Abstract
Mesenchymal stem and progenitor cells (MSPCs) contribute to bone marrow (BM) homeostasis by generating multiple types of stromal cells. MSPCs can be labeled in the adult BM by Nestin-GFP, whereas committed osteoblast progenitors are marked by Osterix expression. However, the developmental origin and hierarchical relationship of stromal cells remain largely unknown. Here, using a lineage-tracing system, we describe three distinct waves of contributions of Osterix+ cells in the BM. First, Osterix+ progenitors in the fetal BM contribute to nascent bone tissues and transient stromal cells that are replaced in the adult marrow. Second, Osterix-expressing cells perinatally contribute to osteolineages and long-lived BM stroma, which have characteristics of Nestin-GFP+ MSPCs. Third, Osterix labeling in the adult marrow is osteolineage-restricted, devoid of stromal contribution. These results uncover a broad expression profile of Osterix and raise the intriguing possibility that distinct waves of stromal cells, primitive and definitive, may organize the developing BM.
INTRODUCTION
The bone marrow (BM) environment is composed of multiple cell types, most of which are thought to be derived from mesenchymal stem and progenitor cells (MSPCs) (Bianco et al., 2013; Caplan, 1991; Frenette et al., 2013). Stromal progenitor activity in the BM was initially isolated from clonal populations of fibroblastic colony-forming units (CFU-F) that exhibit self-renewal and the capacity to differentiate into the major mesenchymal lineages (Friedenstein et al., 1968; Mendez-Ferrer et al., 2010; Sacchetti et al., 2007). Although surface markers have been suggested to mark MSPCs (Dominici et al., 2006), these were based on cultured stromal cells, but not on prospectively isolated native stroma, and lack specificity to identify native bone marrow MSPCs (Bianco et al., 2013). In the mouse BM, transgenic mice expressing GFP under the Nestin promoter (Nes-GFP) select for MSPC activity, and so do stromal cells with CD45− Tie2− CD90− CD51+ CD105+ phenotype (Chan et al., 2009), CXCL12 abundant reticular (CAR) cells (Omatsu et al., 2010), PDGFRα+ Sca-1+ (Morikawa et al., 2009), CD51+ PDGFRα+ (Pinho et al., 2013), and Prx-1-derived CD45− Ter119− PDGFRα+ Sca-1+ populations (Greenbaum et al., 2013). There is evidence that these stromal cell populations display some significant overlap with each other and comprise important cellular constituents of the hematopoietic stem cell (HSC) niche. For example, Nes-GFP+ cells highly overlap with leptin receptor (Lepr)-expressing perivascular cells (Pinho et al., 2013), which were shown to be a major source of Stem Cell Factor (SCF) and CXCL12 in the BM (Ding and Morrison, 2013; Ding et al., 2012). These reports thus suggest that MSPCs organize the BM environment by contributing to osteolineage cells and regulating HSC self-renewal and differentiation.
Additionally, other studies have suggested a role for osteoblasts as a constituent of the HSC niche. Gain- and loss-of function approaches have shown that alterations in osteoblast numbers correlate with the number of HSCs (Calvi et al., 2003; Visnjic et al., 2004; Zhang et al., 2003), although the correlation was not observed in other models (Kiel et al., 2007; Lymperi et al., 2008). Osteoblasts have been suggested to regulate the HSCs via secretion of angiopoietin-1 (Arai et al., 2004), osteopontin (Nilsson et al., 2005; Stier et al., 2005), and noncanonical Wnt signaling (Sugimura et al., 2012). However, the expression of these factors is not specific to osteoblasts and there is no evidence thus far that specific deletion of these factors in committed osteoblasts affects HSC maintenance.
One of the promoters expressed in the bone marrow thought to be specific to the osteolineage is Osterix (Osx), a transcription factor shown to be required for bone formation (Nakashima et al., 2002). During bone development, Osx+ osteoblast precursors appear around the perichondrium and subsequently migrate into the developing primary ossification center along with blood vessels, giving rise to mature osteolineage cells (Karsenty and Wagner, 2002; Maes et al., 2010). In the adult, Osx+ cells provide a transient source of osteoblasts (Park et al., 2012), implying the presence of a more primitive source sustaining osteolineage cells throughout the lifetime. Here we show, unexpectedly that Osx marks successive waves of progenitors during ontogeny, including bona fide MSPCs at the perinatal stage. In addition, our studies have uncovered temporally distinct stromal precursors, termed primitive and definitive stroma, that differentially contribute to skeletal development.
RESULTS AND DISCUSSION
Neonatal Osx+ cells give rise to long-lived BM stromal cells
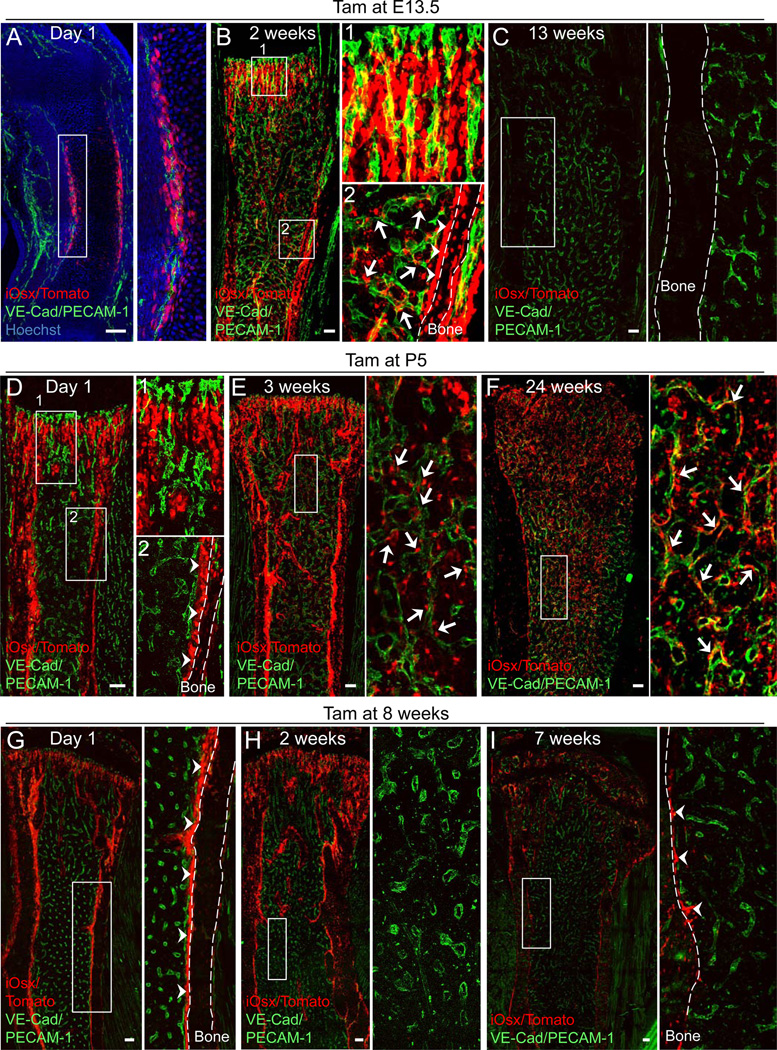
To trace lineages of Osx-expressing cells in the developing bone and BM, we generated double-transgenic Osx-creERT2 /ROSA26-loxP-stop-loxP-tdTomato (iOsx/Tomato) reporter mice where cre expression in Osx+ cells can be induced at different developmental stages by administration of tamoxifen (Tam). Consistent with previous observations (Maes et al., 2010), Osx+ cells were labeled in the perichondrium 1 day after Tam injection in embryonic day 13.5 (E13.5) mice, before the formation of a BM cavity (Figure 1A). The iOsx expression was consistent with staining of the endogenous protein using an anti-Osx antibody confirming the specificity of transgenic expression (Figure S1A). After a chase of 2 weeks, Osx+ cell progeny (designated E13.5-iOsx/Tomato+ cells) were detected in the primary spongiosa adjacent to blood vessels (Figure 1B1) labeled by VE-cadherin and PECAM-1 staining, and around the cortical bone (Figure 1B2). Osx+ cells labeled during fetal BM development gave rise to the full spectrum of osteolineage cells in the growing bone (Maes et al., 2010). Interestingly, our results revealed that 2 weeks after labeling, E13.5-iOsx/Tomato+ cells were not only detected in bone tissues, but also in stromal cells which did not express osteocalcin, in contact with the vasculature (Figures 1B2 and S1C). However, following a chase of 13 weeks, the osteolineage and BM stromal cells were dramatically decreased, along with the longitudinal growth, suggesting that embryonic Osx+ progenitor cells transiently contribute to the developing bone and marrow stroma (Figures 1C and S1D).
Figure 1. Lifelong contribution of Osx+ cells to the BM cells in developing bones.
(A–I) Z-stack confocal images of thick bone sections of iOsx/Tomato mice administered with tamoxifen (Tam) at embryonic day 13.5 (E13.5) (A–C), postnatal day 5 (P5) (D–F), and 8 week-old (G–I) mice analyzed at the indicated periods. Bone sections were stained with VE-cadherin (VE-Cad), PECAM-1 antibodies (green) and Hoechst 33342 (blue). Right panels are magnified views of the boxed areas. Scale bars: 100 µm. Arrows: iOsx-derived Tomato+ (iOsx/Tomato+) stromal cells. Arrowheads: iOsx/Tomato+ osteolineage cells. See also Figures S1 and S2A-G.
We then marked Osx+ cells at post-natal day 5 (PND5; designated P5-iOsx/Tomato+ cells), and found that they included cells of the primary spongiosa and around the cortical bone, but not in the BM cavity 1 day after Tam injection (Figure 1D). The specificity of the iOsx-marked cells was also confirmed at this time point using anti-Osx antibody staining (Figure S1B). FACS analysis revealed that P5-iOsx/Tomato+ cells in the bone tissue were CD45− Ter119− CD31− stromal cells (97.6 ± 0.4%) and that these cells were rarely detected in the BM 1 day after Tam injection (Figure S1E). However, after a chase of 3 weeks, P5-iOsx/Tomato+ osteoblasts and osteocytes were detected around the bone and, unexpectedly, P5-iOsx/Tomato+ stromal cells were also observed adjacent to blood vessels in the BM cavity (Figure 1E). In addition, the stromal cell labeling persisted for at least 32 weeks throughout the BM (Figure 1F: chase of 24 weeks; Figure S1F: chase of 32 weeks). FACS analysis of P5-iOsx/Tomato+ cells after a chase for 15 weeks indicated that they remained confined to the stromal CD45− Ter119− CD31− compartment (97.7 ± 0.4%; Figure S1G). Further analyses of the characteristics of the P5-iOsx/Tomato+ BM cells revealed that the cells became quiescent during bone growth (Figure S1H and I). The contribution of P5-iOsx/Tomato+ cells to the BM stroma increased in a time-dependent manner as seen by the progressive increased frequency within total stroma and their absolute number per femur (Figure S2A and B). By contrast, iOsx/Tomato mice pulsed in the adult stage (8 weeks of age) displayed restricted labeling to the bone tissues 1 day post-Tam injection (Figure 1G), and did not generate BM stromal cells after 2 or 7 weeks chase (Figure 1H and I). Consistent with recent studies (Park et al., 2012), iOsx labeling was reduced after 7 weeks (Figure 1G–I), suggesting that iOsx marks non-self-renewing osteoprogenitors in the 8 weeks pulsed iOsx/Tomato mice. Indeed, after a chase of 12–15 weeks, a significantly higher number of iOsx/Tomato+ bone-lining cells was found in the P5 pulsed mouse bone tissue than in the 8 week-old pulsed mice (Figure S2C-E; 38.6 ± 6.5% vs 8.0 ± 1.4%, p<0.05), and these cells were sustained for up to 32 weeks (Figure S1F), suggesting that the P5-iOsx/Tomato+ cells contain long-lived stromal cells that contribute to osteolineages. These stromal cells can self-renew in vitro since clonally expanded P5-iOsx/Tomato+ cells were able to form primary spheres in non-adherent culture conditions (Mendez-Ferrer et al., 2010), and when dissociated, could form secondary clonal spheres with a similar efficiency as the primary spheres (Primary: 0.6 ± 0.1%; Secondary: 0.5 ± 0.1%; Figure S2F and G). These results thus suggest that iOsx temporally marks at least three distinct mesenchymal precursor cells in the fetal, perinatal, and adult bone marrow, and that the perinatal Osx-expressing cells, which become quiescent during bone growth, also exhibit self-renewal capacity.
P5-iOsx-derived BM stromal cells possess MSPC characteristics
To test whether P5-iOsx/Tomato+ stromal cells contained MSPC activity, we generated Nes-Gfp/iOsx/Tomato triple-transgenic mice. One day after Tam injection, a fraction of P5-iOsx/Tomato+ cells in the primary spongiosa and around the cortical bone area were Nes-GFP+ (Figure 2A). FACS analysis showed that 38.2 ± 2.2% of P5-iOsx/Tomato+ cells in the bone tissue expressed Nes-GFP (Figure 2C). However, most (84.9 ± 6.5%) of P5-iOsx/Tomato+ BM cells were Nes-GFP+ by histological and FACS analyses 15 weeks after Tam injection (Figure 2B and D). P5-iOsx/Tomato+ BM stromal cells were highly enriched in CFU-F activity (Figure 2E) whose colonies exhibited Tomato fluorescence (Figure 2F), thereby confirming that they were derived from Osx+ cells marked at P5. Furthermore, the clonally expanded P5-iOsx/Tomato+ BM cells exhibited tri-lineage differentiation potential (Figure 2G–I). The persistence of perinatally marked iOsx stromal cells in the bone marrow is consistent with recently published data in which E14.5 Osx-expressing cells marked long-lived stroma (Liu et al., 2013). Thus, definitive MSPCs may be established late in gestation.
Figure 2. Osx+ cells in the neonatal bone give rise to Nes-GFP+ MSPCs.
Analysis of Nes-Gfp/iOsx/Tomato mice administered with tamoxifen (Tam) at P5. (A and B) Z-stack confocal images of thick bone sections at 1 day (A) and 15 weeks (B) after Tam injection. Bone sections were stained with VE-cadherin (VE-Cad) and PECAM-1 antibodies (white). Right panels show the magnified confocal images within the area defined by the rectangle. Arrows: Nes-GFP and iOsx-derived Tomato (iOsx/Tomato) double-positive cells. (C and D) Representative FACS plots showing the percentages of the Nes-GFP+ cells within the iOsx/Tomato+ (CD45−Ter119−CD31−) population in the bone (C) (n=3) and in the BM (D) (n=3) after 1 day and 15 weeks of chase, respectively. Blue and red lines represent the WT control and Nes-GFP, respectively. (E and F) CFU-F activity of sorted iOsx/Tomato+ and iOsx/Tomato− populations in the BM stroma harvested 4 weeks after Tam injection. Quantification (E) and representative image of CFU-F colony (F; Giemsa staining on the left panel and Tomato fluorescence on the right panel). n=3 independent experiments. *P<l0.05. (G–I) Differentiated phenotypes of clonal iOsx/Tomato+ BM stromal cells shown by Alizarin Red S: osteoblasts (G), lipid droplets and staining with FABP4 antibody: adipocytes (H), and Alcian Blue: chondrocytes (I). Nuclei were detected by DAPI (blue). (J) Confocal image of Nes-GFP and iOsx/Tomato double-positive BM stromal cells stained with Lepr antibody in 15 week-old mice (white). Arrows: Nes-GFP, iOsx/Tomato, and Lepr triple-positive cells. (K) Representative FACS plots showing the percentages of Nes-GFP and Lepr double-positive cells and the expression of PDGFRαand PDGFRβ in the iOsx/Tomato+ (CD45−Ter119−CD31−) BM stromal population in 15 week-old mice. Blue and red lines represent isotype controls and antibodies against the markers indicated, respectively. n=3. In (C, D, E, and K), data are represented as mean ± SEM. Scale bar: 5000 µm (G), 500 µm (F: left panel and I), 200 µm (A and B: left panels), 100 µm (F: right panel), 30 µm (A and B: right panels, H, and J). See also Figure S2H-J.
Recent studies reported that leptin receptor+ (Lepr)+ stromal cells contribute to HSC maintenance as a major source of SCF (Ding et al., 2012). Our recent analyses indicate that Lepr+ cells in the BM largely overlap with Nes-GFP+ cells (Pinho et al., 2013). Based on these results, we analyzed the expression of Lepr in P5-iOsx/Tomato+ BM cells with an anti-Lepr antibody. We found that most P5-iOsx/Tomato and Nes-GFP double-positive cells at 15 weeks after Tam injection expressed Lepr by immunostaining (Figure 2J) and flow cytometry (Figure 2K; 78 ± 4.5%), and that this population also expressed the MSPC markers PDGFRα (~89%) and PDGFRβ (~83%) (Figure 2K) (Crisan et al., 2008; Morikawa et al., 2009; Pinho et al., 2013). On the other hand, the expression of Lepr was very low in P5-iOsx/Tomato+ cells at 1 day after Tam injection (Figure S2H), suggesting that the Lepr expression increases in the P5-iOsx/Tomato+ BM cells during bone maturation. Interestingly, E13.5-iOsx/Tomato+ BM cells also express Nes-GFP, Lepr, PDGFRα, and PDGFRβ after a chase of 2 weeks (Figure S2I and J). The accompanying manuscript by Ono et al. suggests that some of the Osx+ cells labeled at E12.5 overlap with Nes-GFP+ cells (Ono et al., 2013). Collectively, these results thus support the idea that Osx+ cells in the neonatal bone marrow are the precursors of Nes-GFP+ Lepr+ MSPCs in the adult BM, although whether Osx+ cells were initially Nes-GFP+ cannot be excluded.
Lepr-cre-derived BM stromal cells contribute to the osteolineage in the adulthood
Lepr-marked stromal cells were suggested to be restricted to perivascular areas and do not contribute to osteolineage cells (Ding et al., 2012). Since we found that Lepr was expressed in Nes-GFP+ MSPCs, we analyzed the contribution of Lepr+ cells to bone tissues in Nes-Gfp/Lepr-cre/Tomato triple transgenic mice. Consistent with the above antibody staining experiments (Figure 2J and K), Lepr-cre-derived Tomato+ cells (hereafter Lepr/Tomato+) expressed Nes-GFP, PDGFRα, and PDGFRβ (Figure S3A). Furthermore, clonally expanded Lepr/Tomato+ cells exhibited tri-lineage differentiation capacity (Figure S3B-D), indicating that these populations highly overlap with the P5-iOsx/Tomato+ cells in the BM. During early limb development, Lepr/Tomato+ cells were not present in the primary ossification center at E15.5, whereas Nes-GFP+ and Osx+ cells were expressed at this time in this location (Figure 3A). Lepr/Tomato+ cells were first detected in the primary spongiosa and in the periosteum of the bone tissue at E17.5, and subsequently were present throughout the BM cavity in 1 week-old mice (Figure 3A). Immunofluorescence analyses revealed that Lepr/Tomato+ cells were positive for Nes-GFP and Osx in the primary spongiosa in 1 week-old mice (Figure S3E). By FACS analysis, most (~92%) of Lepr/Tomato+ cells in the BM were also positive for Nes-GFP at 1 week of age (Figure S3F).
Figure 3. Lepr-cre -derived BM stromal cells give rise to bone-lineages in the adult stage.
(A) Z-stack confocal images of thick bone sections of Nes-Gfp/Lepr-cre/Tomato mice in the indicated stage, stained with Osx antibody (white). *: Primary ossification center. Arrows: Lepr-cre-derived Tomato+ (Lepr/Tomato+) cells in the epiphysis. Arrowheads: Lepr/Tomato+ cells in the periosteum. (B) Z-stack confocal images of thick bone sections of Lepr-cre/Tomato mice in the indicated stages. Arrows: Lepr/Tomato+ osteoblasts. Arrowheads: Lepr/Tomato+ osteocytes. (C and D) Confocal images of bone tissues from 15 week-old Lepr-cre/Tomato mice stained with osteocalcin (green) (C) and DMP1 antibodies (green) (D). (E) Z-stack confocal images of thick bone sections in 6 week-old Col1(2.3)-Gfp mice stained with Lepr antibody (red). Right panels show a magnified view the area of around the cortical bone. Arrows: Col1(2.3)-GFP-positive mature osteoblasts. (F) Z-stack confocal images of thick bone sections in 15 week-old Leprcre/Tomato mice stained with Lepr antibody (white). Arrows: Lepr/Tomato+ osteocytes. Arrowheads: Lepr/Tomato+ osteoblasts. Nuclei were detected by Hoechst 33342 (blue). Scale bar: 500 µm (E: left panel), 200 µm (B), 100 µm (A), 30 µm (E: right panels and F), 10 µm (C and D). See also Figure S3.
Next, we analyzed bone tissues of 3 week- and 15 week-old Lepr-cre/Tomato mice to investigate the osteolineage contribution of Lepr/Tomato+ BM stromal cells. In 3 week-old animals, Lepr/Tomato+ cells were distributed throughout the BM cavity, but were not present along the endosteum (Figure 3B, left). By contrast, in the 15 week-old bone tissues, Lepr/Tomato+ cells were observed not only in the BM cavity, but also along the cortical bone (Figure 3B, right). Immunofluorescence staining showed that Lepr/Tomato+ cells in the bone tissue were osteocalcin- and dentin matrix protein 1 (DMP1)-expressing mature osteoblasts and osteocytes, respectively (Figure 3C and D). Lepr/Tomato+ osteolineage cells were also observed in the trabecular bone of 15 week-old mice (Figure S3G). To examine whether Lepr/Tomato+ mature osteolineage cells express Lepr, we performed immunofluorescence staining with anti-Lepr antibody in Col1(2.3)-Gfp and Lepr-cre/Tomato mice. We found that Col1(2.3)-GFP+ mature osteoblasts and Lepr/Tomato+ osteolineage cells were negative for Lepr (Figure 3E and F). In addition, Lepr mRNA was not detectable by quantitative real-time PCR in the osteoblasts (Figure S3H-J). These results suggest that Lepr/Tomato+ mature osteolineage cells do not autonomously express Lepr, but are descendant of Lepr+ precursors. Further, these data indicate that BM cells marked by Lepr-cre highly overlap with P5-iOsx/Tomato+ BM stromal cells that are a permanent source of osteoprogenitors contributing to adult bone homeostasis.
P5-iOsx-derived BM stromal cells contribute to tissue regeneration after injury
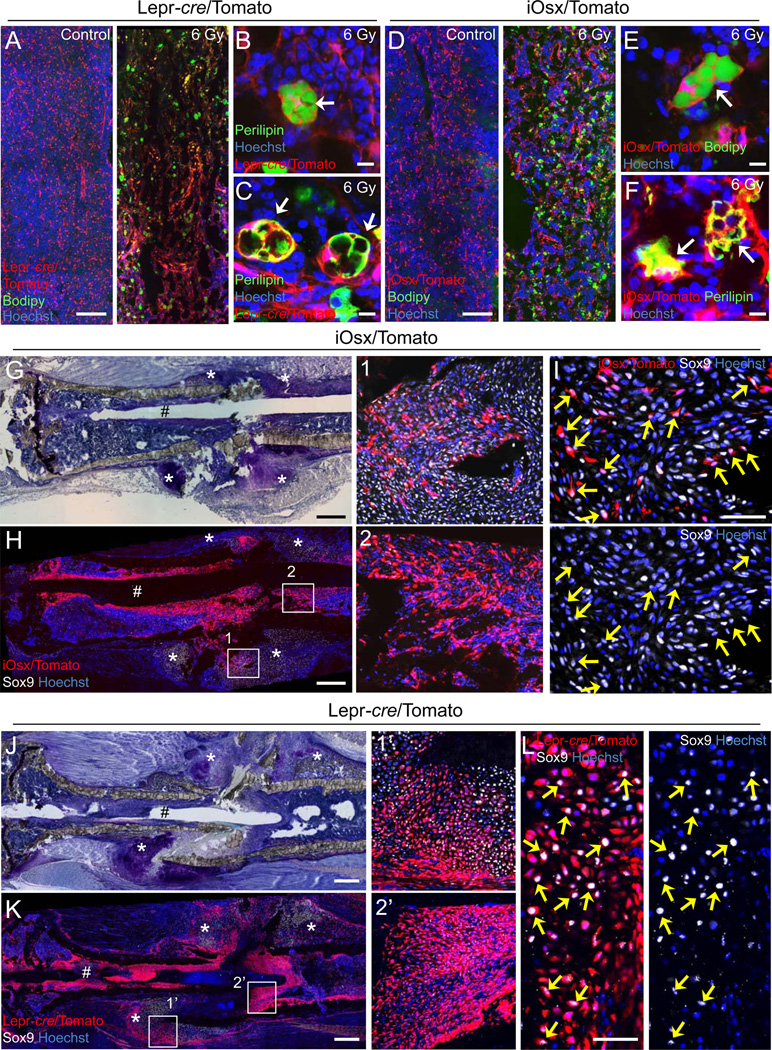
Although MSPCs are thought to have the potential to differentiate into osteoblasts and adipocytes (Takada et al., 2009), their contribution to these lineages in vivo remains unclear. To test whether Lepr/Tomato+ and P5-iOsx/Tomato+ BM stromal cells had the potential to generate adipocytes in vivo, we challenged 8 week-old Lepr-cre/Tomato and 15 week-old iOsx/Tomato mice pulsed at P5 with 6 Gy irradiation, an injury known to induce fatty infiltration in the BM (Bryon et al., 1979). Adipocytes, identified by staining with BODIPY (493/503), a dye that stains lipid droplets (Spangenburg et al., 2011), and perilipin, an essential protein for adipogenesis (Martinez-Botas et al., 2000) were dramatically increased in the BM 6 days after irradiation (Figures 4A–F and S4A). Importantly, these adipocytes were also Tomato+ (Figure 4B, C, E, and F). We next intercrossed Lepr-cre with an inducible diphtheria toxin (DT) receptor (iDTR) line, and examined the effect of the depletion of Lepr+ cells on the adipogenesis. The number of irradiation-induced adipocytes was significantly decreased by the depletion of Lepr+ cells (Figure S4B-E). However, no effect was observed in the number of adipocytes after DT injection in the control groups (Figure S4D and E). These results thus suggest that irradiation-induced adipocytes are derived from Lepr/Tomato+ BM cells as well as P5-iOsx/Tomato+ BM stromal cells. Interestingly, consistent with our results, a recent report has shown that the deletion of Wnt/β-catenin-signaling (an inhibitory signal of adipogenesis) in Osx-expressing cells leads to increased numbers of BM adipocytes (Song et al., 2012).
Figure 4. P5-iOsx-derived BM stromal cells contribute to tissue remodeling after tissue injury.
(A–F) Z-stack confocal (A and D) and confocal (B, C, E, and F) images of thick bone sections at day 6 post-irradiation stained with BODIPY (493/503) (green) (A, B, D, and E) or Perilipin antibody (green) (C and F) from 8 weeks Lepr-cre/Tomato mice (A–C) or P5 labeled iOsx/Tomato mice after 15 weeks chase (D–F). Arrows: Tomato+ adipocytes. (G–L) Images of bone sections at day 8 post-bone fracture from P5 labeled iOsx/Tomato mice after 32 weeks chase (G–I) or 15 week Lepr-cre/Tomato mice (J–L). Serial sections stained with Toluidine blue (G and J). Z-stack confocal (H and K) and confocal (I and L) images of thick bone sections stained with Sox9 antibody (white). The numbered squares indicate the area of respective zoomed right panels (H and K). Arrows: Tomato and Sox9 double-positive chondrocytes. #: Mark of the needle used for bone stabilization during bone fracture. *: Fracture callus. Magnified confocal images in H1 and K1’ panels (I and L). Nuclei were detected by Hoechst 33342 (blue). Scale bar: 500 µm (G, H, J, and K), 200 µm (A and D), 50 µm (I and L), 10 µm (B, C, E, and F). See also Figure S4.
To determine whether P5-iOsx/Tomato+ and Lepr/Tomato+ BM stromal cells participate in the regenerative healing process after bone fracture, we used the semi-stabilized tibia fracture model (Maes et al., 2006), and subjected 32 week-old P5 pulsed iOsx/Tomato and 15 week-old Lepr-cre/Tomato mice to bone fracture. Tomato label-retaining chondrocytes were not observed in P5 pulsed iOsx/Tomato mice after a chase for 3 weeks (data not shown). Eight days after bone fracture, P5-iOsx/Tomato+ cells were observed in the newly formed chondrogenic area of the fracture callus (Figure 4G and H). Some of the P5-iOsx/Tomato+ cells were also positive for the chondrocyte marker Sox9 indicating that P5-iOsx/Tomato+ cells differentiated into chondrocytes and contributed to the bone fracture healing process (Figure 4G–I). Lepr/Tomato+ cells were also detected as Sox9-positive cells in the fractured callus in Lepr-cre/Tomato mice (Figure 4J–L). We confirmed that Lepr/Tomato+ cells in the fracture callus did not express Lepr with anti-Lepr antibody (Figure S4F), suggesting that these cells are the progeny of Lepr+ cells.
A previous report showed that periosteal cells contribute to the woven bone callus formation in the fractured bone healing process (Grcevic et al., 2012). Since P5-iOsx/Tomato+ cells and Lepr/Tomato+ cells were also observed in the periosteum (Figures S1F and 3A), it suggests that these cells may also contribute to the callus formation. Interestingly, P5-iOsx/Tomato+ cells and Lepr/Tomato+ cells accumulated around the needle inserted into the BM for the tibia stabilization after bone fracture, suggesting that MSPC progeny also contributed to the granulation tissue surrounding the foreign body (Figure 4H2 and K2’) (Singer and Clark, 1999). Collectively, these data indicate that Lepr+ P5-iOsx/Tomato+ BM stromal cells are bona fide MSPCs and that they contribute to tissue regeneration as a source of osteo-, adipo-, and chondro-precursors in vivo.
It is temping to speculate that the hematopoietic stroma may undergo a makeover similar to the transition of primitive to definitive hematopoiesis during development (Dzierzak and Speck, 2008). Although both primitive and definitive stromal precursors are endowed with the capacity to contribute to bone and stromal lineages, it is possible that they play important functions in regulating the proliferative and quiescent phases of HSCs. HSCs indeed proliferate markedly in the BM during development and become highly quiescent after weaning (Bowie et al., 2006). Further studies will be needed to explore the differential properties of these stromal precursors.
EXPERIMENTAL PROCEDURES
Experimental Animals
C57BL/6, B6.129-Leprtm2(cre)Rck/J (Lepr-cre), B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, and C57BL/6-Gt(ROSA)26 Sortm1 (HBEGF) Awai/J mice were purchased from Jackson Laboratory. Osx-creERT2 mice (Maes et al., 2010) were provided by author H.M.K. and backcrossed with C57BL/6 for 5 generations. Nes-Gfp mice (Mignone et al., 2004) were bred in our facilities. Col1(2.3)-Gfp mice (Kalajzic et al., 2002) were provided from D. Rowe, J. Butler, and S. Rafii. All mice were maintained in pathogen-free conditions, and the Animal Care and Use Committees of Albert Einstein College of Medicine approved all experimental procedures.
Antibodies and reagents
The primary antibodies used were Alexa Fluor 647-anti-VE-Cadherin (BV13) (Biolegend); APC-anti-CD31/PECAM-1 (MEC13.3), APC-eFluor 780-anti-CD45 (30-F11), APC-eFluor 780-anti-Ter119 (Ter119), biotin-anti-PDGFRα (APA5), biotin-anti-PDGFRβ (APB5), and eFluor 660-anti-Ki-67 (SolA15) (all from eBioscience); anti-Lepr and anti-fatty acid binding protein 4 (FABP4) (all from R&D systems); anti-Osx (Abcam); anti-Osteocalcin (mOC1–20 and R21C-01A) and anti-DMP-1 (all from TAKARA); anti-Perilipin (D1D8) (Cell Signaling); anti-Sox9 (Millipore). The secondary antibodies used were Alexa Fluor 647 donkey anti-goat IgG, Alexa Fluor 488 donkey anti-goat IgG, Alexa Fluor 488 donkey anti-rat IgG, and Alexa Fluor 633 goat anti-rabbit IgG (all from Molecular probes); Streptavidin eFluor 450 (eBioscience). Alexa Fluor 488-anti-GFP (Molecular Probes) was used for enhancement of the Nes-GFP signal. Nuclei were stained with Hoechst 33342 or DAPI (4',6-diamino-2-phenylindole) (all from Sigma-Aldrich). Lipid droplets were stained with BODIPY 493/503 (Molecular Probes).
Immunofluorescence staining
Mice were perfused with 4% paraformaldehyde (PFA) for fixation, and bone tissue were further fixed with 4% PFA for 30 min at 4°C, and incubated in 10%, 20%, and 30% sucrose each for 1 hr at 4°C for cryoprotection and embedded in 5% carboxymethyl cellulose (SECTION-LAB). Sections, 10–30-µm thick, were prepared using Kawamoto’s film method (Kawamoto and Shimizu, 2000). Images were acquired using a laser-scanning confocal microscope (SP5 AOBS, Leica), Leica LAS-AF software (Leica), and image J (Schneider et al., 2012). In the mouse fractured bone, sections were stained with Toluidine blue (Sigma-Aldrich) and imaged by Light microscope Zeiss Axio Observer (Zeiss) and Axiovision software (Zeiss).
Cell sorting and flow cytometry
Cell sorting experiments were performed using an Aria Cell Sorter (BD Biosciences). Flow cytometric analyses were carried out using an LSRII flow cytometer equipped with FACS Diva 6.1 software (all BD Biosciences). Dead cells and debris were excluded by FSC, SSC and DAPI (Sigma-Aldrich) staining profiles. Data were analysed with FlowJo (Tree Star) or FACS Diva 6.1 software.
Statistics
Data were evaluated by unpaired Student’s t-tests. Experiments were performed three times and similar results were obtained. Statistical analyses were performed with Graph Pad Prism 6. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We thank D. Rowe, J. Butler, and S. Rafii for providing Col1(2.3)-Gfp mice. We would like to acknowledge C. Prophete, P. Ciero, L. Schiff, and A. Zahalka for technical assistance, L. Tesfa and O. Uche in the Einstein Flow Cytometry Core Facility for technical assistance of cell sorting, and P. Guo in the Analytical Imaging Facility for technical assistance in confocal microscope imaging. S.P. is a New York Stem Cell Foundation-Druckenmiller Fellow and M.H. is a German Research Foundation (DFG) Fellow. This work was supported by The New York Stem Cell Foundation and by R01 grants from National Institutes of Health (DE022564 to N.O., DK056246 to H.M.K., and DK056638 and HL069438 to P.S.F.). The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryon PA, Gentilhomme O, Fiere D. Histomorphometric analysis of bone-marrow adipose density and heterogeneity in myeloid aplasia and dysplasia (author's transl) Pathol Biol (Paris) 1979;27:209–213. [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- Grcevic D, Pejda S, Matthews BG, Repic D, Wang L, Li H, Kronenberg MS, Jiang X, Maye P, Adams DJ, et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30:187–196. doi: 10.1002/stem.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalajzic Z, Liu P, Kalajzic I, Du Z, Braut A, Mina M, Canalis E, Rowe DW. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone. 2002;31:654–660. doi: 10.1016/s8756-3282(02)00912-2. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Shimizu M. A method for preparing 2- to 50-micron-thick fresh-frozen sections of large samples and undecalcified hard tissues. Histochem Cell Biol. 2000;113:331–339. doi: 10.1007/s004180000149. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, Maye P. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8:e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperi S, Horwood N, Marley S, Gordon MY, Cope AP, Dazzi F. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood. 2008;111:1173–1181. doi: 10.1182/blood-2007-03-082800. [DOI] [PubMed] [Google Scholar]
- Maes C, Coenegrachts L, Stockmans I, Daci E, Luttun A, Petryk A, Gopalakrishnan R, Moermans K, Smets N, Verfaillie CM, et al. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J Clin Invest. 2006;116:1230–1242. doi: 10.1172/JCI26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Ono N, Ono W, Mizoguchi T, Nagasawa T, Frenette PS, Kronenberg HM. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell. 2013 doi: 10.1016/j.devcel.2014.03.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27:2344–2358. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenburg EE, Pratt SJ, Wohlers LM, Lovering RM. Use of BODIPY (493/503) to visualize intramuscular lipid droplets in skeletal muscle. J Biomed Biotechnol. 2011;2011:598358. doi: 10.1155/2011/598358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R, He XC, Venkatraman A, Arai F, Box A, Semerad C, Haug JS, Peng L, Zhong XB, Suda T, et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell. 2012;150:351–365. doi: 10.1016/j.cell.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.