Abstract
Aims
Atrial fibrillation (AF) ablation is generally performed after patients fail antiarrhythmic drug (AAD) therapy. Some patients have drug contraindications or choose to avoid a lifetime of drug therapy. Little is known about the impact of previous drug therapy on ablation outcomes. We evaluated AAD use before AF ablation and its impact on ablation outcomes.
Methods and results
We evaluated freedom from AF after ablation and patients' clinical characteristics by number of AADs failed in 1125 patients undergoing 1504 ablations. We also evaluated reasons why some patients did not receive prior drug therapy. Cox multivariate analysis examined factors predicting ablation failure. Patients failing more drugs before ablation were older (P = 0.001), had a longer duration of AF (P = 0.0001), were more likely female (P = 0.037), had more repeat ablations (P = 0.045), and less paroxysmal AF (P = 0.003). For patients with either paroxysmal or persistent AF, the number of drugs failed predicted AF recurrence (P = 0.0001). Other factors predicting AF recurrence following final ablation included age (P = 0.004), left atrial size (P = 0.002), female gender (P = 0.0001), and persistent AF (P = 0.0001). The reason for not receiving prior drug therapy was medical in 21.5% and patient choice in 78.5%. Number of drugs failed did not influence ablation outcome for patients with long-standing persistent AF (P = 0.352).
Conclusions
For paroxysmal and persistent AF patients undergoing ablation, those failing fewer AADs have different clinical characteristics than those who fail more drugs. Our study also suggests that the more drugs failed pre-ablation, the lower the freedom from AF post-procedure, possibly due to AF progression during drug trials.
Keywords: Atrial fibrillation, Ablation, Antiarrhythmic drugs, AF ablation outcomes
Introduction
Catheter ablation is an established treatment for symptomatic atrial fibrillation (AF).1–5 In the 2006 American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guidelines for the management of AF, catheter ablation is a Class IIa recommendation as an alternative to pharmacological therapy to prevent symptomatic, recurrent AF in patients with little to no left atrial (LA) enlargement.1 This recommendation was endorsed by the Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society (HRS/EHRA/ECAS) guidelines in 2007 and was not updated in the 2011 focused update.2,3 In 2010, the European Society of Cardiology (ESC) guidelines were the first to suggest that some patients with paroxysmal AF (AF1) may proceed to ablation without failing antiarrhythmic drugs (AADs) if they were symptomatic despite rate control and had no underlying heart disease.4 Similarly, the 2011 Canadian Cardiovascular Society (CCS) guidelines recommend ablation for symptomatic patients after failing AAD therapy, but conditionally suggest that first-line ablation may be performed for symptom relief in highly selected patients with AF1.5 Despite the failure of AADs to provide advantages over rate-control therapy6 and data from randomized clinical trials suggesting improved freedom from AF after catheter ablation compared to AADs,7 ablation remains second-line therapy to be considered after failing AADs. There are few studies directly comparing ablation with AADs as first-line therapy8 and most available outcomes data are from patients who failed prior AAD therapy.9–17 The present study examines AAD use prior to catheter ablation with regard to the reasons why some patients did not receive AADs before AF ablation, the differences in clinical characteristics depending upon number of prior AADs failed and the impact of previous AAD therapy on ablation outcomes.
Methods
Patient population
The subjects were consecutive symptomatic patients undergoing AF ablation at Sequoia Hospital, Redwood City, California from 10 October 2003 to 31 December 2010. All signed written informed consent for the ablation procedures. Data collection was prospective, data analysis was retrospective, and use of the data was approved by the hospital Institutional Review Board. Atrial fibrillation type was categorized as paroxysmal (AF1: lasting <1 week), persistent (AF2: lasting >1 week and <1 year or requiring pharmacological or electrical cardioversion in <1 week), and long-standing persistent (AF3: lasting >1 year).1
Ablation protocol
Our ablation protocol18 and anticoagulation strategy19 have been previously published and some of the patients in this study were included in previous publications.16,17 Briefly, AADs were stopped ≥5 half-lives and amiodarone >3 months before ablation. General anaesthesia and venous access from the right groin was used in most ablations. A 7F duodecapolar catheter (Livewire™, St Jude, St Paul, MN) was placed around the tricuspid valve annulus with the distal poles in the coronary sinus. A 9F Boston Scientific (Natick, MA) Ultra Ice™ catheter guided the transseptal puncture, done using a 71 cm St Jude BRK™ or Baylis (Montreal, QC) NRG™ needle.20 Patients had a femoral or radial arterial line. The St Jude NavX™ system was used in all cases. Prior to January 2006, we used a closed-tip catheter [Boston Scientific Blazer II™ or Webster (Diamond Bar, CA) Celsius™ 8 mm] and thereafter, an open-irrigated tip catheter (Webster Thermocool™ 3.5 mm or St Jude Cool Path™ or Sapphire-Blu™ 4.0 mm). Most irrigated tip catheter ablations were done using 50 W and the technique of ‘perpetual motion’ where the catheter is moved back and forth across a small area and not left at single sites for extended times.18 All patients underwent circumferential atrial ablation around all pulmonary veins (PVs) and an LA roof-line ablation. Only patients with right or LA isthmus flutter underwent caval-tricuspid or mitral isthmus line ablation. Many patients had low posterior LA lines and those with LA complex-fractionated electrograms had them ablated. Some patients underwent ablation in the coronary sinus (at 30–35 W) and superior vena cava isolation. A circumferential mapping catheter (7F Webster Lasso™ or St Jude Reflexion Spiral™) was used to isolate all PVs defined as complete local electrical silence indicating entrance block. After May 2010 all patients underwent pacing from the PVs to document exit block. NavX™ activation and entrainment mapping were used to ablate flutters and tachycardias. Isoproterenol was given and non-PV triggers were mapped and ablated. Repeat ablations were done >3 months after initial ablation.
Anticoagulation
Patients receiving warfarin continued it until 5 days pre-procedure. Three days pre-procedure, they began enoxaparin 1 mg/kg for every 12 h with the last dose 24 h pre-ablation. Patients with AF2, AF3, or frequent AF1 underwent transoesophageal echocardiogram. When the transseptal sheath entered the LA, patients received systemic heparinization to a target activated clotting time of 225 s.19 Post-ablation, we used enoxaparin 0.5 mg/kg q12h until warfarin (continued ≥3 months) achieved an International Normalized Ratio of 2.0–3.0.
Data collection and analysis
For each patient we recorded age, AF duration and type, prior AAD therapy, CHADS2 score, body mass index (BMI), strokes/transient ischaemic attacks (TIAs), and the presence of hypertension, diabetes, coronary artery disease, and cardiomyopathy. Antiarrhythmic drugs were defined as flecainide, propafenone, amiodarone, sotalol, dofetilide, dronedarone, dysopyramide, quinidine, and procainamide. For patients not failing AADs prior to ablation, we determined the reasons they had not received AADs. Outcome was determined for each AF type after the initial and after the final ablation in all patients.
Follow-up
Many patients were treated with AADs during the 3-month blanking period post-ablation. Patients transmitted daily electrocardiogram (ECG) strips for 1–3 months post-ablation and were seen at 3 months when an echocardiogram and ambulatory ECG monitor for ≥24 h were performed. A successful ablation was defined as no AF, flutter, or tachycardia exceeding 30 s after a 3-month blanking period off of AADs2 and failures were encouraged to undergo a repeat ablation.16 Patients were seen or contacted frequently from 3 to 12 months and came for a 1-year follow-up with echocardiogram and 24 h ECG. Thereafter, every 6–12 months patients were seen directly or contacted by phone by research nurses or the attending physician and arrhythmia records obtained from hospitals and referring physicians. Patents were advised to call for arrhythmia symptoms and ECG recorders were reissued to them. Late pacemaker AF data were utilized when available. All patients classified as AF free for >1 year post-final ablation were encouraged to undergo a 1-week continuous monitor. One patient was lost to follow-up.
Statistical analysis
Statistical analysis was done using XLSTAT 2010 (Paris, France). Continuous data were described as mean ± standard deviation and counts and per cent if categorical. Analysis of variance and the Cochran–Armitage trend analysis were used to compare the clinical parameters of age, gender, BMI, AF duration and type, LA size, CHADS2 score, and the presence of coronary artery disease, dilated cardiomyopathy, hypertension, and prior stoke/TIA between patients grouped by number of pre-ablation AADs failed. Cox multivariate regression analysis was used to examine the clinical variables of age, gender, LA size, AF duration and type, BMI, the number of AADs failed and the presence of diabetes, hypertension, or coronary artery disease to determine predictors of AF recurrence following both the initial and the final ablations. Kaplan–Meier curves were generated for AF-free survival by AF type and by number of AADs failed for the initial and the last ablation. All tests were two sided and P < 0.05 was considered statistically significant.
Results
Patient population
The subjects of this study were 1125 consecutive patients who underwent 1504 ablations for symptomatic AF. The underlying rhythm was AF1 in 348 (30.9%), AF2 in 594 (52.8%), and AF3 in 183 (16.3%). The mean age of all patients was 62.3 ± 10.3 and 28.8% were female. The mean duration of follow-up was 2.5 ± 1.7 years.
Patient characteristics by prior antiarrhythmic use
Table 1 shows the clinical characteristics of the AF1 and AF2 patients by number of AADs failed. Drug use prior to initial ablation was categorized as no AADs failed (n = 195), one AAD failed (n = 400), two AADs failed (n = 232), and ≥3 AADs failed (n = 115). Patients who failed more AADs tended to be older (P = 0.001), have a longer duration of AF (P = 0.001), were more likely female (P = 0.037), required more repeat ablations (P = 0.045), and were more likely to have AF2 than AF1 (P = 0.003). Patients who failed more drugs compared with those who failed fewer drugs had no difference in LA size, CHADs2 score, BMI, and the incidence of hypertension, diabetes, coronary artery disease, dilated cardiomyopathy, and prior stroke/TIA.
Table 1.
Clinical characteristics by number of antiarrhythmic drugs failed prior to initial ablation for the combined group of patients with AF1 and AF2
| Number of drugs failed | No drugs | One drug | Two drugs | Three or more drugs | P value |
|---|---|---|---|---|---|
| (n = 195) | (n = 400) | (n = 232) | (n = 115) | ||
| LA size (cm) | 4.20 ± 0.72 | 4.26 ± 0.69 | 4.21 ± 0.66 | 4.26 ± 0.60 | 0.675 |
| Age (years) | 61.1 ± 10.2 | 61.8 ± 10.6 | 62.7 ± 10.9 | 65.7 ± 9.6 | 0.001a |
| Duration of AF (years) | 5.2 ± 7.5 | 5.5 ± 6.4 | 7.4 ± 6.3 | 9.5 ± 8.9 | 0.0001a |
| Repeat ablations | 26.2% | 26.8% | 31.9% | 35.7% | 0.045a |
| % Female | 27.7% | 30.0% | 30.1% | 40.9% | 0.037a |
| Average CHADS2 score | 0.76 ± 0.95 | 0.82 ± 0.89 | 0.90 ± 1.05 | 1.03 ± 1.06 | 0.083 |
| Hypertension | 45.6% | 47.2% | 46.6% | 47.8% | 0.778 |
| Diabetes | 7.7% | 8.5% | 10.3% | 6.1% | 0.967 |
| Coronary artery disease | 11.3% | 13.0% | 15.5% | 16.5% | 0.115 |
| Dilated cardiomyopathy | 7.7% | 8.5% | 10.3% | 6.1% | 0.967 |
| BMI | 29.3 ± 5.1 | 29.2 ± 5.2 | 29.1 ± 5.5 | 29.7 ± 6.5 | 0.797 |
| AF1 | 43.1% | 64.2% | 35.0% | 34.7% | 0.003a |
| Prior stroke/TIA | 7.2% | 5.3% | 7.3% | 9.6% | 0.414 |
aStatistically significant.
AF, atrial fibrillation; TIA, transient ischaemic attack.
Outcome of initial and final atrial fibrillation ablation by number of prior antiarrhythmic drugs failed
Kaplan–Meier analysis showed that post-ablation freedom from AF was significantly better when fewer drugs were failed prior to ablation after both the initial ablation and final ablation for patients with AF1 (initial ablation P = 0.031; final ablation P = 0.010) and AF2 (initial ablation P = 0.001; final ablation P < 0.0001). The difference was not statistically significant for AF3 patients (initial ablation P = 0.505; final ablation P = 0.352).
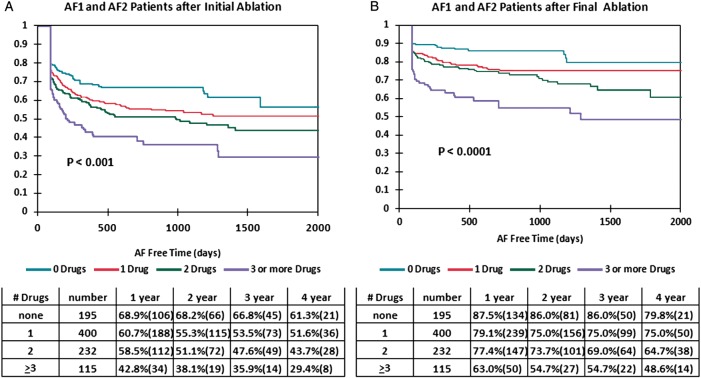
Figure 1A shows the Kaplan–Meier curve for freedom from AF after the initial ablation by number of AADs failed for the combined group of 942 patients with AF1 and AF2. The initial ablation success was greater for those patients failing fewer prior AADs (P < 0.0001). The 1- and 4-year freedom from AF rates after the initial ablation were 68.9 and 61.3% for those failing no AADs compared with 42.8 and 29.4% for those failing ≥3 AADs.
Figure 1.
Kaplan–Meier curves by number of antiarrhythmic drugs failed (no drugs: top curve; one drug: second curve; two drugs: third curve; and three or more drugs: bottom curve) after the initial ablation (A) and after the final ablation (B) for all patients with AF1 (paroxysmal atrial fibrillation) and AF2 (persistent atrial fibrillation) combined.
Figure 1B shows the Kaplan–Meier curve for freedom from AF after the final ablation by number of AADs failed for the combined AF1 and AF2 patient groups. Similar to the initial ablation, the fewer AADs failed prior to ablation the better the AF-free survival rate after the final ablation (P < 0.0001). The 1- and 4-year AF-free rates were 87.5 and 79.8% for those failing no AADs compared with 63.0 and 48.6% for those failing ≥3 AADs.
Prediction of ablation failure
Table 2 shows the Cox multivariate regression analysis for the 942 AF1 and AF2 patients after the initial and final ablation. A greater number of AADs failed pre-ablation was associated with a higher rate of AF recurrence post-ablation (P = 0.0001). Other factors predictive of AF recurrence included LA size (P = 0.0001), female gender (P = 0.0001), and AF2 vs. AF1 (P = 0.0001). The number of AADs failed was also predictive of AF recurrence after the final AF ablation (P = 0.0001). Following the final ablation, other predictors of ablation failure included older age (P = 0.004), larger LA size (P = 0.002), female gender (P = 0.0001), and AF2 vs. AF1 (P = 0.0001).
Table 2.
Cox Multivariate regression analysis of factors affecting atrial fibrillation recurrence after initial atrial fibrillation ablation and after final atrial fibrillation ablation for the combined group of patients with AF1 and AF2
| Variable | AF recurrence after initial ablation |
AF recurrence after final ablation |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | Hazard ratio 95% CI | P value | Hazard ratio | Hazard ratio 95% CI | P value | |
| Age | 0.998 | 0.988–1.008 | 0.704 | 1.022 | 1.007–1.038 | 0.004a |
| LA size | 1.354 | 1.154–1.306 | 0.0001a | 1.381 | 1.121–1.701 | 0.002a |
| BMI | 0.989 | 0.989–1.008 | 0.250 | 1.001 | 0.977–1.026 | 0.940 |
| Atrial fibrillation duration | 1.010 | 0.997–1.023 | 0.137 | 1.003 | 0.985–1.021 | 0.769 |
| Gender (female vs. male) | 1.579 | 1.274–1.956 | 0.0001a | 2.043 | 1.541–2.707 | 0.0001a |
| AF type (paroxysmal vs. persistent) | 0.668 | 0.537–0.830 | 0.0001a | 0.457 | 0.333–0.629 | 0.0001a |
| CAD (absent vs. present) | 0.801 | 0.606–1.058 | 0.118 | 0.747 | 0.529–1.054 | 0.097 |
| Hypertension (absent vs. present) | 0.894 | 0.723–1.105 | 0.299 | 0.891 | 0.673–1.179 | 0.420 |
| Diabetes (absent vs. present) | 0.732 | 0.526–1.018 | 0.064 | 0.798 | 0.521–1.221 | 0.298 |
| Total no. of antiarrhythmics failed | 1.193 | 1.089–1.306 | 0.0001a | 1.317 | 1.167–1.486 | 0.0001a |
aStatistically significant.
AF, atrial fibrillation; CAD, coronary artery disease; CI, confidence interval.
Prior antiarrhythmic drug use
Prior to the initial ablation, patients had failed an average 1.30 ± 1.05 AADs. Yearly analysis from 2003 to 2010 showed no change in the number of drugs failed prior to initial ablation (P = 0.336). Table 3 shows the individual AADs failed for the combined group of AF1 and AF2 patients by number of AADs failed prior to catheter ablation. Propafenone was the most frequently failed drug across all groups. Table 4 summarizes the reasons why 195 patients with AF1 and AF2 proceeded to ablation without first failing AADs. In 42 (21.5%) patients, there was a physician and/or medical decision not to use an AAD. The most commonly documented medical reasons included pre-existing sinoatrial node disease, which may have been exacerbated by AADs or may have necessitated permanent pacemaker implantation to facilitate AAD therapy. Primary AF ablation rather than initial drug therapy was chosen by 153 (78.5%) patients. Among AF3 patients, 63 (33.9%) had not failed a drug. The most common reason the AF3 patients did not receive AADs as first-line therapy was a prior physician decision for a rate-control strategy in 30 patients who subsequently elected primary ablation once the decision was made to pursue rhythm control. The other reasons for no AADs in AF3 patients was patient preference (n = 27), bradycardia (n = 2), and cardiomyopathy (n = 3).
Table 3.
Individual antiarrhythmic drugs failed for the combined group of patients with AF1 and AF2
| Antiarrhythmic drug | Patient groups by total number of drugs failed |
||
|---|---|---|---|
| One drug | Two drugs | Three or more drugs | |
| (n = 400) (%) | (n = 232) (%) | (n = 115) (%) | |
| Amiodarone | 17.8 | 43.5 | 63.5 |
| Propafenone | 42.5 | 59.5 | 80 |
| Flecainide | 17.0 | 38.8 | 54.8 |
| Sotalol | 14.0 | 29.3 | 54.8 |
| Dronedarone | 4.3 | 9.1 | 25.2 |
| Quinidine | 0.0 | 1.7 | 13.9 |
| Dofetilide | 2.5 | 12.9 | 27.0 |
| Procainamide | 0.5 | 3.0 | 7.0 |
| Disopyramide | 1.5 | 2.2 | 11.3 |
Table 4.
Reasons why patients did not fail an antiarrhythmic drug before atrial fibrillation ablation
| Reason for no prior AAD | Total (N = 195) |
|---|---|
| Physician or medical decision | |
| Sinus node disease/bradycardia | 25 |
| Conduction system disease | 1 |
| Coronary artery disease | 6 |
| Cardiomyopathy | 6 |
| Unexplained syncope | 1 |
| Liver or kidney disease | 3 |
| Total physician or medical decision | 42 (21.5%) |
| Patient decision | |
| Patient preference | 153 |
| Total patient decision | 153 (78.5%) |
Complications
Minor complications occurred in 1.4% of ablations and major complications in 1.6% of ablations [pericaridal tamponade in nine, groin complications requiring transfusion or surgery in eight, strokes in four (two with minor residual), and a 70% PV stenosis not requiring intervention, a severe tongue haematoma and a severe protamine reaction in one each]. There were no procedure-related deaths, PV stenoses requiring intervention or atrial-oesophageal fistulae.
Discussion
Our study reveals two important findings. First, patients with AF1 and AF2 who undergo catheter ablation instead of one or more AADs as first-line therapy have improved rates of freedom from AF post-ablation. Second, AF1 and AF2 patients who failed AADs before ablation were older, had a longer duration of AF, were more likely female, had more repeat ablations, and more AF2.
The various AF guidelines differ with regard to their recommendations for AAD therapy prior to ablation.1–5 These differences have been highlighted by Gillis and Skanes.21 The current ACC/AHA/HRS guidelines1–3 provide no pathway to ablation without AAD failure and suggest that patients consider ablation after failing ≥1 drug only if they have minimal structural heart disease and undergo ablation at an experienced centre (≥50 cases/year).3 The CCS guidelines provide a weak recommendation for ablation in some AF1 patients who have not yet failed an AAD.5 They mention tachy-brady syndrome patients who might avoid a pacemaker with a successful ablation and suggest most patients should fail ≥2 drugs before ablation.5 Exceptions might be younger patients desiring to avoid amiodarone, patients with drug contraindications, or those failing one drug in a class where another drug in that class is unlikely to be effective. The ESC guidelines provide a conditional recommendation for primary ablation in symptomatic AF1 patients with no or minimal heart disease.4
There are little data to support the guideline's recommendations that AADs should be first-line therapy with ablation offered primarily to those patients who fail ≥1 AADs. A small, randomized trial of ablation vs. AADs as first-line treatment for AF1 indicated that long-term outcomes were better in patients randomized to ablation (87% AF free with ablation vs. 37% with drugs).8 The Catheter Ablation vs. Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA), a National Institutes of Health sponsored study (clinicaltrials.gov identifier # NCT00911508), prospectively randomizes patients to ablation vs. AAD therapy, but is still recruiting patients. A multicentre registry reported 7% of patients did not receive AAD prior to ablation but provides no outcomes for this subgroup.22 Another single-centre study in 434 patients undergoing ablation compared 17% of patients never treated with an AAD with those failing ≥1 AAD trial prior to ablation.23 The results were similar to the present study. Patients undergoing ablation therapy prior to AAD failure had a shorter duration of AF pre-ablation, improved post-ablation freedom from AF, and required fewer repeat ablations. Our study indicates that not only do those patients who had never failed an AAD have better ablation outcomes than those who failed ≥1 drugs, but also that each drug failed is associated with a worse AF ablation outcome.
There are several possible reasons why patients undergoing ablation as first-line therapy have less AF post-procedure than those previously failing AADs. Patients failing AADs may constitute a subgroup that is more difficult to treat with ablation. It is most likely that the additional time required to fail ≥1 drugs allows for disease progression and a transition from paroxysmal to more persistent AF, a factor well known to reduce ablation efficacy.5–15 We as physicians have no control of patient age, gender, or LA size at the time of AF presentation. We do, however, control how many drugs a patient must fail before ablation. If a physician and patient choose to follow the path of multiple drug trials, they need to recognize the potential adverse effect this may have on ablation outcome down the road.
All currently available AADs carry United States Food and Drug Administration mandated ‘black box’ warnings regarding their potential dangers, which can be frightening to patients. Many patients have either relative or absolute contraindications to some or all AADs, and many patients with AF wish to avoid either the potential danger of AADs and/or a lifetime of drug therapy. Although we have always advised patients that it is recommended that they fail at least one AAD prior to ablation, we have not insisted on this in patients with relative contraindications or patients who, after careful consideration, desire to avoid AADs and proceed directly to ablation therapy.
Without a large randomized trial, it is hard to support a strong recommendation that all patients be offered ablation therapy as first-line treatment. However, our data suggest that we should not actively discourage patients from first-line ablation if they have made an informed decision based on their desire to avoid AADs. Referring physicians should also be aware that delaying referral and serially trying multiple AADs may decrease a patient's likelihood of freedom from AF post-ablation. Proceeding to ablation earlier in the disease process may ultimately reduce ablation costs by preventing repeat ablations and improving the likelihood of procedural success. Although the ESC and CCS guidelines4,5 permit the option of proceeding directly to ablation for selected patients with AF1, our data suggest that this should also be considered for all patients with AF1 as well as those with AF2. Once a patient has progressed to AF3, the issues of how many AADs a patient has failed may be moot, since the number of AADs failed does not appear to predict ablation outcome in this subgroup and it is also unlikely that trials of AADs will prevent AF in many of these patients.
Limitations
Our data are from a single-centre case series which has inherent limitations including selection bias, referral bias, and lack of a control group. The efficacy of ablation therapy as first-line therapy vs. AADs would be best answered by a randomized trial. As in all long-term follow-up studies our ability to monitor patients decreased over time and we could have missed asymptomatic AF over time despite our attempts at regular follow-up and frequent contact by our trained research personnel. Despite its shortcomings, our method of long-term patient follow-up is essentially the same as that used by most other referral centres reporting on long-term outcomes of AF ablations.11,12,24,25 Any missed asymptomatic AF should have occurred equally across all subgroups and is not likely to have changed the conclusions of our study.
Conclusions
AF1 and AF2 patients who have failed more AADs prior to ablation are older, have a longer duration of AF, are more likely to be female, require more repeat ablations, and have more AF2 than those who have failed fewer AADs. The number of AADs failed is an independent predictor of freedom from AF post-ablation possibly due to AF progression while drugs are being tried. The number of AADs failed does not influence ablation outcome of AF3 patients. It is unclear as to whether all AF1 and AF2 patients should proceed directly to ablation without failing an AAD; however, we should permit primary ablation in those who desire to avoid AADs.
Conflict of interest: R.H.M. serves on advisory boards for Medtronic, iRhythm, Proteus Biomedical, and Voyage Medical, has stock options for iRhythm, Voyage Medical, and Proteus Biomedical, and is a director for iRhythm and Voyage Medical. R.A.W. and R.A. are investigators for Cardio Robotics and CABANA Trial. R.A.P. is a consultant for and has stock options in Voyage Medical and is a speaker for St. Jude Medical. G.E. is a speaker for Medtronic. M.H.K. is on Advisory Boards for Medtonic and Biotronik. R.A.W., R.H.M., G.E. and R.A.P. are Investigators for Medtronic, Cameron Health, and Sanofi-Aventis.
Funding
Funding for this study, including the Open Access publication charges, was provided by Silicon Valley Cardiology.
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Brugada J, Packer DL, Capppato R, Chen S, Crijns H, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Europace. 2007;9:335–79. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 3.Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NAM, et al. ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation updating the 2006 guideline) Heart Rhythm. 2011;8:157–76. doi: 10.1016/j.hrthm.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Kirchhof P, Lip GYJ, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation. Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 5.Verma A, Macle L, Cos J, Skanes AC. Canadian cardiovascular society atrial fibrillation guidelines 2010: catheter ablation for atrial fibrillation/atrial flutter. Can J Cardiol. 2011;27:60–6. doi: 10.1016/j.cjca.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 6.The Atrial Fibrillation Follow-up Investigation of Rhythm Management Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 7.Piccini JP, Lopes RD, Kong MH, Hasselblad V, Jackson KJ, Al-Khatib SM. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized controlled trials. Circ Arrhythm Electrophysiol. 2009;2:626–33. doi: 10.1161/CIRCEP.109.856633. [DOI] [PubMed] [Google Scholar]
- 8.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, et al. Radiofrequency ablation vs. Antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation : a randomized trial. JAMA. 2005;293:2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 9.Shah AN, Mittal S, Sichrovsky TC, Cotiga D, Arshad A, Maleki K, et al. Long-term outcome following successful pulmonary vein isolation: pattern and prediction of very late recurrence. J Cardiovasc Electrophysiol. 2008;19:661–7. doi: 10.1111/j.1540-8167.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 10.Gerstenfeld EP, Sauer W, Callans DJ, Dixit S, Lin D, Russo AM, et al. Predictors of success after selective pulmonary vein isolation of arrhythmogenic pulmonary veins for treatment of atrial fibrillation. Heart Rhythm. 2006;3:165–70. doi: 10.1016/j.hrthm.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, Callans DJ, et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:237–42. doi: 10.1161/CIRCEP.109.923771. [DOI] [PubMed] [Google Scholar]
- 12.Wokhlu A, Hodge DO, Monahan KH, Asirvatham SJ, Friedman PA, Munger TM, et al. Long-term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J Cardiovasc Electrophysiol. 2010;21:1071–6. doi: 10.1111/j.1540-8167.2010.01786.x. [DOI] [PubMed] [Google Scholar]
- 13.Patel D, Mohanty P, Di Biase L, Sanchez JE, Shaheen MH, Burkhardt JD, et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010;7:167–72. doi: 10.1016/j.hrthm.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Bhargava M, Di Biase L, Mohanty P, Prasad S, Martin DO, Andrew MW, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom of atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009;6:1403–12. doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Forleo GB, Tondo C, De Luca L, Dello Russo A, Casella M, De Sanctis V, et al. Gender-related differences in catheter ablation of atrial fibrillation. Europace. 2007;9:613–20. doi: 10.1093/europace/eum144. [DOI] [PubMed] [Google Scholar]
- 16.Winkle RA, Mead RH, Engel G, Patrawala RA. Long term results of atrial fibrillation ablation: the importance of all initial ablation failures undergoing a repeat ablation. Am Heart J. 2011;162:193–200. doi: 10.1016/j.ahj.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Winkle RA, Mead RH, Engel G, Patrawala RA. Relation of early termination of persistent atrial fibrillation by cardioversion or drugs to ablation outcomes. Am J Cardiol. 2011;108:374–9. doi: 10.1016/j.amjcard.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 18.Winkle RA, Mead RH, Engel G, Patrawala RA. Atrial fibrillation ablation: perpetual motion of open irrigated tip catheters at 50 watts is safe and improves outcomes. Pacing Clin Elecrophysiol. 2011;34:531–9. doi: 10.1111/j.1540-8159.2010.02990.x. [DOI] [PubMed] [Google Scholar]
- 19.Winkle RA, Mead RH, Engel G, Patrawala RA. Safety of lower activated clotting times during atrial fibrillation ablation using open irrigated tip catheters and a single transseptal puncture. Am J Cardiol. 2011;107:704–8. doi: 10.1016/j.amjcard.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Winkle RA, Mead RH, Engel G, Patrawala RA. The use of a radiofrequency needle improves the safety and efficacy of transseptal puncture for atrial fibrillation ablation. Heart Rhythm. 8:411–15. doi: 10.1016/j.hrthm.2011.04.032. 2011. [DOI] [PubMed] [Google Scholar]
- 21.Gillis AM, Skanes AC. Comparing the 2010 North American and European Atrial Fibrillation Guidelines. Can J Cardiol. 2011;27:7–13. doi: 10.1016/j.cjca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Cappato R, Calkins H, Chen SA, Chen S, Davies W, Iesaka Y, et al. Worldwide survey on the methods, efficacy and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–5. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 23.Tanner H, Makowki K, Roten L, Seiler J, Schwick N, Müller C, et al. Catheter ablation of atrial fibrillation as first-line therapy – a single-centre experience. Europace. 2011;13:646–53. doi: 10.1093/europace/eur065. [DOI] [PubMed] [Google Scholar]
- 24.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, et al. Catheter ablation for atrial fibrillation. Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 25.Cheema A, Vasamraddy CR, Dalal D, Dalal D, Marine JE, Dong J, et al. Long-term single procedure efficacy of atrial fibrillation ablation. J Interv Card Electrophysiol. 2006;15:145–55. doi: 10.1007/s10840-006-9005-9. [DOI] [PubMed] [Google Scholar]