staining intensity (brownness) and positive (brown) and negative (blue) areas were quantified using an ACIS Automated Cellular Imaging System (Dako, Glostrup, Denmark). With standardized tissue processing and immunostaining protocols, as used in this study, ACIS provides a sensitive, reproducible and accurate quantification of the expression of surface markers on and numbers of infiltrating leukocyte populations in LNs.30–31 Positive (light and dark brown) and negative (blue) color thresholds were set for each antibody. Free-hand regions were drawn to include the entire lymph node. Staining intensity measurements gave the average brownness of all positive pixels within the region, expressed as a number on a continuous scale from 0 to 255 (grayscale). Intensity measurements reflect the level of antigen expression within the tissue and provide an indication of functional status of that particular antigen.32 The percentage of the total region area which was positively stained was determined from the brown and blue area measurements by the following:
This area measurement is a quantitative representation of the number of positive cells.33
Regions for CD10 analysis were carefully drawn to exclude germinal centres within lymphoid follicles as some immature T and B precursors, as well as proliferating B cells and mature neutrophils, may weakly express CD10.34 Remaining CD10+ cells displayed morphology and size consistent with endometrial stromal cells rather than lymphocytes.
Statistical Analyses
Statistical analyses were performed using SPSS 17.0 Statistical Analysis Software. The Mann-Whitney U test was used to compare intensity and percentage area between groups. Statistical significance was established at P values of less than .05.
Results
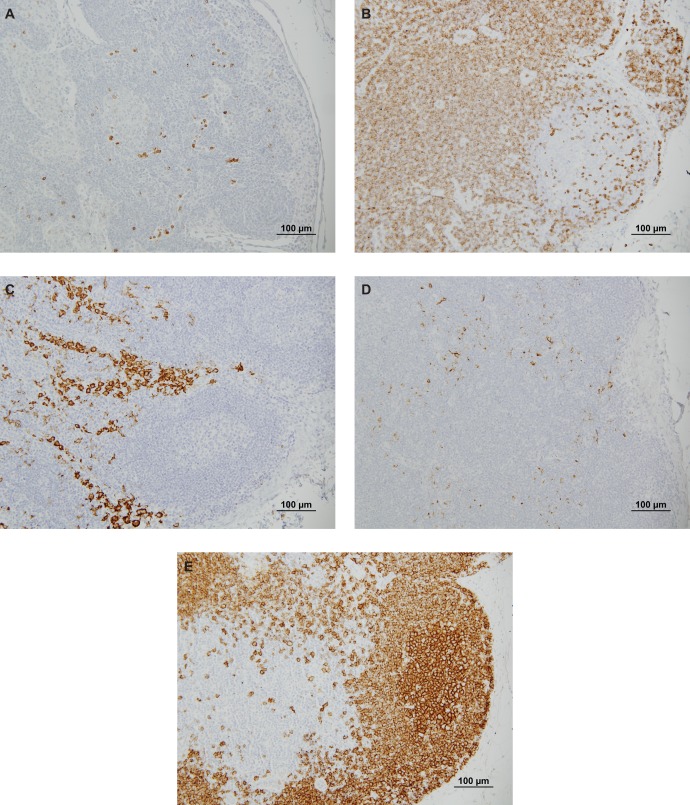
CD10+ endometrial stromal cells, CD3+ T cells, DC-Lamp+ and DC-Sign+ DCs, and CD20+ B cells were observed in baboon LNs (Figure 1 ). The numbers and level of antigen expression of endometrial stromal cells and several immune cell populations were assessed in femoral LNs from animals with and without induced endometriosis, as detailed in Table 2 .
Figure 1.
CD10+ endometrial stromal cells (A), CD3+ T cells (B), DC-Sign+ immature dendritic cells (C), DC-Lamp+ mature dendritic cells (D) and CD20+ B cells (E) in femoral lymph nodes from a baboon with induced endometriosis stained brown with DAB+ chromagen (×200 magnification).
Table 2.
Mean ± SD Percentage Area (Cell Numbers) and Intensity (Grayscale Units; Level of Antigen Expression) of Endometrial Stromal Cells and Several Immune Cell Populations in Femoral Lymph Nodes From Baboons With Induced Endometriosis (n = 11) and Control Animals (n = 11)a
| Control Animals | Induced Endometriosis Animals | ||||
|---|---|---|---|---|---|
| Normal Cycling Control (n = 5) | Surgical Control (n = 6) | Endometriosis Without Lesion Excision (EWE) (n = 6) | Endometriosis With Lesion Excision (ELE) (n = 5) | ||
| CD10 | Area | 0.5 ± 0.6 | 1.2 ± 1.2 | 2.3 ± 2.4 | 1.6 ± 1.2 |
| Intensity | 98.2 ± 2.1 | 95.3 ± 4.0 | 97.8 ± 6.1 | 95.6 ± 5.8 | |
| CD3 | Area | 68.1 ± 11.7 | 56.7 ± 25.9 | 72.1 ± 10.5 | 60.8 ± 12.4 |
| Intensity | 91.3 ± 4.7 | 94.3 ±6.3 | 93.4 ± 9.3 | 96.9 ± 10.6 | |
| DC-Sign | Area | 5.3 ± 3.5 | 16.9 ± 9.9 | 12.6 ± 9.1 | 9.8 ± 5.1 |
| Intensity | 129.7 ± 18.0 | 141.1 ± 6.0 | 131.9 ± 10.6 | 134.7 ± 9.4 | |
| DC-Lamp | Area | 3.3 ± 0.9 | 2.6 ± 0.7 | 11.4 ± 10.3 | 10.9 ± 15.4 |
| Intensity | 99.6 ± 1.6 | 98.5 ± 2.4 | 98.5 ± 5.3 | 100.2 ± 2.2 | |
| CD20 | Area | 36.6 ± 10.3 | 40.9 ± 14.5 | 48.0 ± 12.9 | 42.6 ± 13.5 |
| Intensity | 118.4 ± 16.5 | 119.1 ± 11.6 | 125.4 ± 11.7 | 122.9 ± 11.4 | |
a These groups were further subdivided into 2 control and endometriosis treatment groups.
Effect of Induced Endometriosis on Lymph Node Cell Composition
The total group of animals with induced endometriosis (n = 11) was compared with all control animals (n = 11) in order to investigate the effects of the disease on CD10+ endometrial stromal cell area in femoral LNs. CD10+ cells were significantly increased in animals with induced endometriosis (area mean ± SD = 1.9 ± 1.9 %), compared to controls without the disease as illustrated in Figure 2 (0.8 ± 1.0; P = .033, Mann-Whitney U Z = –2.137).
Figure 2.
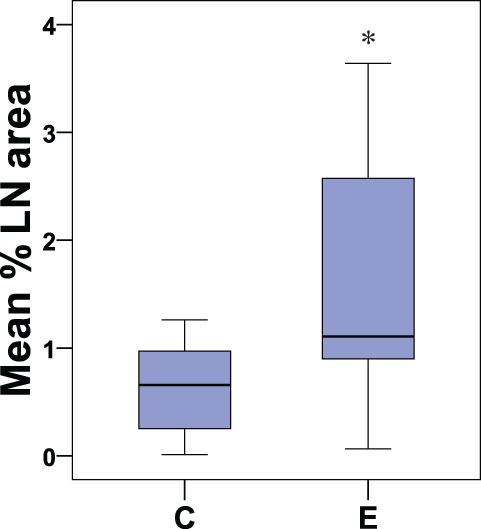
Boxplot showing the percentage of the lymph node area occupied by CD10+ cells in control animals (C; n = 11) and in all animals with induced endometriosis (E; n = 11). The middle line in the boxplot represents the median % lymph node area occupied by CD10+ cells. The lower and upper parts of the box represent 25th and 75th percentiles of data distribution. The length of the box represents the interquartile range and the whiskers (lines above and below the box) represent the range of values that fall within 1.5 interquartile range (IQR; highest and lowest % lymph node area that are not outliers); *P = .033.
The numbers of CD3+, DC-Sign+, DC-Lamp, and CD20+ cells were generally higher in EWE compared to all control baboons; however, due to small numbers within groups, these trends did not reach significance (Figure 3 ). A significant increase in DC-LAMP intensity was observed between ELE (100.2 ± 2.2) and normal cycling control animals (96.6 ± 1.6; P = .012, Z = –2.514)
Figure 3.
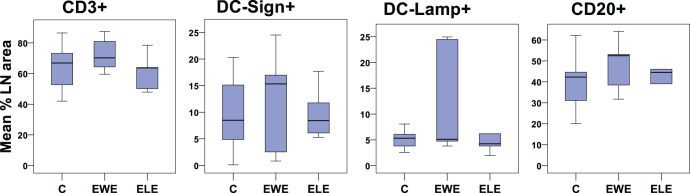
Boxplot showing the percentage of the lymph node area occupied by immune cell populations in control animals and those with induced endometriosis (with and without lesion excision). C indicates control animals (n = 11), EWE, animals with induced endometriosis but without lesion excision (n = 6), ELE, endometriosis-induced animals treated with lesion excision (n = 5).
Effect of Endometriosis Lesion Excision on Lymph Node Cell Composition
Lesion excision did not appear to significantly alter lymph node cell composition in endometriosis-induced animals (ELE vs EWE groups; Table 2).
Effect of Surgical Intervention on Lymph Node Cell Composition
The effect of surgical intervention on lymph node cell composition was determined by comparing normal cycling controls (n = 5) with all animals that had undergone surgery (ie, surgical controls, ELE and EWE groups; n = 17). A strong trend suggested that DC-Sign+ cell numbers were greater in LNs in all animals who had undergone surgery (13.3 ± 8.5) in comparison to normal cycling controls (5.3 ± 3.5; P = .055, Z = –1.919). The numbers of DC-Sign+ cells were significantly increased in the surgical control group (16.9 ± 9.9; n = 6) compared to normal cycling controls (5.3 ± 3.5; n = 5; P = .028, Z = –2.191), as shown in Figure 4 .
Figure 4.
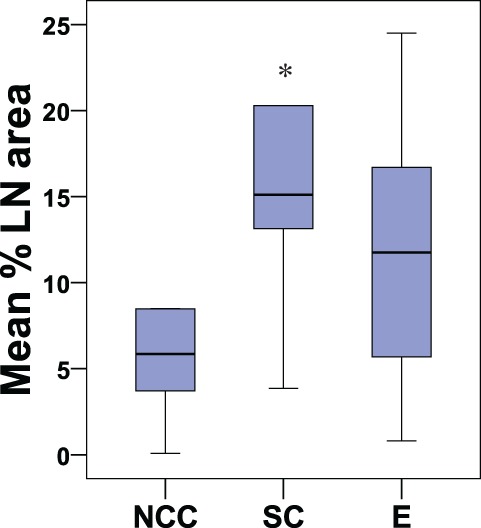
Boxplot showing the percentage of the lymph node area occupied by DC-Sign+ cells in control animals (normal cycling controls [NCC; n = 5] and surgical controls [SC; n = 6]) and animals with induced endometriosis (E; n = 11); *P = .028.
Discussion
This preliminary study has for the first time investigated endometrial stromal and immune cell numbers and levels of antigen expression in uterine- and peritoneal-draining LNs from a nonhuman primate model of endometriosis. Our results suggest that subtle changes are occurring in femoral LNs following endometriosis induction, as well as in response to other interventions, such as evaluative laparoscopy and endometriotic lesion excision. In terms of the immunology of endometriosis, a major part of the value of the nonhuman primate model of induced endometriosis is the unique opportunity to investigate early response to lesion invasion. It is still unknown how the immune system responds to initial and early disease establishment in the human. The initiation of endometriosis in women is difficult to investigate, as there is a considerable delay from onset of lesion development to clinical diagnosis.35–37 Furthermore, it is ethically difficult to address these questions in humans, and thus studies of animals with induced disease are beneficial in allowing us to characterize and better understand early immunological reactions associated with the pathophysiology of endometriosis.
We have demonstrated significantly increased presence of CD10+ endometrial stromal cells in femoral LNs of baboons with induced endometriosis compared to control animals. We recently made a similar statistically significant observation in uterine draining LNs from women with endometriosis.23 Increased CD10+ cells in femoral LNs of baboons may be attributed to one of the following possibilities. Firstly, in addition to the transit of shed endometrial cells from the uterus to uterine-draining LNs, lymphatic drainage from the lesion site within the peritoneal cavity may directly contribute to delivery of CD10+ endometrial stromal cells to femoral LNs. Alternatively, induced endometriotic lesions in the baboon model of endometriosis may cause molecular changes within the eutopic endometrium of these animals. While the disease process is not identical, some evidence suggests that the eutopic endometrium of baboons with induced endometriosis exhibits some similar characteristics to the eutopic endometrium of women with endometriosis, in terms of reduced apoptosis,26 and increased angiogenesis and proliferation.24 This implies that shed endometrial fragments in animals with induced endometriosis may be more resistant to apoptosis and consequently remain viable in the LN environment for longer periods of time.
Analysis of immune cell composition in baboon LNs indicated a trend toward increased numbers of CD3+ T cells, DC-Sign+ and DC-Lamp+ DCs, and CD20+ B cells in animals with induced endometriosis, when compared to control animals. Changes in the level of antigen expression between baboons with induced endometriosis and control animals suggest an increased CD3, CD20, and DC-Lamp antigen expression in animals with induced endometriosis. It may be speculated that this is indicative of immune cell activation during initial stages of disease, however direct in vitro studies of cell activity and signaling are required to elucidate the physiological significance of small changes in the level of antigen expression.
These observations in the baboon model of induced endometriosis are in contrast to the finding of decreased immune cell numbers in obturator LNs from women with endometriosis in comparison to women without the disease. The decrease in immune cell numbers in obturator nodes from women with endometriosis is hypothesized to result from recruitment of these cells to eutopic endometrium and ectopic lesion sites.23 The main reason for the different observations in the current study is likely to simply be that while femoral LNs receive some drainage from the uterus, these LNs also drain the peritoneal cavity and other structures. By contrast, obturator LNs mainly drain the uterus. In animals with induced disease, which undergo multiple surgical procedures, the peritoneal cavity is inflamed and there is a major influx of immune cells.38,39 Increased numbers of immune cells would almost certainly also be evident in the LNs draining the area.
Unlike samples from women with endometriosis, where the disease has been established for a length of time and lesion progression is advanced, LNs from the nonhuman primate model of induced endometriosis represent much earlier stages of disease establishment. The immune response to endometriosis almost certainly varies with the stage of disease/lesion development. In the early stages of endometriosis lesions, it may be expected that certain immune cells would be heavily recruited to the area in an attempt to clear the endometrial tissue from the peritoneum and prevent lesion progression. In the later stages of the disease, when attempts to combat lesion establishment have failed, we may expect decreased numbers and activation of certain immune cell populations in LNs.23
Our results indicate that surgical intervention significantly increased the numbers of DC-Sign+ cells within the animal LN. DC-Sign+ cells are a population of immature DCs that play roles in initial stages of antigen recognition and capture. Previous studies have shown that intrapelvic injection of endometrium into the peritoneal cavity of baboons may increase the numbers of peritoneal leukocytes.39 Serial laparoscopic evaluations over a number of months may cause inflammation of the peritoneal cavity in the baboon.38 Increased presence of immature DCs in endometriosis-inoculated animals and in surgical-control group animals, which were subjected to laparoscopic evaluation, is likely to be directly influenced by the surgically-induced peritoneal inflammatory response.
It is important to acknowledge the limitations associated with animal models of endometriosis. The animals from the present study had induced rather than spontaneously occurring endometriosis. Previous studies have indicated that the immune response to induced endometriosis is not identical to that of spontaneous disease.40 Furthermore, sstatistical significance of results was difficult to establish due to small numbers within subgoups. It is very challenging to acquire optimum numbers of specimens from large nonhuman primates, not only because of the ethical concerns but also because of the cost-related issues.
Our study provides evidence for subtle but significant systemic immune changes in the nonhuman primate model of induced endometriosis. We have demonstrated significantly increased numbers of CD10+ endometrial stromal cells in femoral LNs of baboons with induced endometriosis compared to control animals. We have also documented increased presence of immune cells into LNs draining the uterus and the peritoneal cavity in animals with induced disease. It is thought that this influx may be reflecting a primary response to the early months of disease establishment, a process which is extremely difficult to study in women. The nonhuman primate model of induced endometriosis is crucial in enabling us to understand how early immune responses may attempt to prevent or modify endometriotic lesion establishment and how immune parameters may alter following lesion excision.
Acknowledgements
The authors wish to acknowledge technical support from Patricia Mavrogianis (Imaging and Microscopy Core, University of Illinois at Chicago) and Mr Lawrence Young (Dako, Denmark) and statistical advice from Dr Georgina Luscombe.
This study was performed at the Department of Obstetrics, Gynaecology and Neonatology, The University of Sydney and the University of Illinois at Chicago.
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: Thyne Reid Foundation, by the Department of Obstetrics, Gynaecology and Neonatology, The University of Sydney and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD 40093 to ATF as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research
References
- 1. Vinatier D, Cosson M, Dufour P. Is endometriosis an endometrial disease? Eur J Obstet Gynecol Reprod Biol. 2000;91(2):113–125 [DOI] [PubMed] [Google Scholar]
- 2. Halban J. Hysteroadenosis metastatica. Wien klin Woschensch. 1924;37:1205–1206 [Google Scholar]
- 3. Sampson J. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–109 [PMC free article] [PubMed] [Google Scholar]
- 4. Javert CT. Pathogenesis of endometriosis based on endometrial homeoplasia, direct extension, exfoliation and implantation, lymphatic and hematogenous metastasis. Cancer. 1949;2(3):399–410 [DOI] [PubMed] [Google Scholar]
- 5. Ota H, Igarashi S, Tanaka T. Expression of γδ T cells and adhesion molecules in endometriotic tissue in patients with endometriosis and adenomyosis. Am J Reprod Immunol. 1996;35(5):477–482 [DOI] [PubMed] [Google Scholar]
- 6. Bulmer JN, Jones RK, Searle RF. Intraepithelial leukocytes in endometriosis and adenomyosis: Comparison of eutopic and ectopic endometrium with normal endometrium. Hum Reprod. 1998;13(10):2910–2915 [DOI] [PubMed] [Google Scholar]
- 7. Akoum A, Metz CN, Al-Akoum M, Kats R. Macrophage migration inhibitory factor expression in the intrauterine endometrium of women with endometriosis varies with disease stage, infertility status, and pelvic pain. Fertil Steril. 2006;85(5):1379–1385 [DOI] [PubMed] [Google Scholar]
- 8. Al-Jefout M, Tokushige N, Hey-Cunningham AJ, et al. Microanatomy and function of the eutopic endometrium in women with endometriosis. Expert Rev Obstet Gynecol. 2009;4(1):61–79 [Google Scholar]
- 9. Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod. 2009;24(2):325–332 [DOI] [PubMed] [Google Scholar]
- 10. Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod. 2009;24(7):1695–1703 [DOI] [PubMed] [Google Scholar]
- 11. Berbic M, Hey-Cunningham AJ, Ng C, et al. The role of FoxP3+ regulatory T-cells in endometriosis: A potential controlling mechanism for a complex, chronic immunological condition. Hum Reprod. 2010;25(4):900–907 [DOI] [PubMed] [Google Scholar]
- 12. Beliard A, Donnez J, Nisolle M, Foidart JM. Localization of laminin, fibronectin, e-cadherin, and integrins in endometrium and endometriosis. Fertil Steril. 1997;67(2):266–272 [DOI] [PubMed] [Google Scholar]
- 13. Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13(6):1686–1690 [DOI] [PubMed] [Google Scholar]
- 14. Vinatier D, Orazi G, Cosson M, et al. Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2001;96(1):21–34 [DOI] [PubMed] [Google Scholar]
- 15. Hull ML, Charnock-Jones DS, Chan CLK, et al. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88(6):2889–2899 [DOI] [PubMed] [Google Scholar]
- 16. Sampson JA. Intestinal adenomas of endometrial type: Their importance and their relation to ovarian hematomas of endometrial type (perforating hemorrhagic cysts of the ovary). Arch Surg. 1922;5(1):217–280 [Google Scholar]
- 17. Irani S, Atkinson L, Cabaniss C, Danovitch SH. Pleuroperitoneal endometriosis. Obstet Gynecol. 1976;47(Suppl. 1):72–74 [PubMed] [Google Scholar]
- 18. Roesner N, Boeger A. Endometriosis of the ureter. Eur Urol. 1979;5(1):294–297 [DOI] [PubMed] [Google Scholar]
- 19. Mascaretti G, Patacchiola F, Di Berardino C, Moscarini M. Isolated inguinal endometriosis. A clinical case. Minerva Ginecol. 2000;52(6):249–252 [PubMed] [Google Scholar]
- 20. Abrao MS, Podgaec S, Dias Jr JA, et al. Deeply infiltrating endometriosis affecting the rectum and lymph nodes. Fertil Steril. 2006;86(3):543–547 [DOI] [PubMed] [Google Scholar]
- 21. Mechsner S, Weichbrodt M, Riedlinger WFJ, et al. Estrogen and progestogen receptor positive endometriotic lesions and disseminated cells in pelvic sentinel lymph nodes of patients with deep infiltrating rectovaginal endometriosis: A pilot study. Hum Reprod. 2008;23(10):2202–2209 [DOI] [PubMed] [Google Scholar]
- 22. Noel JC, Chapron C, Fayt I, Anaf V. Lymph node involvement and lymphovascular invasion in deep infiltrating rectosigmoid endometriosis. Fertil Steril. 2008;89(5):1069–1072 [DOI] [PubMed] [Google Scholar]
- 23. Berbic M, Hey-Cunningham AJ, Ng C, et al. A novel study of endometrial stromal cells and immune-cell populations in sentinel uterine-draining lymph nodes during the menstrual cycle and in endometriosis. J Reprod Immunol. 2011 in submission. [DOI] [PubMed] [Google Scholar]
- 24. Gashaw I, Hastings JM, Jackson KS, Winterhager E, Fazleabas AT. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol Reprod. 2006;74(6):1060–1066 [DOI] [PubMed] [Google Scholar]
- 25. Hastings JM, Fazleabas AT. A baboon model for endometriosis: Implications for fertility. Reprod Biol Endocrinol. 2006;4(Suppl. 1):article number S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hastings JM, Jackson KS, Mavrogianis PA, Fazleabas AT. The estrogen early response gene FOS is altered in a baboon model of endometriosis. Biol Reprod. 2006;75(2):176–182 [DOI] [PubMed] [Google Scholar]
- 27. Ellis H. Anatomy of the uterus. Anaesth Intensive Care. 2008;9(2):107–109 [Google Scholar]
- 28. Grevious MA, Cohen M, Shah SR, Rodriguez P. Structural and functional anatomy of the abdominal wall. Clin Plast Surg. 2006;33(2):169–179 [DOI] [PubMed] [Google Scholar]
- 29. Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann NY Acad Sci. 2002;955:308–317 [DOI] [PubMed] [Google Scholar]
- 30. Becker S, Becker-Pergola G, Fehm T, et al. Image analysis systems for the detection of disseminated breast cancer cells on bone-marrow cytospins. J Clin Lab Anal 2005; 19(3): 115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Descazeaud A, De La Taille A, Allory Y, et al. Characterization of ZAG protein expression in prostate cancer using a semi-automated microscope system. 2006; 66(10):1037–1043 [DOI] [PubMed] [Google Scholar]
- 32. Tawfik OW, Kimler BF, Davis M, et al. Comparison of immunohistochemistry by automated cellular imaging system (ACIS) versus fluorescence in-situ hybridization in the evaluation of HER-2/neu expression in primary breast carcinoma. Histopathology. 2006;48(3):258–267 [DOI] [PubMed] [Google Scholar]
- 33. McKay JS, Bigley A, Bell A, et al. A pilot evaluation of the use of tissue microarrays for quantitation of target distribution in drug discovery pathology. Exp Toxicol Pathol. 2006;57(3):181–193 [DOI] [PubMed] [Google Scholar]
- 34. Le Gouill S, Talmant P, Touzeau C, et al. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-myc rearrangement. Haematologica. 2007;92(10):1335–1342 [DOI] [PubMed] [Google Scholar]
- 35. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258 [DOI] [PubMed] [Google Scholar]
- 36. Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: A survey of women from the USA and the UK. Hum Reprod. 1996;11(4):878–880 [DOI] [PubMed] [Google Scholar]
- 37. Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296–1301 [DOI] [PubMed] [Google Scholar]
- 38. D'Hooghe TM, Bambra CS, Raeymaekers BM, Hill JA. Pelvic inflammation induced by diagnostic laparoscopy in baboons. Fertil Steril. 1999;72(6):1134–1141 [DOI] [PubMed] [Google Scholar]
- 39. D'Hooghe TM, Bambra CS, Xiao L, Peixe K, Hill JA. Effect of menstruation and intrapelvic injection of endometrium on inflammatory parameters of peritoneal fluid in the baboon (Papio anubis and Papio cynocephalus). Am J Obstet Gynecol. 2001;184(5):917–925 [DOI] [PubMed] [Google Scholar]
- 40. D'Hooghe TM, Hill JA, Oosterlynck DJ, Koninckx PR, Bambra CS. Effect of endometriosis on white blood cell subpopulations in peripheral blood and peritoneal fluid of baboons. Hum Reprod. 1996;11(8):1736–1740 [DOI] [PubMed] [Google Scholar]