Abstract
The significance of endothelin-1 (ET-1) in platelet-activating factor (PAF)-induced fetal growth restriction (FGR) was evaluated in timed-pregnant rats receiving intravenous carbamyl-PAF (c-PAF; 0.5, 1.0, or 2.5 µg/kg per h) or vehicle, with or without ET-1 receptor A (ETA) antagonist (10 or 20 mg/kg per d) for 7 days beginning on gestation day 14. Tissues were collected on day 21. Carbamyl-PAF reduced fetal weights dose dependently. Placental weights were significantly reduced but not dose dependently. ETA antagonism prevented FGR at the 0.5, but not the 1.0 and 2.5 µg/kg per h c-PAF doses. Correspondingly, placental, but not uterine, preproET-1 messenger RNA (mRNA) expression (determined by reverse transcription–polymerase chain reaction) was increased at 0.5 µg/kg per h but not at higher c-PAF doses. In summary, c-PAF infusion results in fetal and placental growth restriction in the rat. At low doses of c-PAF, ET-1 is central to the pathophysiology of PAF-induced FGR. At higher c-PAF doses, FGR is induced by mechanisms other than ET-1 action.
Keywords: Endothelin, fetal growth restriction, platelet-activating factor, pregnancy, rat
Introduction
Fetal growth restriction (FGR) is a leading cause of perinatal morbidity and mortality in humans.1–5 Suboptimal uteroplacental perfusion is a commonly identified cause of FGR, and histologic evidence of placental ischemia and inflammation is commonly observed. Because the placental vasculature is not regulated by the autonomic nervous system, vasoactive mediators play a prominent role in the regulation of placental perfusion.
Platelet-activating factor (PAF) is a potent vasoactive mediator which has both proinflammatory and hemodynamic effects. Platelet-activating factor may cause either vasoconstriction or vasodilation, depending on the vascular bed involved.6,7 Systemic administration of PAF leads to hypotension and other manifestations of shock.8 Several investigators have demonstrated an interaction between PAF and the potent vasoconstrictor endothelin (ET-1). Endothelin-1 has been shown to increase the production of PAF in a variety of cells and tissues.9–15 Conversely, PAF has been shown to activate ET-1 receptors.16 These mediators have been shown to act synergistically to decrease perfusion and increase inflammation in several animal models, including bacteremia-induced lung injury,17 acute pancreatitis,18 posttransplant lung ischemia/reperfusion,19 small intestine microcirculation,20 and peritonitis.21 These models all demonstrate that ET-1 and PAF act synergistically to mediate responses to ischemic insult.
In a previous study, we demonstrated that PAF infusion during pregnancy in the rat leads to FGR in a dose-dependent manner.22 We have also previously evaluated the role of ET-1 and PAF in ischemia/reperfusion-induced FGR in the rat.23 In that study, we found that antagonism of either mediator resulted in normalization of fetal growth, demonstrating that both are important mediators in ischemia/reperfusion-induced FGR. The purpose of this study was to evaluate the significance of ET-1 in PAF-induced FGR.
Methods
Animals
Seventy-two female Sprague-Dawley rats (6 in each of 12 experimental groups) were purchased from Harlan Sprague Dawley (Madison, Wisconsin), housed in the NorthShore University HealthSystem Research Institute animal care facility, and bred with males from the same source. All rats received a standard laboratory rodent diet (PMI Feeds, St Louis, Missouri) and water ad libitum and were kept on a 12-hour light/12-hour dark cycle. Animal care and the conduct of all experiments were in accord with guidelines approved by the Research Institute Animal Care and Use Committee. For timed pregnancy, female rats in estrus were mated overnight and mating confirmed by the presence of sperm in the vaginal smear the following morning. Date of sperm positivity was designated day 0 with expected delivery during the night between days 21 and 22.
Carbamyl-PAF Administration
On day 14 of a 22-day gestation in timed pregnant rats, general anesthesia was accomplished with a single intraperitoneal injection of xylazine 8 mg/kg, ketamine 40 mg/kg, and acepromazine 1.3 mg/kg in combination. An indwelling catheter (Tygon, OD = 0.76 mm) was then placed into the left jugular vein through which vehicle (1.5% ethyl alcohol in normal saline) or 0.5, 1.0 or 2.5 μg/kg per h carbamyl-PAF (c-PAF, Alexis Biochemicals, San Diego, California), a stable analog of PAF, was infused via an attached, subcutaneous Alzet osmotic pump (Durect Corp., Cupertino, California) at a rate of 10 µL/h. The 0.5 µg/kg per h dose was the lowest dose of c-PAF that was shown previously to cause FGR.22 The highest dose of c-PAF was only 25% of the threshold dose of c-PAF that would lower maternal blood pressure.22
Endothelin Receptor Antagonist Administration
The ET receptor antagonist, ABT-546 (Abbott Laboratories., Abbott Park, Illinois), is a nonpeptide compound with a high affinity for ETA receptors (Ki = 0.46 nmol/L) and a low affinity for ETB receptors (Ki = 13 000 nmol/L), thus it has a 28 000-fold selectivity for ETA. In separate groups of 6 pregnant rats, this antagonist was administered subcutaneously at 10 or 20 mg/kg per d (based on efficacy in other rat models of FGR), via a second Alzet osmotic pump, beginning on gestation day 14, simultaneously with either c-PAF at the doses indicated above or its vehicle. Control rats received vehicle (20% ethyl alcohol, 40% propylene glycol, and 0.04 mol/L NaOH in H2O) at 10 µL/h subcutaneously by osmotic pump, as well as the vehicle for c-PAF.
Pregnancy Outcome and Tissue Collection
On gestation day 21, a hysterotomy was performed. Litter size was recorded, fetal viability was determined for each pup and fetal and placental weights were obtained for each live pup. Uterine and placental tissues and kidneys were collected and stored at −80°C for molecular analysis. The kidneys were included for comparison to reproductive tissues because they are a rich source of and are responsive to ET-1, and they are involved in pregnancy-related pathology that is closely related to FGR, for example preeclampsia.
Real-Time Quantitative Reverse Transcription–Polymerase Chain Reaction
Tissue samples stored at −80°C were homogenized in RNA STAT-60 (a phenol/guanidinium thiocyanate reagent, TEL-TEST, Friendswood, Texas) on ice, for extraction of total RNA. RNA was extracted from whole tissues, therefore the homogenates included a mixture of cell types. The concentration of RNA was determined by absorbance at 260 nm and the purity was checked by the 260/280 nm ratio (greater than 1.8). RNA integrity was verified by electrophoresis in a 1% agarose gel. For each sample, 3 μg total RNA was reverse transcribed at 37°C for 1 hour in a total of 20 μL reaction mixture: 50 mmol/L Tris-HCl, 75 mmol/L KCl, 2.0 mmol/L MgCl2, 10 mmol/L dithiothreitol, 1.25 mmol/L of each deoxynucleotide triphosphate, 7.5 pmol/L random hexamer, 1 U/μL RNaseOUT (RNase inhibitor, Invitrogen, Carlsbad, California), and 10 U/μL Moloney-murine leukemia virus reverse transcriptase (Invitrogen). The resulting complementary DNA (cDNA) was amplified by polymerase chain reaction (PCR) with 0.1 U/μL AmpliTaq DNA polymerase on a Corbett Rotor-Gene real-time quantitative PCR instrument in a total volume of 50 μL consisting of 1.0 to 3.0 μL RT product, 10 mmol/L Tris-HCl, 50 mmol/L KCl, 2 mmol/L MgCl2, 0.1 mmol/L of each dNTP, 0.2 to 1.0 μmol/L of each primer, sense and antisense, respectively, and 0.2 to 1.0 μmol/L TaqMan-MGB fluorescent detection probe (Applied Biosystems, Foster City, California). For each PCR reaction, a probe and primer titration was performed to determine optimal concentrations for each. The reaction mixtures were heated at 95°C for 30 seconds and then immediately carried through 40 cycles of PCR using the following schedule: 30 seconds denaturation at 94°C, and 2 minutes of annealing plus extension at 60°C. Primers and probes specific for rat preproET-1 were designed by Primer Express (Applied Biosystems). The following are the primer sense and antisense, and TaqMan probe sequences, respectively, for rat preproET-1: 5′-GACCAGCGTCCTTGTTCCAA-3′, 5′-TTGCTACCAGCGGATGCAA-3′, 5′-(6FAM™)-TCCAAGAGAGGTTGAGGTGT-(MGBNFQ)-3′. 18S rRNA control reagents (Applied Biosystems) were used for normalization. No template controls were used to exclude the possibility of primer dimer formation.
Statistical Analysis
Results are reported as mean ± standard error of mean (SE). Comparisons among multiple treatment groups were made using an analysis of variance (ANOVA) followed by a Newman-Keuls post hoc test, or using a Kruskal-Wallis nonparametric ANOVA with post hoc Dunn multiple comparison test, as appropriate. All tests were 2-tailed with P < .05 representing statistical significance.
Results
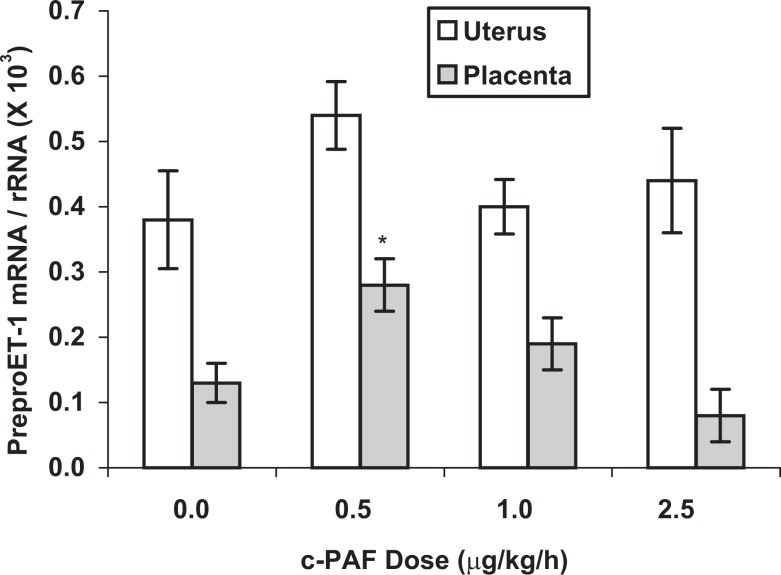
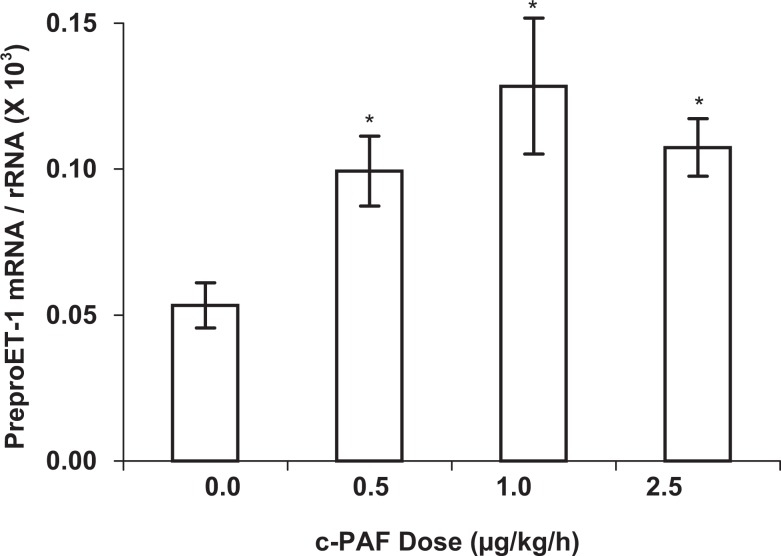
PreproET-1 expression in the placenta was significantly increased (P < .05) after 7 days of c-PAF infusion at 0.5 µg/kg per h but was not elevated at higher doses of c-PAF (Figure 1). In uterine tissue, preproET-1 messenger RNA (mRNA) expression was neither increased nor decreased in response to any of the c-PAF doses tested (Figure 1). In the kidney (Figure 2), a rich source of ET-1 and often involved in reproductive pathophysiologic processes, preproET-1 mRNA expression was increased approximately 2-fold in response to all doses of c-PAF tested (P < .05). These mRNA expression results were determined in whole homogenates, which contained a mixture of cell types including epithelium, endothelium, and, in the case of the uterus, myometrium, as well as interstitial cell types. Results were not differentiated for subsets of cell types.
Figure 1.
PreproET-1 mRNA expression in uterine and placental tissues from rats treated with carbamyl-PAF or vehicle for 7 days (gestation days 14-21) at the indicated doses. Comparative mRNA expression was determined by real-time quantitative reverse transcription–polymerase chain reaction and results were normalized to the internal control gene, the 18S subunit of ribosomal RNA. PreproET-1 mRNA expression was significantly increased in the placenta by the lowest dose of c-PAF but not by either of the higher doses. There was no significant change in preproET-1 mRNA expression in the uterus in response to c-PAF. All data are based on mRNA extraction from whole tissue homogenates, therefore they represent several cell types in each tissue. Data are presented as mean ± SE; n = 6 rats in each treatment group. *P < .05 compared to vehicle control (0 c-PAF dose), by Kruskal-Wallis nonparametric ANOVA. ET-1 indicates endothelin-1; mRNA, messenger RNA; PAF, platelet-activating factor; c-PAF, carbamyl-PAF; SE, standard error; ANOVA, analysis of variance.
Figure 2.
PreproET-1 mRNA expression in renal tissue of rats treated with carbamyl-PAF or vehicle for 7 days (gestation days 14-21) at the indicated doses. Comparative mRNA expression was determined by real-time quantitative reverse transcription–polymerase chain reaction and results were normalized to the internal control gene, the 18S subunit of ribosomal RNA. PreproET-1 mRNA expression was significantly elevated in the kidney in response to all doses of c-PAF tested. Data are presented as mean ± SE; n = 6 rats in each treatment group. *P < .05 compared to vehicle control (0 c-PAF dose) by Kruskal-Wallis nonparametric ANOVA. ET-1 indicates endothelin 1; mRNA, messenger RNA; PAF, platelet-activating factor; c-PAF- carbamyl-PAF; SE, standard error; ANOVA, analysis of variance.
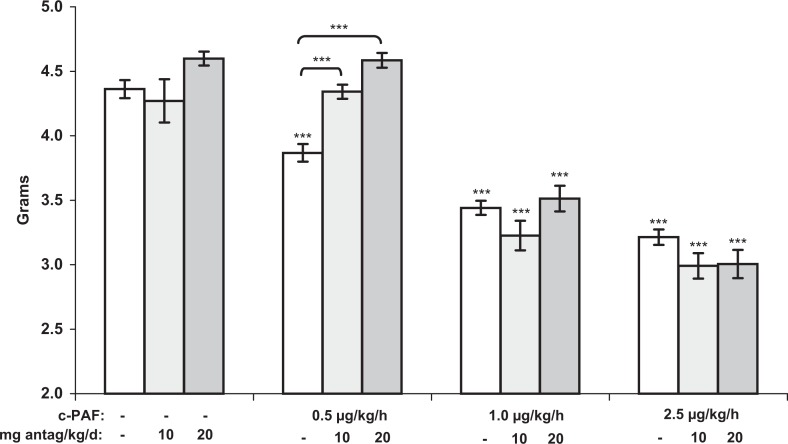
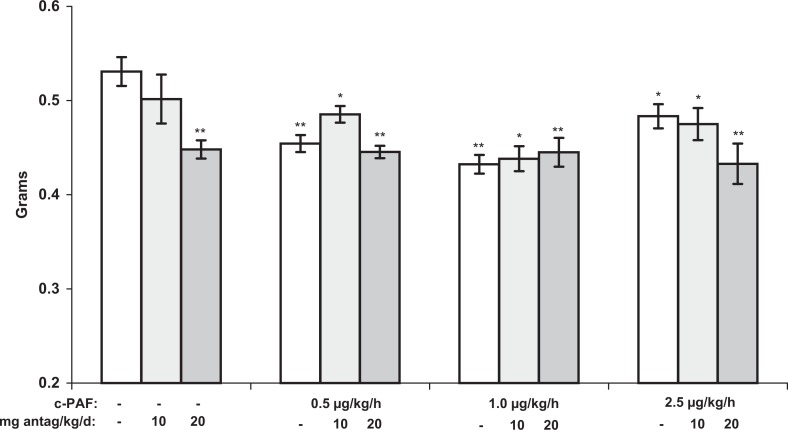
Both fetal and placental weights decreased significantly in response to 7 days of c-PAF infusion (Figures 3 and 4). The decrease in fetal weight (P <.001) was dose dependent. In contrast, placental growth, though significantly restricted at 0.5, 1.0, and 2.5 µg/kg per h c-PAF (P <.01, P <.01, and P <.05, respectively), compared to vehicle-treated controls, was not dose dependent (Figure 4). The ETA antagonism at both doses prevented FGR in the 0.5 µg/kg per h c-PAF treatment group (P < .001) but not in the higher dose c-PAF groups, even at twice the antagonist dose that has proven effective in other FGR models. Placental weights in the ETA antagonist-treated groups were not significantly different from placental weights in the respective c-PAF-treated groups but remained significantly less than the weights in the vehicle control group. Only in the 20 mg/kg per d ETA antagonist-alone treatment group were the placental weights significantly less compared to those in rats treated with vehicle alone (P < .01). Interestingly, these placentas were apparently completely capable of supporting normal fetal growth as evidenced by the normal fetal weights in this group (Figure 3).
Figure 3.
Fetal weights (grams) from maternal rats treated with carbamyl-PAF or vehicle, for 7 days (gestation days 14-21), with and without the ETA antagonist, ABT-546, at the indicated doses. Fetal weights decreased dose dependently in response to c-PAF. The ETA antagonism was effective to prevent FGR at the lowest dose of c-PAF but not at higher doses. Data are presented as mean ± SE; n = 6 rats in each treatment group. ***P < .001 compared to vehicle controls, by ANOVA. ET indicates endothelin; mRNA, messenger RNA; PAF, platelet-activating factor; c-PAF, carbamyl-PAF; FGR, fetal growth restriction; SE, standard error; ANOVA, analysis of variance.
Figure 4.
Placental weights (grams) from maternal rats treated with c-PAF or vehicle, for 7 days (gestation day 14-21), with and without the ETA antagonist, ABT-546, at the indicated doses. Placental weights decreased in response to c-PAF treatment but the decreases were not dose dependent. This placental growth restriction was not significantly improved by treatment with an ETA antagonist. Data are presented as mean ± SE; n = 6 rats in each treatment group. *P < .05, **P < .01 compared to vehicle controls, by ANOVA. ET indicates endothelin; mRNA, messenger RNA; PAF, platelet-activating factor; c-PAF, carbamyl-PAF; SE, standard error; ANOVA, analysis of variance.
There were no significant differences in litter sizes among the experimental groups, which averaged 13.4 ± 0.3 fetuses per litter. Fetal demise was highly variable within each group; especially those treated with c-PAF. It was not statistically significantly different among the treatment groups, averaging 5% ± 2% per litter in the control groups (vehicle and antagonist alone), 20% ± 6% in the PAF-treated rats and 21% ± 6% in the PAF and antagonist treatment groups. While it was more pronounced with c-PAF treatment, fetal demise was neither c-PAF dose dependent nor significantly affected by ETA antagonist.
Discussion
Platelet-activating factor is an inflammatory mediator that is commonly associated with ischemia. The placental ischemic insult that is often observed in cases of FGR suggests a potential link between PAF and this pregnancy disorder. We previously demonstrated that PAF infusion during pregnancy in the rat leads to FGR in a dose-dependent manner.22 The amelioration of FGR with an ETA antagonist demonstrates that ET-1 is important to the pathophysiology of FGR in this model. Our previous work has revealed a primary role for the vasoconstrictor ET-1 in several other models of FGR.23–25
Our current results show that PAF stimulates ET-1 expression, as has also been shown by others.17 This is true in reproductive organs as well as in the kidneys, a known rich source of ET-1 production also involved in reproductive pathophysiologic processes such as preeclampsia. Additionally, increased levels of both PAF and ET-1 have been associated with pre-eclampsia,26–29 a condition that is often accompanied by FGR.
Both PAF and ET-1 may act synergistically to increase the intensity of vascular pathology produced by either mediator alone. This possibility is implied from our previous study of ET-1 and PAF in ischemia/reperfusion-induced FGR. In this model, antagonism of either mediator was effective to ameliorate the FGR.23 Interaction between ET-1 and PAF, however, is not the only mechanism for producing FGR by these 2 mediators. In the PAF infusion model, ETA antagonism did not affect FGR at the higher PAF doses; therefore other mechanisms, such as direct effects of PAF are operative at these higher doses of PAF. It is noteworthy that PAF infusion in sheep increases vascular resistance and reduces uterine perfusion,30 thus PAF-induced FGR may be due to placental insufficiency, whether by direct action of PAF or indirectly by other mediators including ET-1.
A possible systemic effect of PAF needs to be considered in the pathophysiology of FGR in this model. PAF can cause hypotension in the rat as well as in other species,7,31 and hypotension would be the most likely systemic effect related to FGR in this model. However, the doses of c-PAF used in the current study were as low as 10-fold below those previously shown to produce hypotension in the rat. Therefore, the FGR seen in this model is not likely to be secondary to systemic effects but is more likely to be secondary to direct effects on the uterine and placental vasculature.
With increasing PAF doses, we observed that placental weight is reduced and then is maintained at the reduced level across all c-PAF doses while fetal weight dose dependently continues to decline. This could be due either to a compensatory response of the placenta to growth stimuli as the functional capability to support fetal metabolism is increasingly compromised by a high level of PAF, or interstitial edema from inflammatory changes in the placenta. Placental edema was not evaluated in this study. The significantly lower placental mass in the 20 mg/kg per d antagonist-alone treated rats, along with fully normal fetal growth in this group, suggests improved placental efficiency in spite of decreased placental weights in maternal rats treated with the ETA antagonist. This same mechanism could be operative in the rats treated with 0.5 µg/kg per h c-PAF where normal fetal weights were evident in spite of reduced placental weights. ETA antagonist-mediated increased blood flow and the resultant improved placental efficiency apparently provide for adequate nutrition and gas exchange even with a smaller placental mass. At higher doses of c-PAF, other mechanisms unrelated to ET-1 prevent ETA antagonism from positively impacting fetal growth.
Platelet-activating factor normally increases in the uterus and decreases in the placenta during the final week of rat pregnancy.32 Though this natural shift in PAF expression is likely related to the initiation of parturition, the elevation in PAF levels by infusion of low-dose c-PAF did not cause premature labor or delivery but produced consistent FGR. The initiation of labor, then, is not strictly secondary to increased PAF expression, but increased PAF expression is part of a complex interplay of molecular signals observed with the initiation of parturition.
Infusion of PAF consistently produces FGR in a dose-dependent manner, and ET-1 significantly contributes to the pathophysiology of FGR seen in this model at least at lower doses of PAF infusion. These findings suggest that ETA and/or PAF antagonism are worthy of consideration as therapeutic options for the treatment of FGR.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number HD046968).
References
- 1. McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. New Engl J Med. 1999;340(16):1234–1238 [DOI] [PubMed] [Google Scholar]
- 2. Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001;184(5):946–953 [DOI] [PubMed] [Google Scholar]
- 3. Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. Am J Obstet Gynecol. 2000;182(1 pt 1):198–206 [DOI] [PubMed] [Google Scholar]
- 4. Marconi AM, Ronzoni S, Vailati S, Bozzetti P, Morabito A, Battaglia FC. Neonatal morbidity and mortality in intrauterine growth restricted (IUGR) pregnancies is predicated upon prenatal diagnosis of clinical severity. Reprod Sci. 2009;16(4):373–379 [DOI] [PubMed] [Google Scholar]
- 5. Narchi H, Skinner A, Williams B. Small for gestational age neonates--are we missing some by only using standard population growth standards and does it matter? J Matern Fetal Neonatal Med. 2010;23(1):48–54 [DOI] [PubMed] [Google Scholar]
- 6. Hanahan DJ. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem 1986;55:483–509 [DOI] [PubMed] [Google Scholar]
- 7. Handley DA, Tomesch JC, Saunders RN. Inhibition of PAF-induced systemic responses in the rat, guinea pig, dog and primate by the receptor antagonist SRI63-441. Thromb Haemost. 1986;56(1):40–44 [PubMed] [Google Scholar]
- 8. Snyder F. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am J Physiol. 1990;259(5 pt 1):C697–C708 [DOI] [PubMed] [Google Scholar]
- 9. Zouki C, Baron C, Fournier A, Filep JG. Endothelin-1 enhances neutrophil adhesion to human coronary artery endothelial cells: role of ET(A) receptors and platelet-activating factor. Br J Pharmacol. 1999;127(4):969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schramek H, Wang Y, Konieczkowski M, Simonson MS, Dunn MJ. Endothelin-1 stimulates cytosolic phospholipase A2 activity and gene expression in rat glomerular mesangial cells. Kidney Int. 1994;46(6):1644–1652 [DOI] [PubMed] [Google Scholar]
- 11. Mustafa SB, Gandhi CR, Harvey SAK, Olson MS. Endothelin stimulates platelet-activating factor synthesis by cultured rat Kupffer cells. Hepatol. 1995;21(2):545–553 [PubMed] [Google Scholar]
- 12. Abdel-Latif AA, Zhang Y, Yousufzai SYK. Endothelin-1 stimulates the release of arachidonic acid and prostaglandins in rabbit iris sphincter smooth muscle: activation of phospholipase A2. Curr Eye Res. 1991;10(3):259–265 [DOI] [PubMed] [Google Scholar]
- 13. Montero A, Rodriguez-Barbero A, López-Novoa JM. A role for platelet-activating factor in endothelin-1-induced rat mesangial cell proliferation. Eur J Pharmacol. 1993;243(3):235–240 [DOI] [PubMed] [Google Scholar]
- 14. Filep JG, Fournier A, Földes-Filep É. Endothelin-1-induced myocardial ischaemia and oedema in the rat: involvement of the ETA receptor, platelet-activating factor and thromboxane A2 . Br J Pharmacol. 1994;112(3):963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collado MP, Latorre E, Fernandez I, Aragones MD, Catalan RE. Endothelin-1 decreases ethanolamine plasmalogen levels and evokes PAF production in brain microvessels. Microvasc Res. 2003;66(3):197–203 [DOI] [PubMed] [Google Scholar]
- 16. Yoshizumi M, Kurihara H, Morita T, et al. Interleukin 1 increases the production of endothelin-1 by cultured endothelial cells. Biochem Biophys Res Commun. 1990;166(1):324–329 [DOI] [PubMed] [Google Scholar]
- 17. Clavijo LC, Carter MB, Matheson PJ, et al. Platelet-activating factor and bacteremia-induced pulmonary hypertension. J Surg Res. 2000;88(2):173–180 [DOI] [PubMed] [Google Scholar]
- 18. Foitzik T, Hotz HG, Eibl G, Hotz B, Kirchengast M, Buhr HJ. Therapy for microcirculatory disorders in severe acute pancreatitis: effectiveness of platelet-activating factor receptor blockade vs. endothelin receptor blockade. J Gastrointest Surg. 1999;3(3):244–251 [DOI] [PubMed] [Google Scholar]
- 19. Stammberger U, Carboni GL, Hillinger S, Schneiter D, Weder W, Schmid RA. Combined treatment with endothelin- and PAF-antagonists reduces posttransplant lung ischemia/reperfusion injury. J Heart Lung Transplant. 1999;18(9):862–868 [DOI] [PubMed] [Google Scholar]
- 20. King-VanVlack CE, Mewburn JD, Chapler CK, MacDonald PH. Hemodynamic and proinflammatory actions of endothelin-1 in guinea pig small intestine submucosal microcirculation. Am J Physiol Gastrointest Liver Physiol. 2003;284(6):G940–G948 [DOI] [PubMed] [Google Scholar]
- 21. Getting SJ, Di Filippo C, Lam CW, Rossi F, D'Amico M. Investigation into the potential anti-inflammatory effects of endothelin antagonists in a murine model of experimental monosodium urate peritonitis. J Pharmacol Exp Ther. 2004;310(1):90–97 [DOI] [PubMed] [Google Scholar]
- 22. Thaete LG, Neerhof MG, Jilling T, Caplan MS. Infusion of exogenous platelet-activating factor produces intrauterine growth restriction in the rat. J Soc Gynecol Investig. 2003;10(3):145–150 [DOI] [PubMed] [Google Scholar]
- 23. Thaete LG, Neerhof MG. Endothelin and platelet-activating factor: Significance in the pathophysiology of ischemia/reperfusion-induced fetal growth restriction in the rat. Am J Obstet Gynecol. 2006;194(5):1377–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thaete LG, Neerhof MG, Caplan MS. Endothelin receptor A antagonism prevents hypoxia-induced intrauterine growth restriction in the rat. Am J Obstet Gynecol. 1997;176(1 pt 1):73–76 [DOI] [PubMed] [Google Scholar]
- 25. Thaete LG, Neerhof MG, Silver RK. Differential effects of endothelin A and B receptor antagonism on fetal growth in normal and nitric oxide-deficient rats. J Soc Gynecol Investig. 2001;8(1):18–23 [PubMed] [Google Scholar]
- 26. Rowland BL, Vermillion ST, Roudebush WE. Elevated circulating concentrations of platelet activating factor in preeclampsia. Am J Obstet Gynecol. 2000;183(4):930–932 [DOI] [PubMed] [Google Scholar]
- 27. Taylor RN, Varma M, Teng NNH, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71(6):1675–1677 [DOI] [PubMed] [Google Scholar]
- 28. Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am J Obstet Gynecol. 1991;165(3):724–727 [DOI] [PubMed] [Google Scholar]
- 29. Kraayenbrink AA, Dekker GA, van Kamp GJ, van Geijn HP. Endothelial vasoactive mediators in preeclampsia. Am J Obstet Gynecol. 1993;169(1):160–165 [DOI] [PubMed] [Google Scholar]
- 30. Greenberg SG, Clark KE. Hemodynamic effects of platelet-activating factor in nonpregnant and pregnant sheep. Am J Physiol. 1999;277(4 pt 2):R996–R1001 [DOI] [PubMed] [Google Scholar]
- 31. Shibamoto T, Liu W, Cui S, Zhang W, Takano H, Kurata Y. PAF, rather than histamine, participates in mouse anaphylactic hypotension. Pharmacol. 2008;82(2):114–120 [DOI] [PubMed] [Google Scholar]
- 32. Matsubara T, Yasuda K, Johnston JM, et al. Platelet-activating factor (PAF) and PAF acetylhydrolase activity in rat uterus and placenta during the late stages of pregnancy. Biol Reprod. 1997;56(4):885–890 [DOI] [PubMed] [Google Scholar]