Abstract
Severe calcification of the bone microvascular network was observed in rats, whereby the bone marrow blood vessels appeared ossified. This study sought to characterize the magnitude of ossification in relation to patent blood vessels and adipocyte content in femoral diaphyses. Additionally, this study confirmed the presence of ossified vessels in patients with arteriosclerotic vascular disease and peripheral vascular disease and cellulitis. Young (4–6 mon; n=8) and old (22–24 mon; n=8) male Fischer-344 rats were perfused with barium sulfate to visualize patent bone marrow blood vessels. Femoral shafts were processed for bone histomorphometry to quantify ossified (Goldner’s Trichrome) and calcified (Alizarin Red) vessels. Adipocyte content was also determined. Additional femora (n=5/age group) were scanned via µCT to quantify microvascular ossification. Bone marrow blood vessels from rats and the human patients were also isolated and examined via microscopy. Ossified vessels (rats and humans) had osteocyte lacunae on the vessel surfaces and “normal” vessels were transitioning into bone. The volume of ossified vessels was 4800% higher (p <0.05) in old vs. young rats. Calcified and ossified vessel volumes per tissue volume and calcified vessel volume per patent vessel volume were augmented (p <0.05) 262%, 375% and 263%, respectively, in old vs. young rats. Ossified and patent vessel number was higher (171%) and lower (40%), respectively, in old vs. young rats. Finally, adipocyte volume per patent vessel volume was higher (86%) with age. This study is the first to report ossification of bone marrow blood vessels in rats and humans. Ossification presumably results in “microvascular dead space” in regards to loss of patency and vasomotor function as opposed to necrosis. The progression of bone microvascular ossification may provide the common link associated with age-related changes in bone and bone marrow. The clinical implications may be evident in the difficulties treating bone disease in the elderly.
Keywords: microvascular dead space, bone marrow blood vessel ossification, human, rat, adipocytes
INTRODUCTION
The vascular system is important for bone and bone marrow. Immune cells are produced in the bone marrow [1] and blood vessels serve a role in hematopoietic stem cell niches [2]. In addition, capillaries are integral components of bone basic multicellular units and may coordinate the activities of osteoblasts and osteoclasts [3, 4]. Further, alterations in skeletal perfusion have been linked with changes in bone cell metabolism [5]. Thus, a properly functioning bone vascular system is critical for healthy bone and bone marrow and vascular dysfunction is presumed to contribute to osteopathology.
Dysfunction or dysregulation of the bone vascular network may rest internal to the skeleton (i.e., bone marrow blood vessels) or external (i.e., nutrient arteries and veins that originate outside and penetrate the skeleton). Thus, the potential influence of bone vascular dysfunction on the development of bone disease is multifaceted and complex. Previously, the roles of skeletal perfusion [6, 7], spatial redistribution of bone marrow blood vessels [8], bone vascular density [8] and vasomotor responsiveness of the femoral principal nutrient artery (i.e., the primary conduit for blood flow to long bones) [6, 7, 9, 10] have been considered as contributing factors to altered bone metabolism and mass. What has received little attention, however, has been the role of bone vascular calcification and its potential influence on vascular regulation of skeletal blood flow.
Calcification of blood vessels occurs in several diseases such diabetes, end stage renal disease, atherosclerosis and calciphylaxis [11–16] and is present in different sized vessels (e.g., aorta, dermal microvessel, etc.)[17]. Calcification is a complex process and the mechanisms are currently under investigation [17]. During calcification, small crystals of hydroxyapatite deposit in the intima and/or media and often contain pockets of marrow and cartilagenous tissue [11–17]. Arteriosclerosis in human bone marrow was first reported in the 1960s and Ramseier suggested that this disease occurs at least a decade earlier in bone marrow in comparison to blood vessels in other organs [18]. In primates fed an atherogenic diet for 20 months, minimal changes occurred in bone arterial morphology of the maxilla and mandible, which presented as fibro-fatty intimal plaques and the occasional replacement of smooth muscle fibers with collagen [18]. These alterations, however, only caused minimal lumen occlusion in the effected bone blood vessels and therefore the declines in blood flow to the maxilla and mandible was attributed to the gross lesions observed in the afferent vasculature (i.e., carotid artery) [18]. Further, some evidence of intraosseous blood vessel calcification presented as heterogeneous staining of the adventitial layer; however, no further physiological or pathological details of the sample were given [19].
The totality of evidence in the literature invited further clarification as to the extent of microvascular calcification in bone. Examination of bone marrow blood vessels from young and old rats revealed severe pathology that extended beyond calcium deposition, whereby the vessels appeared ossified and bone-like in morphology. Thus, the purpose of this investigation was to characterize the magnitude of bone marrow blood vessel ossification in relation to patent bone marrow blood vessels and adipocyte volume in the femoral diaphyses of young and old rats. In addition, this study confirmed the presence of ossified bone marrow blood vessels in human long bones.
MATERIALS AND METHODS
The procedures employed in this study were approved by the University of Delaware Institutional Animal Care and Use Committees, and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23, revised 1996). Young (4–6 months) and old (22–24 months) male Fischer-344 rats were doubly housed in a temperature-controlled (23±2°C) room with a 12:12 h light/dark cycle. Tap water and rat chow were provided ad libitum.
Experimental Protocol
Perfusion of the vascular network
To visualize the bone microvascular network, young (n=8) and old (n=8) male Fischer-344 rats were injected with heparinized saline (50 IU/kg, subcutaneously) 1 hour prior to sacrifice and subsequently perfused with a barium sulfate solution as previously described [8]. Briefly, under anesthesia (isoflurane 3% to oxygen balance), the thoracic cavity was opened; a guided cannula was inserted into the left ventricle and a small incision was made in the right atrium. The cannula was connected to Tygon tubing (Masterflex 14) and the tubing was secured onto the rollers of a perfusion pump (Masterflex L/S, Cole-Parmer, Vernon Hills, IL, USA). Using the perfusion pump, blood was flushed from the vascular system with physiological phosphate-buffered saline (37°C) containing sodium nitroprusside. A cannula was then inserted into the abdominal aorta and barium sulfate solution pumped into the circulation. The vascular system was considered entirely perfused when the liver and feet of the animal appeared white. Right femora were then dissected and stored in 10% formalin at 4°C for 3 days.
Evaluation of the femoral diaphysis for patent bone marrow blood vessels and bone marrow blood vessel ossification
Bone Histomorphometry
Right femora were removed from the formalin, serially dehydrated in increasing concentrations of ethanol, and the femoral shafts were embedded in methyl methacrylate at low temperature as previously described [8]. Femoral shafts were sectioned frontally with a microtome (Leica RM2255, Leica Microsystems, Buffalo Grove, Illinois, United States) and histological slides created. Goldner’s Trichrome or Alizarin Red staining of barium sulfate-perfused femoral shafts allow for the simultaneous visualization of bone marrow blood vessels that are patent and those that stain as bone (Goldner’s Trichrome) or for calcium deposition (Alizarin Red). Two 9-µm-thick histological sections stained with Alizarin Red were analyzed for the determination of calcification (i.e., calcified vessel volume per tissue volume ratio and calcified vessel volume per patent vessel volume ratio). In addition, two 9-µm-thick histological sections stained with Goldner’s Trichrome were used for the determination of ossification (i.e., ossified vessel volume per tissue volume ratio and the number of ossified vessels). In addition, patent bone marrow blood vessels in the femoral shaft were quantified (i.e., patent bone marrow blood vessel volume per tissue volume ratio and the number of patent bone marrow blood vessels). Adipocyte content of the femoral shaft was also quantified for adipocyte volume per tissue volume ratio, adipocyte volume per patent vessel volume ratio and adipocyte number. For each slide, the entire femoral shaft was analyzed and the data was averaged per rat.
High-resolution microtomography (µCT)
A separate group of young (n=5) and old (n=5) male Fischer-344 rats were used to determine bone marrow blood vessel ossification via µCT. Rats were anesthetized (isoflurane 3% to oxygen balance) and euthanized by removing the heart. Right femora were scanned ex vivo using high-resolution (8 µm) µCT Viva CT40 (Scanco Medical AG, Wayne PA) at 55 kVP and acquired. To isolate bone marrow blood vessels, the distal end (i.e., distal metaphysis and epiphysis) was cut free from the left femur with a bone saw and the intact cortical shaft and proximal end (i.e., proximal metaphysis and epiphysis with the femoral head) was placed in a 1.5 mL microcentrifuge tube containing small amounts of phosphate buffered saline. Subsequently, the tube was spun briefly in a microcentrifuge until the marrow dislodged from the bone and into the phosphate buffered saline. Next, utilizing a stereomicroscope and microsurgical forceps, bone marrow blood vessels were isolated from the marrow, cleared of bone marrow cells, stained with Goldner’s Trichrome and examined by light and stereomicroscopy.
Isolation of human bone marrow blood vessels from the femoral and fibular diaphyses
An amputated distal femur from a 59-year-old female patient with arteriosclerotic vascular disease and an amputated fibula from a 75-year-old female patient with peripheral vascular disease and cellulitis were obtained through a material transfer agreement with Christiana Care Health Systems (courtesy of Dr. Mark Mitchell) and fixed in 10% formalin for 3 weeks. The amputated limbs were considered medical waste and Institutional Review Board approval and subject’s consent were not required. For the distal femur, a 3-cm cross-section of the diaphysis was cut with a Dremel Saw-Max™. To locate bone marrow in the fibula, the cortical shell was sectioned on both sides with a Dremel Saw-MaxTM and the bone separated into two halves along the longitudinal axis. Utilizing a stereomicroscope and microsurgical forceps, bone marrow blood vessels were isolated from bone marrow located in the middle of the cross- and longitudinal-sections. The bone marrow blood vessels were cleared of bone marrow cells, stained with Goldner’s Trichrome and examined by light and stereomicroscopy.
Statistical Analysis
One-way ANOVA was used to determine the significance of differences in body mass, patent, ossified and calcified bone marrow blood vessel parameters, adipocyte parameters and bone marrow blood vessel ossification via µCT. Data are presented as mean ± S.E. Significance was defined a priori as p ≤ 0.05.
RESULTS
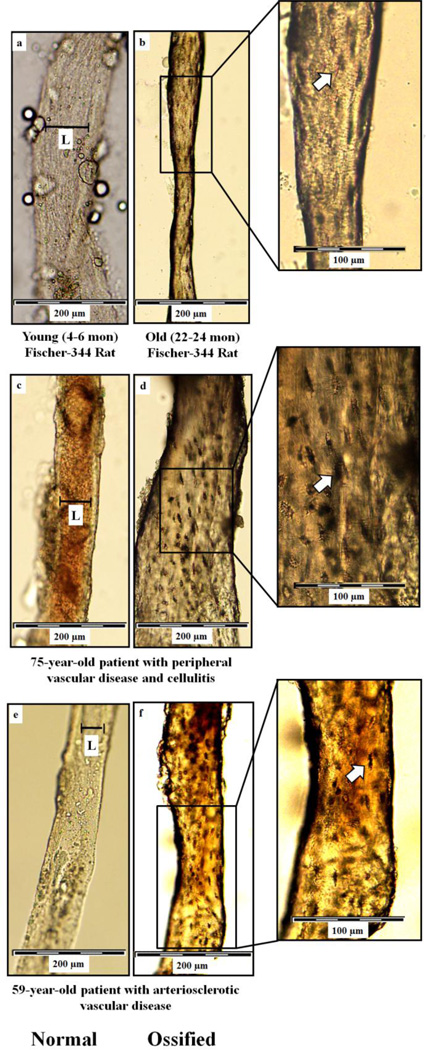
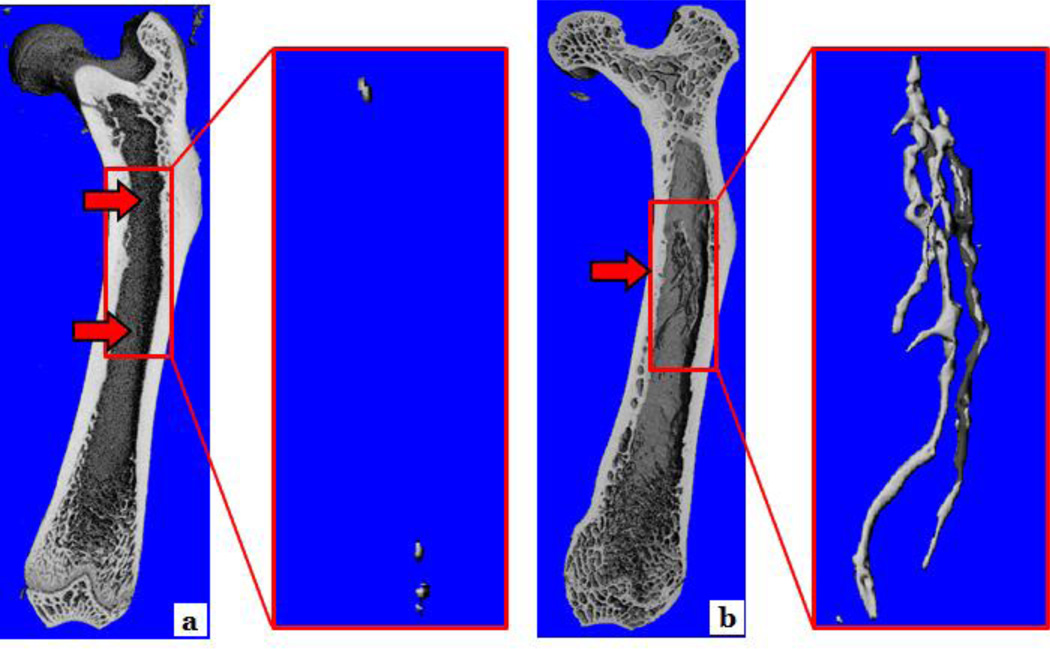
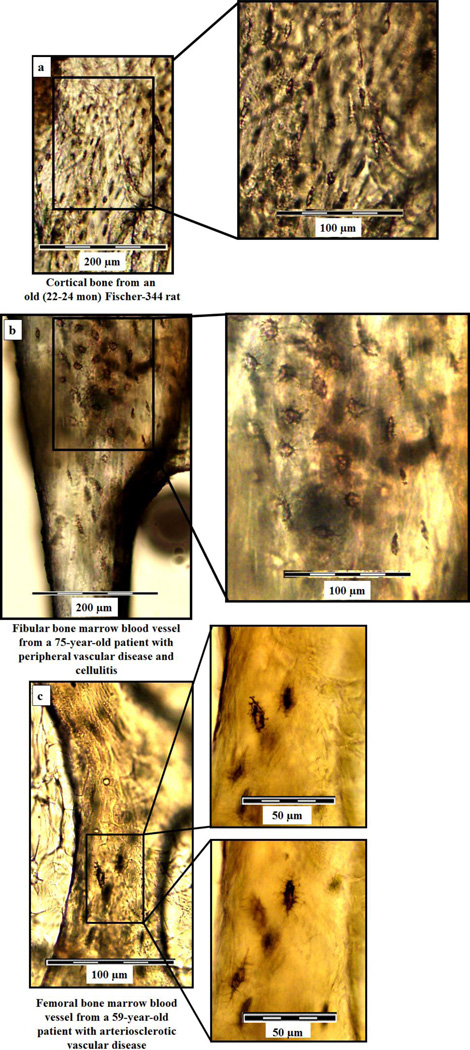
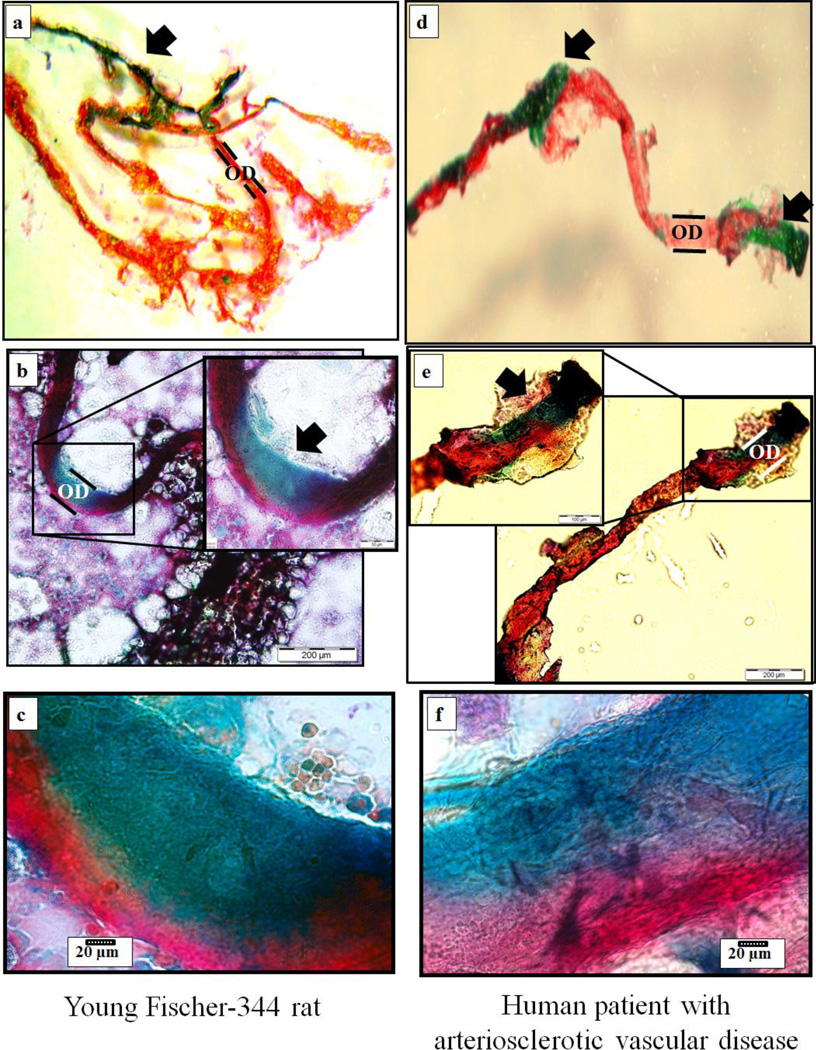
Mean body mass did not differ between young and old rats (378 ± 7g vs. 410 ± 13g, respectively). Figure 1 illustrates normal (a, c and e) and ossified (b, d and f) bone marrow blood vessels taken from the femoral diaphyses of young and old rats and from the fibular and femoral diaphyses of the human subjects. Light microscopy revealed the presence of osteocyte lacunae on the surfaces of the old rat and human bone marrow blood vessels (Figure 1 b, d and f). Even though not depicted in Figure 1, ossified vessels were observed in the young animals (as shown in Figure 2A). Additionally, µCt data revealed that the volume of ossified bone marrow blood vessels was significantly (p < 0.05) higher in old vs. young rats (0.49 ± 0.11% vs. 0.01 ± 0.01%, respectively, and Figure 2). Figure 3 depicts photomicrographs of cortical bone from an old Fisher-344 rat (a) and bone marrow blood vessels isolated from the fibular (b) and femoral (c) diaphyses of the human patients. Higher magnifications (insets) reveal the details and similarities of osteocyte lacunae on the surfaces of the cortical bone and bone marrow blood vessels. The similarities in morphology of the ossified bone marrow blood vessels between species (i.e., human and rat; Figure 1) and the bone tissue (Figure 3) is striking and confirm the presence of bone marrow blood vessel ossification in humans. Goldner’s Trichrome staining of “normal” bone marrow blood vessels from a young rat and the human patient with arteriosclerotic vascular disease revealed a transition of vascular cells to bone (Figure 4a and d; stereomicroscopy and Figure 4b, c, e and f; light microscopy).
Figure 1. Bone marrow blood vessels isolated from femoral and fibular diaphyses.
Light microscopic images of bone marrow blood vessels isolated from femoral diaphyses of young (a) and old (b) male Fischer-344 rats and fibular and femoral diaphyses from human patients with peripheral vascular disease and cellulitis (c and d) and arteriosclerotic vascular disease (e and f). Panels a, c and e illustrate normal bone marrow blood vessels. Blood is evident in the normal human bone marrow blood vessel in panel c. Arrows denotes the presence of osteocyte lacunae on the surfaces of the ossified bone marrow blood vessels from the old rat (b, inset) and the human patients. (d and f, insets). The L denotes the lumen of the rat and human normal bone marrow blood vessels.
Figure 2. µCT imaging of young and old femora.
Panels (a) and (b) represent femora from young and old rats, respectively. The arrows denote ossification of the bone microvascular network in young and old femora, which is marked in the old rat.
Figure 3. Osteocyte lacunae on rat cortical bone and bone marrow blood vessels isolated from human fibular and femoral diaphyses.
Light microscopic images of cortical bone from the femoral diaphysis of an old male Fischer-344 rat (a) and fibular and femoral bone marrow blood vessels from human patients with peripheral vascular disease and cellulitis (b) and arteriosclerotic vascular disease (c). Higher magnifications illustrate the similarities in osteocyte lacunar morphology between the cortical bone and ossified blood vessels.
Figure 4. Histological staining of “normal” bone marrow blood vessels.
Goldner’s Trichrome staining of “normal” bone marrow blood vessels from a young male Fischer-344 rat (a, b and c) and a human patient with arteriosclerotic vascular disease (d, e and f). Stereo- (a and d) and light (b, c, e and f) microscopy revealed transitioning of vascular cells to bone (arrows) in both the rat and human vessels. Panels c and f are higher magnifications of the areas denoted by the arrows in the insets of panels b and e. Vascular smooth muscle is stained red and bone is stained green. OD denotes the outer diameter of the blood vessels.
Quantifying Patent, Calcified and Ossified Bone Marrow Blood Vessels in the Femoral Diaphyses of Rats
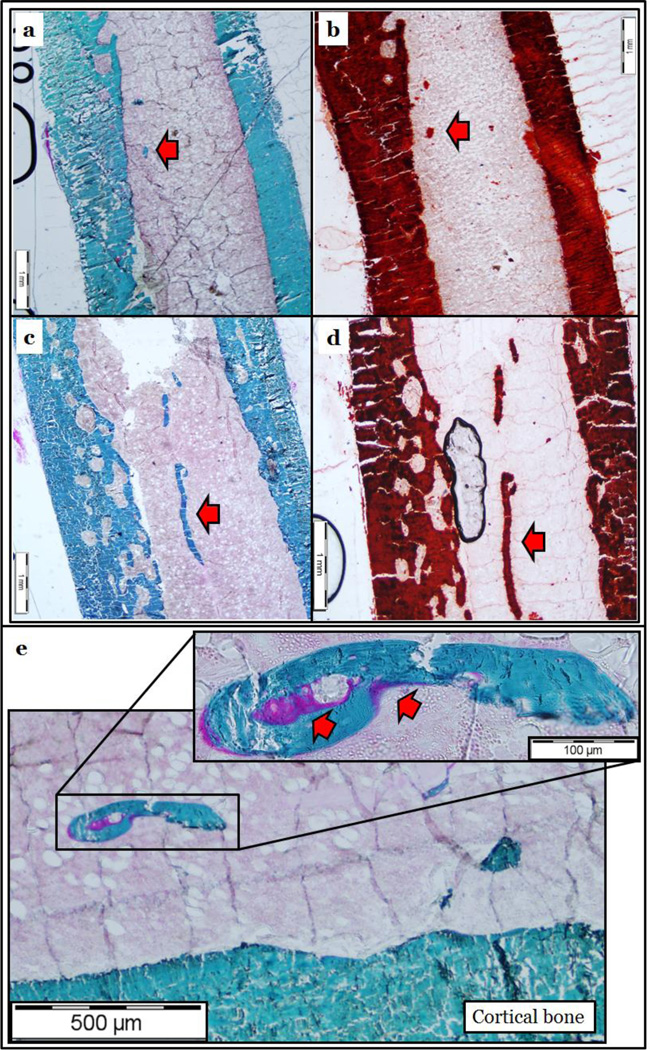
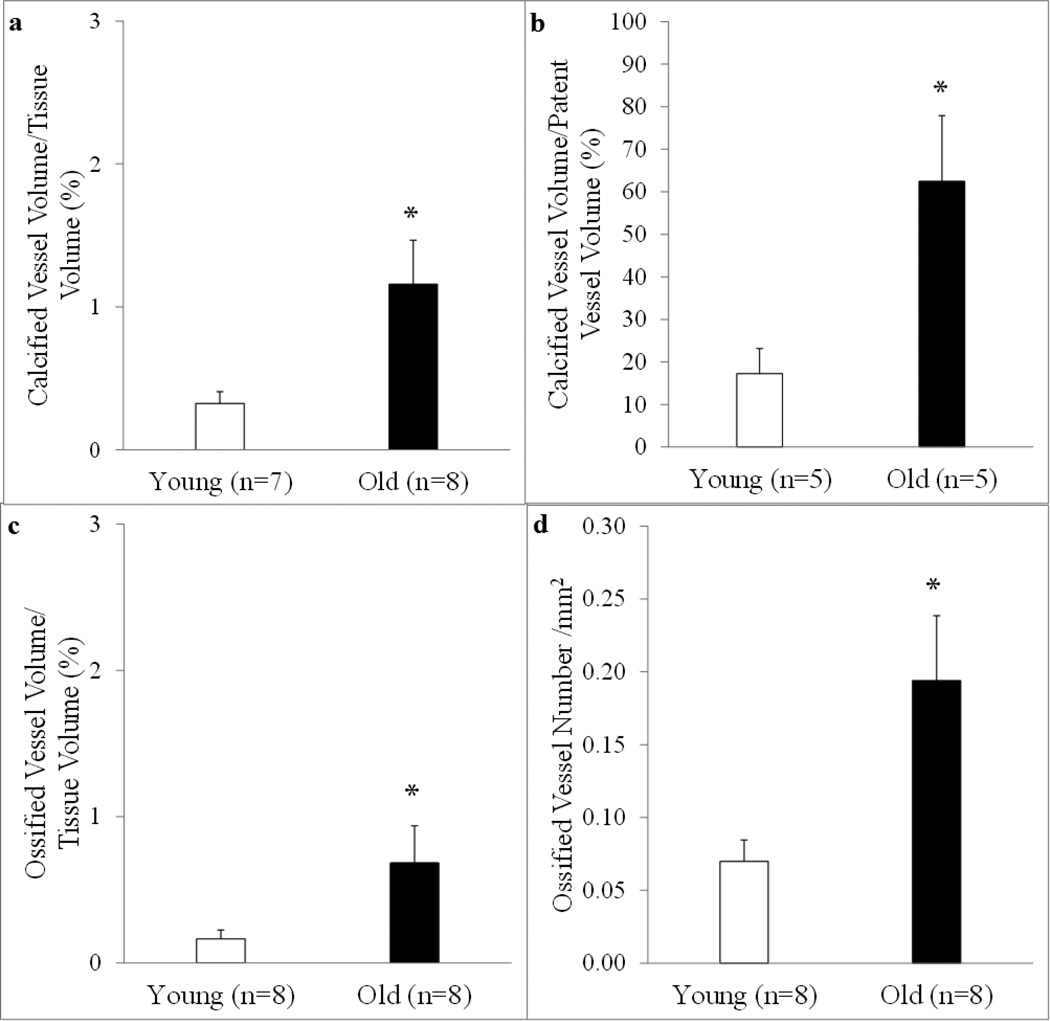
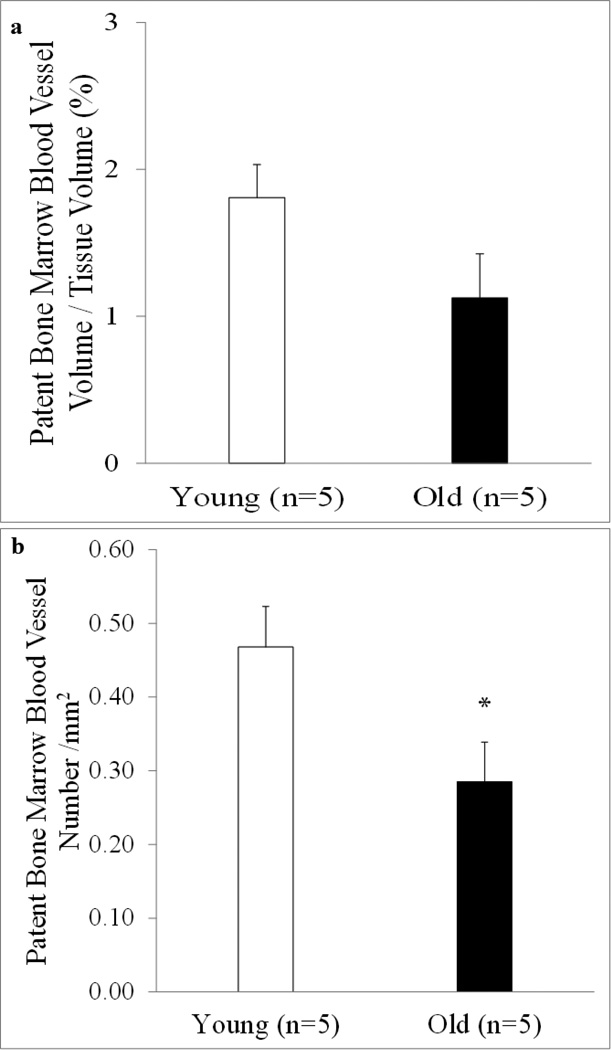
Goldner’s Trichrome (Figure 5a and c) and Alizarin Red (Figure 5b and d) staining of femoral shafts revealed ossification and calcification of the bone marrow blood vessels in both young (Figure 5a and b, arrows) and old (Figure 5c and d, arrows) rats. As observed in Figure 5e, osteoid seams (which represents newly formed but unmineralized bone) are present on the ossified bone marrow blood vessel. When quantified, the volumes of calcification per tissue volume and per patent vessel volume were higher (p < 0.05) in old rats (Figure 6a and 6b). Further, there was a higher (p < 0.05) volume (Figure 6c) and number (Figure 6d) of ossified bone marrow blood vessels in old rats. Examination of barium sulfate-perfused vessels revealed similar volumes of patent bone marrow blood vessels between young and old rats (Figure 7a); however, overall number was diminished (p < 0.05) in old age (Figure 7b).
Figure 5. Bone histomorphometry of rat femoral diaphyses.
Femoral diaphyses of young (a and b) and old (c and d) male Fischer-344 rats stained with Goldner’s Trichrome for bone (a and c) and Alizarin Red for calcium (b and d). Note that bone marrow blood vessels stain similar to cortical bone and completely for calcium (arrows). Additionally, the presence of osteoid seams are visible on the bone marrow blood vessel (e, arrows), indicating that the vessel is undergoing active bone formation.
Figure 6. Quantification of calcified and ossified bone marrow blood vessels.
The calcified vessel volume per tissue volume ratio (a), calcified vessel volume per patent vessel volume ratio (b), ossified vessel volume per tissue volume ratio (c), and the number of ossified vessels (d) in the femoral diaphyses from young and old male Fischer-344 rats were quantified from the Goldner’s Trichrome- and Alizarin Red-stained sections. The volumes of calcification (a) and ossification (c) per tissue volume are higher in old vs. young rats. In addition, as the volume of calcified vessels increased, the volume of patent vessels declined (b) and the overall number of ossified bone marrow blood vessels (d) is higher with old age. Values are means ± S.E. *Denotes a significant (p < 0.05) difference from young rats.
Figure 7. Quantification of patent bone marrow blood vessels.
The volume of patent bone marrow blood vessels per tissue volume ratio and the number of patent bone marrow blood vessels from young and old male Fischer-344 rats were quantified from barium sulfate-perfused sections. The volume of patent bone marrow blood vessel per tissue volume (a) did not differ between ages; however, the number of patent bone marrow blood vessels (b) was lower with old age. Values are means ± S.E. *Denotes a significant (p < 0.05) difference from young rats.
Adipocyte Content in the Femoral Diaphysis
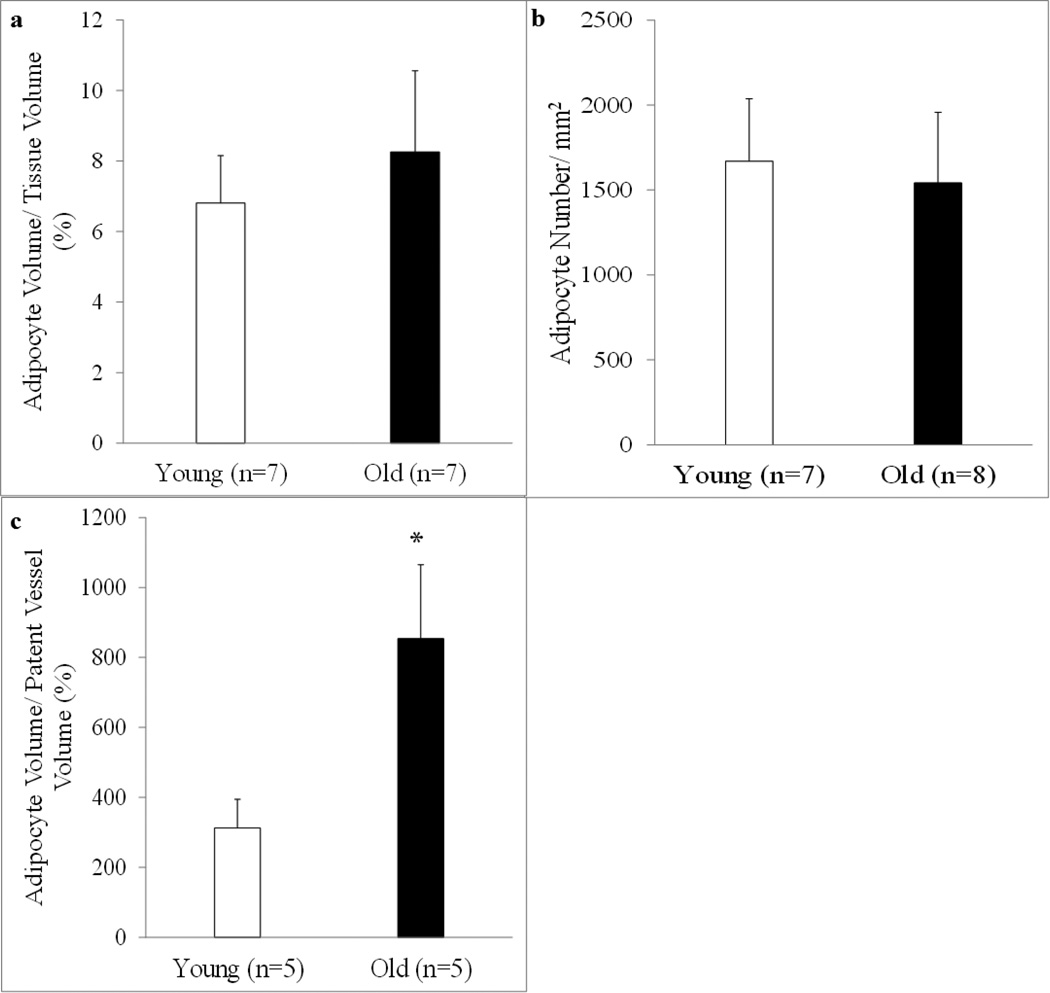
Overall, adipocyte volume per tissue volume and adipocyte number was similar between young and old rats (Figure 8a and b, respectively); however, adipocyte volume per patent vessel volume was higher (p < 0.05) in the old group (Figure 8c).
Figure 8. Quantification of bone marrow adipocytes.
Adipocyte volume per tissue volume, adipocyte volume per patent vessel volume and overall adipocyte number in the femoral diaphyses from young and old male Fischer-344 rats was quantified via bone histomorphometry. Adipocyte volume per tissue volume (a) and adipocyte number (b) were similar between ages; however, as the volume of adipocytes increased, the volume of patent bone marrow blood vessels declined (c). Values are means ± S.E. *Denotes a significant (p < 0.05) difference from young rats.
DISCUSSION
This study is the first to report ossification of bone marrow blood vessels in young and old rats and in patients with arteriosclerotic vascular disease and peripheral vascular disease with cellulitis. Bone marrow blood vessel ossification and calcification drastically progressed as a function of age in rats (Figure 2 and Figure 6) and presumably resulted from a transition of vascular cells to an osteogenic phenotype (Figure 4 and Figure 5). Further, the number of patent bone marrow blood vessels declined with age (Figure 7b) and with increased adipocyte volume (Figure 8c). The pathology presented in this manuscript differs from vascular calcification reported in several disease states [11–16]. As observed, bone marrow blood vessels from both rat and human long bones lose a vascular appearance (except for the rod-like structure) and resemble bone, with osteocyte lacunae on the vessel surface (Figures 1b, d and f and Figure 3b and c). Histological staining revealed that these blood vessels are similar to cortical bone (Figure 5) and are undergoing active bone formation, as evidenced by the presence of osteoid seams (Figure 5E). To date, however, the cell type responsible for the ossification of bone marrow blood vessels is unknown but may be attributable to adventitial reticular cells, which form a subendothelial layer on the abluminal surface of sinusoidal walls [20]. Adventitial reticular cells (i.e., skeletal stem cells) express alkaline phosphatase and are osteogenic in nature [21, 22]. The presence of this pathology in both young and old animals suggests that it initiates in youth. The human femoral diaphyses came from elderly individuals with arteriosclerotic vascular disease and peripheral vascular disease with cellulitis; thus, examination of additional samples varying in age and health status will need to occur.
It seems implausible that ossified vessels maintain patency and the ability to regulate blood flow via vasodilation and/or vasoconstriction. Since bone vascular density does not always correspond to bone perfusion [23], the loss of vasomotor function in bone resistance arteries and its impact on bone health cannot be overstated. Declines in vasodilator capacity of the femoral principal nutrient artery were associated with reduced bone mass and vice versa [6, 7, 9, 24]. Ossification of bone marrow blood vessels presumably results in “microvascular dead space” in regards to loss of vasomotor function as opposed to blood vessel necrosis, since the described pathology is a cellularbased transformation into bone. Coupled with age-related declines in vasodilator capacity of the nutrient arterial system, the clinical significance of these findings may have therapeutic implications regarding systemic hormonal regulation of bone, delayed fracture healing and the delivery of oxygen, nutrients and pharmaceuticals to bone.
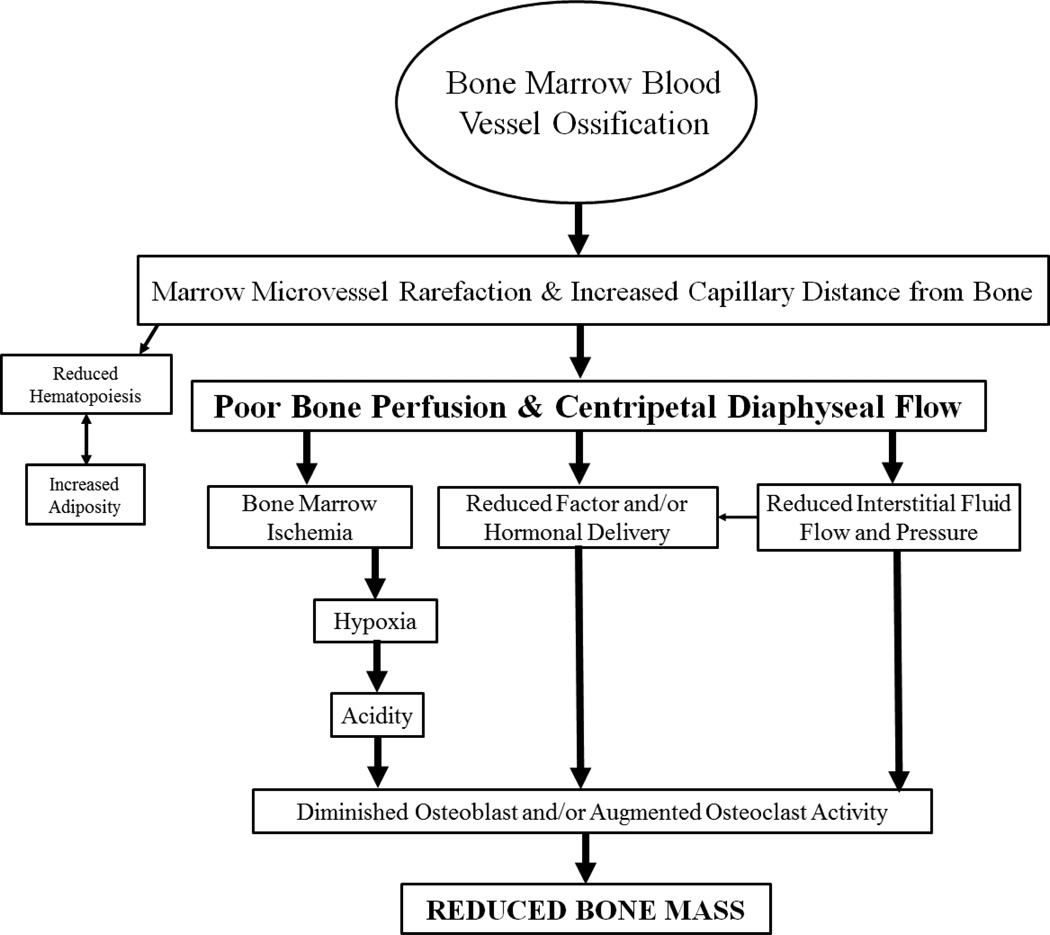
In context of these novel findings, what are the clinical ramifications associated with an enlarging “microvascular dead space” in bone? Researchers have long recognized the importance of vascular supply for biological activity, including for the skeleton [10, 25, 26]. Since angiogenesis often, but not always [8], precedes osteogenesis, the circulation of bone is substantively involved in bone cellular regulation and activity [27, 28]. As hypothetically illustrated in Figure 9, which extends upon theories previously put forth by Colleran et al. (2000) [29], bone marrow blood vessel ossification presumably results in rarefaction and increased capillary distance from bone. This, in turn, would result in diminished bone blood flow and a centripetal direction to flow. Subsequently, bone marrow ischemia would ensue along with diminished interstitial fluid flow and pressure, and declines in hormonal and factor delivery to bone. These physiological changes may create hypoxic and acidic conditions and alter osteoblast and osteoclast activity, culminating in reduced bone mass. Theoretically, the conversion of hematopoietic to fatty marrow may indirectly result from microvascular rarefaction. Many of the physiological and morphological alterations presented in Figure 9 have been characterized in bone and bone marrow with advanced age. Thus, it is plausible that these alterations are secondary consequences of blood vessel ossification; consequences that would induce a microenvironment unfavorable for bone accrual and ultimately contribute to declines in bone mass.
Figure 9. Schematic of presumed consequences associated with bone marrow blood vessel ossification.
Extending beyond the theories presented previously that illustrates how declines in bone blood flow can modulate bone mass29, the hypothetical sequences of events presented in this investigation includes the centralized role of bone marrow blood vessel ossification and the presumed physiological and morphological consequences associated with this pathology. Several of these alterations occur with advancing age. The central role of bone marrow blood vessel ossification may provide a common mechanism linking these physiological and morphological alterations in bone and bone marrow that ultimately leads to reduced bone mass in aged individuals.
The commonality of medullary ischemia in aging long bone has been recognized for several decades [10, 30–35]. Bridgeman and Brookes (1996) reported marrow ischemia in femoral diaphyses of aged women and men and a greater reliance upon the periosteal blood supply (i.e., centripetal blood flow) for survival of the diaphyseal cortex [30]. Conversion of diaphyseal flow from centrifugal in youth to centripetal in old age represents an abnormal blood flow pattern [36] and corresponds with declines in bone perfusion reported in animal models and humans [6, 7, 33, 37–39]. Diminished blood flow to bone marrow [6, 7, 33, 40] and bone [6, 7], reduced vasodilator capacity of bone arteries [6, 7], diminished bone volume [6, 7] and mineral density [40], and replacement of hematopoietic marrow with adipocytes [26, 33, 40] have been reported in aged rats and humans. Further, the number of bone marrow sinusoids was higher in young healthy individuals vs. individuals >70 years and those with osteopenia [26]. The term sinusoids refer to capillaries in hematopoietic marrow [27]. Thus, the bone vascular system, the volume of hematopoietic marrow and bone mass may be causally related.
The relation between hematopoietic and fatty marrow was not examined in the current investigation; however, higher volumes of marrow adipocytes corresponded with reduced patent bone marrow blood vessel number. Adventitial reticular cells cover the abluminal surface of marrow sinusoids and conversion of these cells into adipocytes cause sinusoidal collapse, presumably resulting in loss of blood flow [41]. Thus, the agerelated decline in the number of patent bone marrow blood vessels observed in the current study can be explained by increased ossification of bone marrow blood vessels coupled with an augmented volume of marrow adipocytes. Low capillarity is associated with poor bone vitality [25, 42] and declines in bone vascular density with advanced age have been reported in humans [26, 31]. Additionally, we demonstrated the importance of the spatial location of bone marrow blood vessels, whereby the smallest vessels (≤ 29 µm in diameter) were spatially closer to sites of new bone formation [8]. This spatial closeness may permit increased nutrient and oxygen exchange between vessels and bone [8] and allow for enhanced delivery of osteoblast and osteoclast precursors to bone remodeling sites [43, 44]. The closeness and prevalence of capillaries next to cancellous bone remodeling was subsequently confirmed in human bone biopsies [45]. There is continuity among the microvascular beds of the bone marrow, cortex and periosteum [46–48] and ossification may disrupt this continuity, creating ischemic and hypoxic areas between bone compartments.
Finally, the vascular supply to bone provides more than just the delivery of oxygen and nutrients. Bone cell modulators (e.g., fibroblast growth factors, colony stimulating factors, endothelin-1, interleukin-1, nitric oxide and prostacyclin) are released by endothelial cells [27, 49, 50]. Additionally, the vascular endothelium may modulate bone cellular activity through alterations in interstitial fluid flow and pressure [29, 49–54]. Nitric oxide and prostacyclin released from endothelial cells in response to shear stress may serve to increase bone formation and diminish bone degradation [55–59]. Since the volume and speed of blood delivered to the skeleton is attenuated with advancing age [33, 36], these reductions may significantly influence the shear forces acting upon bone and endothelial cell membranes, reducing the release of bone regulating factors [29].
In conclusion, this investigation demonstrates, for the first time, ossification of bone marrow blood vessels in both rodent and human long bone. The creation of “microvascular dead space” would impair the regulation of bone perfusion via vasodilation and/or vasoconstriction or impede the passage of blood. This pathology may arise from a transitioning of vascular cells to an osteogenic phenotype. Ossification of bone marrow blood vessels with advancing age may provide the central link associated with declines in bone perfusion, the centripetal nature of diaphyseal flow, increased bone marrow ischemia, decreased hematopoiesis, augmented bone marrow adiposity and ultimately reduced bone mass (Figure 9). The clinical consequences arising from such pathology may be evident in the difficulties associated with treating bone disease and delayed fracture healing in the elderly.
Highlights.
-
➢
Bone marrow blood vessels of long bones in rats progressively ossify as a function of advancing age
-
➢
The pathology appears to be a cellular-based transformation of vascular cells into bone
-
➢
Such pathology has been confirmed to exist in human subjects.
-
➢
Ossification presumably results in “microvascular dead space,” i.e., loss of vessel patency and function as opposed to vascular necrosis.
-
➢
This pathology may provide the common link associated with age-related changes in bone and bone marrow.
ACKNOWLEDGEMENTS
A special thanks to Dr. Mark Mitchell and Christiana Care Health Systems (Newark, DE) for supplying the amputated human long bones.
A special thanks to Jean Ross at the Delaware Biotechnology Institute’s Bioimaging Center for aid in generating some of the photomicrographs.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (Award Number 1R15AR062882-01) supported the research reported in this manuscript. The content presented in this manuscript is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The author has no conflicts of interest.
Authors’ roles: Study design: RDP. Study conduct: RDP. Data collection: RDP. Data analysis: RDP. Data interpretation: RDP. Drafting manuscript: RDP. Revising manuscript content: RDP. Approving final version of manuscript: RDP. RDP takes responsibility for the integrity of the data analysis.
REFERENCES
- 1.Goldsby RA, Kindt TJ, Osborne BA. Immunology. 4th ed. New York: W.H.: Freeman and Company; 2000. [Google Scholar]
- 2.Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- 3.Eriksen EF, Eghbali-Fatourechi GZ, Khosla S. Remodeling and vascular spaces in bone. J Bone Miner Res. 2007;22:1–6. doi: 10.1359/jbmr.060910. [DOI] [PubMed] [Google Scholar]
- 4.Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16:1575–1582. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- 5.Grundnes O, Reikerås O. Blood flow and mechanical properties of healing bone. Acta Orthop Scand. 1992;63:487–491. doi: 10.3109/17453679209154720. [DOI] [PubMed] [Google Scholar]
- 6.Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM, Donato AJ, Allen MR, Delp MD. Aging reduces skeletal blood flow, endothelium-dependent vasodilation and nitric oxide bioavailability in rats. J. Bone Miner Res. 2007;22:1280–1288. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez JM, Prisby RD, Muller-Delp JM, Allen MR, Delp MD. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone. 2010;46:813–819. doi: 10.1016/j.bone.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prisby R, Guignandon A, Vanden-Bossche A, Mac-Way F, Linossier MT, Thomas M, Laroche N, Malaval L, Langer M, Peter ZA, Peyrin F, Vico L, Lafage-Proust MH. Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res. 2011;26:2583–2596. doi: 10.1002/jbmr.459. [DOI] [PubMed] [Google Scholar]
- 9.Prisby RD, Dominguez JM 2nd, Muller-Delp J, Allen MR, Delp MD. Aging and estrogen status: a possible endothelium-dependent vascular coupling mechanism in bone remodeling. PLoS One. 2012;7:e48564. doi: 10.1371/journal.pone.0048564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookes M, Revell WJ. Blood Supply of Bone: Scientific Aspects. London: Great Britain: Springer-Verlag; 1998. [Google Scholar]
- 11.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 12.Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63–70. doi: 10.1016/S1054-8807(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen NX, Moe SM. Arterial calcification in diabetes. Curr Diab Rep. 2003;3:28–32. doi: 10.1007/s11892-003-0049-2. [DOI] [PubMed] [Google Scholar]
- 14.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 15.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 16.Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial. 2002;15:172–186. doi: 10.1046/j.1525-139x.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 17.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 18.Brenneise CV, Squier CA. Blood flow in maxilla and mandible of normal and atherosclerotic rhesus monkeys. J Oral Pathol. 1985;14:800–808. doi: 10.1111/j.1600-0714.1985.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 19.Laroche M. Intraosseous circulation from physiology to disease. Joint Bone Spine. 2002;69:262–269. doi: 10.1016/s1297-319x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- 20.Sacchetti BFA, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Westen H, Bainton DF. Association of alkaline-phosphatase-positive reticulum cells in bone marrow with granulocytic precursors. J Exp Med. 1979;150:919–937. doi: 10.1084/jem.150.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianco P, Boyde A. Confocal images of marrow stromal (Westen-Bainton) cells. Histochemistry. 1993;100:93–99. doi: 10.1007/BF00572894. [DOI] [PubMed] [Google Scholar]
- 23.Roche B, Vanden-Bossche A, Normand M, Malaval L, Vico L, Lafage-Proust MH. Validated Laser Doppler protocol for measurement of mouse bone blood perfusion - response to age or ovariectomy differs with genetic background. Bone. 2013;55:418–426. doi: 10.1016/j.bone.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Prisby R, Menezes T, Campbell J. Vasodilation to PTH (1–84) in bone arteries is dependent upon the vascular endothelium and is mediated partially via VEGF signaling. Bone. 2013;54:68–75. doi: 10.1016/j.bone.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Burkhardt R, Bartl R, Frisch B, Jager K, Mahl G, Hill W, Kettner G. The structural relationship of bone forming and endothelial cells of the bone marrow. In: Arlet J, Ficat RP, Hungerford DS, editors. Bone Circulation. Baltimore: Williams and Wilkins; 1984. pp. 2–14. [Google Scholar]
- 26.Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone-marrow vessels in aplastic-anemia, primary osteoporosis, and old-age: a comparative histomorphometric study. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am. 2006;88:4–9. doi: 10.2106/JBJS.F.00890. [DOI] [PubMed] [Google Scholar]
- 28.Trueta J. The role of the blood vessels in osteogenesis. J Bone Joint Surg Br. 1993;45:402–418. [Google Scholar]
- 29.Colleran PN, Wilkerson MK, Bloomfield SA, Suva LJ, Turner RT, Delp MD. Alterations in skeletal perfusion with simulated microgravity: A possible mechanism for bone remodeling. J Appl Physiol. 2000;89:1046–1054. doi: 10.1152/jappl.2000.89.3.1046. [DOI] [PubMed] [Google Scholar]
- 30.Bridgeman G, Brookes M. Blood supply to the human femoral diaphysis in youth and senescence. J Anat. 1996;188:611–621. [PMC free article] [PubMed] [Google Scholar]
- 31.Brookes M. The vascular reaction of tubular bone to ischaemia in peripheral occlusive vascular disease. J Bone and Joint Surg. 1960;42:110–125. doi: 10.1302/0301-620X.42B1.110. [DOI] [PubMed] [Google Scholar]
- 32.Brookes M. Sequelae of experimental partial ischaemia in long bones of the rabbit. J Anat. 1960;94:552–561. [PMC free article] [PubMed] [Google Scholar]
- 33.Kita K, Kawai K, Hirohata K. Changes in bone marrow blood flow with aging. J Orthop Res. 1987;5:569–575. doi: 10.1002/jor.1100050412. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Curto JA, Bassingthwaighte JB, Kelly PJ. Anatomy of the microvasculature of the tibial diaphysis of the adult dog. J Bone Joint Surg Am. 1980;62:1362–1369. [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson GEJ, Kelly PJ, Peterson LF, Janes JM. Blood supply of the human tibia. J Bone Joint Surg Am. 1960;42-A:625–636. [PubMed] [Google Scholar]
- 36.Brookes M. La circulation osseuse normale and pathologique. In: Arlet J, Ficat P, editors. Circulation Osseuse. Paris: INSERM; 1973. pp. 3–13. [Google Scholar]
- 37.Bloomfield S, Hogan HA, Delp MD. Decreases in bone blood flow and bone material properties in aging Fischer-344 rats. Clin Orthop Related Res. 2002;396:248–257. doi: 10.1097/00003086-200203000-00036. [DOI] [PubMed] [Google Scholar]
- 38.Lahtinen T, Alhava EM, Karjalainen P, Romppanen T. The effect of age on blood flow in the proximal femur in man. J Nucl Med. 1981;22:966–972. [PubMed] [Google Scholar]
- 39.Hamaguchi H, Fujioka M, Takahashi KA, Hirata T, Ishida M, Sakao K, Ushijima Y, Kubota T, Nishimura T, Kubo T. Age-related changes in the hemodynamics of the femoral head as evaluated by early phase of bone scintigraphy. Ann Nucl Med. 2006;20:35–40. doi: 10.1007/BF02985588. [DOI] [PubMed] [Google Scholar]
- 40.Griffith JF, Yeung DK, Tsang PH, Choi KC, Kwok TC, Ahuja AT, Leung KS, Leung PC. Compromised bone marrow perfusion in osteoporosis. J Bone Miner Res. 2008;23:1068–1075. doi: 10.1359/jbmr.080233. [DOI] [PubMed] [Google Scholar]
- 41.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117:5281–5288. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- 42.Rhinelander F, Stewart CL, Wilson JW. Skeletal Research. New York: Academic Press; 1979. Bone vascular supply; pp. 367–395. [Google Scholar]
- 43.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 44.Kassem M, Risteli L, Mosekilde L, Melsen F, Eriksen EF. Formation of osteoblast-like cells from human mononuclear bone marrow cultures. APMIS. 1991;99:269–274. doi: 10.1111/j.1699-0463.1991.tb05149.x. [DOI] [PubMed] [Google Scholar]
- 45.Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse JM. Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J Bone Miner Res. 2013;28:574–585. doi: 10.1002/jbmr.1760. [DOI] [PubMed] [Google Scholar]
- 46.De Bruyn PP, Breen PC, Thomas TB. The microcirculation of the bone marrow. Anat Rec. 1970;168:55–68. doi: 10.1002/ar.1091680105. [DOI] [PubMed] [Google Scholar]
- 47.De Saint-Georges L, Miller SC. The micocirculation of bone and marrow in the diaphysis of the rat hemopoietic long bones. Anat Rec. 1992;233:169–177. doi: 10.1002/ar.1092330202. [DOI] [PubMed] [Google Scholar]
- 48.Trias A, Fery A. Cortical circulation of long bones. J Bone Joint Surg Am. 1979;61:1052–1059. [PubMed] [Google Scholar]
- 49.Johnson DL, McAllister TN, Frangos JA. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol. 1996;271:E205–E208. doi: 10.1152/ajpendo.1996.271.1.E205. [DOI] [PubMed] [Google Scholar]
- 50.Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J. Bone Miner. Res. 2006;21:183–192. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- 51.McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun. 2000;270:643–648. doi: 10.1006/bbrc.2000.2467. [DOI] [PubMed] [Google Scholar]
- 52.Bergula AP, Haidekker MA, Huang W, Stevens HY, Frangos JA. Venous ligation-mediated bone adaptation is NOS 3 dependent. Bone. 2004;34:562–569. doi: 10.1016/j.bone.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Bergula AP, Huang W, Frangos JA. Femoral vein ligation increases bone mass in the hindlimb suspended rat. Bone. 1999;24:171–177. doi: 10.1016/s8756-3282(98)00165-3. [DOI] [PubMed] [Google Scholar]
- 54.Revell WJ, Brookes M. Haemodynamic changes in the rat femur and tibia following femoral vein ligation. J Anat. 1994;184:625–633. [PMC free article] [PubMed] [Google Scholar]
- 55.Ralston SH. Nitric oxide and bone: what a gas! Br J Rheumatol. 1997;36:831–838. doi: 10.1093/rheumatology/36.8.831. [DOI] [PubMed] [Google Scholar]
- 56.Hikiji H, Shin WS, Oida S, Takato T, Koizumi T, Toyo-Oka T. Direct action of nitric oxide on osteoblastic differentiation. FEBS Lett. 1997;410:238–242. doi: 10.1016/s0014-5793(97)00597-8. [DOI] [PubMed] [Google Scholar]
- 57.Chambers TJ, Ali NN. Inhibition of osteoclastic motility by prostaglandins I2, E1, E2 and 6-oxo-E1. J Pathol. 1983;139:383–397. doi: 10.1002/path.1711390313. [DOI] [PubMed] [Google Scholar]
- 58.Kasten TP, Collin-Osdoby P, Patel N, Osdoby P, Krukowski M, Misko TP, Settle SL, Currie MG, Nickols GA. Potentiation of osteoclast bone-resorption activity by inhibition of nitric oxide synthase. Proc Natl Acad Sci U.S.A. 1994;91:3569–3573. doi: 10.1073/pnas.91.9.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van't Hof RJ, Ralston SH. Cytokine-induced nitric oxide inhibits bone resorption by inducing apoptosis of osteoclast progenitors and suppressing osteoclast activity. J Bone Miner Res. 1997;12:1797–1804. doi: 10.1359/jbmr.1997.12.11.1797. [DOI] [PubMed] [Google Scholar]