Abstract
An important action of progesterone during pregnancy is to maintain the uterus in a quiescent state and thereby prevent preterm labor. The causes of preterm labor are not well understood, so progesterone action on the myometrium can provide clues about the processes that keep the uterus from contracting prematurely. Accordingly, we have carried out Affymetrix GeneChip analysis of progesterone effects on gene expression in immortalized human myometrial cells cultured from a patient near the end of pregnancy. Progesterone appears to inhibit uterine excitability by a number of mechanisms, including increased expression of calcium and voltage-operated K+ channels, which dampens the electrical activity of the myometrial cell, downregulation of agents, and receptors involved in myometrial contraction, reduction in cell signal components that lead to increased intracellular Ca2+ concentrations in response to contractile stimuli, and downregulation of proteins involved in the cross-linking of actin and myosin filaments to produce uterine contractions.
Keywords: progesterrone, myometrium, quiescence, gene array, quantitative PCR, estrogen
Introduction
During human pregnancy, the myometrium is relatively quiescent until near term, when it becomes more sensitive to contractile stimuli. Thus, begins the development of coordinated rhythmic contractions that increase in size and frequency until the initiation of labor. Factors that maintain uterine quiescence prevent the myometrium from contracting prematurely and hence are critical for allowing the normal course of gestation. It is generally accepted that progesterone plays an important role in the maintenance of uterine quiescence until the perinatal period, when estrogen action dominates.1 However, the molecular mechanisms by which progesterone reduces uterine contractility in human gestation are not completely known; and a better understanding of the processes could lead to the development of improved progesterone receptor (PR) modulators that might be useful in preventing preterm labor.
The effects of progesterone on smooth muscle likely occur at multiple levels. Nongenomic effects, which are to be seen within minutes, have been described in the studies using smooth muscle from several organ sources (Xiao et al2 and references therein). These actions appear to be mediated by nontranscriptional activator type, membrane-associated PRs.3 However, most effects of progesterone are thought to be mediated through its specific binding to nuclear hormone receptors, which acting as transcriptional regulators cause changes in the expression of target genes. Several laboratories have used genomic approaches on human myometrium and compared gene networks expressed before and during parturition in the transition from relative quiescence to the contractile state.4–8 Salomonis and coworkers compared the mouse myometrial transcriptome during the quiescent, midgestation phase with other defined physiological phases such as nonpregnant, late gestation, and postpartum states.9 These workers demonstrated a number of specific genes whose expression is changed during midgestation, but it was not possible to attribute the changes to specific activators or repressors, such as estradiol or progesterone. It also was not possible to attribute the changes in gene expression to myometrial smooth muscle cells or to other cell types in the myometrium.
The gene targets of progesterone related to myometrial quiescence or activation remain to be clarified.10 Progesterone might modulate uterine physiology by acting directly on myometrial cells or indirectly by stimulating the release of humoral factors from extrauterine cell types that in turn regulate myometrial function. To elucidate the direct genomic effects of progesterone on human myometrial cells, we treated immortalized myometrial cells prepared from patients in late pregnancy,11 with increasing doses of progesterone and performed Affymetrix GeneChip analyses. Cultured human myometrial cells rapidly lose the expression of both endogenous estrogen and PRs.12 However, by engineering ectopic expression of estrogen receptor α (ER-α) in these cells, we were able to induce the expression of functional endogenous PRs with estrogen treatment. Such an induction in uterine tissue has been widely demonstrated in animal species and in humans.13–15 The present findings show that progesterone can act either by opposing the effects of estrogen, enhancing the effects of estrogen, or independently of estrogen. Among these actions, progesterone can stimulate the transcription of genes that normally suppress myometrial contractions or repress genes that serve to activate uterine smooth muscle contraction.
Experimental Procedures
Materials
Minimum essential medium (MEM), l-glutamine, antibiotic-antimycotic (penicillin, streptomycin, and amphotericin), and G-418 were purchased from GIBCO (Life Technologies, Carlsbad, California). Estradiol-17β and progesterone were purchased from Sigma-Aldrich (St. Louis, Missouri). Enzyme-linked immunosorbent assay (ELISA) kits for endothelin 1 and cyclic adenosine monophosphate (cAMP) were purchased from BD Biosciences (San Jose, California) and used according to the manufacturer’s directions. Antibody to human estrogen receptor-α (hER-α) was obtained NeoMarkers (Freemont, California). Antibody to AKAP12 was purchased from Bethyl Laboratories Montgomery, Texas. Antibodies to insulin receptor substrate 1 (IRS-1) and IRS-2 were purchased from Cell Signaling Technology (Boston, Massachusetts). Monoclonal antibody to KCNMA1 (slo1 Maxi-K+ channel was obtained from NeuroMab (UCDavis, Davis, California). Monoclonal antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was purchased from Millipore Corp (Billerica, Massachusetts). Promegestrone (R5020), (17α-methyl-3H) [3H]R5020), 2.83 TBq/mmol, was purchased from PerkinElmer, Boston, Massachusetts.
Human Estrogen Receptor-α Expression Construct
The constitutive hER-α expression vector was generated by appending EcoRI sites to hER-α complementary DNA (cDNA), using forward 5′-CAAGAATTCGGATCCATGACCATGACCCT-3′ and reverse 5′-CATGAATTCTTCGAAGTCGACTCAGACTGTGGCAG-3′ PCR primers. The approximate 1800 nt polymerase chain reaction (PCR) fragment was digested with EcoRI and inserted into EcoRI-linearized pQCXIN retroviral vector (Clontech, Mountain View, California) to generate pQCXIN-hER-α. The appropriate orientation of hER-α cDNA was verified by DNA sequencing. The construct was transfected into AmphoPack-293 packaging cells (BD Biosciences Clontech), using Fugene (Roche Diagnostics, Indianapolis, Indiana). Retroviruses were harvested and used to infect telomerase immortalized human myometrial cells (HM6) from a pregnant women.11 The infected myometrial cells were subjected to antibiotic selection using G-418 (Invitrogen, Carlsbad, California), 125 μg/mL. After cloning to establish cell lines, ER-α expression levels in each line were determined by quantitative PCR (qPCR) and immunoblotting. One cell line, HM6ERMS2, was used for subsequent studies.
Cell Culture
Cells were maintained in culture dishes in MEM containing 10% v/v fetal bovine serum (FBS), 4 mmol/L l-glutamine, penicillin G (100 IU/mL), streptomycin sulfate (100 μg/mL), and amphotericin B (15 μg/mL) at 37°C (95% humidity) in the presence of 5% CO2. Cells were maintained confluent in 6-cm plates for 1 to 2 weeks before treatment to ensure that they were in stationary phase. The medium was replaced with medium containing estradiol (20 nmol/L) and bovine serum albumin, 0.5%, in place of the FBS. The following day, the medium was replaced with a medium in which the estradiol concentration was lowered to 2 nmol/L. After 24 hours, the cells were then treated with 2 nmol/L estradiol and either 25, 100, or 500 nmol/L progesterone and for 2 successive days with daily changes of medium. RNA was extracted using the Ambion RNAqueous kit (Ambion, Austin, Texas), according to the manufacturer’s instructions. The quality of the purified RNA was checked using an Agilent 2100 Bioanalyzer 2100 (Agilent Technologies, Palo Alto, California).
Quantification of PRs by Radioligand Uptake
Specific uptake of [3H]R5020 was determined using HM6ERMS2 cells. Prior to assay, the cells in 24-well culture dishes were incubated with or without 10 nmol/L estradiol-17β in serum-free medium containing 0.1% bovine serum albumin for 4 days. Increasing concentrations of [3H]R5020 either with or without 500 nmol/L progesterone were added, and after a 2-hour incubation period at room temperature, the cells were rinsed free of medium, and the amounts of radioactivity and DNA concentration in each well were determined. Each well contained about 280 000 cells. Specific uptake was determined by subtracting the disintegrations per minute (dpm) from wells containing progesterone from their counterparts lacking progesterone. The data were analyzed using a nonlinear, single-binding site model using GraphPad Prism software (GraphPad Software, La Jolla, California).
Quantitative Reverse Transcriptase–Polymerase Chain Reaction
RNA samples for were quantified using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, Delaware) and qualified by the analysis on an RNA Nano chip using the Agilent 2100 Bioanalyzer. Synthesis of cDNA was performed with 1 μg of total RNA in a 20-μL reaction using the reagents in the Taqman Reverse Transcription Reagents kit from Applied Biosystems (ABI, Carlsbad, California). Quantitative reverse transcriptase–polymerase chain reaction (qPCR) amplifications, performed in triplicate, were done using 2 μL of cDNA in a total volume of 25 μL using the SYBR Green PCR Master Mix (ABI) as specified by the manufacturer. The final concentrations of probes and primers were 250 and 900 nmol/L, respectively. Primers were designed to span intron junctions to eliminate reverse transcription of genomic DNA. In the case of genes lacking introns, the RNA was treated with RNase-free DNase I after deproteinization with protease A treatment and ethanol precipitation. DNase activity was removed by heat inactivation in the presence of 0.5 mol/L EDTA. The PCR assays were run in the ABI Prism 7000 or the ABI Prism 7500 Sequence Detection System. Relative cDNA values were determined using 18S RNA as a normalizer, using the 2−ΔΔCT method.16 One sample in the assay set served as the calibrator or reference sample.
GeneChip Microarray
Total RNAs from triplicate samples were used for first-strand cDNA synthesis and preparation of biotin-labeled complementary RNA (cRNA). Hybridizations were carried out using the Affymetrix Human Genome U133 Plus 2.0 Array, which contains essentially the whole human genome (ca 38 500 genes). Detailed protocols are provided at www.scms.utmb.edu/genomics/index.htm and at www.affymetrix.com.
Bioinformatic Processing of Affymetrix GeneChip Data
Results from GeneChip analysis of 1 of the cell lines, HM6ERMS2, were processed using the S-PLUS Array Analyzer (Insightful Corp, Seattle, Washington). After robust multichip averaging (RMA) and quantile normalization, the summarized data were subjected to differential expression testing using 1-way analysis of variance (ANOVA) to filter out nonresponders. Genes classified as “absent” across all the chips were eliminated from consideration. Multitest comparison tests were then performed using Benjamini and Hochberg (BH) and Bonferroni methods to filter out false positives.
Immunoblotting
Cell lysates were prepared in radioimmunoprecipitation (RIPA) buffer (Thermo Scientific, Rockford, Illinois) supplemented with Halt Protease Inhibitor Cocktail and Halt Phosphatase Inhibitor Cocktail (Thermo Scientific), and protein concentrations were determined using a Pierce BCA Protein Assay kit (Thermo Scientific). The lysates were adjusted to 1× Laemmli sample preparation buffer and subjected to sodium dodecyl sulfate–polyacryamide gel electrophoresis (SDS-PAGE) followed by transfer of the proteins to polyvinylidene difluoride membranes (Millipore, Bedford, Massachusetts). The membranes were probed with 1:1000 dilutions of primary antibodies. Following rinsing, the blots were incubated with a 1:10 000 dilution of horseradish peroxidase (HRP)-conjugated antirabbit or antimouse immunoglobulin G ([IgG] Southern Biotech, Birmingham, Alabama), depending on the first antibody. Analysis was performed using the Enhanced Chemiluminescence Detection Kit (Thermo Scientific). Images were analyzed by densitometry using a FluorChem Imager (Alpha Innotech, San Leandro, California). The membranes were stripped using Restore Western Blot Stripping Buffer (Thermo Scientific) and reprobed with antibody to IRS-1 or GAPDH.
Statistical Analysis
Experiments were carried out using the mean ± standard error (SE) of triplicate samples. Comparison among groups was made by 1-way ANOVA and the Holm-Sidak post test, using Sigma-Stat software (Systat, Point Richmond, California). A value of P ≤ .05 was considered significant.
Results and Discussion
Development of Immortalized Human Myometrial Cell Lines That Constitutively Express hER-α, and Estrogen Induction of PRs
A myometrial cell line derived from a patient in late pregancy, HM6ERMS2, expressed ectopic ER-α, as determined by qPCR. The expression level was 3.6 times greater than the endogenous ER-α expression level in MCF-7 cells. The appropriately sized ER-α (66.2 kDa) was demonstrated by immunoblot analysis, using a specific antibody to ER-α (Figure 1). A band of the same mass was also seen in amphopak 293 cells transiently transfected with the expression vector DNA (Figure 1). In contrast, there was no antibody staining in the extracts from uninfected myometrial or nontransfected 293 cells.
Figure 1.
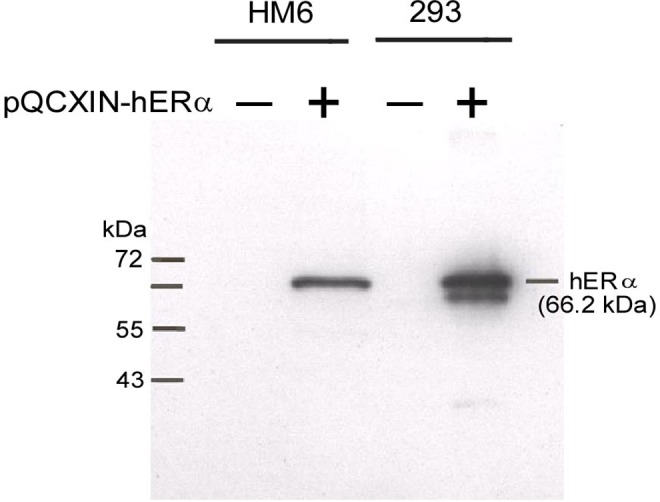
Immunoblot analysis of hER-α in immortalized human myometrial cells (HM6) and Amphopack 293 (293) cells. A single 66.2-kDa band corresponding in size to hER-α was demonstrated in both cell lines transfected with the vector, pQCXIN-hERα (+). In contrast, there was no band present in cells that were not transfected (–).
Treatment with estradiol, 20 nmol/L, for 24 hours followed by 2 nmol/L for an additional 24 hours resulted in a 5.6-fold increase in PR B messenger RNA (mRNA) levels, as determined by qPCR. These receptors were shown to be functional, as assessed by the cellular uptake of [3H]promegastone (R5020). There was about a 36-fold increase in the uptake of radioactivity (2-hour incubation period). Estrogen treatment increased the estimated number of PRs per cell from about 2250 (without estrogen treatment) to about 81 000 (Figure 2). The apparent K d, about 30 nmol/L, is about 5 times greater than that reported using cell-free, calf uterine cytosol17 and may be a reflection of less than optimal binding conditions found in intact cell assays. In the only other studies of this nature, Sandovsky et al18 stably expressed the hER-α in a transformed myometrial cell line from nonpregnant hamster. Treatment of these cells with estradiol resulted in about a 9-fold increase in the concentration of PRs (from 3700 to 33 000 receptors per cell).
Figure 2.
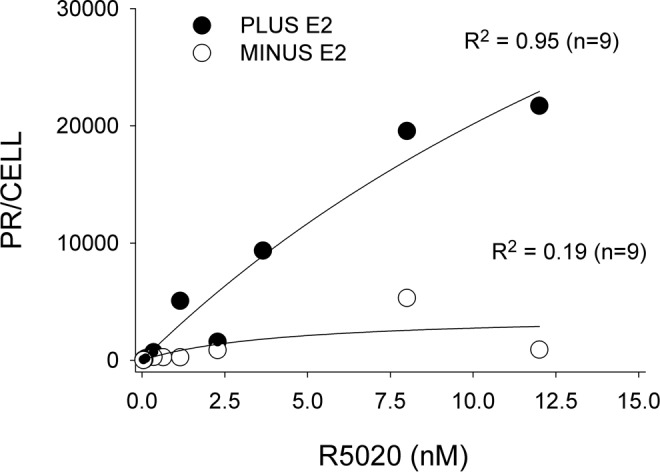
Specific uptake of [3H]R5020 by immortalized human myometrial cells constitutively expressing ER-α(HM6ERMS2). Cells were incubated either in the presence or absence of estradiol (plus E2 and minus E2, respectively) prior to and during assay. The number of progesterone receptors (PRs) are expressed per cell versus the concentration of [3H]R5020 in the medium. The correlation coefficient (R 2) for each curve is shown.
Genomics Approach Used for the Identification of Genes and Pathways Responsive to Progesterone Treatment
Following induction of PRs by treatment with estradiol for 2 days, cells were incubated for 2 days with 25, 100, or 500 nmol/L progesterone in the presence of 2 nmol/L estradiol. Whole-genome array hybridizations were performed after the extraction of total RNA. The effects of progesterone on genes of interest were confirmed by qPCR and also assessed by immunoassays and immunoblotting.
Clustering of Expression Changes With Estrogen and Estrogen Plus Progesterone Treatments
Differential expression testing using the Local Pooled Error Test19 showed 2040 and 794 probe sets were expressed in cells treated with estradiol at P ≤ .01 without and with Bonferroni corrections, respectively. One-way ANOVA of the estradiol plus progesterone-treated data set identified 444 probe sets at P ≤ .05 with the Bonferrroni correction. Gene clusters that differentiate treatment groups are presented graphically using heat maps with hierarchical clustering (Figure 3).
Figure 3.
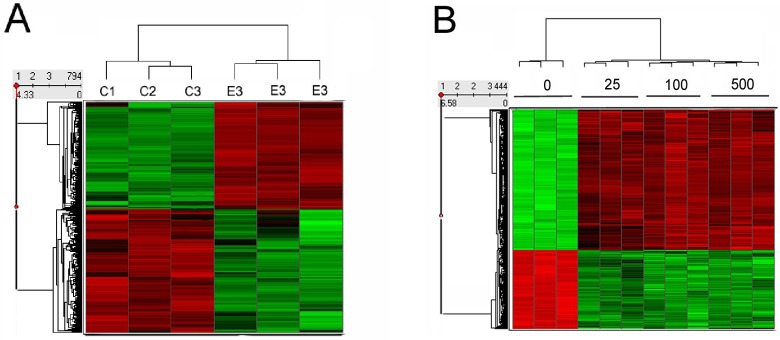
Heat map analysis of differentially regulated genes showing intersample variability of triplicate replicates and lack of differences between 3 doses of progesterone. Red indicates upregulated messenger RNA (mRNA) expression and green indicates downregulated mRNA expression from baseline averages . A, Estrogen (2 days) versus vehicle treatment. B, Estrogen (4 days) versus progesterone (pretreatment for 2 days with estrogen followed by 2 days of estrogen plus either 0, 25, 100, or 500 nmol/L progesterone).
These data show that there were distinct differences between control and experimental groups, with the treatments resulting in gene clusters characterized as having either increased (red) or decreased (green) expression. The heat map also shows that the 3 replicates within each treatment group were closely related to each other (Figure 3). There were no marked qualitative or quantitative differences in gene expression between the 3 doses of progesterone used (Figure 3 and Supplement Table 1). Setting the filter at changes ≥2-fold further reduced the number of genes to 49 and 270 in cells treated with estrogen and estrogen plus progesterone, respectively (Supplement Table 1).
General Effects of Progesterone
Many of the differentially expressed probe sets lie in the range of 1.5 to 2.0 absolute fold change. In instances where the GeneChip data were compared with qPCR results, a 1.5-fold change in the former was almost always found to be a significant difference when examined by qPCR. Using a filter of 1.5-fold change (P ≤ .05), 3 general categories of genes that responded to progesterone were clearly seen: those whose expression was affected (1) in the opposite direction (enhanced/repressed) to that seen with estrogen treatment, (2) in the same direction as changes seen with the effects of estrogen, either enhanced or repressed, or (3) separately from the effects of estrogen (Supplement Tables 2-4). Most of the affected genes were in the third category, with progesterone treatment affecting a greater number of genes than estradiol (Supplement Table 4). This might be due to the fact that the cells were maintained in the confluent state during the addition of steroids, to mimic myometrial conditions around mid-pregnancy after hyperplasia has occurred. Accordingly, genes involved in DNA synthesis, cell cycle progression and cytokinesis were marginally changed with estrogen treatment (Supplement Table 1). In contrast, the expression of these same genes was downregulated by progesterone treatment. As a case in point, anillin, an actin-binding protein that acts as a scaffold protein to link RhoA, actin, and myosin during cytokinesis, was downregulated about 4.5-fold by progesterone treatment (Supplement Table 1).
Genes involved in transport and cell adhesion were upregulated by progesterone treatment, as were genes expressing proteolytic enzymes (Supplement Table 1). Expression of metallothioneins, including metallothionein-2A, was also upregulated by progesterone (Supplement Table 1). These findings agree with the observations showing that endogenous metallothionein-2A is induced by progestins in human cell lines.20
The utility of the cell culture system was established by correlating the changes in gene expression with the known actions of estrogen and progesterone on specific genes. GREB1 and PDZK1 are estrogen-regulated genes expressed in hormone-responsive breast cancer cells and tissues.21 Estrogen treatment caused more than a 19-fold increase in GREB1 and 4.5-fold increase in PDZK1 expression in the myometrial cells (Supplement Table 1). Stanniocalcin 2 (STC2) mRNA expression is induced 3-fold with estrogen treatment of MCF-7 breast tumor cells, and the induction is totally reversed with anti-estrogen treatment.22 In agreement with these findings, STC2 was induced 3-fold with estrogen treatment of human myometrial cells, and this effect was completely reversed with progesterone treatment (Supplement Table 1).
Progesterone treatment downregulated ER-α and PR expression 1.4- and 2.4-fold, respectively. The Affymetrix probe set on which the estrogen receptor data are based is localized to the extreme 3′ untranslated region,23 representing the endogenous ER-α and not the ectopically expressed receptor, which does not contain untranslated sequences. As the untransfected cells were virtually devoid of endogenous ER-α, estrogen priming of ER-α-transfected cells induced endogenous ER-α to a relatively small, albeit statistically significant level. The direction of change in ER-α and PR expression is commensurate with that reported for the uterus in whole animal studies.24–27 Progesterone receptor membrane component 2 (PGRMC2) mRNA and the associated protein serpine 128 were increased 1.6 and about 12 times, respectively, after treating the human myometrial cells with progesterone (Supplement Table 4). These findings agree with the studies on mouse uterus in which it was shown that pgrmc2 and pgrmc1 expression were upregulated during proestrus and metestrus (when blood progesterone levels are elevated) in intact mice, or after treating ovariectomized mice with progesterone.28 The major progesterone-modulated proteins secreted into the sheep uterus are members of the serpin superfamily of serine protease inhibitors.29 Parenthetically, as the PGRMC-serpine 1 complex mediates certain nongenomic effects of progesterone,28,30 the upregulation of the complex by the genomic actions of progesterone might be required for some of its nongenomic actions.
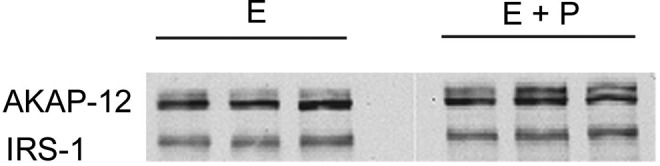
FKBP-51 is a PR-associated immunophilin that forms part of the inactive, hetero-oligomeric complex of unliganded PR and components of the molecular chaperone machinery.31 Progesterone increases the expression of FKBP-51 in T-47D cells, a breast cancer cell line.32 In human myometrial cells, progesterone induced a 5.7- to 7.1-fold increase in FKBP-51 (FKBP5) expression (Supplement Table 1). Human insulin receptor substrate 2 (IRS-2) has been shown to be a primary progesterone response gene in HeLa33 and MCF-7 cells.34 Human IRS-2 expression in human skeletal muscle is increased 1.5- to 2-fold near the end of pregnancy in women.35 These findings would be expected, given elevated concentrations of progesterone produced during pregnancy. Treatment of the human myometrial cells with progesterone increased IRS-2 expression by about 3-fold (Supplement Table 1), which was confirmed by real-time PCR (3.6-fold; 0.79 ± 0.1 vs 2.87 ± 0.07, n = 3, for estrogen vs estrogen + progesterone treatment). There was also an increase in immunoreactive IRS-2 with progesterone treatment, as shown by immunoblot analysis (Figure 4). However, the increase was only 2-fold (P ≤ .001). There were no changes in either IRS-1 mRNA or protein levels (Figure 4) with progesterone treatment.
Figure 4.
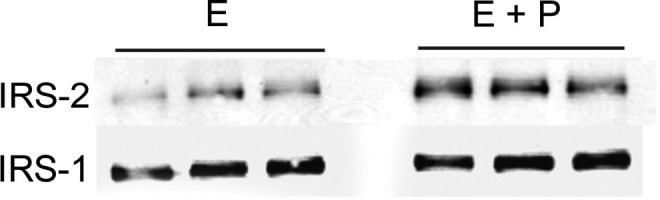
Immunoblot of insulin receptor substrate (IRS)-2 and IRS-1 in human myometrial cells with estrogen (E) and estrogen plus progesterone (E + P) treatment of triplicate samples.
Progesterone-treated human myometrial cells showed a 1.5-fold increase in the regulator of G-protein signaling 2 ([RGS2] Supplement Table 2), in agreement with the previous study that showed that progesterone treatment increased RGS2 mRNA levels in the rat myometrium.36 A new finding was that progesterone treatment reduced the level of RGS4 and RGS7 expression in human myometrial cells by 2.3- and 1.7-fold, respectively (Supplement Table 3). Progesterone caused almost an 8-fold reduction in the expression of stromelysin 3, a matrix metalloproteinase encoded by the MMP11 gene (Supplement Table 1). While there are no comparable findings on these effects of progesterone in human myometrium, progesterone is considered the principal suppressor of endometrial matrix metalloproteinase production.37 Progesterone induced a 3.7-fold increase in interleukin 8 (IL-8) expression, in line with the previous observations using human myometrial cells.38 These findings collectively show that both estrogen and progesterone genomic actions in the myometrial cell system are typical, validating the use of these cells to elucidate the PR transcriptome.
Gene Networks
Certain effects of progesterone might be attributable to the induction of key regulatory entities such as the IL-1 system. Although there was no apparent rise in IL-1 expression per se, progesterone increased the expression of the IL-1 receptor type 1 5- to 6.2-fold (Supplement Table 1). Expression of an IL-1 target gene, such as the pentraxin-related gene, has been shown to be rapidly induced by IL-1β in endothelial39 and human myometrial40 cells, and the mRNA levels were elevated about 25-fold in HM6ERMS2 cells (Supplement Table 1). Chevillard and coworkers40 have identified mRNAs of 198 known genes that were changed more than 2-fold after treating human myometrial cells with IL-1β for 1 hour. Increased IL-1β stimulation could account for the 3.0- to 3.7-fold increased expression of IL-8 (Supplement Table 1), as seen previously in human myometrial cells,41 as well as increased IL-6 (3-fold) expression (Supplement Table 1). Additional myometrial genes shown to be controlled by IL-1β40 that were increased more than 2-fold in the present studies include PTX3, CXCL2, EGR1, CEBPD, ZFP36, DUSP1, PAPPA, and NFKBIZ, IL6 (see Supplement Table 1 for symbol description). None of the genes negatively regulated by IL-1β were y noted.
Despite elevated IL-1 activity, there was no increased expression of prostaglandin-endoperoxide synthase 2 (PTGS2), a known IL-1 target gene in the human myometrium.41–44 PTGS2 catalyzes the rate-limiting step in the inducible production of prostaglandins in a number of cell types, including cultured human myometrial cells. Because prostaglandins can stimulate uterine contractions, it has been suggested that induction of PTGS2 expression in the myometrium at the end of pregnancy is involved in labor initiation.45–47 It seems likely that the absence of PTGS2 induction in the presence of increased IL-1 action is the result of progesterone costimulation of factors that negate the effects of IL-1 on PTGS2 induction.
Many of the effects of progesterone likely are also mediated by the transforming growth factor (TGF)-β signaling system. Progesterone treatment increased the expression of thrombospondin 1 (THBS1), an extracellular matrix protein, 2.1- to 2.3-fold (Supplement Table 1). Thrombospondin 1 interacts with the TGF-β precursor complex composed of latency-associated peptide in noncovalent association with mature TGF-β to effect conversion into active TGF-β.48 Prior to activation of TGF-β, the latency complex is targeted by latent transforming growth factor by binding protein 1 (LTBP1) to the extracellular matrix where the latent cytokine is subsequently activated.49 Progesterone treatment caused a 5.5- to 6.0-fold increase in the expression of LTBP1 (Supplement Table 1). Progesterone also stimulated a 2.1-fold increase in the expression of TGF-β receptor II ([TGFB2] Supplement Table 1). Expression of SMAD6, a negative regulator of TGF-β signaling,50 was reduced 1.8-fold with progesterone treatment (Supplement Table 1), consistent with progesterone stimulation of TGF-β signaling pathways. Increased TGF-β signaling likely accounts for the expression of genes associated with cell cycle arrest, a hallmark of TGF-β action.51,52 Inhibin βB, a member of the TGF-β superfamily, also was upregulated (2.2- to 2.5-fold) with progesterone treatment (Supplement Table 1).
Specific Effects of Progesterone on Uterine Quiescence
The possible actions of progesterone in eliciting and/or maintaining uterine quiescence can be divided into the following categories: membrane ion channels that hyperpolarize myometrial cells and diminish contractile activity; modified expression of agonists and receptors involved in uterine contractions; increased intracellular cAMP concentrations and protein kinase A (PKA) signaling, reduced Ca2+ signaling; and changes in the concentration of the cellular contractile components.
Effects on Potassium Channels
The resting membrane potential and corresponding excitability of uterine myocytes, and the frequency and duration of action potentials are differentially influenced by the enhancing effects of estrogen and suppressive actions of progesterone.53 During gestation, potassium channels are thought to maintain the uterus in a state of quiescence by contributing to the resting membrane potential and counteracting contractile stimuli. Potassium selective channels are the largest and most diverse group of ion channels represented by some 70 subunit encoding genes.54 Their main function is to maintain the membrane potential close to the reversal potential of K+ ions. Thus, drugs that open K+ channels inhibit uterine contractility.55–57 Conversely, K+ channel blockers stimulate uterine contractions.58,59
Potassium channels are widely expressed on the uterine smooth muscle cell surface.60–62 BK channels, also called Maxi-K or slo1, are opened by changes in membrane electrical potential and or by increases in concentrations of intracellular Ca2+.63 These channels are essential for the regulation of several key physiological processes including smooth muscle tone.63 Calcium-activated large-conductance potassium current has been demonstrated in human myometrial cells both in nonpregnant64 and pregnant58,65,66 states, but the channel becomes relatively insensitive to calcium during labor, likely contributing to augmented myometrial contractility.66 In addition, myometrial expression of Maxi-K channels is significantly reduced in women in labor compared with those not in labor.67
Maxi-K channels can contain 2 distinct subunits: a pore-forming α-subunit and a modulatory β-subunit. Each complete Maxi-K channel contains four copies of the α-subunit and up to 4 β-subunits. Treatment of human myometrial cells with progesterone resulted in greater than a 4-fold increase in mRNA levels of the α1-subunit (KCNMA1) and greater than a 5-fold decrease in the β2-subunit (KCNMB2; Supplement Table 1). These results were substantiated by qPCR, which showed that the expression of KCNMA1 was increased more than 4-fold with progesterone treatment (0.76 ± 0.12 vs 3.12 ± 0.13; P < .05) and the expression of KCNMB2 was decreased about 3.5-fold (0.73 ± 0.12 vs 0.21 ± 0.02; P < .05). Progesterone treatment increased the amount of KCNMA1 protein about 2-fold, as shown by immunoblot analysis (Figure 5).
Figure 5.
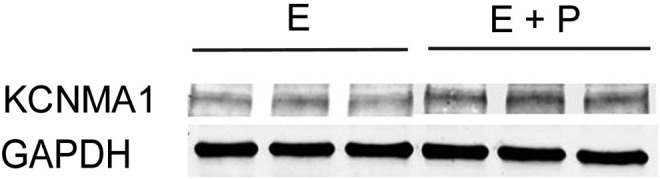
Immuoblot of Maxi-K channel α-subunit (KCNMA1) in triplicate samples of cells treated either with estrogen (E) or estrogen plus progesterone (E + P). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) staining illustrates the uniformity of gel loading and blotting.
The β2 subunit, 1 of the 4 β-subunit types, is an auxiliary subunit that influences calcium sensitivity and, following activation of Maxi-K current, causes persistent inactivation.68,69 The changes seen in the expression levels of both α1 and β2 subunits with progesterone treatment are consistent with augmented Maxi-K currents and inhibition of uterine contractility. Aside from genomic effects, estradiol interacts with β1 and β4 subunits to potentiate Maxi-K channel gating,70–73 but progesterone does not appear to have an appreciable postgenomic effect.73
Messenger RNAs expressing other types of potassium channels were also upregulated more than 2-fold by progesterone treatment. These include KCTD12, KCNE4, KCNG1, KCNS3, and KCNT2, (Supplement Table 1). It is noteworthy that none of the channels upregulated by progesterone treatment is among the well-characterized calcium- or voltage-activated K+ channels. KCTD12 expresses the tetramerization domain of a channel that has been proposed to participate in voltage-gated K+ channel activity. KCNE4 encodes a member of the K+ channel, voltage-gated, isk-related subfamily. KCNG1 and KCNS3 also express voltage-gated K+ channels, and the KCNT2 protein is a member of the calcium-activated K+ channel protein family (see NCBI AceView, http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/, for references). These channels, if functional, along with the Maxi-K channels would be expected to maintain the myometrium in a more quiescent state, and perhaps account for the major part of progesterone’s effects’ on uterine contractility.
VGCNL1, encoding a nonselective cation channel, was elevated over 5-fold with progesterone treatment (Supplement Table 1). Proteins comprising the family in which this channel is a member have 6 transmembrane helices in which the last 2 flank a loop that determines ion selectivity (NCBI, AceView). Genes with the same motif or that are more related by function include the Maxi-K channel, and glutamate and serotonin receptors (NCBI, AceView) which were all elevated with progesterone treatment. Aside from evidence of channel activity, little is known of the VGCNL1 channel operation or its functional significance.74 However, given its progesterone-associated upregulation with other ion channel proteins, it is probable that VGCNL1 plays a role in modulating uterine contractile activity.
Increased Expression of Receptors and Agonists Associated With Uterine Quiescence
Atrial natriuretic peptide
Atrial natriuretic peptide (ANP), a powerful vasodilator, is produced by human myometrial cells and causes myometrial relaxation in a dose-dependent manner.75 Myometrial ANP concentrations increase with advancing gestational age.75 Functional ANP receptors have been demonstrated in pregnant human myometrium, as the treatment of the myometrial membrane fraction from uteri taken during the second trimester with ANP markedly augmented the production of cyclic guanosine monophosphate (cGMP).76 Atrial natriuretic peptide is synthesized as a prohormone, and the precursor, pro-atrial natriuretic peptide (pro-ANF), is converted into ANP by the actions of corin, a transmembrane serine protease.77 The expression of corin by the human myometrial cells was upregulated almost 8-fold with progesterone treatment (Supplement Table 1). Implicit in these findings is that the concentration of an endogenous inhibitor of uterine contractility, ANP, is elevated following progesterone treatment.78,79 However, as the tocolytic effects of ANP were absent in pregnant rats or were negated by progesterone administration,80 it remains to be determined whether ANP plays an important role in maintaining uterine quiescence during pregnancy in humans.
Reduced Expression of Receptors and Agonists Associated With Increased Uterine Contractility
Endothelin and endothelin receptors.
Endothelin 1 (ET-1) is a potent vasoconstrictor peptide, originally believed to be expressed only in endothelial cells. Subsequently, it has been shown that it is widely distributed in different cell types. Endothelin1 has been shown to be produced by the rat myometrium during pregnancy, with the greatest concentrations found in the early postpartum period.81 Endothelin 1 induces phasic contractions of rat uterus in a manner similar to those induced by oxytocin.82 Endothelin 1 also increases the frequency of contractions of human uterine smooth muscle strips in vitro,83 being even more potent than oxytocin itself.84 It also increases cytoplasmic intracellular Ca2+ concentrations83,85 and myosin light-chain phosphorylation83 in primary cultures of human myometrial cells.
Estrogen treatment for 2 days elevated ET-1 mRNA expression 2.6-fold, and the effects of estrogen were reversed by progesterone treatment (Supplement Table 1). After 1 day of estrogen treatment, the amount of ET-1 in the medium was increased almost 10 times over control levels (Figure 6). Endothelin 1 in the medium declined thereafter to 3.7 times the control level on the second day of incubation. The addition of progesterone to the medium on day 3 reduced ET-1 levels to control values (Figure 6), confirming the mRNA GeneChip results. By day 4, the amount of ET-1 released into the medium with estrogen treatment was reduced to control levels (Figure 6).
Figure 6.
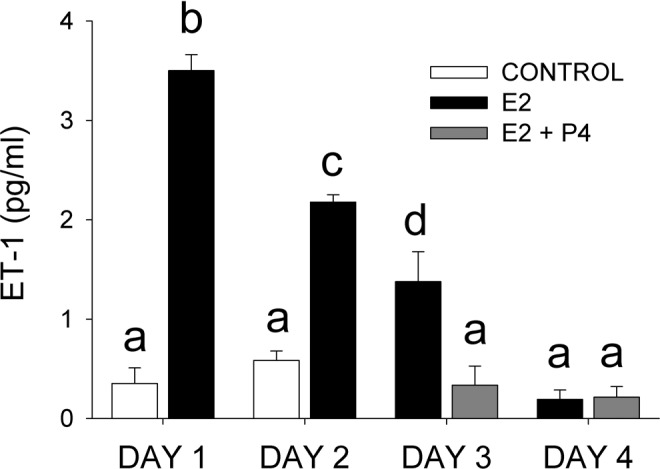
Endothelin 1 (ET-1) concentration in the incubation medium after culturing human myometrial cells in the absence (control) or presence of estradiol (E2) on days 1 and 2 of culture, or estradiol + progesterone, 100 nmol/L (E2 + P4) on days 3 and 4. The medium was replaced with fresh medium each day. Values are expressed as the mean ± standard error (SE) of triplicate samples. Symbols a, b, and c indicate significant differences (P ≤ .05) from each other.
Endothelin 1 receptors have been demonstrated in rat myometrium by ligand-binding studies.82 Endothelin 1 receptor concentrations in the rat myometrium are significantly elevated during labor when the uterus is under estrogen domination, likely accounting for increased uterine sensitivity to ET-1.86 These findings suggest that ET-1 is an important physiological modulator of uterine activity. There are at least 3 known endothelin receptors, ETA, ETB1, and ETB2, all of which are G-protein-coupled receptors whose activation results in the elevation of intracellular free-calcium ion concentration.87 ET-1-induced uterine contractions in humans are increased during pregnancy and are mediated through ETA, which also is elevated.88 In contrast, ETB receptor levels are decreased.88 Treatment of human myometrial cells with progesterone resulted in about a 3-fold reduction in the expression of ETA, as shown by GeneChip analysis (Supplement Table 1), or a 1.5-fold reduction as shown by qPCR (endothelin receptor type A [EDNRA], Figure 7). The reduction in both ET-1 and ETA with progesterone treatment indicates that progesterone treatment would cause a reduced contractile response to endogenously produced ET-1. In support of this idea, progestin treatment significantly attenuated exogenous ET-1-induced increases in intracellular Ca2+ concentrations in isolated human myometrial cells.89
Figure 7.
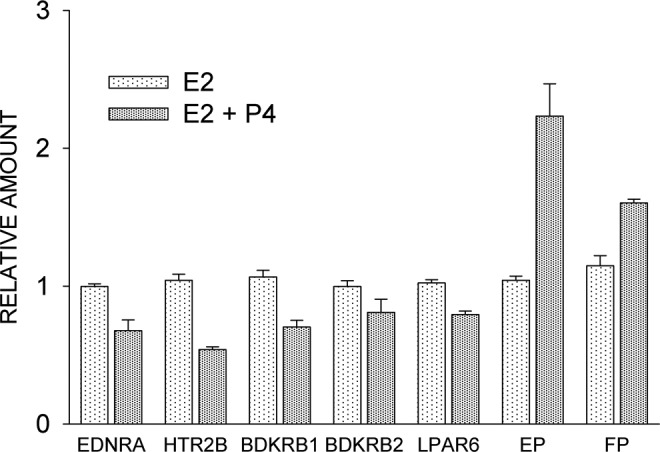
Effect of estradiol (E2) and progesterone, 100 nmol/L (E2 + P4) on receptor expression, as determined by quantitative reverse transcriptase–polymerase chain reaction (qPCR). Cells were incubated for 4 days with E2 or 2 days with E2 followed by another 2 days with E2 plus P4. EDNRA indicates endothelin receptor A; HTR2B, 5-hydroxytryptamine 2B receptor; BDKRB1, bradykinin B1 receptor; BDKRB2,, bradykinin B2 receptor; LPAR6, lysophosphatidic acid receptor 6; EP, prostaglandin E2 receptor; FP, prostaglandin F2α receptor. Each values is the mean ± standard error (SE) of triplicate determinations. With the exception of BDKRB2, E2 + P4 treatment groups were significantly different (P ≤ 0.05) from E2 treatment.
Oxytocin receptors
Oxytocin is one of the most potent uterotonic agents. Myometrial oxytocin receptor (OXTR) concentrations are highly regulated and are maximal at or near the time of labor in a variety of species examined, including humans.90–92 Although it is clear that OXTR levels are upregulated by estrogen and downregulated by progesterone in the rat myometrium,93,94 factors regulating OXTR concentrations in the human myometrium are less clear. Basal levels of OXTR expression in cultured human myometrial cells are relatively high and can be downregulated by removal of FBS.95 Surprisingly, the treatment of serum-starved cells with estradiol downregulated OXTR expression by 50%, and progesterone treatment resulted in a further 50% reduction (data not shown).
The effects of progesterone on OXTR expression appear to be mediated by IL-1. Interleukin 1 treatment of human myometrial cells reduces OXTR steady-state mRNA levels.96–98 Interleukin 1 acts on the OXTR gene at the transcriptional level through recruitment of nuclear factor (NF)-κB.99 Although progesterone treatment had no apparent effect on the level of IL-1 expression in human myometrial cells, there was almost a 6-fold increase in the level of expression of the IL1 receptor type 1 (Supplement Table 1).40 Mechanisms accounting for the effects of estrogen on human myometrial OXTR are currently unknown.
Studies have shown that progesterone inhibits the expression of connexin 43, a contraction-associated protein, and gap junction formation in cultured human myometrial cells.100,101 However, connexin 43 expression was unaffected by either estrogen or progesterone treatments in the present studies (data not shown).
Serotonin receptors
Progesterone treatment of human myometrial cells caused about a 2-fold reduction in the expression of the 5-hydroxytryptamine (5-HT) receptor 2B, as determined by GeneChip (Supplement Table 1) and confirmed by qPCR analysis (Figure 7). The 5-HT2 receptors are a subfamily of 5-HT receptors that bind the endogenous neurotransmitter. This subfamily consists of 3 G-protein-coupled receptors, 5-HT2A, 5-HT2B, and 5-HT2C, that are coupled to Gq/G11.102 The 5-HT2 receptors mediate excitatory neurotransmission and are involved in multiple physiological functions in smooth muscle including proliferation, differentiation, and contraction.102 In myometrium, 5-HT2 receptors not only play a role in contraction but also regulate the activity of hypertrophic genes during pregnancy.103 The sensitivity of the contractile response to serotonin of the isolated rat uterus is increased either during estrus or by administration of estradiol to ovariectomized rats, and estradiol treatment more than doubles the number of 5-HT analogue-binding sites.104 Contractions of the rat uterus in response to 5-HT-is greatest at the end of pregnancy, in association with the upregulation of 5-HT2A transcript (approximately 5-fold) and protein (approximately 6-fold) levels.104 Circulating levels of progesterone fall off sharply at the end of pregnancy, likely removing inhibitory influences on 5-HT2A expression.
Bradykinin receptor B1
Bradykinin stimulates contractions in isolated rat uterus preparations.105 Progesterone treatment of human myometrial cells caused a 1.5-fold reduction in B1 receptor expression, as determined initially by GeneChip analysis and confirmed by qPCR (Figure 7). Expression of the second bradykinin receptor, BDKRB2, was not significantly changed with progesterone treatment (Figure 7).
Lysophospholipid receptor
Progesterone treatment resulted in a significant 1.3-fold reduction in the expression of P2Y5, as confirmed by qPCR (Figure 7). P2Y5 originally was an orphan receptor with low-sequence homology to the purinergic P2Y receptor family.106 It turned out instead to be a novel lysophospholipid (LPA) receptor, renamed LPAR,107 that is coupled to Gαi and Gα12/13.108 Lysophospholipids stimulate isolated uterine smooth muscle (rat) contractions in a dose-dependent manner,109 so it is conceivable that downregulation of at least 1 LPA receptor type with progesterone treatment could contribute to reduced uterine activity.
Neurotensin
Neurotensin has also been shown to stimulate contractile activity of the isolated rat uterus.105 This 13 amino acid neuropeptide is distributed in both the nervous system and the peripheral tissues and displays a wide spectrum of biological activities.110 Neurotensin and neurotensin receptor 1 are expressed in connective tissue cells of the human myometrium.111 However, both hormone and receptor are expressed in smooth muscle cells of leiomyomas.111 In the present studies, estradiol increased the level of neurotensin expression over 7-fold and progesterone was found to cause about a 5-fold reduction in neurotensin mRNA levels in the human myometrial cells (Supplement Table 1).
Prostaglandin E2 receptor (EP2)
Prostanoid receptors activate either contractile or relaxant responses in uterine smooth muscle.112 The expression of EP2 mRNA, which mediates uterine relaxation, was upregulated about 4-fold with progesterone treatment (Supplement Table 1). Quantitative PCR indicated about a 2-fold increase (Figure 7). Activation of the EP2 results in relaxation of the myometrium in association with the stimulation of adenylyl cyclase activity and the generation of intracellular cAMP.112 Our findings are consistent with an observation showing that EP2 receptor mRNA expression was found to be maximal in pregnant rat myometrium113 at the time when blood progesterone levels are greatest.114 Furthermore, the treatment of ovariectomized rats with progesterone causes a significant increase in EP2 mRNA levels.115
Progesterone increased prostaglandin-endoperoxide synthase 1 (PTGS1) expression by 1.6- and 1.3-fold in the GeneChip and qPCR assays, respectively (P ≤ .05). It is likely that the capacity for increased prostaglandin synthesis by myometrial cells is related to the production of prostacyclin (PGI2). Thus, in addition to the upregulation of EP2, the prostaglandin produced by the myometrium is mainly PGI2,116–118 which also causes uterine quiescence.119,120
The prostaglandin F2α receptor, FP, was also significantly upregulated 1.4-fold (Figure 7). These results are inconsistent with the prostaglandin E2 receptor (EP) findings as FP is associated with a contractile response. In addition, PTGFRN, the prostaglandin F2α receptor negative regulator, was downregulated about 2-fold (Supplement Table 1), seemingly reducing the inhibition of FP signaling. This apparent disparity between FP upregulation and myometrial quiescence may be secondary to the fact that the major prostaglandin produced by the myometrium, PGI2, is a uterine relaxant.
Additional Receptor Types Downregulated by Progesterone
Progesterone treatment also downregulated the expression of the somatostatin receptor 1 (more than 2-fold) and the activin a receptor type 1 (about 2-fold; Supplement Table 1). The significance of these findings with respect to uterine contractile activity is not clear at the present time.
Intracellular cAMP Concentrations and PKA Signaling
Likely associated with EP2-elicited increases in cAMP synthesis, progesterone also caused a 2.5-fold reduction in the level of expression of the cAMP-specific degradative enzyme phosphodiesterase 4B, and a 1.8- to 2-fold decrease in calcium/calmodulin-dependent 3′,5′-cyclic nucleotide phosphodiesterase 1C expression (Supplement Table 1). The reduction in PDE4B expression was confirmed by qPCR (data not shown).
These findings agree with those of Kofinas et al, who showed that treatment of human myometrial cells with progesterone inhibits PDE4 catalytic activity121 and explain, at least in part, with the observation that progesterone treatment results in increased cAMP release and accumulation by isolated human pregnant myometrium.122 Indeed, treatment of human myometrial cells with progesterone resulted in almost a doubling of basal cAMP levels when compared with nontreated (control) or estrogen-treated cells (Figure 8). Stimulation of adenylyl cyclase activity by the addition of forskolin (final concentration 20 μmol/L) to progesterone-treated cells did not increase intracellular cAMP levels over basal levels after 15 minutes, unlike the case with untreated and estrogen-treated cells (Figure 8). Thus, basal levels of intracellular cAMP with progesterone treatment appear to resemble actively stimulated levels. Insofar as elevated cAMP levels activate PDE4B4 by serine phosphorylation,123 decreased expression of PDE4B4 caused by progesterone can apparently reduce the effects of cAMP on PDE activity. It is notable that during pregnancy the amount of myometrial PDE4 activity is 75% of that found in the nonpregnant myometrium.124 Furthermore, inhibition of PDE4 activity causes uterine relaxation.125,126
Figure 8.
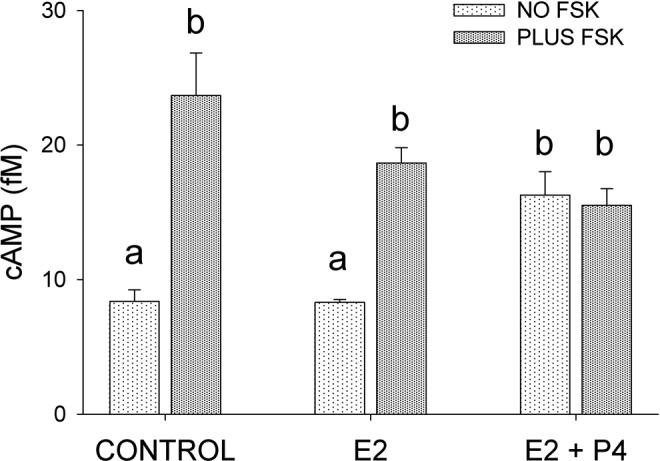
Effect of estradiol (E2) and progesterone, 100 nmol/L (E2 + P4) on intracellular cyclic adenosine monophosphate (cAMP) concentration, with and without forskolin ([FSK], 20 μmol/L) administration. Cells were incubated for 4 days with E2 or 2 days with E2 followed by another 2 days with E2 plus P4. FSK treatment was for 15 minutes. The values are expressed at the mean ± standard error (SE) of triplicate samples. Symbols a, b, and c indicate significant differences (P ≤ .05) from each other.
Cyclic AMP appears to effect smooth muscle relaxation at several different target sites.127 The regulatory unit of the protein kinase A complex, which is activated by cAMP, binds to a group of structurally diverse A-kinase anchoring proteins (AKAPs) that function both to confine the holoenzyme to discrete locations within the cell and to assemble components of the signaling complex. At the end of pregnancy, PKA and AKAP79/150 (AKAP5) are dissociated from myometrial membranes in the rat128 and human,129 but progesterone treatment maintains the association.129 It has been postulated that the decline in AKAP/PKA association with the myometrial plasma membrane causes the loss of cAMP inhibitory effects on phosphatidylinositide turnover and, by extension, an increase in uterine contractility.130 Treatment of human myometrial cells with progesterone resulted in a 2.7- to 3.5-fold increase in AKAP12 mRNA levels (Supplement Table 1). These results were confirmed by immunoblot analysis (Figure 9). AKAP12 migrates as a doublet on SDS-PAGE, but progesterone caused about a 5-fold increase in expression of the larger form, without affecting the expression of the smaller form (Figure 9). AKAP12 (also designated gravin) is a scaffold protein that specifically interacts with β2-adrenergic receptors (β2AR) to form a supramolecular signaling complex with protein kinases and phosphatases.131,132 It has recently been shown that the glutamate receptor subunit AMPA-1 (also known as GluR1) forms part of the β2AR complex.133 In line with its effects on AKAP12 expression, progesterone increased the expression of AMPA1 between 4.4- and 5.1-fold (Supplement Table 1). Conceivably, the upregulation of APAK12 and AMPA1 could lead to enhanced β2AR signaling and the well-characterized β-mimetic relaxation of uterine smooth muscle, despite the lack of any apparent increase in β2AR expression per se. Human myometrial strips treated with progesterone in vitro have been shown to be sensitized to the relaxant effects of the β2 agonist ritodrine.134 However, these effects have been attributed by the authors to nongenomic actions of progesterone.
Figure 9.
Immunoblot of AKAP12 in triplicate samples of cells treated either with estrogen (E) or estrogen plus progesterone (E + P). Insulin receptor substrate (IRS)-1 staining illustrates the uniformity of gel loading and blotting.
Calcium Signaling
G-protein receptor agonists that activate contractile activity stimulate increases in intracellular Ca2+ concentrations via phospholipase Cs, which catalyze the hydrolysis of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate (InsP3) and 1,2-diacylglycerol (DAG). Both InsP3 and DAG have important second messenger functions. Inositol 1, 4, 5-trisphosphate activates its receptor on the endoplasmic reticulum (ER) or sarcoplasmic reticulum in the case of muscle cells, opening a calcium channel that allows the release of Ca2+ into the cytoplasm. Cytosolic Ca2+ in combination with calmodulin causes the phosphorylation of myosin regulatory light chains. This enables myosin to form cross-bridges with actin filaments and the initiation of a contraction cycle.
The phospholipase C (PLC) family is divided into several subtypes of which PLCβ is primarily activated by Gq/11 and PLCγ is activated in response to a variety of growth factor and immune system signals. Progesterone treatment caused a modest 1.4-fold reduction in PLCβ1, but a notable 2-fold decrease in PLC∊1 expression (Supplement Table 1). Like the well-characterized PLCβ isozymes, PLC∊ is activated by Gβγ.135 In addition, PLC∊ can be stimulated by Gα12/13-coupled receptors, Ras and Rho, to generate InsP3 and increased intracellular Ca2+ concentrations.135 Thus, the progesterone-induced reduction in the expression of PLC∊ might be expected to reduce the Ca2+ signal response to contractile activators. The agonist-elicited depletion of Ca2+ stores from the ER activates the influx of Ca2+ from outside the cell136 by a mechanism known as store-operated Ca2+entry (SOCE).
Progesterone treatment increased the expression of inositol monophosphatase 2 (IMPA2) between 7.5- and 8.7-fold (Supplement Table 1). Increased expression of IMPA2 has also been shown to increase during pregnancy (not in labor) in the human myometrium.137 Both IMPA2 and IMPA1 catalyze the hydrolysis of myositol 1 (or 4) monophosphate to form free myoinositol, maintaining a supply of the precursor for inositol phospholipid second messenger signaling systems. Conceivably, the upregulation of IMPA2 is an attempt to compensate for reduced InsP3 formation resulting from the downregulation of PLCs.
Contractile Proteins
Smooth muscle contraction is initiated by the phosphorylation of myosin light chain kinase by calcium/calmodulin, allowing the activation of myosin Mg2+-ATPase by actin and cross-bridge cycling between actin and myosin filaments. Maximal activation of the Mg2+-ATPase by actin requires Ca2+ and tropomyosin, a protein located on the thin filament.138 Several mRNAs encoding proteins of the contractile apparatus of smooth muscle cells were downregulated with progesterone treatment, but the change fell below the 2-fold cutoff. Reexamination of mRNA levels using qPCR (Figure 10) showed the following fold changes that were statistically significant (P ≤ .05): tropomyosin 1α (−2.3); myosin light chain 6B subunit, a pair of which comprise the nonphosphorylatable alkai light chains of myosin (−1.4); myosin regulatory light chain interacting protein (−1.6), which regulates the activity of the actomyosin complex by interacting with the myosin regulatory light chain B139; and smoothelin, a structural protein found exclusively in contractile smooth muscle cells in association with stress fibers and α-actin (−1.6). A decrease in myosin light chain kinase (MYLK) was not statistically significant (Figure 10). Paradoxically, there was about a 2-fold decrease in the expression of calponin 1, which inhibits actin-activated myosin ATPase activity140 and has been shown to be present in increased concentrations in the myometrium of pregnant women.141 As a decrease in calponin 1 levels would be expected to enhance contractile activity, it seems likely that the effects of progesterone on calponin 1 expression are overridden by other regulators.
Figure 10.
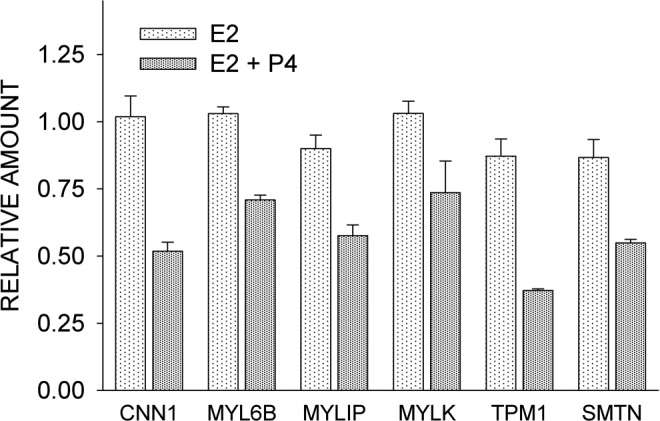
Comparison of the effects of estrogen alone (E2) and estrogen plus progesterone (E2 + P4) on the expression of contraction-associated proteins, as determined by quantitative reverse transcriptase–polymerase chain reaction (qPCR). CNN1 indicates calponin 1; MYL6B, myosin light chain 6B; MYLIP, myosin regulatory light chain interacting protein; MYLK, myosin light chain kinase; TPM1, tropomyosin 1α; SMTN, smoothelin. Each value is the mean ± standard error (SE) of triplicate determinations. With the exception of MYLK, E2 + P4 treatment caused a significant (P ≤ 0.05) reduction in expression of the genes indicated.
Tissue-Specific Effects of Progesterone and Association of Expression Clusters With Previously Associated Uterine Quiescence Genes
Comparison of the present microarray results obtained using myometrial cells with the published data on progesterone-treated breast cancer cell lines142 indicates little agreement in the genes influenced. In a few instances, the results are opposite to each other. Based on these findings, limited as they are to only 2 progesterone target tissue types, the actions of progesterone are clearly cell-type specific. Our findings are compatible with GeneChip results of the studies on the quiescent (day 14.5 of pregnancy) mouse myometrium.9 In general, decreased contractile signaling was attributed to decreased calcium influx/mobilization and increased cGMP/cAMP stimulation, while decreased contractile propagation was due to cell contact remodeling with respect to increased expression of serine proteases.
Concluding Remarks
The present studies offer some unexpected findings regarding the ability of progesterone to play a role in maintaining uterine quiescence. Previous studies depicting the transcriptome of the noncontractile myometrium during pregnancy have elucidated some of the same genes described in the present studies. We would not expect to find complete correspondence because the myometrium is not made up exclusively of smooth muscle cells, and gene expression by other cell types would complicate interpretation of the results. More significantly, despite the fact that the myometrium is “progesterone dominated,” it would be difficult to ascribe specific effects of progesterone on myometrial gene expression in vivo, given elevations in a number of other hormones of pregnancy. Thus, a clonal population of myocytes offers the means by which to determine the specific and direct effects of progesterone. A criticism that has arisen with the use of cultured cells is that they might not reflect a true picture of biological events seen in vivo. As a case in point, cultured human myometrial cells rapidly lose estrogen and thus PRs. However, the findings using cells expressing ER-α ectopically responded to estrogen and progesterone treatment in much the same manner as has been reported in in vivo studies (examples are cited throughout the text), giving good reason for concluding that the results reported here are representative of the actions of progesterone in vivo. In any case, our findings provide the basis for further investigations using the intact myometrium in vivo.
It is generally accepted that many of the functions of progesterone are to oppose the actions of estrogens. Yet, as shown in Supplement Tables 1 and 4, most of the effects of progesterone were separate from those of estradiol. The downregulation of receptors involved in uterine contractility, especially EP2 and OXTR, would have been expected, but the nonstimulatory effects of estrogen on OXTR expression in human myocytes indicate that the regulation of OXTR concentrations in the human myometrium is very different from that of the rat93,94,114 and other species. Physiological roles for downregulation of bradykinin and serotonin signaling in uterine quiescence were not anticipated. In addition, a possible role for neurotensin and ANP on uterine contractility is suggested from the microarray data.
Given the myometrial production of ET-1, the efficacy of ET-1 as an uterotonic agent, and the rise in myometrial ET-1 receptors near the time of labor, ET-1 might turn out to be critical for the initiation of labor, as has been suggested in other studies. The present studies lend credence to the importance of ET-1 and ETA.
It has been generally accepted that potassium channels play a role in uterine quiescence. Quantitatively, progesterone had the greatest effect on the Maxi-K channel. Downregulation of Maxi-K channel α-subunit mRNA and protein in the rat myometrium has, in fact, been proposed as a mechanism by which uterine excitability is increased at term.143 Other types of K+ channels might also be involved in mediating the effects of progesterone on uterine quiescence, as indicated by our findings. Primary cultures of human myometrial cells possess both delayed rectifier (IKV) and A-like (IKA) voltage-gated K+ currents.61 Treatment of these cells with estradiol for 24 hours inhibits myometrial K+ channel activity, whereas progesterone treatment had the opposite effect.61 These results are consistent with the respective procontractile and proquiescence roles for 17β-estradiol and progesterone in the human uterus during pregnancy. Acute treatment of cells61 or uterine strips144 with progesterone has no effect on K+ channel activity, indicating that the effects of progesterone are not direct but are genomic, in accordance with the findings in the present study.
In summary, any one of a number of potential genomic targets allows progesterone to suppress uterine activity. They include increases in K+ transport as the result of increased expression of calcium- and voltage-operated K+ channels, which dampens the electrical activity of the myometrial cell, downregulation of agents, and receptors involved in myometrial contraction, reduction in cell signal components that lead to increased intracellular Ca2+ concentrations in response to contractile stimuli, and downregulation of proteins ultimately involved in the cross-linking of actin and myosin filaments to produce uterine contractions. Any one of these processes might reduce uterine activity, but collectively they ensure that the uterus is maintained in a quiescent state throughout pregnancy until the initiation of labor, when these very same processes are reversed under the influence of estrogen action.
Supplementary Material
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: supported by award number R21HD056399 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
References
- 1. Casey ML, MacDonald PC. The endocrinology of human parturition. Ann N Y Acad Sci. 1997;828:273–284 [DOI] [PubMed] [Google Scholar]
- 2. Xiao Z-L, Cao WB, Biancani P, Behar J. Nongenomic effects of progesterone on the contraction of muscle cells from the guinea pig colon. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G1008–G1015 [DOI] [PubMed] [Google Scholar]
- 3. Lange CA. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol. 2004;18(2):269–278 [DOI] [PubMed] [Google Scholar]
- 4. Aguan K, Carvajal JA, Thompson LP, Weiner CP. Application of a functional genomics approach to identify differentially expressed genes in human myometrium during pregnancy and labour. Mol Hum Reprod. 2000;6(12):1141–1145 [DOI] [PubMed] [Google Scholar]
- 5. Charpigny G, Leroy MJ, Breuiller-Fouche M, et al. A functional genomic study to identify differential gene expression in the preterm and term human myometrium. Biol Reprod. 2003;68(6):2289–2296 [DOI] [PubMed] [Google Scholar]
- 6. Bethin KE, Nagai Y, Sladek R, et al. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol Endocrinol. 2003;17(8):1454–1469 [DOI] [PubMed] [Google Scholar]
- 7. Esplin MS, Fausett MB, Peltier MR, et al. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am J Obstet Gynecol. 2003;193(2):404–413 [DOI] [PubMed] [Google Scholar]
- 8. Havelock JC, Keller P, Muleba N, et al. Human myometrial gene expression before and during parturition. Biol Reprod. 2005;72(3):707–719 [DOI] [PubMed] [Google Scholar]
- 9. Salomonis N, Cotte N, Zambon AC, et al. Identifying genetic networks underlying myometrial transition to labor. Genome Biol. 2005;6(2):R12–R12.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown AG, Leite RS, Strauss JF. Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann N Y Acad Sci. 2004;1034:36–49 [DOI] [PubMed] [Google Scholar]
- 11. Soloff MS, Jeng YJ, Ilies M, et al. Immortalization and characterization of human myometrial cells from term-pregnant patients using a telomerase expression vector. Mol Hum Reprod. 2004;10(9):685–695 [DOI] [PubMed] [Google Scholar]
- 12. Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod. 2006;12(3):187–207 [DOI] [PubMed] [Google Scholar]
- 13. Snijders MP, de G, bets-Te B, Rousch MJ, Koudstaal J, Bosman FT. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J Reprod Fert. 1992;94(2):363–371 [DOI] [PubMed] [Google Scholar]
- 14. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KSJ. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67(2):334–340 [DOI] [PubMed] [Google Scholar]
- 15. Graham JD, Roman SD, McGowan E, Sutherland RL, Clarke CL. Preferential stimulation of human progesterone receptor B expression by estrogen in T-47D human breast cancer cells. J Biol Chem. 1995;270(51):30693–30700 [DOI] [PubMed] [Google Scholar]
- 16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 17. Hurd C, Moudgil VK. Characterization of R5020 and RU486 binding to progesterone receptor from calf uterus. Biochemistry. 1988;27(10):3618–3623 [DOI] [PubMed] [Google Scholar]
- 18. Sadovsky Y, Kushner PJ, Roberts JM, Riemer RK. Restoration of estrogen-dependent progesterone receptor expression in a uterine myocyte cell line. Endocrinology. 1993;132(4):1609–1613 [DOI] [PubMed] [Google Scholar]
- 19. Jain N, Thatte J, Braciale T, Ley K, O'Connell M, Lee JK. Local-pooled-error test for identifying differentially expressed genes with a small number of replicated microarrays. Bioinformatics. 2003;19(15):1945–1951 [DOI] [PubMed] [Google Scholar]
- 20. Slater EP, Cato AC, Karin M, Baxter JD, Beato M. Progesterone induction of metallothionein-IIA gene expression. Mol Endocrinol. 1988;2(6):485–491 [DOI] [PubMed] [Google Scholar]
- 21. Ghosh MG, Thompson DA, Weigel RJ. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 2000;60(22):6367–6375 [PubMed] [Google Scholar]
- 22. Bouras T, Southey MC, Chang AC, et al. Stanniocalcin 2 is an estrogen-responsive gene coexpressed with the estrogen receptor in human breast cancer. Cancer Res. 2002;62(5):1289–1295 [PubMed] [Google Scholar]
- 23. Stec J, Wang J, Coombes K, et al. Comparison of the predictive accuracy of DNA array-based multigene classifiers across cDNA arrays and Affymetrix GeneChips. J Mol Diagn. 2005;7(3):357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leavitt WW, Cobb AD, Takeda A. Progesterone-modulation of estrogen action: rapid down regulation of nuclear acceptor sites for the estrogen receptor. Adv Exp Med Biol. 1987;230:49–78 [DOI] [PubMed] [Google Scholar]
- 25. Okulicz WC, Balsamo M, Tast J. Progesterone regulation of endometrial estrogen receptor and cell proliferation during the late proliferative and secretory phase in artificial menstrual cycles in the rhesus monkey. Biol Reprod. 1993;49(1):24–32 [DOI] [PubMed] [Google Scholar]
- 26. Savouret JF, Muchardt C, Quesne M, Mantel A, De T, Milgrom E. Regulation of the progesterone receptor functions. Ann N Y Acad Sci. 1998;839:138–142 [DOI] [PubMed] [Google Scholar]
- 27. Medlock KL, Forrester TM, Sheehan DM. Progesterone and estradiol interaction in the regulation of rat uterine weight and estrogen receptor concentration. Proc Soc Exp Biol Med. 1994;205(2):146–153 [DOI] [PubMed] [Google Scholar]
- 28. Zhang L, Kanda Y, Roberts DJ, et al. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol Cell Endocrinol. 2008;287(1-2):81–89 [DOI] [PubMed] [Google Scholar]
- 29. Ing NH, Roberts RM. The major progesterone-modulated proteins secreted into the sheep uterus are members of the serpin superfamily of serine protease inhibitors. J Biol Chem. 1989;264(6):3372–3379 [PubMed] [Google Scholar]
- 30. Peluso JJ, Romak J, Liu X. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008;149(2):534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nair SC, Rimerman RA, Toran EJ, et al. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol Cell Biol. 1997;17(2):594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144(6):2380–2387 [DOI] [PubMed] [Google Scholar]
- 33. Vassen L, Wegrzyn W, Klein-Hitpass L. Human insulin receptor substrate-2 (IRS-2) is a primary progesterone response gene. Mol Endocrinol. 1999;13(3):485–494 [DOI] [PubMed] [Google Scholar]
- 34. Cui X, Lazard Z, Zhang P, Hopp TA, Lee AV. Progesterone crosstalks with insulin-like growth factor signaling in breast cancer cells via induction of insulin receptor substrate-2. Oncogene. 2003;22(44):6937–6941 [DOI] [PubMed] [Google Scholar]
- 35. Friedman JE, Ishizuka T, Shao J, Huston L, Highman T, Catalano P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes. 1999;48(9):1807–1814 [DOI] [PubMed] [Google Scholar]
- 36. Suarez VR, Park ES, Hankins GD, Soloff MS. Expression of regulator of G protein signaling-2 in rat myometrium during pregnancy and parturition. Am J Obstet Gynecol. 2003;188(4):973–977 [DOI] [PubMed] [Google Scholar]
- 37. Wahab M, Taylor AH, Pringle JH, Thompson J, Al-Azzawi F. Trimegestone differentially modulates the expression of matrix metalloproteinases in the endometrial stromal cell. Mol Hum Reprod. 2006;12(3):157–167 [DOI] [PubMed] [Google Scholar]
- 38. Senturk LM, Sozen I, Gutierrez L, Arici A. Interleukin 8 production and interleukin 8 receptor expression in human myometrium and leiomyoma. Am J Obstet Gynecol. 2001;184(4):559–566 [DOI] [PubMed] [Google Scholar]
- 39. Breviario F, d’Aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267(31):22190–22197 [PubMed] [Google Scholar]
- 40. Chevillard G, Derjuga A, Devost D, Zingg HH, Blank V. Identification of interleukin-1β regulated genes in uterine smooth muscle cells. Reproduction. 2007;134(6):811–822 [DOI] [PubMed] [Google Scholar]
- 41. Soloff MS, Cook DL, Jr, Jeng YJ, Anderson GD. In situ analysis of interleukin-1-induced transcription of cox-2 and il-8 in cultured human myometrial cells. Endocrinology. 2004;145(3):1248–1254 [DOI] [PubMed] [Google Scholar]
- 42. Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1β: involvement of p38 mitogen-activated protein kinase. J Physiol (Lond). 1999;520(pt 2):399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belt AR, Baldassare JJ, Molnár M, Romero R, Hertelendy F. The nuclear transcription factor NF-κB mediates interleukin-1β-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181(2):359–366 [DOI] [PubMed] [Google Scholar]
- 44. Rauk P, Chiao J. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol. 2000;43(3):152–159 [DOI] [PubMed] [Google Scholar]
- 45. Zuo J, Lei ZM, Rao CV, Pietrantoni M, Cook VD. Differential cyclooxygenase-1 and -2 gene expression in human myometria from preterm and term deliveries. J Clin Endocrinol Metab. 1994;79(3):894–899 [DOI] [PubMed] [Google Scholar]
- 46. Slater DM, Dennes WJ, Campa JS, Poston L, Bennett PR. Expression of cyclo-oxygenase types-1 and -2 in human myometrium throughout pregnancy. Mol Hum Reprod. 1999;5(9):880–884 [DOI] [PubMed] [Google Scholar]
- 47. Tsuboi K, Sugimoto Y, Iwane A, Yamamoto K, Yamamoto S, Ichikawa A. Uterine expression of prostaglandin H2 synthase in late pregnancy and during parturition in prostaglandin F receptor-deficient mice. Endocrinology. 2000;141(1):315–324 [DOI] [PubMed] [Google Scholar]
- 48. Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-β. J Biol Chem. 2003;279(36):38032–38039 [DOI] [PubMed] [Google Scholar]
- 49. Saharinen J, Hyytiainen M, Taipale J, Keski-Oja J. Latent transforming growth factor-β binding proteins (LTBPs)—structural extracellular matrix proteins for targeting TGF-β action]. Cytokine Growth Factor Rev. 1999;10(2):99–117 [DOI] [PubMed] [Google Scholar]
- 50. Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275(23):17647–17652 [DOI] [PubMed] [Google Scholar]
- 51. Moses HL, Yang EY, Pietenpol JA. TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990;63(2):245–247 [DOI] [PubMed] [Google Scholar]
- 52. Hannon GJ, Beach D. p15INK4B is a potential effector of TGF−β-induced cell cycle arrest. Nature. 1994;371(6494):257–261 [DOI] [PubMed] [Google Scholar]
- 53. Kuriyama H, Suzuki H. Changes in electrical properties of rat myometrium during gestation and following hormonal treatments. J Physiol [Lond]. 1976;260(2):315–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gutman GA, Chandy KG, Grissmer S, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57(4):473–508 [DOI] [PubMed] [Google Scholar]
- 55. Piper I, Minshall E, Downing SJ, Hollingsworth M, Sadraei H. Effects of several potassium channel openers and glibenclamide on the uterus of the rat. Br J Pharmacol. 1990;101(4):901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cheuk JM, Hollingsworth M, Hughes SJ, Piper IT, Maresh MJ. Inhibition of contractions of the isolated human myometrium by potassium channel openers. Am J Obstet Gynecol. 1993;168(3 pt 1):953–960 [DOI] [PubMed] [Google Scholar]
- 57. Morrison JJ, Ashford ML, Khan RN, Smith SK. The effects of potassium channel openers on isolated pregnant human myometrium before and after the onset of labor: potential for tocolysis. Am J Obstet Gynecol. 1993;169(5):1277–1285 [DOI] [PubMed] [Google Scholar]
- 58. Anwer K, Oberti C, Perez GJ, et al. Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am J Physiol. 1993;265(4 pt 1):C976–C985 [DOI] [PubMed] [Google Scholar]
- 59. Smith RC, McClure MC, Smith MA, Abel PW, Bradley ME. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol. 2007;5:41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Khan RN, Matharoo-Ball B, Arulkumaran S, Ashford ML. Potassium channels in the human myometrium. Exp Physiol. 2001;86(5):255–264 [DOI] [PubMed] [Google Scholar]
- 61. Knock GA, Tribe RM, Hassoni AA, Aaronson PI. Modulation of potassium current characteristics in human myometrial smooth muscle by 17β-estradiol and progesterone. Biol Reprod. 2001;64(5):1526–1534 [DOI] [PubMed] [Google Scholar]
- 62. Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Sem Cell Dev Biol. 2007;18(2):332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66(5):852–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tritthart HA, Mahnert W, Fleischhacker A, Adelwohrer N. Potassium channels and modulating factors of channel functions in the human myometrium. Z Kardiol. 1991;80(suppl 7):29–33 [PubMed] [Google Scholar]
- 65. Khan RN, Smith SK, Morrison JJ, Ashford ML. Properties of large-conductance K+ channels in human myometrium during pregnancy and labour. Proc R Soc Lond B. 1993;251(1330):9–15 [DOI] [PubMed] [Google Scholar]
- 66. Khan RN, Smith SK, Morrison JJ, Ashford ML. Ca2+ dependence and pharmacology of large-conductance K+ channels in nonlabor and labor human uterine myocytes. Am J Physiol. 1997;273(5 pt 1):C1721–C1731 [DOI] [PubMed] [Google Scholar]
- 67. Gao L, Cong B, Zhang L, Ni X. Expression of the calcium-activated potassium channel in upper and lower segment human myometrium during pregnancy and parturition. Reprod Biol Endocrinol. 2009;7:27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci USA. 1999;96(7):4137–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Torres YP, Morera FJ, Carvacho I, Latorre R. A marriage of convenience: β-subunits and voltage-dependent K+ channels. J Biol Chem. 1924;282(34):24485–24489 [DOI] [PubMed] [Google Scholar]
- 70. Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000;474(1):99–106 [DOI] [PubMed] [Google Scholar]
- 71. Dick GM, Sanders KM. (Xeno)estrogen sensitivity of smooth muscle BK channels conferred by the regulatory beta1 subunit: a study of beta1 knockout mice. J Biol Chem. 1930;276(48):44835–44840 [DOI] [PubMed] [Google Scholar]
- 72. Valverde MA, Rojas P, Amigo J, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit.[see comment]. Science. 1999;285(5435):1929–1931 [DOI] [PubMed] [Google Scholar]
- 73. King JT, Lovell PV, Rishniw M, Kotlikoff MI, Zeeman ML, McCobb DP. β2 and β4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. J Neurophysiol. 2006;95(5):2878–2888 [DOI] [PubMed] [Google Scholar]
- 74. Nash HA, Scott RL, Lear BC, Allada R. An unusual cation channel mediates photic control of locomotion in Drosophila. Curr Biol. 2002;12(24):2152–2158 [DOI] [PubMed] [Google Scholar]
- 75. Cootauco AC, Murphy JD, Maleski J, Blakemore KJ, Slodzinski MK. Atrial natriuretic peptide production and natriuretic peptide receptors in the human uterus and their effect on myometrial relaxation. Am J Obstet Gynecol. 2009;199(4):429e1–429e6 [DOI] [PubMed] [Google Scholar]
- 76. Itoh H, Sagawa N, Hasegawa M, et al. Expression of biologically active receptors for natriuretic peptides in the human uterus during pregnancy. Biochem Biophys Res Commun. 1994;203(1):602–607 [DOI] [PubMed] [Google Scholar]
- 77. Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75(2):142–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Watanabe H, Lau DC, Guyn HL, Wong NL. Effect of progesterone therapy on arginine vasopressin and atrial natriuretic factor in premenstrual syndrome. Clin Invest Med. 1997;20(4):211–223 [PubMed] [Google Scholar]
- 79. Hong M, Yan Q, Tao B, et al. Estradiol, progesterone and testosterone exposures affect the atrial natriuretic peptide gene expression in vivo in rats. Biol Chem Hoppe Seyler. 1992;373(4):213–218 [DOI] [PubMed] [Google Scholar]
- 80. Potvin W, Varma DR. Refractoriness of the gravid rat uterus to tocolytic and biochemical effects of atrial natriuretic peptide. Brit J Pharmacol. 1990;100(2):341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kajihara T, Tomioka Y, Hata T, Ghazizadeh M, Asano G. Synthesis of endothelin-1 in rat uterus during pregnancy. J Histochem Cytochem. 1996;44(9):953–957 [DOI] [PubMed] [Google Scholar]
- 82. Kozuka M, Ito T, Hirose S, Takahashi K, Hagiwara H. Endothelin induces two types of contractions of rat uterus: phasic contractions by way of voltage-dependent calcium channels and developing contractions through a second type of calcium channels. Biochem Biophys Res Commun. 1989;159(1):317–323 [DOI] [PubMed] [Google Scholar]
- 83. Word RA, Kamm KE, Stull JT, Casey ML. Endothelin increases cytoplasmic calcium and myosin phosphorylation in human myometrium. Am J Obstet Gynecol. 1990;162(4):1103–1108 [DOI] [PubMed] [Google Scholar]
- 84. Wolff K, Nisell H, Modin A, Lundberg JM, Lunell NO, Lindblom B. Contractile effects of endothelin 1 and endothelin 3 on myometrium and small intramyometrial arteries of pregnant women at term. Gynecol Obstet Invest. 1993;36(3):166–171 [DOI] [PubMed] [Google Scholar]
- 85. Maher E, Bardequez A, Gardner JP, et al. Endothelin- and oxytocin-induced calcium signaling in cultured human myometrial cells. J Clin Invest. 1991;87(4):1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yallampalli C, Garfield RE. Uterine contractile responses to endothelin-1 and endothelin receptors are elevated during labor. Biol Reprod. 1994;51(4):640–645 [DOI] [PubMed] [Google Scholar]
- 87. Davenport AP. International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54(2):219–226 [DOI] [PubMed] [Google Scholar]
- 88. Osada K, Tsunoda H, Miyauchi T, Sugishita Y, Kubo T, Goto K. Pregnancy increases ET-1-induced contraction and changes receptor subtypes in uterine smooth muscle in humans. Am J Physiol. 1997;272(2 pt 2):R541–R548 [DOI] [PubMed] [Google Scholar]
- 89. Fomin VP, Cox BE, Word RA. Effect of progesterone on intracellular Ca2+ homeostasis in human myometrial smooth muscle cells. Am J Physiol. 1999;276(2 pt 1):C379–C385 [DOI] [PubMed] [Google Scholar]
- 90. Soloff MS, Alexandrova M, Fernstrom MJ. Oxytocin receptors: triggers for parturition and lactation? Science. 1979;204(4399):1313–1315 [DOI] [PubMed] [Google Scholar]
- 91. Fuchs AR, Fuchs F, Husslein P, Soloff MS, Fernstrom MJ. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science. 1982;215(4538):1396–1398 [DOI] [PubMed] [Google Scholar]
- 92. Kimura T, Takemura M, Nomura S, et al. Expression of oxytocin receptor in human pregnant myometrium. Endocrinology. 1996;137(2):780–785 [DOI] [PubMed] [Google Scholar]
- 93. Soloff MS, Fernstrom MA, Periyasamy S, Soloff S, Baldwin S, Wieder M. Regulation of oxytocin receptor concentration in rat uterine explants by estrogen and progesterone. Can J Biochem Cell Biol. 1983;61(7):625–630 [DOI] [PubMed] [Google Scholar]
- 94. Fuchs AR, Periyasamy S, Alexandrova M, Soloff MS. Correlation between oxytocin receptor concentration and responsiveness to oxytocin in pregnant rat myometrium: effects of ovarian steroids. Endocrinology. 1983;113(2):742–749 [DOI] [PubMed] [Google Scholar]
- 95. Jeng YJ, Soloff SL, Anderson GD, Soloff MS. Regulation of oxytocin receptor expression in cultured human myometrial cells by fetal bovine serum and lysophospholipids. Endocrinology. 2003;144(1):61–68 [DOI] [PubMed] [Google Scholar]
- 96. Rauk PN, Friebe-Hoffmann U. Interleukin-1β down-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am J Reprod Immunol. 2000;43(2):85–91 [DOI] [PubMed] [Google Scholar]
- 97. Schmid B, Wong S, Mitchell BF. Transcriptional regulation of oxytocin receptor by interleukin-1β and interleukin-6. Endocrinology. 2001;142(4):1380–1385 [DOI] [PubMed] [Google Scholar]
- 98. Helmer H, Tretzmuller U, Brunbauer M, Kaider A, Husslein P, Knofler M. Production of oxytocin receptor and cytokines in primary uterine smooth muscle cells cultivated under inflammatory conditions. J Soc Gynecol Invest. 2002;9(1):15–21 [DOI] [PubMed] [Google Scholar]
- 99. Soloff MS, Izban MG, Cook DLJ, Jeng YJ, Mifflin RC. Interleukin-1-induced NF-κB recruitment to the oxytocin receptor gene inhibits RNA polymerase II-promoter interactions in cultured human myometrial cells. Mol Hum Reprod. 2006;12(10):619–624 [DOI] [PubMed] [Google Scholar]
- 100. Zhao K, Kuperman L, Geimonen E, Andersen J. Progestin represses human connexin43 gene expression similarly in primary cultures of myometrial and uterine leiomyoma cells. Biol Reprod. 1996;54(3):607–615 [DOI] [PubMed] [Google Scholar]
- 101. Di WL, Lachelin GC, McGarrigle HH, Thomas NS, Becker DL. Oestriol and oestradiol increase cell to cell communication and connexin43 protein expression in human myometrium. Mol Hum Reprod. 2001;7(7):671–679 [DOI] [PubMed] [Google Scholar]
- 102. Hoyer D, Clarke DE, Fozard JR, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46(2):157–203 [PubMed] [Google Scholar]
- 103. Minosyan TY, Lu R, Eghbali M, Toro L, Stefani E. Increased 5-HT contractile response in late pregnant rat myometrium is associated with a higher density of 5-HT2A receptors. J Physiol. 2007;581(pt 1):91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ichida S, Oda Y, Tokunaga H, Hayashi T, Murakami T, Kita T. Mechanisms of specific change by estradiol in sensitivity of rat uterus to serotonin. J Pharmacol Exp Therap. 1984;229(1):244–249 [PubMed] [Google Scholar]
- 105. Lebrun I, Camargo AC, Correa FM. Pharmacological effects and metabolism of neurotensin and bradykinin in the isolated rat uterus. Eur J Pharmacol. 1988;148(2):231–237 [DOI] [PubMed] [Google Scholar]
- 106. Webb TE, Kaplan MG, Barnard EA. Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem Biophys Res Commun. 1996;219(1):105–110 [DOI] [PubMed] [Google Scholar]
- 107. Yanagida K, Masago K, Nakanishi H, et al. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284(26):17731–17741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lee M, Choi S, Hallden G, Yo SJ, Schichnes D, Aponte GW. P2Y5 is a Gαi, Gα12/13 G protein-coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion. Am J Physiol Gastrointest Liver Physiol. 2009;297(4):G641–G654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tokumura A, Fukuzawa K, Yamada S, Tsukatani H. Stimulatory effect of lysophosphatidic acids on uterine smooth muscles of non-pregant rats. Arch Int Pharmacodyn Ther. 1980;245(1):74–83 [PubMed] [Google Scholar]
- 110. Leeman SE, Carraway RE. Neurotensin: discovery, isolation, characterization, synthesis and possible physiological roles. Ann N Y Acad Sci. 1982;400:1–16 [DOI] [PubMed] [Google Scholar]
- 111. Rodriguez Y, Almeida TA, Valladares F, et al. Neurotensin and neurotensin receptor 1 expression in human myometrium and uterine leiomyomas. Biol Reprod. 2010;83(4):641–647 [DOI] [PubMed] [Google Scholar]
- 112. Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46(2):205–229 [PubMed] [Google Scholar]
- 113. Brodt-Eppley J, Myatt L. Changes in expression of contractile FP and relaxatory EP2 receptors in pregnant rat myometrium during late gestation, at labor, and postpartum. Biol Reprod. 1998;59(4):878–883 [DOI] [PubMed] [Google Scholar]
- 114. Alexandrova M, Soloff MS. Oxytocin receptors and parturition. I. Control of oxytocin receptor concentration in the rat myometrium at term. Endocrinology. 1980;106(3):730–735 [DOI] [PubMed] [Google Scholar]
- 115. Dong YL, Yallampalli C. Pregnancy and exogenous steroid treatments modulate the expression of relaxant EP2 and contractile FP receptors in the rat uterus. Biol Reprod. 2000;62(3):533–539 [DOI] [PubMed] [Google Scholar]
- 116. Bamford DS, Jogee M, Williams KI. Prostacyclin formation by the pregnant human myometrium. Br J Obstet Gynaecol. 1980;87(3):215–218 [DOI] [PubMed] [Google Scholar]
- 117. Sanger GJ, Hensby CN, Stamford IF, Bennett A. Identification of arachidonic acid metabolites extracted from human uterus, and their effects on the isolated myometrium. J Pharm Pharmacol. 1981;33(9):607–610 [DOI] [PubMed] [Google Scholar]
- 118. Satoh K, Yasumizu T, Kawai Y, Ozaki A, Wu T, Kinoshita K, Sakamoto S. In vitro production of prostaglandins E, F, and 6-keto prostaglandin F1α by human pregnant uterus, decidua and amnion. Prostaglandins Med. 1981;6(4):359–368 [DOI] [PubMed] [Google Scholar]
- 119. Omini C, Folco GC, Pasargiklian R, Fano M, Berti F. Prostacyclin (PGI2) in pregnant human uterus. Prostaglandins. 1979;17(1):113–120 [DOI] [PubMed] [Google Scholar]
- 120. Lye SJ, Challis JR. Inhibition by PGI2 of myometrial activity in vivo in non-pregnant ovariectomized sheep. J Reprod Fert. 1982;66(1):311–315 [DOI] [PubMed] [Google Scholar]
- 121. Kofinas AD, Rose JC, Koritnik DR, Meis PJ. Progesterone and estradiol concentrations in nonpregnant and pregnant human myometrium. Effect of progesterone and estradiol on cyclic adenosine monophosphate-phosphodiesterase activity. J Reprod Med. 1990;35(11):1045–1050 [PubMed] [Google Scholar]
- 122. Fu X, Backstrom T, Ulmsten U. Progesterone increases cAMP release and accumulation in isolated term human myometrium. Gynecol Obstet Invest. 1998;45(4):242–246 [DOI] [PubMed] [Google Scholar]
- 123. Shepherd M, McSorley T, Olsen AE, et al. Molecular cloning and subcellular distribution of the novel PDE4B4 cAMP-specific phosphodiesterase isoform. Biochem J. 2003;370(pt 3):429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kofinas AD, Rose JC, Meis PJ. Changes in cyclic adenosine monophosphate-phosphodiesterase activity in nonpregnant and pregnant human myometrium. Am J Obstet Gynecol. 1987;157(3):733–738 [DOI] [PubMed] [Google Scholar]
- 125. Leroy MJ, Cedrin I, Breuiller M, Giovagrandi Y, Ferre F. Correlation between selective inhibition of the cyclic nucleotide phosphodiesterases and the contractile activity in human pregnant myometrium near term. Biochem Pharmacol. 1989;38(1):9–15 [DOI] [PubMed] [Google Scholar]
- 126. Mehats C, Tanguy G, Paris B, et al. Pregnancy induces a modulation of the cAMP phosphodiesterase 4-conformers ratio in human myometrium: consequences for the utero-relaxant effect of PDE4-selective inhibitors. J Pharmacol Exp Ther. 2000;292(2):817–823 [PubMed] [Google Scholar]
- 127. Abdel-Latif AA. Cross talk between cyclic nucleotides and polyphosphoinositide hydrolysis, protein kinases, and contraction in smooth muscle. Exp Biol Med. 2001;226(3):153–163 [DOI] [PubMed] [Google Scholar]
- 128. Ku CY, Sanborn BM. Progesterone prevents the pregnancy-related decline in protein kinase A association with rat myometrial plasma membrane and A-kinase anchoring protein. Biol Reprod. 2002;67(2):605–609 [DOI] [PubMed] [Google Scholar]
- 129. Ku CY, Word RA, Sanborn BM. Differential expression of protein kinase A, AKAP79, and PP2B in pregnant human myometrial membranes prior to and during labor. J Soc Gynecol Invest. 2005;12(6):421–427 [DOI] [PubMed] [Google Scholar]
- 130. Dodge KL, Carr DW, Yue C, Sanborn BM. A role for AKAP (A kinase anchoring protein) scaffolding in the loss of a cyclic adenosine 3′,5′-monophosphate inhibitory response in late pregnant rat myometrium. Mol Endocrinol. 1999;13(12):1977–1987 [DOI] [PubMed] [Google Scholar]
- 131. Shih M, Lin F, Scott JD, Wang HY, Malbon CC. Dynamic complexes of β2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem. 1999;274(3):1588–1595 [DOI] [PubMed] [Google Scholar]
- 132. Fan G, Shumay E, Wang H, Malbon CC. The scaffold protein gravin (cAMP-dependent protein kinase-anchoring protein 250) binds the β2-adrenergic receptor via the receptor cytoplasmic Arg-329 to Leu-413 domain and provides a mobile scaffold during desensitization. J Biol Chem. 2001;276(26):24005–24014 [DOI] [PubMed] [Google Scholar]
- 133. Joiner ML, Lise MF, Yuen EY, et al. Assembly of a β2-adrenergic receptor—GluR1 signalling complex for localized cAMP signalling. EMBO J. 2010;29(2):482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chanrachakul B, Pipkin FB, Warren AY, Arulkumaran S, Khan RN. Progesterone enhances the tocolytic effect of ritodrine in isolated pregnant human myometrium. Am J Obstet Gynecol. 2005;192(2):458–463 [DOI] [PubMed] [Google Scholar]
- 135. Wing MR, Bourdon DM, Harden TK. PLC-∊: a shared effector protein in Ras-, Rho-, and Gαβγ-mediated signaling. Mol Interv. 2003;3(5):273–280 [DOI] [PubMed] [Google Scholar]
- 136. Putney JW ., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7(1):1–12 [DOI] [PubMed] [Google Scholar]
- 137. Breuiller-Fouche M, Germain G. Gene and protein expression in the myometrium in pregnancy and labor. Reproduction. 2006;131(5):837–850 [DOI] [PubMed] [Google Scholar]
- 138. Chacko S, Heaslip RJ, Fillers WS, Kaminski EA. Regulation of actomyosin ATPase in smooth muscle. Prog Clin Biol Res. 1986;219(51):169–185 [PubMed] [Google Scholar]
- 139. Olsson PA, Korhonen L, Mercer EA, Lindholm D. MIR is a novel ERM-like protein that interacts with myosin regulatory light chain and inhibits neurite outgrowth. J Biol Chem. 1999;274(51):36288–36292 [DOI] [PubMed] [Google Scholar]
- 140. Winder SJ, Allen BG, Clement-Chomienne O, Walsh MP. Regulation of smooth muscle actin-myosin interaction and force by calponin. Acta Physiol Scand. 1998;164(4):415–426 [DOI] [PubMed] [Google Scholar]
- 141. Cornwell TL, Li J, Sellak H, Miller RT, Word RA. Reorganization of myofilament proteins and decreased cGMP-dependent protein kinase in the human uterus during pregnancy. J Clin Endocrinol Metab. 2001;86(8):3981–3988 [DOI] [PubMed] [Google Scholar]
- 142. Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5218 [DOI] [PubMed] [Google Scholar]
- 143. Song M, Zhu N, Olcese R, Barila B, Toro L, Stefani E. Hormonal control of protein expression and mRNA levels of the MaxiK channel alpha subunit in myometrium. FEBS Lett. 1999;460(3):427–432 [DOI] [PubMed] [Google Scholar]
- 144. Anderson L, Martin W, Higgins C, Nelson SM, Norman JE. The effect of progesterone on myometrial contractility, potassium channels, and tocolytic efficacy. Reprod Sci. 2009;16(11):1052–1061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


