Abstract
Urogenital diseases, especially infection and cancer, are major causes of death and morbidity in females. Yet, millions of women in the developing world have no access to basic urogynecological care, and the diagnosis and treatment of widespread aberrant bacterial conditions (bacterial vaginosis [BV] and aerobic vaginitis [AV]) remain suboptimal the world over. Samples from women living in resource-disadvantaged and developed countries have been analyzed by high-throughput sequencing to reveal the diversity of bacteria in the vagina, how rapidly the bacterial population fluctuates over time, and how rapidly the switch occurs between healthy and aberrant conditions. Unfortunately, clinical diagnostic methods are inefficient and too often outdated therapies are administered. The net result is suboptimal care and recurrent disease that adversely affects the quality of life. This viewpoint outlines a scientific and translational road map designed to improve the cervicovaginal health and treatment of disease. This comprises (1) improving education of women and physicians on the vaginal microbiota; (2) having agencies target funding for research to improve diagnosis and test new therapies; and (3) making sure that new approaches are accessible in developing countries, empowering to women, and are acceptable and appropriate for different populations.
Keywords: cervicovagina, bacteria, vaginosis, diagnosis, gardnerella, lactobacillus, Nugent, Amsel, microbiome
Which Bacteria are Associated With Health and Disease?
A series of high-throughput sequencing studies have recovered over 250 different bacterial taxonomic units from the vagina of women of differing ages, health status, and country of origin.1–4 Longitudinal sampling has revealed that communities sometimes change markedly over short periods of time, while others are relatively stable, independent of the vaginal community compositions. Five types of temporal patterns of variation have been observed, including stable communities that do not contain any Lactobacillus sp. Of those healthy women dominated by Lactobacillus, the species L crispatus, L iners, L jensenii, and L gasseri are most commonly found.5,6 This raises the question of what is normal? Further studies suggest some degree of variation in abundance profiles of bacterial types depending upon race,7 but essentially there is tremendous commonality in the species of bacteria and community types detected across populations around the world, even though their frequencies can differ.
The acquisition of sequence data might appear to some clinicians to be far removed from everyday practice, but those wishing to obtain such data will be interested in the process. The articles cited in this review1–7 and others are not universal as there is variation in the physiological collection site, in whether the samples were self-collected versus collected by a caregiver, or in the methods to determine which organisms are present. (DNA extraction, which primers were used, which sequencing method). Yet, collectively there is little disagreement about the findings, namely the dominance of lactobacilli in most healthy cases and its depletion in disease (as will be discussed). In essence, when bacterial DNA is extracted from the vagina and surrounding areas, and then sequenced and compared to databases of known bacterial sequences to determine its species it provides a road map of the organisms and their relative abundance present at that time and location. If these organisms could be cultured and quantified, the clinicians would be better informed as to how optimally to manage the patient in terms of infection; but many of these bacteria are not easy to culture. If the microbiota profiles (based upon DNA sequencing) are obtainable, preferably from several samplings over a given timeframe like 1 week or month, it becomes easier to understand what, if any, medical intervention is needed, based upon the patient's history and presentation.
The importance of having more than a single sample is highlighted by the finding that a healthy microbiota can switch to an aberrant one rapidly within days and in a portion of women it can also resolve quickly and without treatment.8,9 These fluctuations and the fact that many women are asymptomatic with what would be called an aberrant vaginal microbiota based upon microscopic analysis, increase confusion over what is meant by a healthy or bacterial vaginosis (BV) or aerobic vaginitis (AV) condition, independently from risk of acquisition of secondary diseases. There are some similarities to Escherichia coli and urinary tract infections (UTIs), where pathogens with the armamentarium to cause symptomatic illness can be found in the bladder of asymptomatic women.10 Likewise, around 25% of UTI cases spontaneously resolve.11 If the bacteria do not produce symptoms and rapidly disappear, are they pathogens at that time? Does their presence constitute infection? Some might say yes, because the organisms have virulence factors and their presence meets the general definition of infection: “invasion by and multiplication of pathogenic microorganisms in a bodily part or tissue, which may produce subsequent tissue injury and progress to overt disease through a variety of cellular or toxic mechanisms.” Some clinicians might say no, because the condition does not warrant treatment. For BV, it is not so much that the organisms regarded as pathogens, namely Gardnerella vaginalis, Atopobium vaginae, Prevotella bivia, some Clostridiales species, including 3 newly recognized bacteria provisionally named bacterial vaginosis–associated bacteria (BVAB) 1, 2, and 3,12 are only present at appreciable levels when the microbiota is aberrant. Rather, it is their abundance that appears to diminish significantly when the vaginal status returns to normal. In addition, gross taxonomic classification is insufficient since small difference in genomic content between strains could alter pathogenic capability.
In the case of AV, a condition associated with red inflammation, yellow discharge, and vaginal dyspareunia, spontaneous resolution has not been investigated, and with causative or associated agents being E coli, Staphylococcus aureus, and Group B streptococci,13 and parabasal epithelial cells, and/or positive for increased numbers and activity of vaginal leucocytes visible under the microscope,14 this would be classified as an infectious condition. concerningly, AV is seldom diagnosed or even looked for during the examination of women having a routine examination or reporting symptoms and signs of vaginal discomfort, and wet mount examinations for accurate diagnosis of AV are almost never performed outside of specialized centers. Yet, the combination of the assessment of the microbiota with the associated host-response findings offers unseen opportunities to better understand the pathogenesis and potential complications of the disease and facilitates tailoring of treatment.
Therefore, the determination of the status of a woman's vagina with regard to its bacterial content is not universally performed, and likewise the diagnosis of an infectious condition is often arbitrarily decided based upon methods that are outdated and fairly inaccurate, as will be discussed in the next section. The single factor that is apparent is that lactobacilli are the dominant organism in the vast majority of healthy vaginas, and their depletion in favor of what is believed to be pathogenic species could correspond to an aberrant condition. As it is unclear what BV is, how best to diagnose and treat it, and whether it coincides or is separate from AV, it seems a good time to reevaluate what is meant by vaginal health and the risk of infection posed by various microbiota profiles.
More Problems With Diagnosis and Therapy
If we cannot define normal then how can we determine whether someone has an aberrant microbiota? In essence we do not. First, because high-throughput microbiota sequencing is not yet part of the diagnosis of disease or routine health check; and second, without symptoms and signs of disease patients would rarely be treated, unless an aberrant profile (as yet undefined) puts them at risk of another disease. This of course assumes that clinical checkups occur (not the case in most developing countries)15 and that they are informative. In a recent study, a small proportion of women reported vaginal malodor (8%) but 42% had odor when a clinician used the 10% KOH test (Boon et al, unpublished data). This is part of the Amsel test first established in 1983,16 28 years after Gardnerella vaginalis was reported to be the cause of BV.17 The remainder of the assessment includes vaginal pH elevated above 4.5, the presence of “clue” cells seen under the microscope, and a thin milky vaginal discharge that is different in form from normal discharge and that associated with vulvovaginal candidiasis. However, the specificity of abnormal vaginal discharge is not higher than 49%,18 and vaginal pH in normal asymptomatic women ranges from 4.5 to 4.8 in different ethnic groups.19
Other attempts have been made to differentiate an aberrant from healthy vagina, based upon Gram staining of vaginal smears and enumerating the number of gram-positive rods, presumed to be lactobacilli, in comparison to gram-positive cocci, as well as gram-variable and gram-negative rods presumed to be anaerobic pathogens. Two scoring systems have been proposed, one by Nugent et al20 called the Nugent system, and one by Hay et al,21 which is a modification of a method first described by Speigel et al.22 The Nugent system has been the most widely adopted to diagnose BV, however, more and more problems have been identified with it in recent times. The discovery of gram-positive rod-shaped Atopobium vaginae associated with BV23 and its ability to produce lactic acid means that it could potentially sway the score toward normal when in fact the patient is not colonized with lactobacilli. It should be stated that health and susceptibility to disease are 2 different things; in the latter case, a woman may have a stable, nonlactobacilli microbiota which for her is asymptomatic and “healthy,” but increases her risk of sexually transmitted infections.
These diagnostic systems do not take into account abundant aerobic organisms that account for over 10% of all aberrant samples.13 In this case, the diagnosis should be AV not BV. Furthermore, concerns over the fields of view that are analyzed under the Gram stain methods and the difficulty in reproducibility between microscopes, especially for intermediate scoring, have been raised.24
In short, the most often used methods of diagnosing BV, the Amsel and Nugent scores, are often not in agreement with each other or accurate, nor do they correspond in any significant way with the self-reported symptoms.25,26
What are the Bacteria Doing?
To date, our understanding of what the bacteria do in the vagina is quite primitive and simplistic. The finding that most lactobacilli strains isolated from the vagina produce hydrogen peroxide (H2O2),27 and this defense against pathogens28 is flawed for several reasons. First, the ability of bacteria to produce this compound in a laboratory setting under aerobic conditions does not mean it can do this in a mostly anaerobic vaginal environment. Second, it is these H2O2 strains that are displaced when BV occurs,1 suggesting the protection offered by this compound is ineffective even in allowing the lactobacilli to outcompete the pathogens. Third, the species most adapted to survival in the vagina, L iners, produces little or no H2O2; thus, from an ecological point of view, H2O2 does not appear to provide a major advantage to lactobacilli.
On the other hand, H2O2-producing species, such as L crispatus are clearly dominant in some healthy women.1–3 Is this because the species or strains have certain properties different from less commonly found types like L reuteri? This has not been determined in full; but hypothesizing that adhesins could be important for persistent colonization, a comparison of known genomes has not revealed any major properties found exclusively in L crispatus (Table 1).
Table 1.
A comparison of known genomes of vaginal lactobacilli with S. aureus a
| # proteins in each organism containing adhesin-related domains | |||||||
|---|---|---|---|---|---|---|---|
| Pfam ID | Annotation | L. reuteri RC-14 | L. jensenii 1153 | L. rhamnosus GR-1 | L. crispatus ST1 | S. aureus Newman | |
| General adhesion | PF01468 | GA module | 2 | 2 | |||
| PF02216 | B domain | 2 | |||||
| PF03642 | MAP domain | 3 | |||||
| PF07501 | G5 domain | 1 | |||||
| PF01473 | Putative cell wall–binding repeat | 1 | |||||
| Ig | PF02368 | Bacterial Ig-like domain (group 2) | |||||
| PF07523 | Bacterial Ig-like domain (group 3) | 1 | 3 | 3 | 1 | ||
| Mucus | PF06458 | MucBP domain | 3 | 8 | 1 | 4 | |
| Collagen | PF05738 | Cna protein B-type domain | 2 | 5 | 3 | ||
| PF05737 | Collagen binding domain | ||||||
| PF01391 | Collagen triple helix repeat (20 copies) | 1 | 3 | ||||
| PF02352 | Decorin binding protein | 1 | |||||
| Fibronectin /fibrinogen | PF02986 | Fibronectin binding repeat | |||||
| PF05833 | Fibronectin-binding protein A N-terminus (FbpA) | 1 | 1 | 1 | 1 | ||
| PF10425 | C-terminus of bacterial fibrinogen-binding adhesin | 1 | 7 | ||||
| Peptidoglycan | PF01471 | Putative peptidoglycan binding domain | 1 | 2 | 1 | ||
| PF01476 | LysM domain | 9 | 1 | 4 | 1 | 5 | |
| Other | PF07554 | Uncharacterized sugar-binding domain | 1 | 4 | 1 | 1 | 2 |
| PF07564 | Domain of unknown function (DUF1542) | 1 | 3 | ||||
| PF08428 | Rib/alpha-like repeat | 2 | 5 | ||||
| PF00041 | Fibronectin type III domain | 1 | |||||
| PF03217 | Bacterial surface layer protein | 11 | |||||
| Cell wall anchor | PF00746 | Gram positive anchor | 5 | 6 | 5 | 4 | 13 |
| PF04650 | YSIRK type signal peptide | 1 | 7 | 5 | 17 | ||
a Using Pfam predictions at an e-value cutoff of 1E-3.
A study of the genome of L iners, the Lactobacillus most universally found in the vagina,29 including when patients have BV or are treated with antibiotics,1 has revealed specialized adaptation mechanisms such as an iron–sulfur cluster assembly system, several unique σ factors to regulate gene transcription in the vagina, and a highly expressed homolog of a cholesterol-dependent cytolysin (CDC); all may contribute to persistence.27 This species upregulates carbohydrate utilization genes during BV, as revealed by transcriptomic analysis (Macklaim et al, unpublished data), again illustrating its unique ecological and evolutionary adaptation to the vaginal environment. It remains to be determined whether all L iners, or just certain strains, use the CDC which is also significantly upregulated in BV (Macklaim et al, unpublished data) to extract nutrients from epithelial cells and thereby play a role in the onset or recovery from BV, or if this system simply takes advantage of an environment created by the dense BV pathogen biofilms on the epithelial cells. The difference in the function of L iners in a BV environment compared to a healthy vagina highlight our need to understand the conditional response and activity of the vaginal bacteria and not just their presence or abundance.
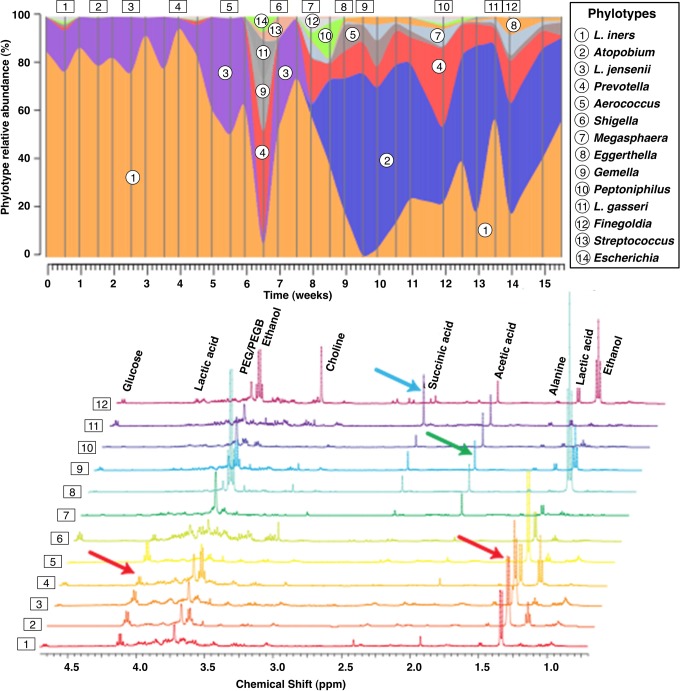
Preliminary metabolomic studies of vaginal samples have confirmed the presence of succinate and butyrate, products of anaerobic metabolism but not by L iners (McMillan et al, unpublished data; Ravel et al, unpublished data). This succinate peak also arises in patients who move from a normal to an Atopobium-donimated BV microbiota (Figure 1), again suggesting it is due to bacterial metabolism. Spear’s group30 have quantified the immunomodulatory short chain fatty acids and shown increased levels of acetate, propionate, and butyrate corresponding to increased anaerobes. Meta-transcriptomic analysis has revealed that the genes necessary for the production of these metabolites exist, and are active, in the organisms classically associated with BV (Macklaim et al, unpublished data).
Figure 1.
Courtesy of Dr. J. Ravel.6
The manner in which the host responds to the vaginal microbiota has been examined using human gene arrays in 3 studies. In premenopausal woman, the administration of L rhamnosus GR-1 to the vagina resulted in the upregulation of some antimicrobial genes.31 On the contrary, studies of women with BV have shown impairment in cervicovaginal immune responses; and for postmenopausal women with vaginal dryness, there was also downregulation of epithelial integrity genes.13,32,33 Such epithelial damage may be visible upon genital examination, and one such study found an increased prevalence in women with BV.34
Toll-like receptor 4 is stimulated, presumably by gram-negative anaerobes, in BV,30 and this may play a role in bacterial binding and stimulation of interleukin (IL)-1b, but it is in AV that IL-1b and IL-6 are most significantly stimulated.13 Higher levels of proinflammatory cytokines in cervicovaginal fluid may affect local HIV replication and increase the risk of acquisition or transmission of HIV35 by attracting CD4 cells to the mucosa. Interestingly, glycogen accumulation in the epithelial cells is down during BV events, for unknown reasons, and as this is believed to be a key substrate for Lactobacillus, it may in part explain that genera’s decline.40 Supporting this hypothesis, studies of the vaginal microbiota of macaques have revealed lower glycogen levels than in humans and a correspondingly more “BV-like” microbiota with fewer lactobacilli.36
Another intriguing issue is the role that organisms such as G vaginalis have in the vagina, since they appear to be present in almost all women. It seems increasingly clear that there is more than 1 G vaginalis, and indeed 4 clones at least have been discovered.37 The genus Gardnerella comprises a single species, G vaginalis, and a distinct clade within the Bifidobacteraceae family. A genomic comparison of 2 G vaginalis strains isolated from BV patients with almost identical 16S rDNA, a strain sharing only 98% 16S rDNA identity from a healthy woman, noted significant differences,38 and supported the concept that detection of virulence expression should accompany detection of G vaginalis in the vagina, in order to know whether it is involved in infection. Whether the difference between being commensal and pathogenic related to adhesion to vaginal epithelial cells and cytotoxicity remains to be tested in vivo but is a possibility.39
What Do Probiotics Do?
As listed in Table 2, significant advances have been made in our understanding of probiotic lactobacilli and their potential to improve vaginal health. Some of the findings, such as upregulation of barrier function of the epithelium have been shown in intestinal epithelial models,40 human amnion cells (Koscik et al, unpublished data), immortalized vaginal epithelial cells (http://www.ncbi.nlm.nih.gov/pubmed/22072832), and gene microarray studies (G. Reid, personal communication, April 20, 2012). While numerous products claim to be for vaginal health, 3 strains, L rhamnosus GR-1, L reuteri RC-14, and L crispatus CTV05 have been well studied in humans. The decision to select these strains is quite different: for the GR-1 and RC-14 combination, the rationale was that the organisms should be able to interfere with gram-positive and gram-negative pathogen growth and encourage restoration of the indigenous lactobacilli, while the CTV05 was selected because the species is commonly found in the vagina and produces large quantities of H2O2. Many properties of the GR-1/RC-14 strains have been investigated and recently summarized in a review41: these include their ability to modulate host defences,31 produce antiadhesion and antivirulence factors,42 and disrupt urogenital pathogen biofilms.43 In human studies, the strains administered orally have been shown to reach the vagina, reduce pathogen ascension into the vagina, reduce recurrences of BV, prevent recurrences of UTI, enhance antimicrobial cure of BV and vulvovaginal candidosis, and shift the microbiota to be dominated by Lactobacillus sp.41,44,45
Table 2.
What Can Probiotics Do?
|
|
|
|
|
|
Phase 1 and 2 studies using an intravaginal drug containing L crispatus CTV05 have shown that when the organism persists for 28 days, number of pathogens G vaginalis, A vaginae, Megasphaera sp, Leptotrichia/Sneathia spp, and BVAB2 falls significantly.46 Another pilot study showed higher colonization was associated with reduced recurrences of UTI.47 However, when the organism does not persist, likely because indigenous L crispatus are present, or antimicrobial therapy has failed to alter uropathogen counts, or there has been exposure to semen,48 the effect on pathogens is marginal at best.
Another strain, L acidophilus KS400 has been used in conjunction with 0.03 mg estriol for local application to the vagina and shown to help restoration of lactobacilli after antibiotic treatment, as well as provide beneficial short-term effects on symptomatic BV.49–51 In some of these effects, it is not clear how much is contributed by the addition of the small dosage of estriol.
In other studies, lactobacilli have been tested to deliver microbicides for the prevention of HIV spread through intercourse. Various concepts have been explored using L reuteri RC-14 expressing CD4D1D2-antibody-like fusion proteins,52 and L jensenii 1153 expressing anti-HIV-1 chemokine RANTES, and mutated analogue C1C5 RANTES.53 In addition, microbicides such as VivaGel (3% w/w SPL7013 in Carbopol-based aqueous gel), a potent inhibitor of HSV-2 and HIV has been tested, but it induced vulvar and cervical erythema, cervical lesions, symptomatic BV, urinary frequency, and metrorrhagia.54 As BV is a risk factor for HIV, rather than use microbicides that are toxic to the epithelium, the administration of lactobacilli and extended periods of maintenance of microbial homeostasis might also be worth pursuing to prevent HIV. Whether probiotics can also decrease the risk of HIV in women infected with human papilloma virus (HPV), itself a risk factor (new ref 55) remains to be seen, but one study suggests that HPV positive women treated with L. rhamnosus GR-1 and L. reuteri RC-14 have lower incidence of BV (new ref 56). The potential for long-term maintenance of a lactobacilli-dominated vagina is being explored with L crispatus CTV05.55
The Road Map
The plight of women with urogenital disease is massive and major efforts are needed to address this burden. The following is a road map that identifies 3 areas in need of urgent attention: (1) improvement in education of women and health care practitioners on the vaginal microbiota; (2) targeted funding for research to improve diagnosis and test new therapies; and (3) ensure that new approaches are accessible in developing countries and empowering to women, as well as being acceptable and appropriate for different populations.
Education
Educational programs accessible to as many females as possible (by necessity in different languages) are needed to explain urogenital diseases, the natural role that microbes play in maintaining health, and hygienic practices that can help or hinder maintenance of microbial homeostasis. This should differentiate pain, discomfort, and signs such as reddening and discharge from UTI versus vaginal infections by viruses, yeast, and bacteria. Knowledge is the first stage of empowerment, and for many women simple anatomy and issues that arise through the menstrual cycle have never been adequately explained. Such education must avoid any perception of guilt for such conditions.
For physicians, there is an urgent need to update their knowledge of the vaginal microbiome, the different profiles associated with health and with disease, the many limitations of current diagnostic methodologies, the narrow efficacy and poor specificity of antimicrobial therapies, the expectation of recurrences and the burden that lower urogenital symptoms place on well-being and quality of life.56 In addition, there needs to be a consensus drawn on what tests can be done in different settings (private practice, clinics, hospitals, rural areas), what information can be gained from each, and how to interpret the findings. When symptoms are reported, on-site wet mount microscopy is arguably the most revealing in diagnosing AV, BV, or yeast infection and a normal condition,14 and, contrary to common perception, the learning curve of fresh wet mount microscopy is relatively short, provided proper training is administered (Donders et al, submitted for publication). Requesting cultures is unlikely to be revealing except for detection of Trichomonas and the confirmation of Candida in patients suspected of recurrent vulvovaginal candidosis but with negative microscopy. In all other cases, just the isolation of yeast does not necessarily equate to infection. Hopefully, with increased education, physicians seeing women with BV and AV will be able to consider the use of clinically documented probiotics as a therapeutic adjunct.
New Diagnostics and Therapies
Now that there is a better understanding of the types of bacteria present in the vagina, the next steps are to identify the other classes of organisms (viruses, fungi, and protozoa), more fully the environmental metabolic outputs (the metabolome), as well as how the organisms and host function over time. Such research studies are critical if novel approaches to therapy are to be created, and funding for such fundamental science will only be forthcoming if government and philanthropic agencies begin to appreciate the seriousness of urogenital disease and its complications. Such studies can identify longitudinal microbial profiles that correlate with highest risk of disease and its complications (eg, preterm labor, cervical cancer) and how the host can sometimes self-modulate these profiles back to normal. By identifying subgroups of women, it becomes possible to improve responder rates and stratify participants in longitudinal studies.
The potential benefit of probiotic and prebiotic intervention is good, but studies are required to understand how they work and in whom. It may be that different probiotics are required for different microbiota profiles that are clinically deemed to be healthy. Also, in some cases, addition of small amounts of substrate-enhancing products like estriol may improve the effect of probiotic action on vaginal health.
In all clinical samples, the better defined the patient and the more samples that can be obtained, the better will be the data that are acquired. Standardized protocols for reading diagnostic methods (eg, differentiating Atopobium from Lactobacillus gram-positive rods; numbers of fields of view evaluated, or number of epithelial cells examined) would be helpful across centers, with samples appropriately stored for later analysis, for example on the metabolome.
Empowerment
Much can be learned from resource-disadvantaged settings, for example in Africa. There, interventions must be thoroughly explained prior to their introduction and use and an appreciation and respect given to local community and their cultural ways. Thus, for example, women in Western Europe, United States, and Canada might be fully able to collect their own vaginal swabs and deliver it to the laboratory, while women in rural Africa may not be familiar with their urogenital area and be uncomfortable with even a doctor taking a vaginal sample. Some women are very interested in self-testing for vaginal microbiota abnormalities, and positive toward understanding the risks of abnormal test results and accepting treatment to correct this.57 Such differences between people are not confined to African countries and need to be considered in all cases. Sensitivity to cost is also required, and arguably this is becoming more and more relevant in North America where companies charge disproportionately higher fees for drugs and diagnostics, yet more of the population is facing economic challenges.
The ideal interventions are those which any woman can use with ease and comfort while maintaining privacy. These should allow easy interpretation of findings along with an explanation of what any given result means. Thus, if a diagnostic kit accurately identified a pregnant woman at risk of preterm delivery because of microbiota and host changes, what does the recipient of this information need to do next? It might be obvious to visit a doctor, but if they are far away, or unavailable, or the person has no money to go there, what are the consequences for the person and her baby? Are there things she can do to reduce the risk of miscarriage? An advantage of a probiotic yogurt produced locally at a very low cost, and for example shown to improve vaginal microbiome and immune status, is that a woman can gain easy access to it and is thereby empowered. Too often, expensive products are tested on poverty-stricken participants, then after the trial the participants cannot afford to buy them or the products are not available. In the future, neglecting the women who need treatment the most must be regarded as being unacceptable. Likewise, not seeking their input into such interventions makes the likelihood of implementation diminished. The successful integration of cervical cancer prevention in HIV/AIDS treatment and care programs in Zambia58 demonstrates that novel therapies can be transferred to the betterment of women around the world.
Having made these points, it is clear that money drives product development, and industrial partnerships will be needed to create novel and better diagnostic systems and drugs, as well as medical foods and natural health products that are effective. For now, drugs such as metronidazole continue to be used in most cases to treat symptomatic BV, even if it was never created to treat the organisms causing the disease.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no conflict of interest with respect to the research, authorship, and/or publication of this article but declare GD is a consultant for Medinova and Alfa Wassermann, and GR receives funding from Danone and Kimberly Clark.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The support of the International Scientific Association for Probiotics and Prebiotics is acknowledged in making it possible for the authors to convene and discuss these issues.
References
- 1. Hummelen R, Fernandes AD, Macklaim JM, et al. Deep sequencing of the vaginal microbiota of women with HIV. PLoS One. 2010;5(8):e12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(suppl 1):4680–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oakley BB, Fiedler TL, Marrazzo JM, Fredricks DN. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl Environ Microbiol. 2008;74(15):4898–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill JE, Goh SH, Money DM, et al. Characterization of vaginal microflora of healthy, nonpregnant women by chaperonin-60 sequence-based methods. Am J Obstet Gynecol. 2005;193(3 pt 1):682–692 [DOI] [PubMed] [Google Scholar]
- 5. Kim TK, Thomas SM, Ho M, et al. Heterogeneity of vaginal microbial communities within individuals. J Clin Microbiol. 2009;47(4):1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gajer P, Brotman RM, Bai G, Sakamoto J, SchÜtte UME, Zhong X, Koenig SSK, Fu L, Ma Z, Zhou X, Abdo Z, Forney LJ, Ravel J. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4, 132ra52 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou X, Brotman RM, Gajer P, et al. Recent advances in understanding the microbiology of the female reproductive tract and the causes of premature birth. Infect Dis Obstet Gynecol. 2010;2010:737425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol. 2001;32(1):37–41 [DOI] [PubMed] [Google Scholar]
- 9. Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86(4):297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reid G, Zorzitto ML, Bruce AW, Jewett MAS, Chan RCY, Costerton JW. The pathogenesis of urinary tract infection in the elderly: the role of bacterial adherence to uroepithelial cells. Curr Microbiol. 1984;11(2):67–72 [Google Scholar]
- 11. Nicolle LE, Zhanel GG, Harding GK. Microbiological outcomes in women with diabetes and untreated asymptomatic bacteriuria. World J Urol. 2006;24(1):61–65 [DOI] [PubMed] [Google Scholar]
- 12. Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–1911 [DOI] [PubMed] [Google Scholar]
- 13. Donders GG, Vereecken A, Bosmans E, et al. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. 2002;109(1):34–43 [DOI] [PubMed] [Google Scholar]
- 14. Donders GG. Microscopy of the bacterial flora on fresh vaginal smears. Infect Dis Obstet Gynecol. 1999;7(4):177–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Cervical Cancer Screening in Developing Countries Geneva, Switzerland: World Health Organization; 2002 [Google Scholar]
- 16. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22 [DOI] [PubMed] [Google Scholar]
- 17. Gardner HL, Dukes CD. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol. 1955;69(5):962–976 [PubMed] [Google Scholar]
- 18. Sodhani P, Garg S, Bhalla P, Singh MM, Sharma S, Gupta S. Prevalence of bacterial vaginosis in a community setting and role of the pap smear in its detection. Acta Cytol. 2005;49(6):634–638 [DOI] [PubMed] [Google Scholar]
- 19. Fiscella K, Klebanoff MA. Are racial differences in vaginal pH explained by vaginal flora? Am J Obstet Gynecol. 2004;191(3):747–750 [DOI] [PubMed] [Google Scholar]
- 20. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ. 1994;308(6924):295–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spiegel CA, Amsel R, Holmes KK. Diagnosis of bacterial vaginosis by direct Gram stain of vaginal fluid. J Clin Microbiol. 1983;18(1):170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burton JP, Devillard E, Cadieux PA, Hammond JA, Reid G. Detection of Atopobium vaginae in post menopausal women by cultivation-independent methods warrants further investigation. J Clin Microbiol. 2004;42(4):1829–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larsson PG, Carlsson B, Fåhraeus L, Jakobsson T, Forsum U. Diagnosis of bacterial vaginosis: need for validation of microscopic image area used for scoring bacterial morphotypes. Sex Transm Infect. 2004;80(1):63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaijareenont K, Sirimai K, Boriboonhirunsarn D, Kiriwat O. Accuracy of Nugent's score and each Amsel's criteria in the diagnosis of bacterial vaginosis. J Med Assoc Thai. 2004;87(11):1270–1274 [PubMed] [Google Scholar]
- 26. Gallo MF, Jamieson DJ, Cu-Uvin S, Rompalo A, Klein RS, Sobel JD. Accuracy of clinical diagnosis of bacterial vaginosis by human immunodeficiency virus infection status. Sex Transm Dis. 2011;38(4):270–274 [DOI] [PubMed] [Google Scholar]
- 27. Reid G, McGroarty JA, Tomeczek L, Bruce AW. Identification and plasmid profiles of Lactobacillus species from the vagina of 100 healthy women. FEMS Immunol Med Microbiol. 1996;15(1):23–26 [DOI] [PubMed] [Google Scholar]
- 28. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174(5):1058–1063 [DOI] [PubMed] [Google Scholar]
- 29. Macklaim J, Gloor GB, Anukam KC, Cribby S, Reid G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners . Proc Nat Acad Sci USA. 2011;108(suppl 1):4688–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mirmonsef P, Zariffard MR, Gilbert D, et al. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with Toll-like receptor ligands. Am J Reprod Immunol. 2011. doi: 10.1111/j.1600-0897.2011.01089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirjavainen PK, Laine RM, Carter D, Hammond JA, Reid G. Expression of anti-microbial defense factors in vaginal mucosa following exposure to Lactobacillus rhamnosus GR-1. Int J Probiotics. 2008;3:99–106 [Google Scholar]
- 32. Cauci S, Guaschino S, De AD, et al. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003;9(1):53–58 [DOI] [PubMed] [Google Scholar]
- 33. Dahn A, Saunders S, Anukam KC, et al. Vaginal gene expression changes and Lactobacillus presence in women treated with oral Premarin estrogen replacement therapy. Microbes Infect. 2008;10(6):620–627 [DOI] [PubMed] [Google Scholar]
- 34. Hummelen R, Macklaim JM, Bisanz JE, et al. Vaginal microbiome diversity and epithelial cell changes in post-menopausal women with dryness and atrophy. PLoS One. 2011;6(11):e26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spear GT, Gilbert D, Sikaroodi M, Doyle L, Green L, Gillevet PM, Landay AL, Veazey RS. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res Hum Retroviruses. 2010 Feb;26(2):193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schellenberg J.J., Links M.G., Hill J.E., et al. Molecular definition of vaginal microbiota in East African commercial sex workers. Appl. Environ. Microbiol. 2011; 77(12):4066–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeoman CJ, Yildirim S, Thomas SM, et al. Comparative genomics of Gardnerella vaginalis strains reveals substantial differences in metabolic and virulence potential. PLoS One. 2010;5(8):e12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harwich MD, Jr, , Alves JM, Buck GA, et al. Drawing the line between commensal and pathogenic Gardnerella vaginalis through genome analysis and virulence studies. BMC Genomics. 2010;11:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, Spear GT. A comparison of lower genital tract glycogen and lactic acid levels in women and Macaques: implications for HIV and SIV susceptibility. AIDS Res Hum Retroviruses. 2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacPhee RA, Hummelen R, Bisanz JE, Miller WL, Reid G. Probiotic strategies for the treatment and prevention of bacterial vaginosis. Expert Opin Pharmacother. 2010;11(18):2985–2995 [DOI] [PubMed] [Google Scholar]
- 41. Li J, Wang W, Xu SX, et al. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc Natl Acad Sci USA. 2011;108(8):3360–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McMillan A, Dell M, Zellar MP, et al. Disruption of urogenital biofilms by lactobacilli. Colloids Surf B Biointerfaces. 2011;86(1):58–64 [DOI] [PubMed] [Google Scholar]
- 43. Hummelen R, Changalucha J, Butamanya NL, Cook A, Habbema JDF, Reid G. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 to prevent or cure bacterial vaginosis among women with HIV. Int J Gynecol Obstet. 2010;111(3):245–248 [DOI] [PubMed] [Google Scholar]
- 44. Petricevic L, Unger FM, Viernstein H, Kiss H. Randomized, double-blind, placebo-controlled study of oral lactobacilli to improve the vaginal flora of postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2008;141(1):54–57 [DOI] [PubMed] [Google Scholar]
- 45. Ngugi BM, Hemmerling A, Bukusi EA, et al. Effects of bacterial vaginosis-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex Transm Dis. 2011;38(11):1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Antonio MA, Meyn LA, Murray PJ, Busse B, Hillier SL. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous lactobacilli. J Infect Dis. 2009;199(10):1506–1513 [DOI] [PubMed] [Google Scholar]
- 48. Ozkinay E, Terek MC, Yayci M, Kaiser R, Grob P, Tuncay G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG. 2005;112(2):234–240 [DOI] [PubMed] [Google Scholar]
- 49. Donders GG, Van BB, Van de Walle P, et al. Effect of lyophilized lactobacilli and 0.03 mg estriol (Gynoflor(R)) on vaginitis and vaginosis with disrupted vaginal microflora: a multicenter, randomized, single-blind, active-controlled pilot study. Gynecol Obstet Invest. 2010;70(4):264–272 [DOI] [PubMed] [Google Scholar]
- 50. Parent D, Bossens M, Bayot D, et al. Therapy of bacterial vaginosis using exogenously-applied Lactobacilli acidophili and a low dose of estriol: a placebo-controlled multicentric clinical trial. Arzneimittelforschung. 1996;46(1):68–73 [PubMed] [Google Scholar]
- 51. Liu JJ, Reid G, Jiang Y, Turner MS, Tsai CC. Activity of HIV entry and fusion inhibitors expressed by the human vaginal colonizing probiotic Lactobacillus reuteri RC-14. Cell Microbiol. 2007;9(1):120–130 [DOI] [PubMed] [Google Scholar]
- 52. Vangelista L, Secchi M, Liu X, et al. Engineering of Lactobacillus jensenii to secrete RANTES and a CCR5 antagonist analogue as live HIV-1 blockers. Antimicrob Agents Chemother. 2010;54(7):2994–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cohen CR, Brown J, Moscicki AB, et al. A phase I randomized placebo controlled trial of the safety of 3% SPL7013 Gel (VivaGel®) in healthy young women administered twice daily for 14 days. PLoS One. 2011;6(1):e16258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veldhuijzen NJ, Vyankandondera J, van de Wijgert JH. HIV acquisition is associated with prior high-risk human papillomavirus infection among high-risk women in Rwanda. AIDS. 2010;24(14):2289–92 [DOI] [PubMed] [Google Scholar]
- 55. Perisić Z, Perisić N, Golocorbin Kon S, et al. The influence of probiotics on the cervical malignancy diagnostics quality. Vojnosanit Pregl. 2011;68(11):956–60 [PubMed] [Google Scholar]
- 56. Hemmerling A, Harrison W, Schroeder A, et al. Phase 1 dose-ranging safety trial of Lactobacillus crispatus CTV-05 for the prevention of bacterial vaginosis. Sex Transm Dis. 2009;36(9):564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liao YM, Yang CY, Kao CC, et al. Prevalence and impact on quality of life of lower urinary tract symptoms among a sample of employed women in Taipei: a questionnaire survey. Int J Nurs Stud. 2009;46(5):633–644 [DOI] [PubMed] [Google Scholar]
- 58. Mwanahamuntu MH, Sahasrabuddhe VV, Stringer JS, Parham GP. Integrating cervical cancer prevention in HIV/AIDS treatment and care programmes. Bull World Health Organ. 2008;86(8):D–E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mwanahamuntu MH, Sahasrabuddhe VV, Kapambwe S, et al. Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia. PLoS Med. 2011;8(5):e1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]