Abstract
Phospholipid scramblases (PLSCR), stimulated by proinflammatory cytokines, are thought to mediate the loss of lipid asymmetry in cell membranes, allowing for specific reactions in the coagulation cascade. The PLSCR may therefore provide a link between inflammation, coagulation, and, because thrombin is a uterotonic, preterm birth (PTB). To explore the relationship between PLSCR expression and inflammation-related PTB, we utilized reverse transcriptase–polymerase chain reaction and Western blot studies to quantify messenger RNA (mRNA) and protein expression for the 4 PLSCR homologues (PLSCR 1-4). Uteri from day 15 pregnant mice were harvested at several time points after intrauterine lipopolysaccharide (LPS) injection (or normal saline, for controls). Expression of mRNA in all 4 Plscr isoforms was demonstrated. Lipopolysaccharide treatment resulted in increased expression of PLSCR-1 and a decrease in Plscr4 mRNA, thereby demonstrating modulation of PLSCR-1 and PLSCR-4 in LPS-induced PTB. Additionally, protein expression was confirmed for all except PLSCR-4, with increased expression of PLSCR-1 after LPS treatment.
Keywords: inflammation, phospholipid, pregnant mouse, preterm birth, scramblase
Introduction
The plasma membrane of mammalian cells demonstrates asymmetrical distribution of membrane phospholipids, with the choline-containing phospholipids phosphatidyl choline (PC) and sphingomyelin dominating the outer leaflet, and the negatively charged aminophospholipids phosphatidyl ethanolamine (PE) and phosphatidyl serine (PS) dominating the inner leaflet.1 Elimination of this lipid bilayer asymmetry is an important physiological process and appears to occur as a result of elevated intracellular calcium concentration, as can occur with cell activation, injury, and apoptosis.2
Phospholipid scramblases (PLSCRs), comprised of a family of 4 homologous proteins (PLSCR 1-4), are thought to mediate this loss of lipid asymmetry. The PLSCRs are products of apparently highly conserved genes, from Caenorhabditis elegans to humans.3 Human PLSCR was first cloned and sequenced by Zhou et al in 1997.4 In 2000, Wiedmer et al demonstrated that all 4 isoforms, PLSCRs 1-4, are differentially expressed in both mouse and human tissues.5 Our laboratory previously used real-time polymerase chain reaction (RT-PCR) studies to confirm the expression of messenger RNA (mRNA) for PLSCR-3 and PLSCR-4 in both the endometrial and myometrial layers of the near term, pregnant rat uterus. Further, complementary DNA (cDNA) and amino acid sequencing studies showed PLSCR isoforms expressed in rat uterine tissue are close homologues to the isoforms expressed in both mouse and human tissues.
One of the more critical effects of the loss of lipid asymmetry at the plasma membrane is the exposure of PS at the cell surface. PS is normally excluded from the external leaflet of the lipid bilayer, and the surface-exposed PS acts as a platform for specific reactions in the coagulation cascade.1 Because PLSCR expression has been shown to be stimulated by proinflammatory cytokines, PLSCR may provide a link between inflammation and activation of the coagulation cascade. The demonstration of such a link would be particularly instructive in the setting of preterm labor. Thrombin has been shown both in vitro and in vivo to be a potent uterotonic agonist.6–9 As such, PLSCR activity in the pregnant uterus, through its stimulation of the coagulation system and its transcriptional regulation by proinflammatory cytokines, provides a promising potential mechanism for preterm labor in the setting of intrauterine infection.
The current study was undertaken to explore the possible relationship between PLSCR expression and inflammation-related preterm birth (PTB). Using a mouse model, we evaluated the modulation of PLSCR expression in the pregnant mouse uterus during lipopolysaccharide (LPS)-induced preterm delivery. Both mRNA and protein expression for the PLSCR isoforms were investigated.
Materials and Methods
These studies were performed using tissues harvested from timed-pregnant CD-1 mice utilized in an ongoing series of studies to characterize a LPS-induced preterm delivery mouse model (ie, with over 100 intrauterine LPS-treated mice to date). Mice were purchased from Charles River Laboratories (Wilmington, Massachusetts). The animals was shipped on day 9 after mating and acclimated in the University of Vermont’s animal care facility for several days before undergoing surgery on day 15 of gestation. LPS injections were performed on day 15 of gestation in an effort to produce an experimental model that recapitulates preterm delivery at 80% of gestation in human pregnancies (ie, at about 32 weeks of gestation). All experiments were performed in accordance with the National Institutes of Health guidelines for laboratory animals and had approval by the Institutional Animal Care and Utilization Committee at the University of Vermont. After induction of isoflurane anesthesia, a laparotomy was performed using sterile technique, at which time the right uterine horn was exposed. An intrauterine injection of LPS dosed at 250 μg or an equal volume of sterile saline (ie, 100 μL) was given between gestational sacs 2 and 3 in the right uterine horn. At the completion of surgery, mice received 1 dose of buprenorphine (50 mg/kg) analgesic subcutaneously and were allowed to recover. Subsequently, the mice were euthanized at 2, 6, 12, 18, and 24 hours after LPS injection using isoflurane anesthesia and a lethal sodium pentobarbital injection (200 mg/kg intraperitoneally). The time-0 (0 hour) control mice were euthanized without undergoing surgery or intrauterine injection.
Additional uteri were collected from untreated nonpregnant and timed-pregnant mice to evaluate PLSCR expression throughout gestation. Specifically, uterine tissue was harvested from normally cycling, nonpregnant mice, pregnant mice on gestational days 7, 12, 15, and 18, and pregnant mice on postpartum day 1. The uteri were harvested from 3 animals in each group for a total of 18 mice for these studies.
The uterine tissues from the pregnant and nonpregnant mice were then processed. Samples for protein assays were rinsed in normal saline and then immediately frozen in liquid nitrogen and stored at −80°C. Uterine tissue from the right uterine horn was typically designated for protein analysis, while the left uterine horn was used for RNA analysis. Based on a previous report, a comparable inflammatory response is anticipated in both horns of the pregnant mouse uterus.10 Samples for RNA assays were rinsed in normal saline, then placed in RNAlater (Ambion Inc, Austin, Texas), and stored at 4°C for approximately 1 week. The tissue samples were then removed from RNAlater and stored at −80°C.
Reverse transcriptase PCR techniques
Total RNA was extracted from maternal mouse uterus, liver, lung, and kidney tissue using Trizol reagent (Invitrogen, Carlsbad, California). Subsequently, genomic DNA was removed from samples using TURBO DNA free (Ambion Inc). The RNA concentrations were determined using a NanoDrop spectrophotometer (NanoDrop Inc, Wilmington, Delaware). Intact total RNA was confirmed by the analysis of the 18 S and 28 S band patterns after formaldehyde–agarose gel electrophoresis.
Real-time quantitative RT-PCR (qRT-PCR) was performed on an ABI Prism 7000 Sequence Detection System using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, California). For each sample, the cDNA was generated as described previously and used to amplify the 4 mouse PLSCR isoforms. See Table 1 for mouse PLSCR isoform sense and antisense primer sequences. Reactions were performed in triplicate for each sample. Following the qRT-PCR runs, standard curves generated for each primer set were used to calculate the relative quantities expressed as “relative intensity” of isoform expression Three constitutively expressed genes (β-2 macroglobulin, Ywhaz and Hprt) were used to normalize the target gene quantities Negative (water) controls were run for each primer set in the qRT-PCR reaction to ensure the reagents were not contaminated and that no secondary primer structures were amplified. Specific sense and antisense primers used in RT-PCR and qRT-PCR runs were designed to span at least 1 exon–exon junction (see Table 1). The PCR amplicons for all genes were sequence verified by the University of Vermont’s DNA Analysis Core using an AGI 3130x1 Genetic Analyzer (16 Capillary; Applied Biosystems).
Table 1.
Primer Sequences for PLSCR Isoforms 1-4
| Gene | Primer Sequence | Amplicon Length | NCBI Accession Number |
|---|---|---|---|
| PLSCR-1_forward | GCTTGGTGCTTGTTTCCTCATAGATT | 143 bp | NM_011636.2 |
| PLSCR-1_reverse | CTCAGTTTCCATTCAGGGTCCATAA | ||
| PLSCR-2_forward | CTGAGTCGCTGCTGGTGCTA | 145 bp | NM_008880.2 |
| PLSCR-2_reverse | GTCCTTGGACTGCTGCTGGA | ||
| PLSCR-3_forward | ATGCTGATCGCCAACCTGT | 97 bp | NM_023564.4 |
| PLSCR-3_reverse | GATTCATCCTTAGTCTTCACCTCAAAG | ||
| PLSCR-4_forward | GGCACCCATCCAATCCA | 180 bp | NM_178711.3 |
| PLSCR-4_reverse | CGTGTCATCAGTTCCAGAGG |
Abbreviation: PLSCR, phospholipid scramblase.
Western blot techniques
For the Western blots, mouse uterine tissues were homogenized in a protease inhibitor solution (containing 50 mmol/L Tris [pH 7.4], 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 5 μg/mL aprotinin, 3μg/mL leupeptin, 1 mmol/L sodium orthovanadate, and 1 mmol/L phenylmethylsulfonyl fluoride). The homogenate solutions were centrifuged at 800g to remove the cellular debris; and the protein concentrations of the supernatant solutions (crude tissue homogenates) were determined by BCA protein assay. Subsequently, 100 μg protein aliquots were resolved using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and the Biorad Mini-PROTEAN 3 Electrophoresis system (BioRad Laboratories, Hercules, California). The resolved proteins were then electrophoretically transferred to nitrocellulose (NC) membranes. The NC membranes were blocked with 5% powdered milk and subsequently incubated overnight in the appropriate primary antibody solution, which included monoclonal antibody for PLSCR-1 (generously provided by Dr Peter Sims, University of Rochester), monoclonal antibody for PLSCR-2 (from Sigma-Aldrich, St Louis, Missouri), monoclonal antibody for PLSCR-3 (from Novus Biologicals, LLC, Littleton, Colorado), polyclonal antibody for PLSCR-4 (from Proteintech Group Inc, Chicago, Illinois), or monoclonal antibodies for α-smooth muscle actin and for β-actin (Sigma-Aldrich). Immunodetection was performed using HRP-conjugated secondary antibodies (BioRad Laboratories) followed by incubation in Supersignal HRP Substrate (Calbiochem EMB Chem, Gibbstown, New Jersey). Visualization of the chemiluminescent protein bands was performed using the BioRad ChemiDoc XRS chemiluminescence detection system.
Immunofluorescence techniques
Mouse uterine tissue was harvested, rinsed in saline, placed in Tissue-Tek OCT Compound (Fisher Scientific), and frozen in liquid nitrogen. Frozen blocks were stored at −80°C. Tissue was sliced at 25 μm and placed on glass slides (2 sliced per slide) by the UVM Microscopy Department. After reaching room temperature, the tissue sections were fixed in 4% paraformaldehyde, then washed in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) and 0.5% Tween (PBST + BSA). The tissue was then blocked for 1 hour in MOM blocking solution containing PBST + BSA (Vector Laboratories, Burlingame, California). Subsequently, the tissue sections were incubated overnight at 4°C with monoclonal antibody for PLSCR-1, while only blocking buffer was added to negative control tissue sections. After washing in PBST + BSA, the secondary antibody (antimouse Alexafluor 555; Invitrogen) was added to the tissue sections in PBST + BSA and incubated for 1 hour. The tissue was washed again in PBST + BSA, then incubated in 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Invitrogen) for 15 minutes. Cover slips were then placed over the tissue sections using Aqua Polymount (Polysciences, Inc, Warrington, Pennsylvania), and microscopic analysis was performed using a Zeiss LSM 510 META Laser Scanning Microscope and the Zeiss LSM imaging software.
Statistical Analyses
Statistical analyses were performed using the 1-way analysis of variance (ANOVA) on ranks or the nonparametric Kruskal-Wallis ANOVA, followed by multiple comparisons tests including the Student-Newman-Keuls method, the Dunn test, or the Bonferroni test, where appropriate. Statistical significance occurred when P < .05.
Results
With the intrauterine administration of 250 µg of LPS, preterm delivery (delivery of at least one pup) was observed in the majority of pregnant mice within 24 hours following intrauterine injection. In this ongoing series of studies, 10% of mice experienced preterm delivery by 6 hours, 65% by 12 hours, and 81% to 90% by 18 to 24 hours after LPS injection. In contrast, no mice receiving intrauterine saline injections delivered by 24 hours after injection. All pups delivered were nonviable, as expected for the gestational age of 15 to 16 days.11
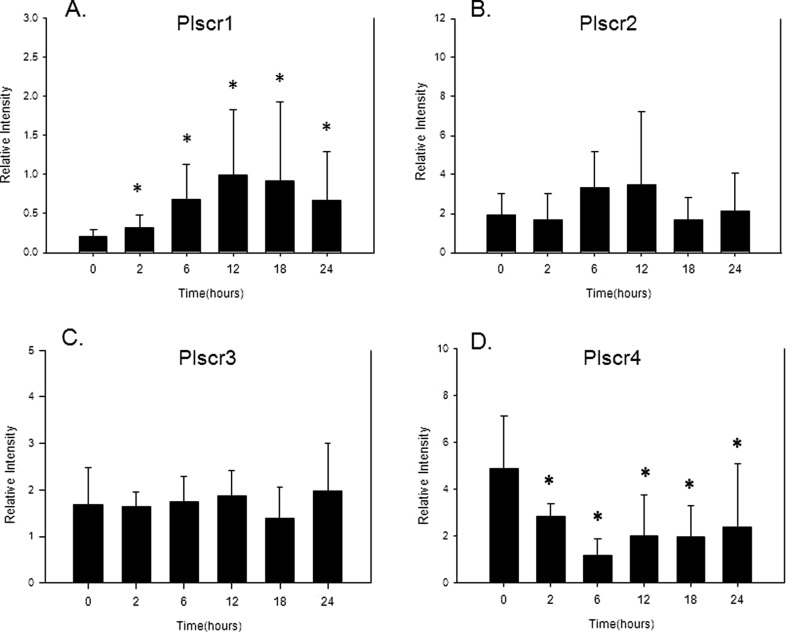
The RT-PCR studies confirmed mRNA expression for all 4 of the PLSCR isoforms in day 15 pregnant mouse uteri, as observed in Figure 1. Using multiple comparisons testing (Student-Newman-Keuls Method), PLSCR-1 mRNA expression was observed to be significantly (P < .05) increased at each time point when compared to the levels seen in the control mouse uterine tissue (time point 0). This increase in PLSCR-1 expression began at 2 hours with a 1.8-fold increase in expression and peaked at 12 hours with a 4-fold increase in response to intrauterine LPS injection (Figure 1A). In contrast, the uterine mRNA expression for the PLSCR-2 and PLSCR-3 isoforms remained stable after intrauterine LPS injection (Figure 1B and C). Interestingly, the mRNA expression for the PLSCR-4 isoform significantly decreased in response to intrauterine LPS with a maximal nadir at 6 hours after LPS injection, as observed in Figure 1D.
Figure 1.
Bar graphs demonstrating RT-PCR results in relative intensity (based on standard curves generated for each primer set) for the level of mRNA expression for PLSCR-1 (A), PLSCR-2 (B), PLSCR-3 (C), and PLSCR-4 (D) at multiple time points (ie, at 2, 6, 12, 18, and 24 hours) after intrauterine LPS injection along with time 0 untreated control mice. Data are presented as mean ± standard deviation, N = 5-7, and (*) represents P < .05 compared to time 0 control samples. RT-PCR indicates reverse transcriptase–polymerase chain reaction; mRNA, messenger RNA; PLSCR, phospholipid scramblase; LPS, lipopolysaccharide.
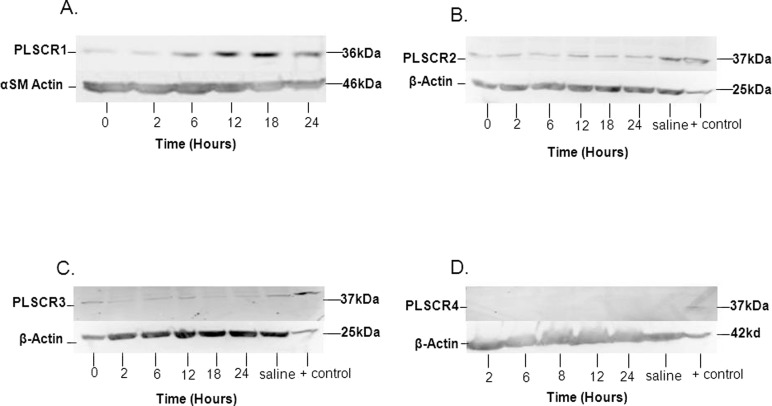
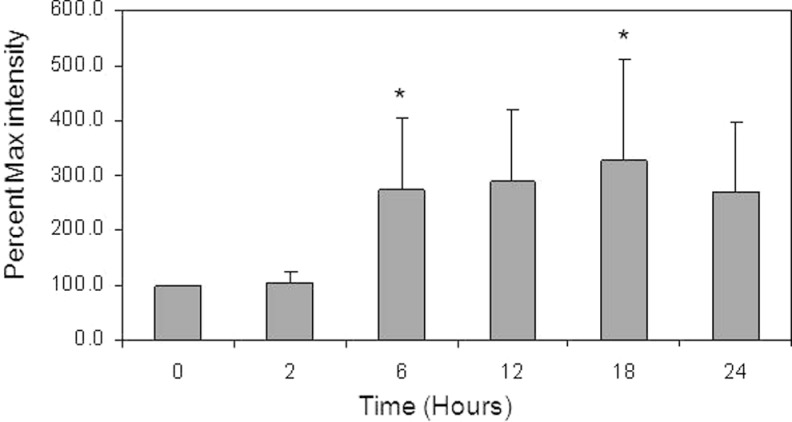
Western blot studies were performed to evaluate the expression of PLSCR protein for all 4 isoforms in mouse uterine tissue (Figure 2). As observed in Figure 2A, PLSCR-1 protein bands increased in intensity by 6 hours after LPS injection; in contrast, there was some variation in band intensity for PLSCR-2 and PLSCR-3 proteins; however, there did not appear to be a significant trend for these isoforms (Figure 2B and C). The Western blot performed for PLSCR-4 failed to demonstrate protein expression in the mouse uterine tissue (Figure 2D), despite the demonstration of PLSCR-4 protein expression in the positive control (mouse testicular tissue; Figure 2D), even when band amplification techniques were used (Qentix Western Blot Signal Enhancer, Thermo Scientific, Rockford, Illinois). Analysis of multiple blots for PLSCR-1 confirmed that the increase in PLSCR-1 protein was significant (P < .05) by 6 hours (a >2-fold increase), with a peak of about 3-fold at 18 hours after LPS intrauterine injection (Figure 3).
Figure 2.
Western blots demonstrating (A) protein expression of PLSCR-1 at time points 6, 12, 18, and 24 hours after intrauterine LPS injection; the lower panel demonstrates expression bands for α-smooth muscle actin (αSM actin), used to confirm even protein loading; (B) expression of PLSCR-2 at the same time points after intrauterine LPS injection, along with expression in a uterine sample from a saline-treated mouse and in the positive control tissue; (C) expression of PLSCR-3 in uterine tissue and the positive control tissue; and (D) expression of PLSCR-4, which was only observed in the positive control tissue. For B-D, the positive control tissue was mouse testis (Proteintech, Chicago, Illinois). The lower panels for B-D demonstrate expression bands for cellular β-actin (β-actin), used to confirm even protein loading. PLSCR indicates phospholipid scramblase; LPS, lipopolysaccharide.
Figure 3.
Bar graph demonstrating the Western blot results for PLSCR-1 protein expression in mouse uterine tissue at 2, 6, 12, 18, and 24 hours after LPS injection. Band intensity measure (percentage max intensity) is the maximal intensity as a percentage of the average control (time 0) value. A significant increase (P < .05) was observed at 6 and 18 hours after LPS when compared to the time 0 control, as denoted by the asterisks. Data are presented as mean ± standard deviation; N = 5. PLSCR indicates phospholipid scramblase; LPS, lipopolysaccharide.
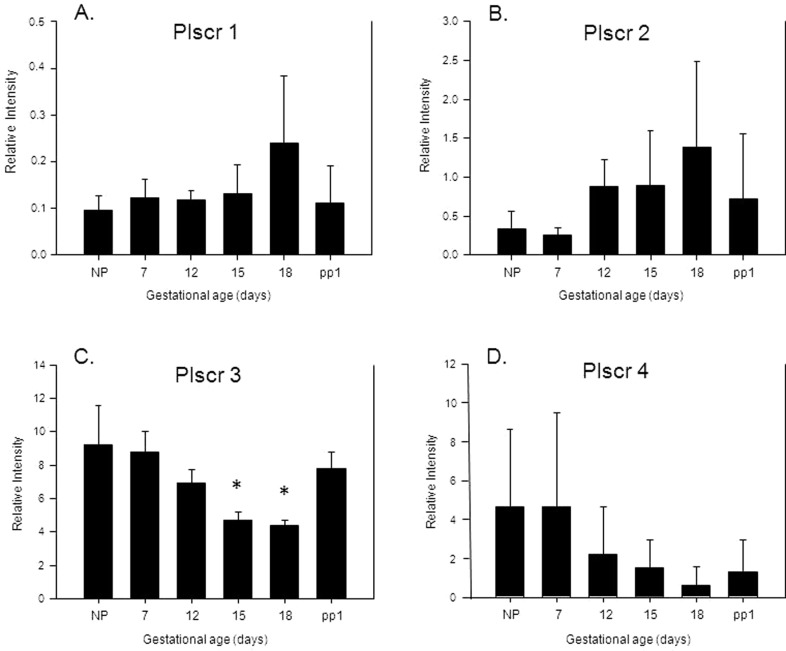
Uterine PLSCR mRNA expression was then evaluated in the absence of LPS administration in nonpregnant, timed pregnant, and postpartum mouse uteri. Modulation of the mRNA expression for the 4 PLSCR isoforms is demonstrated in Figure 4. These RT-PCR studies confirmed stable expression for PLSCR-1 across gestation, along with a trend toward increased PLSCR-2 and decreased PLSCR-4 with progression of pregnancy. None of these changes, however, were significant. In contrast, the RT-PCR studies for PLSCR-3 revealed a 50% reduction in mRNA expression on gestational days 15 and 18 (both Ps < .05, compared to expression in the nonpregnant uteri), which returned to baseline on postpartum day 1 (see Figure 4).
Figure 4.
Bar graphs demonstrating RT-PCR results in relative intensity (based on standard curves generated for each primer set) for the level of mRNA expression for PLSCR-1 (A), PLSCR-2 (B), PLSCR-3 (C), and PLSCR-4 (D) in uterine samples collected from nonpregnant (NP), pregnant (gestational days 7, 12, 15, and 18), and postpartum day 1 (pp1) mice. Data are presented as mean ± standard deviation, N = 3, and (*) represents P < .05 compared to nonpregnant mouse tissue samples. RT-PCR indicates reverse transcriptase–polymerase chain reaction; mRNA, messenger RNA; PLSCR, phospholipid scramblase.
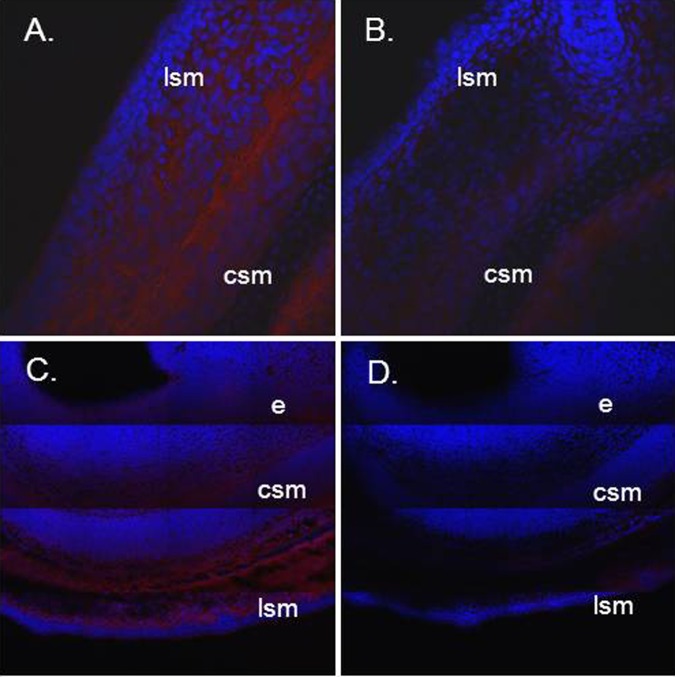
The results of the immunofluorescence studies, which were performed in an effort to localize PLSCR1 protein expression in the mouse uteri, are shown in Figure 5. Using a monoclonal antibody to PLSCR-1 as the primary antibody, these immunofluorescence studies confirmed the expression of PLSCR-1 protein in the uterine wall of the day 14 pregnant mouse. The PLSCR-1 protein appeared to be expressed both in the inner circular and in the outer longitudinal smooth muscle layers of the pregnant mouse uterus. There also appeared to be low-level expression of PLSCR-1 protein in the endometrial layer of the uterine wall (Figure 5).
Figure 5.
Immunofluorescence studies, showing localization of PLSCR-1 protein expression in the tissue layers of the day 14 to 15 pregnant mouse uterus. Pane A shows positive immunofluorescence red stain (Alexafluor 555) in the longitudinal and circular smooth muscle layers (lsm and csm, respectively) of the uterus. Pane B is the negative control showing these 2 uterine layers with minimal autofluorescence. The blue fluorescence is from DAPI staining of cell nuclei. Panes C (positive) and D (negative control) show composite images to better define uterine wall architecture. Longitudinal and circular smooth muscle layers (lsm and csm, respectively) are again seen, along with faint fluorescence in the endometrial layer (E). Magnifications are approximately × 25 for the images shown in panes A and B, and × 6.25 for panes C and D. PLSCR indicates phospholipid scramblase; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
Comment
In the current study, we have demonstrated mRNA expression of all 4 PLSCR isoforms in the pregnant and nonpregnant mouse uterus. PLSCR-1 mRNA expression, and that of its protein product, increase in the setting of preterm delivery initiated by intrauterine LPS administration. These studies were performed on gestational day 15 in order to recapitulate preterm delivery at 80% of gestation in human pregnancies (ie, at about 32 weeks of gestation). PLSCR-1 mRNA expression was significantly increased by the 2-hour time point, with a peak in expression at the 12-hour time point. The Western blot studies confirmed parallel PLSCR-1 protein expression which significantly increased by 6 hours after LPS injection; with continued high levels of expression through to the 24-hour time point. The immunofluorescence studies localized the PLSCR-1 protein to the smooth muscle layers of the mouse uterus. These findings are consistent with a previous report demonstrating transcriptional control of PLSCR-1 expression mediated by a family of proinflammatory cytokines (ie, interferons).12 Key reactions in the coagulation cascade, including the activities of plasma prothrombinase complex (F X/V), tissue prothrombinase (Fgl-2), and the tissue factor (TF)/FVII complex, are dependent upon membrane exposure of PS. The presence of PS at the outer leaflet of the plasma membrane, largely a result of phospholipid redistribution, is essential for the generation of active thrombin. Such phospholipid redistribution occurs in the setting of cell activation, injury, or apoptosis and is mediated by members of the PLSCR family. Our observations of enhanced PLSCR expression in response to LPS, a Toll-like receptor 4 agonist, help confirm the potential link between intrauterine inflammation and activation of important components of the coagulation cascade.
Evidence for substantial intersection and integration of the coagulation system and immune/inflammatory responses is growing rapidly.13 Our findings add to this burgeoning collection of data by providing a specific mechanistic link between intrauterine inflammation and thrombin generation. These findings are particularly instructive when considered in the context of intrauterine bleeding, a common complication of pregnancy, and a risk factor for spontaneous abortion and preterm delivery. Although such complications are associated with increased uterine contractility, the mechanisms responsible are poorly understood. Several reports indicate that actively clotting blood is capable of inducing myometrial contractions in vitro 14 and in vivo in both pregnant and nonpregnant animals, an effect at least partly attributable to the generation of active thrombin.6,9,15,16 Moreover, several studies have provided further evidence to support the role of thrombin in the etiology of premature labor.8,17 Interestingly, thrombin is also capable of stimulating the release of the matrix metalloproteinase (MMP)-1 and thus may be implicated in preterm premature rupture of membranes (PPROM).18 Indeed, Rosen et al reported that women who developed PPROM had a higher median plasma concentration of thrombin–antithrombin complexes in the second and third trimesters than women who had a normal outcome, suggesting that excessive thrombin generation precedes the development of PPROM.19
The mechanisms responsible for the increased generation of thrombin in preterm labor with intact membranes and in PPROM have not been fully determined. It has been postulated that uteroplacental ischemia may lead to tissue factor release, resulting in increased thrombin generation at the decidual–placental interface.20 Alternatively, intrauterine inflammation leading to intravascular thrombin generation and/or tissue factor release may prove to be of greatest consequence.20 Thrombin is well recognized not only as a potent uterotonic, but also as a major inflammatory mediator during localized and systemic inflammatory events.13 Early PTB and PPROM are often associated with maternal intravascular inflammation,21 which may be of a chronic nature. Indeed, elevation of cytokines in amniotic fluid and in maternal plasma has been demonstrated in advance of the development of clinical signs and/or symptoms of PTB.22–25 Increased thrombin generation in preterm labor and PPROM may therefore reflect systemic inflammation.
In summary, we have shown expression of PLSCRs, a family of novel proteins responsible for moving negatively charged membrane phospholipids from the inner to the outer leaf of the cell membrane, in the pregnant mouse uterus. Our results demonstrate the modulation of PLSCR expression with stimulation of the innate immune response in LPS-induced PTB. These observations provide an interesting potential mechanism relating the activation of the coagulation cascade and uterine contractility, with the proinflammatory immune response observed during preterm labor in the setting of intrauterine infection and/or inflammation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was Supported by the National Institute of Child Health and Human Development (grant HD 044747).
References
- 1. Bevers EM, Williamson PL. Phospholipid scramblase: an update. FEBS Lett. 2010;584(13):2724-2730 [DOI] [PubMed] [Google Scholar]
- 2. Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb Haemost. 2001;86(1):266-275 [PubMed] [Google Scholar]
- 3. Sahu SK, Gummadi SN, Manoj N, Aradhyam GK. Phospholipid scramblases: an overview. Arch Biochem Biophys. 2007;462(1):103-114 [DOI] [PubMed] [Google Scholar]
- 4. Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J Biol Chem. 1997;272(29):18240-18244 [DOI] [PubMed] [Google Scholar]
- 5. Wiedmer T, Zhou Q, Kwoh DY, Sims PJ. Identification of three new members of the phospholipid scramblase gene family. Biochim et Biophys Acta. 2000;1467(1):244-253 [DOI] [PubMed] [Google Scholar]
- 6. Elovitz MA, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J Obstet Gynecol. 2000;183(4):799-804 [DOI] [PubMed] [Google Scholar]
- 7. O'Brien M, Morrison JJ, Smith TJ. Expression of prothrombin and protease activated receptors in human myometrium during pregnancy and labor. Biol Reprod. 2008;78(1):20-26 [DOI] [PubMed] [Google Scholar]
- 8. Phillippe M, Wolff D, Saunders T, Thomas L, Chapa J. Intrauterine expression of prothrombin in the sprague-dawley rat. J Soc Gynecol Investig. 2002;9(5):276-281 [DOI] [PubMed] [Google Scholar]
- 9. Elovitz MA, Ascher-Landsberg J, Saunders T, Phillippe M. The mechanisms underlying the stimulatory effects of thrombin on myometrial smooth muscle. Am J Obstet Gynecol. 2000;183(3):674-681 [DOI] [PubMed] [Google Scholar]
- 10. Mussalli GM, Blanchard R, Brunnert SR, Hirsch E. Inflammatory cytokines in a murine model of infection-induced preterm labor: cause or effect? J Soc Gynecol Investig. 1999;6(4):188-195 [DOI] [PubMed] [Google Scholar]
- 11. Diamond AK, Sweet LM, Oppenheimer KH, Bradley DF, Phillippe M. Modulation of monocyte chemotactic protein-1 expression during lipopolysaccharide-induced preterm delivery in the pregnant mouse. Reprod Sci. 2007;14(6):548-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillippe M, Bradley DF, Ji H, Oppenheimer KH, Chien EK. Phospholipid scramblase isoform expression in pregnant rat uterus. J Soc Gynecol Investig. 2006;13(7):497-501 [DOI] [PubMed] [Google Scholar]
- 13. Levi M, Schultz M, van der Poll T. Coagulation biomarkers in critically ill patients. Crit Care Clin. 2011;27(2):281-297 [DOI] [PubMed] [Google Scholar]
- 14. Fareed J, Kindel G, Kumar A. Modulation of smooth muscle responses by serine proteases and related enzymes. Semin Thromb Hemost. 1986;12(4):265-276 [DOI] [PubMed] [Google Scholar]
- 15. Phillippe M, Elovitz M, Saunders T. Thrombin-stimulated uterine contractions in the pregnant and nonpregnant rat. J Soc Gynecol Investig. 2001;8(5):260-265 [DOI] [PubMed] [Google Scholar]
- 16. Uszynski M, Rutkowska L. Oxytocic action of thrombin on the rat uterus in vitro. Ginekologia polska. 1975;46(7):783-786 [PubMed] [Google Scholar]
- 17. Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol. 2001;185(5):1059-1063 [DOI] [PubMed] [Google Scholar]
- 18. Chaiworapongsa T, Yoshimatsu J, Espinoza J, et al. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;11(6):362-367 [DOI] [PubMed] [Google Scholar]
- 19. Rosen T, Kuczynski E, O'Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med. 2001;10(5):297-300 [DOI] [PubMed] [Google Scholar]
- 20. Buhimschi CS, Schatz F, Krikun G, Buhimschi IA, Lockwood CJ. Novel insights into molecular mechanisms of abruption-induced preterm birth. Expert Rev Mol Med. 2010;12:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185(5):1124-1129 [DOI] [PubMed] [Google Scholar]
- 22. Winkler M. Role of cytokines and other inflammatory mediators. BJOG: Int J Obstet Gynaecol. 2003;110(suppl 20):118-123 [DOI] [PubMed] [Google Scholar]
- 23. Buhimschi CS, Dulay AT, Abdel-Razeq S, et al. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG: Int J Obstet Gynaecol. 2009;116(2):257-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185(5):1130-1136 [DOI] [PubMed] [Google Scholar]
- 25. Ghezzi F, Franchi M, Raio L, et al. Elevated amniotic fluid C-reactive protein at the time of genetic amniocentesis is a marker for preterm delivery. Am J Obstet Gynecol. 2002;186(2):268-273 [DOI] [PubMed] [Google Scholar]