Abstract
Introduction:
Fragile X premutations are associated with primary ovarian insufficiency when the patient presents with amenorrhea, but the fragile X mental retardation 1 (FMR1) CGG repeat count among cycling women with low ovarian reserve (diminished ovarian reserve [DOR]) is not yet established.
Patients and Methods:
Sixty-two infertile DOR patients were recruited from 4 US private and academic fertility centers. Results: The prevalence of 35-44 FMR1 CGG repeats was 14.5%. Compared with the general female population estimate from the literature, infertile women with DOR were more likely to have 35-44 FMR1 CGG repeats (14.5% and 3.9%, respectively, P = .0003). Similar findings were noted by 5-repeat bandwidth: 35-39 CGG repeats (9.7% DOR vs 3.2% comparison, P = .012) or 40-44 CGG repeats (4.8% DOR vs 0.7% comparison, P = .024).
Conclusions:
These data suggest that CGG repeats of 35-44 may be markedly overrepresented in women with DOR, whereas the current FMR1 reference range indicates that there is no clinical phenotype with <45 CGG repeats.
Keywords: FMR1, female infertility, diminished ovarian reserve, trinucleotide repeats, epidemiology, FXPOI
Introduction
Fragile X syndrome (FXS) is the most common heritable form of mental impairment. The molecular cause of this abnormality is an expansion of over 200 (CGG) trinucleotide repeats (full mutation) within the 5′-untranslated region of the fragile X mental retardation 1 (FMR1) gene.
Primary ovarian insufficiency (POI) is a term that represents a broad clinical spectrum related to early aging of the ovaries.1 Diminished ovarian reserve (DOR) and premature ovarian failure (POF) both fall within the definition of POI. Clinically, DOR typically presents as infertility, while POF presents as a cessation of menses. POF is related to DOR in that both are diagnosed by levels of high follicle-stimulating hormone (FSH; >40 IU/L for POF2 vs >10 IU/L in cycle days 2-4 for DOR3). POF, however, is accompanied by 4 or more months of secondary amenorrhea and age <40,2 while DOR women are still menstruating and no age limit is applied. DOR is a normal physiologic process when it occurs in the mid to late 40s; and at earlier ages, DOR causes infertility and reduced response to controlled ovarian hyperstimulation (COH) for assisted reproductive therapy.4 Approximately 10% of women seeking fertility assistance are diagnosed with DOR.5 Fragile X premutations (55-199 repeats6) have been associated with POF,7 and older men with this premutation have an increased risk of an ataxia disorder termed FXTAS.8
Based on the available research, committees for the American College of Obstetrics & Gynecology and the American College of Medical Genetics have concluded that an FMR1 CGG repeat count <45 is not associated with an abnormal phenotype and are without reproductive risk to subsequent generations.6,9,10 The “intermediate” zone (45-54 repeats) has not been associated with any phenotype nor risk of expansion large enough to cause FXS within 1 generation,11 although there is a risk of FXS in 2 generations.12
Fu et al13 reported that 30% of males and females combined had 29 CGG triplet repeats; no data by gender were reported. The modal CGG count worldwide varies between 29 and 32, as reviewed by Peprah.14 Detailed data are limited on the frequency of high normal triplet repeats in the general female population,15–18 and the literature on the FMR1 triplet repeat count among DOR cases is limited to 1 report.15 The goal of this article is to report the FMR1 triplet repeat count in 62 infertile women with DOR and to compare these results with the existing literature.
Patients and Methods
This was a prospective multicenter cohort study of infertile women diagnosed with DOR who were enrolled from March 2005 to May 2010. This cohort was enrolled from 4 sites: academic reproductive endocrinology and infertility clinics in Virginia (31% of participants), California (31%), and North Carolina (13%), plus a private fertility practice in Virginia (26%). This study was approved by the Human Ethics Boards at all academic sites.
Eligibility requirements included diagnosis of DOR (cycle days 2-5 FSH >10 mIU/mL, or FSH >12 mIU/mL after 5 days of 100 mg clomiphene citrate medication, or fewer than 6 antral follicles sized 2-10 mm), age at DOR diagnosis ≤42 years, and regular menstrual cycles for the past 6 months. The antral follicle count (AFC) was only used for enrollment at 1 site. The criteria for exclusion were known cause of elevated FSH for one’s age unrelated to fragile X (eg, surgical removal of either one or both ovaries, chemotherapy or radiation therapy, Turner syndrome, and autoimmune disease) or a family history of FXS or premutation.
Knowing that FSH values can vary by assay,19,20 de-identified samples were run at each satellite site and the primary site (University of Virginia). Based on those results, the cycle day 2 to 5 FSH enrollment criteria was increased by 1 point (Immulite 2500 machine; bioMérieux Vidas machine) or decreased by 1.8 points (Ortho 5600 machine) to ensure consistency in the enrollment criteria across sites. All FSH values presented have been adjusted to the corresponding cycle days 2 to 5 value at the University of Virginia.
After signing an informed consent, women provided a single blood sample for FMR1 trinucleotide assessment and received pretest genetic counseling by an experienced certified genetic counselor. Questionnaires completed at the study visit and/or medical record reviews were the source of all demographic, reproductive, and family medical history variables.
The CLIA-certified UVA Molecular Diagnostics Laboratory at the University of Virginia developed a capillary electrophoresis method that can measure FMR1 alleles with an accuracy of ±1 CGG repeat.21 The process commenced with isolation of DNA from peripheral blood leukocytes by conventional techniques. The repeated trinucleotide sequence (CGG)n on the X chromosome at Xq27.3 was amplified by polymerase chain reaction (PCR) using primers that flank the (CGG)n region. The PCR product with a size standard included was subsequently electrophoresed through a capillary tube and the amplified fragments were detected by the 6FAM fluorescent label on one of the PCR primers. The base pairs were graphed and analyzed for dual peaks. If there was only 1 peak, the sample was further assessed by Southern blotting to determine whether there were (a) 2 homozygous alleles, (b) a premutation allele between 108 and 199 repeats, or (c) a full mutation. One third of this cohort required a Southern blot, all of whom were homozygous.
Power analysis was conducted with EpiInfo (Centers for Disease Control, Atlanta, Georgia). A total of 60 DOR (N = 60) cases and 540 comparison women from the general population were needed to have 80% power and 95% confidence interval to detect a statistical difference in the prevalence of 35-44 CGG repeats between the 2 populations (14.5% in the DOR cases and 3.9% in the comparison population). This analysis had 62 DOR cases and 564 comparison individuals from the literature.
Descriptive analyses consisted of means, medians, modes, and standard deviation (SD) calculations using SAS v9.1 (SAS Institute, Cary, North Carolina). Chi-square tests were performed using EpiInfo with Fisher exact P values when any cell had fewer than 5 observations. The comparison cohorts consisted of the females in the reports from Bretherick,16 Otsuka,17 and Streuli15 (controls only), as those reports all had detailed prevalence data at the 35-39 and 40-44 CGG repeat levels. In the DOR cohort, the CGG repeat count was further analyzed by examining the proportion outside the range of 26-34 CGG repeats, as one research group reported that women with repeats above and below that range were equally predisposed toward early ovarian aging.22,23 Using their most recent nomenclature,24 women with both alleles in the 26 to 34 range will be termed “norm,” those with only 1 allele in that range are termed “het-norm/low” or “het-norm/high” depending on whether the second allele is below 26 or above 34, respectively, and those with both alleles outside this range are termed “hom.” Statistical significance was evaluated with α = .05.
Results
Fifty-two participants (84% of the cohort) were enrolled based on FSH laboratory results. Excluding 1 woman with a postmenopausal value (>40 mIU/mL) despite having regular periods, the mean FSH at DOR diagnosis was 16.2 mIU/mL (SD 5.8, median 13.6). More than half the women (58%) had FSH values between 10 and 15 mIU/mL, 21% had FSH between 15.1 and 20.0 mIU/mL, and 21% had FSH >20 mIU/mL. Eight women were enrolled based on AFC; for both ovaries combined, the AFC ranged from 2 to 5 (median 3.5, SD 1.16).
This cohort was primarily of Caucasian race (76%) with 11% of Asian race (Table 1). The median age at DOR diagnosis was 38 years and the median age at menarche was 13 years. The cohort overall had a normal BMI (median 22.6 kg/m2) and 38% had never been pregnant.
Table 1.
Participant Demographics and Reproductive History, 4 US Clinics, 2005 to 2010
| Factor | Distribution |
|---|---|
| Age at DOR diagnosis, mean (SD) | 37.8 (3.3) |
| Median, range | 38.7, 29-42 |
| Age at menarche, mean (SD) | 12.8 (1.4) |
| Median, range | 13.0, 11-18 |
| Ever smoke | 8 (13%) |
| Currently smoking | 1 |
| Race, N (%) | |
| Caucasian | 47 (76%) |
| African American | 3 (5%) |
| Asian | 7 (11%) |
| Other/mixed | 5 (8%) |
| Hispanic ethnicity, N (%) | 1 (2%) |
| Nulligravid, N (%) | 23 (38%) |
| BMI: mean (SD) | 23.9 (4.9) |
| Median, range | 22.6, 18.3-34.0a |
Abbreviation: SD, standard deviation; BMI, body mass index; DOR, diminished ovarian reserve.
a Plus 1 outlier value of 48.5 BMI (265 lbs and 5′2″)
Between one fifth and one quarter of the cohort had a family history of infertility (12 women) and/or menopause before age 45 in their first- or second-degree relatives (12 women, Table 2). Five women had elderly relatives with tremors.
Table 2.
Self-Reported Family History in First- or Second-Degree Relatives, 4 US Clinics, 2005 to 2010a
| Family History Variable | n/N (%) |
|---|---|
| Down syndrome | 0/62 (0%) |
| Infertility | 12/61 (20%) |
| Menopause before age 45 | 12/59 (20%) |
| Menopause before age 40 | 3/60 (5%) |
| Mental impairment, but not FXS | 3/62 (5%) |
| Ovarian cancer | 5/54 (9%) |
| Uterine cancer | 2/54 (4%) |
| Endometriosis | 10/51 (20%) |
| Spontaneous abortion | 5/54 (9%) |
| Tremor in elderly relatives | 5/62 (8%) |
Abbreviation: FXS, fragile X syndrome.
a Some family history variables were not included in the initial participant questionnaire; thus, for 5 variables, the maximum denominator is 54 instead of 60. Any variation in the denominator from 54 to 62 is due to missing responses.
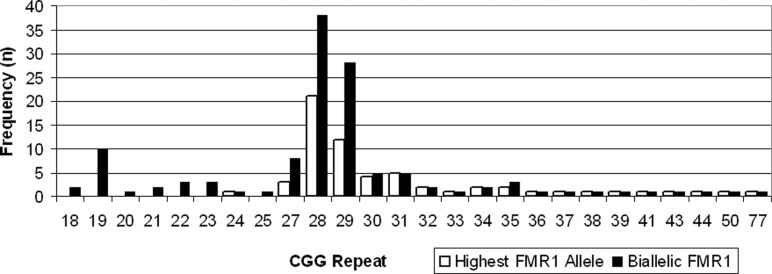
The FMR1 CGG repeat distribution (Figure 1) ranged from 24 to 77. There were 6 (9.7%) participants with an FMR1 trinucleotide repeat count between 35 and 39, 3 (4.8%) between 40 and 44, 0 between 45 and 49, 1 (1.6%) between 50 and 54, and 1 (1.6%) with a CGG repeat ≥55. The most common high allele was 28 (35% of the cohort). As some authors15 reported biallelic frequencies (ie, both alleles instead of the highest allele per woman),15 those are also displayed.
Figure 1.
Discrete distribution of CGG repeats in women with diminished ovarian reserve (DOR), 4 US clinics, 2005 to 2010.
In all, 48% of this cohort had both alleles with 26-34 CGG repeats (norm), and an equal proportion had only 1 allele in that range (n = 9, het-norm/high; n = 20 het-norm/low). In all, 5% of the cohort were hom (n = 1, with both alleles >34; n = 1, with both alleles <26; n = 1, with 1 allele >34 and 1 allele <26). Excluding those enrolled based on AFC, the mean FSH levels did not trend with the FMR1 CGG count: the mean FSH by subgroup was 16.2 IU/L for het-norm/low, 15.6 IU/L for norm, 23.9 IU/L for het-norm/high, and 20.3 IU/L for hom. The FSH level for the het-norm subgroups combined was 18.6 IU/L. (Note that there was no correlation between the adjusted FSH level and the age at diagnosis overall in this cohort nor within the group who had both alleles in the 26 to 34 range nor within the group who had only 1 allele within that range [Spearman P = .26, .72, .36, respectively].)
Table 3 compares the CGG repeat count in this DOR cohort with the Streuli et al15 cohort (n = 27, termed “occult POI” in that report), which was defined as having regular or irregular cycles (63% and 37%, respectively), FSH >10 mIU/mL and/or AMH <7 pmol/L and/or poor response to COH, and no family history of FXS. The proportion of Streuli occult POI cases with 35-44 CGG repeats was 16.7%.
Table 3.
The FMR1 CGG Repeats in Occult POI Women and Female Comparison Cohorts, 4 US Clinics, 2005 to 2010
| Occult POI Cohorts | Comparison Cohorts | |||||
|---|---|---|---|---|---|---|
| Pastore DOR | Streuli Occult POI15 | Streuli15 | Bretherick16 | Otsuka17 | Bodega18 | |
| Sample size | 62 | 27a | 32a | 162 | 370 | 200 |
| <35 repeats | 82.3% | 77.7% | 95.3% | 92.9% | 96.8% | 99.0% |
| 35-39 | 9.7% | 9.3% | 3.1% | 3.7% | 3.1% | |
| 40-44 | 4.8% | 7.4% | 1.6% | 1.9% | 0% | 0% |
| 45-49 | 0% | 0% | 0% | 0.9% | 0.2% | 0% |
| 50-54 | 1.6% | 0% | 0% | 0% | 0% | 1.0% |
| 55-59 | 0% | 0% | 0% | 0% | 0% | 0% |
| 60-199 | 1.6% | 5.6% | 0% | 0.9% | 0% | 0% |
| >200 | 0% | 0% | 0% | 0.9% | 0% | 0% |
Abbreviations: POI, primary ovarian insufficiency; DOR, diminished ovarian reserve.
a Data reported for both alleles in the publication, so these percentages reflect the biallelic distribution; all other columns represent the distribution of the highest allele.
This DOR cohort is further compared with populations of women designed to reflect the general female population (Table 3).15–18 These comparison cohorts are described below. Excluding the Bodega report due to its lack of detail at the 35 to 39 range, the remaining 3 comparison cohorts had 3.2% with 35-39 CGG repeats and 0.7% had 40-44 CGG repeats. Comparing the DOR cohort with those 3 reports,15–17 the infertile women with DOR were more likely to have 35-39 CGG repeats (P = .012), 40-44 CGG repeats (Fisher P = .024), or 35-44 CGG repeats (P = .0003). The comparison cohort descriptions are
Streuli15: women who were referred to genetic counseling for a condition unrelated to fertility or mental impairment; unknown fertility history;
Bretherick16: mostly unaffected female spouses of families with an autosomal dominant genetic disorder; unknown fertility status;
Otsuka17: females from Japan who were a control population for a study of diabetes and cancer; unknown fertility status and unknown family history of mental impairment; and
Bodega18: women with a natural menopause after age 45 and normal menstrual history; unknown fertility status and unknown family history of mental impairment.
Discussion
Within this cohort of infertile women diagnosed with DOR based on elevated basal FSH levels (>10 IU/L) or low AFC (<6), 14.5% had 35-44 CGG repeats in the FMR1 gene, which is a repeat level that is considered normal by genetics committees.9,10,25 In comparison with the literature on general female populations with sufficient FMR1 repeat detail, infertile women with DOR were more likely to have 35-39 CGG repeats (P =.012) or 40-44 CGG repeats (Fisher P = .024). Combining these 2 ranges, infertile women with DOR were more likely to have 35-44 FMR1 CGG repeats (P = .0003) than the general female population estimate (14.5% and 3.9%, respectively).
Strengths and Limitations of This Study
The primary strength of this study was the identification of women with a well-defined phenotype independent of any potential risk factors for an FMR1 genetic alteration. Second, the cohort was identified prospectively. Third, the cohort represents 3 geographic areas (central North Carolina, central Virginia, and northern California) as opposed to only 1 center/geographic region.
In terms of limitations, this cohort has a small volume, although it is twice as large as the Streuli et al cohort.15 Studies of infertile women are often limited to women who present for medical evaluation of their infertility and/or intervention to become pregnant, and this is also applicable to this investigation. It is unknown whether genetics (as opposed to a psychological or motivational end point) are different in a clinical cohort than in women who do not seek specialized fertility assistance or are not aware of their infertility because they were not attempting to become pregnant. It should be noted that the comparison cohorts from the literature are not ideal for our analysis, as none of the reports assessed their population for fertility issues/reproductive history and most did not assess for a family history of fragile X. If the comparison cohorts contained a modest percentage of women with DOR, this would cause bias toward the null. Lastly, this cohort underrepresented African Americans and Latinas, although there was a notable proportion of Asians in this cohort (11%).
Comparison With Other Studies
The frequency of 35-44 FMR1 CGG repeats in this DOR cohort is quite similar to a report by Streuli et al15 (16.7%) that used a slightly broader case definition, as previously described. The similarity of findings in the 2 overlapping cohorts suggests a robust association between the FMR1 repeat count and DOR infertility. Two additional reports have described FMR1 test results in cohorts of women with elevated FSH (defined as 12-49 mIU/mL), neither of which had a menstrual frequency nor lack of FXS family history eligibility requirement. In the Gleicher report26 of women aged <42 years, 17% (4/23) had 31-40 CGG repeats and 13% (3/23) had 41-54 CGG repeats. In the Ficicioglu report27 of women aged ≤40 years without a history of chemo- or radiotherapy, 27% (8 of 30) had 31-40 CGG repeats and 0 had a 41-54 CGG repeat size. An additional report with 158 occult POI cases lacked sufficient FMR1 data for comparison.28
Gleicher et al29 reported elevated FSH in infertility patients with a hom FMR1 pattern compared with norm women: 13.2 versus 9.5 IU/L among those younger than age 38 and 23.8 IU/L versus 10.8 IU/L in those ≥38 years old, respectively. Our data are supportive of their findings in those 2 subgroups, albeit without consideration of age. The FSH levels for their het-norm group was similar to their norm subgroup and was not reported separately for het-norm/low and het-norm/high; our mean FSH values varied by whether the single allele outside the 26-34 CGG range was low or high.
Clinical Impact
The uncertainty about clinical outcomes by FMR1 repeat size makes counseling of patients difficult and an increasing amount of clinical testing makes these questions more common. Fertility clinic patients are given 2 distinct messages as a result of FMR1 allele testing. The first message she learns is whether or not this gene is a likely contributing cause of her ovarian dysfunction; this would be currently limited to premutation carriers. The second message relates to her risk of having a child with a full mutation and FXS, which is also targeted to only premutation carriers, as only those women are at risk of an FXS child in a single generation.12,30 A woman with a CGG repeat <45 is currently told that the FMR1 gene is not a cause of her ovarian dysfunction and does not place her at risk of an affected child. If future research confirms an association between FMR1 CGG repeat sizes in the 35-44 range, then counseling would change considerably to include a likelihood that those repeat sizes contribute to DOR and that female offspring who carry a similar repeat size may also be at risk of DOR.
Importantly, as FMR1 test results do not vary with age, there is the potential to be able to prospectively identify young women at risk of a reduced reproductive window if these results are confirmed. It would be useful to investigate the incidence of infertility of ovarian origin in women with 35-44 FMR1 CGG repeats as the prevalence of this phenotype is unknown in women within this trinucleotide subset.
This study, if confirmed, also has implications for the FMR1 laboratory reference range interpretation, which is based on the studies designed to establish the diagnosis of FXS and the associated risk of expansion to a full mutation during female meiosis. Historically, reference populations were chosen based on the absence of FXS phenotype in males or absence of a family history of FXS in females. The current reference ranges are additionally used to distinguish normality from abnormality for other conditions, namely adult onset FXTAS primarily in males and Fragile-X-related POI in females. The problem lies in assuming that the FXS ranges are appropriate for defining FXTAS and POI. Unless studies establish a common pathogenesis between FXS and FXTAS/POI, using the same reference ranges has questionable validity. For a review of the literature on the pathogenesis of FMR1-related phenotypes, the reader is referred elsewhere.14
Our findings raise questions about the applicability of the current FMR1 reference ranges for cycling infertile women with POI. Our data and that of others15 suggest that DOR may be a phenotype associated with 35-44 FMR1 CGG repeats.
Acknowledgment
The authors thank the study research coordinators (Parchayi Dalal, Angie Morey, and Amy Brown) and the University of Virginia Molecular Diagnostics Lab supervisor (James Bowden).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: supported by the Eunice K. Shriver National Center for Child Health and Human Development at the National Institutes of Health (grant R03 HD052768 to LMP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. McConkie-Rosell A, Abrams L, Finucane B, et al. Recommendations from multi-disciplinary focus groups on cascade testing and genetic counseling for fragile X-associated disorders. J Genet Couns. 2007;16(5):593–606 [DOI] [PubMed] [Google Scholar]
- 2. Nelson LM, Bakalov VK. Mechanisms of follicular dysfunction in 46, XX spontaneous premature ovarian failure. Endocrinol Metab Clin North Am. 2003;32(3):613–637 [DOI] [PubMed] [Google Scholar]
- 3. Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7 ed Philadelphia: Lippincott Williams & Wilkins; 2005 [Google Scholar]
- 4. Scott RT, Jr, , Hofmann GE. Prognostic assessment of ovarian reserve. Fertil Steril. 1995;63(1):1–11 [PubMed] [Google Scholar]
- 5. Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott RT., Jr Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril. 2001;76(4):666–669 [DOI] [PubMed] [Google Scholar]
- 6. Spector E, Kronquist K. Fragile X: Technical standards and guidelines: ACMG Standards and Guidelines for Clinical Genetics Laboratories; 2005 [Google Scholar]
- 7. Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. 2000;97(3):189–194 [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19(R1):R83–R89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7(8):584–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ACOG Committee of Genetics. Carrier Screening for Fragile X Syndrome. Obstet Gynecol. 2010;116(4):1008–1010 [DOI] [PubMed] [Google Scholar]
- 11. Nolin SL, Brown WT, Glicksman A, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet. 2003;72(2):454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zuniga A, Juan J, Mila M, Guerrero A. Expansion of an intermediate allele of the FMR1 gene in only two generations. Clin Genet. 2005;68(5):471–473 [DOI] [PubMed] [Google Scholar]
- 13. Fu YH, Kuhl DPA, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–1058 [DOI] [PubMed] [Google Scholar]
- 14. Peprah E. Fragile X syndrome: the FMR1 CGG repeat distribution among world populations. Ann Hum Genet. 2012;76(2):178–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Streuli I, Fraisse T, Ibecheole V, Moix I, Morris MA, de Ziegler D. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil Steril. 2009;92(2):464–470 [DOI] [PubMed] [Google Scholar]
- 16. Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117(4):376–382 [DOI] [PubMed] [Google Scholar]
- 17. Otsuka S, Sakamoto Y, Siomi H, et al. Fragile X carrier screening and FMR1 allele distribution in the Japanese population. Brain Dev. 2010;32(2):110–114 [DOI] [PubMed] [Google Scholar]
- 18. Bodega B, Bione S, Dalpra L, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006;21(4):952–957 [DOI] [PubMed] [Google Scholar]
- 19. Taieb J, Olivennes F, Birr AS, et al. Comparison of day 3 FSH serum values as determined by six different immunoassays. Hum Reprod. 2002;17(4):926–928 [DOI] [PubMed] [Google Scholar]
- 20. Scriver J, Baker V, Young S, Behr B, Pastore L. Inter-laboratory validation of the measurement of follicle stimulating hormone (FSH) after various lengths of frozen storage. Reprod Biol Endocrinol. 2010;8(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen LA, Grønskov K, Nørgaard-Pedersen B, Brøndum-Nielsen K, Hasholt L, Vuust J. High-throughput analysis of Fragile X (CGG)n alleles in the normal and premutation range by PCR amplification and automated capillary electrophoresis. Hum Genet. 1997;100(5-6):564–568 [DOI] [PubMed] [Google Scholar]
- 22. Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009;19(3):385–390 [DOI] [PubMed] [Google Scholar]
- 23. Gleicher N, Weghofer A, Barad DH. Effects of race/ethnicity on triple CGG counts in the FMR1 gene in infertile women and egg donors. Reprod Biomed Online. 2010;20(4):485–491 [DOI] [PubMed] [Google Scholar]
- 24. Gleicher N, Weghofer A, Lee IH, Barad DH. Association of FMR1 genotypes with in vitro fertilization (IVF) outcomes based on ethnicity/race. PLoS One. 2011;6(4):e18781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maddalena A, Richards CS, McGinniss MJ, et al. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3(3):200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Müllerian hormone. Fertil Steril. 2009;91(5):1700–1706 [DOI] [PubMed] [Google Scholar]
- 27. Ficicioglu C, Yildirim G, Attar R, Kumbak B, Yesildaglar N. The significance of the number of CGG repeats and autoantibodies in premature ovarian failure. Reprod Biomed Online. 2010;20(6):776–782 [DOI] [PubMed] [Google Scholar]
- 28. Gleicher N, Weghofer A, Oktay K, Barad DH. Correlation of triple repeats on the FMR1 (fragile X) gene to ovarian reserve: a new infertility test? Acta Obstet Gynecol Scand. 2009;88(9):1024–1030 [DOI] [PubMed] [Google Scholar]
- 29. Gleicher N, Weghofer A, Barad D. Ovarian reserve determinations suggest new finction of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod Biomed Online. 2010;20(6):768–775 [DOI] [PubMed] [Google Scholar]
- 30. Cronister A, Teicher J, Rohlfs EM, Donnenfeld A, Hallam S. Prevalence and instability of fragile X alleles: implications for offering fragile X prenatal diagnosis. Obstet Gynecol. 2008;111(3):596–601 [DOI] [PubMed] [Google Scholar]