Abstract
BACKGROUND
This is a retrospective study of 136 patients with Cushing disease treated with transsphenoidal microsurgery.
OBJECTIVE
To evaluate factors influencing immediate postoperative results and long-term outcomes.
METHODS
Data regarding clinical presentation, endocrine evaluation, imaging studies, surgical technique, immediate postoperative biochemical remission (IPBR), and long-term results were entered into a database and analyzed statistically. IPBR was based on biochemical evidence of adrenal cortical insufficiency and clinical evidence of such insufficiency.
RESULTS
IPBR for the entire series was 83.4%. In microadenomas, IPBR was 89.8% with a mean immediate postoperative plasma cortisol (IPPC) of 2.1 μg/dL (range, <0.5–5.3). Positive magnetic resonance imaging (MRI) was associated with 18 times greater odds of finding microadenoma at surgery (P < .001) and with 4.1 times greater odds of IPBR (P = .07). In patients with a negative MRI, a positive inferior petrosal sinus sampling (IPSS) test was associated with 93% of IPBR (P = .004). IPBR in macroadenomas was 30.7%. Of patients followed for 12 months or longer, 34.8% required glucocorticoid replacement for the duration of follow-up. The mean follow-up in microadenomas was 68.4 months with a 9.67% incidence of recurrences. The estimated actuarial incidence of recurrences increased with the passage of time and IPPC of greater than 2 μg/dL was associated with higher incidence of recurrences, although without statistical significance (P = .08).
CONCLUSION
In microadenomas, a positive MRI and positive IPSS test were associated with a higher incidence of IPBR. Recurrences increased with the passage of time, and an IPPC of greater than 2 μg/dL may be associated with higher incidence of recurrences.
Keywords: Cushing disease, Immediate postoperative remission, Long-term results, Statistical analysis, Transsphenoidal surgery
Since first described in 1932,1 Cushing disease has challenged endocrinologists and neurosurgeons alike. It is well established that untreated Cushing disease is associated with high mortality.2 After the introduction of transsphenoidal microsurgery in the treatment of pituitary disorders by Hardy,3 this treatment method was applied to the surgical management of Cushing disease with encouraging early results.4–8 Trans-sphenoidal microsurgery has since been established as the preferred treatment choice in the management of Cushing disease. Nevertheless, even treated Cushing disease can be associated with increased mortality compared to the general population.9,10
OBJECTIVE
The objective of this study was to evaluate factors that influence immediate postoperative biochemical remission (IPBR) and long-term outcomes in patients with Cushing disease treated with transsphenoidal microsurgery. The factors analyzed relative to IPBR included preoperative imaging studies, the inferior petrosal sinus sampling (IPSS) test, surgical technique, the size of the adenoma, and the presence or absence of a microadenoma on surgical exploration and on histopathology and immunohistochemistry. Factors analyzed relative to long-term outcomes included the extent of the IPBR and the duration of the follow-up.
MATERIAL AND METHODS
This is a multicenter retrospective study of 136 patients with Cushing disease treated with transsphenoidal microsurgery at the Evanston Hospital by a single surgeon (I.C.) between March 1970 and April 2010. The preoperative and postoperative endocrine evaluations and follow-ups were conducted by endocrinologists, both at the parent institution and at the outside referral centers. The endocrinologists (J.W.F., M.E.M., R.E.W., S.R., and W.D.K.), and the parent institution neurosurgical faculty (I.C. and J.-C.Z.) contributed to the study and to the formulation of opinions rendered in the article. Imaging studies were interpreted by the NorthShore University HealthSystem senior neuroradiologist (J.M.). Statistical analysis was conducted by the NorthShore University HealthSystem statistician (H.D.). Institutional Review Board approval for this study was obtained (no. EH09-522). Patients with both microadenomas (123) and macroadenomas (13) were included in the study. A microadenoma was defined as a pituitary tumor of 1 cm or less. Twelve additional patients had clinically “silent” adrenocorticotropin (ACTH)-secreting macroadenomas; these patients were excluded from this review.
Patient records, both paper charts and, since 2001, electronic records, and referring endocrinologists’ follow-up data were reviewed. In addition, patients were contacted with a form letter. The information extracted, including patient’s age and sex, clinical presentation, preoperative endocrine workup, imaging data, and correlation of the same with the endocrine workup, surgical technique and complications, histopathologic diagnosis and immunohystochemical confirmation for adrenocorticotropic hormone (ACTH) staining, was entered into a database and analyzed statistically. IPBR was defined on the basis of both the biochemical evidence of adrenal cortical insufficiency as witnessed by the drop in the immediate postoperative plasma cortisol (range, < 0.5–5.3 μg/dL) and the clinical evidence of such insufficiency with symptoms such as headache, nausea, general ill feeling, and vital sign changes, including low-grade fever, that required glucocorticoid replacement therapy. IPBR results as well as long-term outcomes were also entered into the database and analyzed statistically. The statistical methods used included the Mantel-Haenszel χ2 test, the Fisher exact test, and logistic regression. A P < .05 is regarded as statistically significant.
Clinical Presentation
There were 109 female and 27 male patients. Seven patients were under the age of 20 (range, 8–20) and 3 were older than 70 (range, 71–80). The median age in the 20- to 70-year age group was 41.
All patients presented with the characteristic, but, to a varying degree, pronounced, cushingoid symptoms and signs of truncal obesity, a round, ruddy, and red face that is slightly hirsute, osteoporosis, tendency toward bruising, proximal muscle weakness, and fatigue. A significant proportion had hypertension and diabetes. Three patients with macroadenomas had visual changes: 2 had visual field defects and 1 had ocular motility impairment (Table 1). One patient with known Cushing disease and with a history of 2 miscarriages, previously treated medically, was operated on in the second trimester. One patient had stunted growth.
TABLE 1.
One Hundred Thirty-Six Patients With Cushing Disease Clinical Presentation
| Hypertension | 51 (37.5%) |
| Diabetes | 41 (30.1%) |
| Morbid obesity | 5 (3.6%) |
| Spontaneous fractures | 12 (8.8%) |
| Depression | 9 (6.6%) |
| Visual changes | 3 (2.2%) |
Preoperative Endocrine Evaluation
The preoperative endocrine workup evolved over the years and depended on evaluation paradigms developed at referring centers. The most frequently used tests to establish endogenous hypercortisolism were 24-hour urine free cortisol, late night salivary cortisol,11 and plasma cortisol determination (midnight to 2 AM: >5 μg/dL) (R. E. Weiss and S. Refetoff, personal communication, June 2002)12,13 and the low-dose dexamethasone suppression testing.13–15 In addition, analysis of 24-hour circadian profiles of ACTH secretion at baseline and on low dexamethasone was used routinely at 1 center.16 Once endogenous hypercortisolism had been established, the confirmation of Cushing disease included plasma ACTH determination, pituitary imaging with magnetic resonance imaging (MRI), and the IPSS test,17–20 which was first used in our study patients in 1988. The prime indication for the IPSS test was a normal-appearing pituitary on the MRI in face of an endocrine evaluation indicative of a pituitary dependent Cushing disease. Loss of diurnal variation, concordance of ACTH and cortisol secretion in 24-hour profiles, and a robust ACTH response to corticotrophin-releasing hormone (CRH) stimulation were considered in some centers as further evidence of pituitary-dependent ACTH hypersecretion (R. E. Weiss and S. Refetoff, personal communication, June 2002).12,13,21 In some patients the endocrine workup was expanded to include adrenal and other organ imaging.
Imaging
Early on in the series, 17 patients with suspected microadenomas were imaged with thin-cuts computed tomography (CT) scan technique, with available data in 16 patients. Findings indicative of a microadenoma were found in 11 (68.7%) patients. Pituitary dedicated magnetic resonance imaging (MRI), utilizing the technique of immediate postinjection scanning, has been the preferred study since 1986. One hundred six patients were imaged with MRI, with data available in 105 patients. In this group, the MRI was positive for a microadenoma in 82 (78%) patients. In the remaining 23 patients, the MRI, at times done more than once, was negative for a distinct, unequivocal microadenoma.
Thirteen patients with macroadenomas had tumors ranging in size from 1.6 to 3.0 cm. Seven (53%) patients had tumors invasive into the cavernous sinus and sphenoid bone.
Transsphenoidal Surgical Technique
All patients were operated on by use of the transsphenoidal micro-surgical technique. Until the year 2000, the sella was approached via the sublabial, ororhinoseptal route. Since 2000, the preferred approach has been the endonasal technique. Image-guided fluoroscopy was used as an adjunct to the surgical technique until the year 2000. Since then, all patients have undergone transsphenoidal surgery in conjunction with intracranial navigation with the Stealth technique, with the use of external anatomic landmarks as fiducial reference points.22 In contrast to image-guided fluoroscopy that allows only for saggital orientation, the use of intracranial navigation is helpful in confirming the sellar and parasellar anatomy in all 3 planes.
At operation, the anterior sella dura was exposed from the floor to the planum and from the medial aspect of one cavernous sinus to the medial aspect of the opposite one. The initial dural opening was done below the sella equator to avoid a potentially large descending anterior arachnoid recess.22 In microadenomas, the dura exposure favored the side as indicated by the location of the microadenoma on the MRI. Further dura opening was done in increments depending on the microadenoma geography and to visualize and, in some cases, explore the rest of the gland, especially if there was a discrepancy between the locations of the microadenoma as seen on the MRI and as lateralized by the IPSS test. When a microadenoma was found at surgery (100 patients, 81.3%) a selective microadenomectomy in conjunction with removal of the adjacent tumor bed, often referred to as the pseudocapsule22–26 (Figure 1), was performed prospectively in each case. Two microadenomas were supradiaphragmatic and required an extended transsphenoidal approach. When a microadenoma was not found, a partial or complete anterior hypophysectomy was performed in accordance with the preoperative consensus between the endocrinologist, neurosurgeon, and patient. Two patients with a negative exploration refused to consider hypophysectomy.
FIGURE 1.
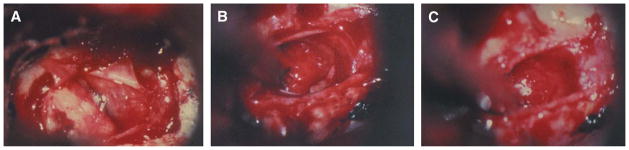
A, ACTH-producing adenoma, on the left side of the picture. B, pseudocapsule. Please note the distinct layer of tissue next to the residual anterior pituitary. C, after removal of the pseudocapsule. ACTH, adrenocorticotropin.
The surgical technique in macroadenomas was similar in terms of approach. Occasionally, as indicated by the patient’s anatomy and the size of the tumor, we have resorted to the transnasal submucous approach, in conjunction with removal of the bony septum that, in our experience, can afford a wider exposure. Surgical technique of macroadenoma removal commenced with internal decompression followed by separation of the tumor surface from the dura of the sella floor, the cavernous durae, and from any laterally positioned residual normal anterior pituitary. The final separation of the decompressed tumor was performed at the diaphragm sellae that is often protected by a thin layer of residual normal anterior pituitary.22 No attempt was made to extract tumors invasive into the cavernous sinus.
Operative Complications
Table 2 lists operative complications for the entire series. There were no mortalities, and there were no complications related to the surrounding neurovascular anatomy. Anterior pituitary insufficiency, not related to the pituitary-adrenal axis, occurred only in conjunction with a complete anterior hypophysectomy. Diabetes insipidus present at the time of discharge was seen in 17 patients (12.5%). Two additional patients required readmission for syndrome of inappropriate antidiuretic hormone secretion. CSF rhinorrhea requiring treatment occurred in 6 patients (4.4%) and was treated with a lumbar drain in 4 and reoperation in 2 patients. Postoperative epistaxis, usually delayed (7–10 days), requiring readmission and treatment, was seen in 5 patients (3.6%). This complication has not occurred since we began using a microsurgical drill armed with a conically shaped bur to remove the sphenoid face. Deep venous thrombosis and/or pulmonary embolism occurred, despite appropriate prophylaxis, in 4 patients (3%). Acute sinusitis requiring treatment occurred in 3 patients (2.2%). One patient each developed meningitis, pancreatitis, and cholecystitis (0.7%).
TABLE 2.
Surgical Complications of 136 Patientsa
| Anterior pituitary insufficiency secondary to hypophysectomy | 10 (7.3%) |
| Diabetes insipidus | 17 (12.5%) |
| SIADH | 2 (1.4%) |
| CSF rhinorrhea | 6 (4.4%) |
| Meningitis | 1 (0.7%) |
| Epistaxis | 5 (3.6%) |
| Sinusitis | 2 (2.2%) |
| DVT and PE | 4 (2.9%) |
| Pancreatitis | 1 (0.7%) |
| Cholecystitis | 1 (0.7%) |
| Neurovascular morbidity | 0 |
| Mortality | 0 |
SIADH, syndrome of inappropriate antidiuretic hormone secretion; DVT, deep vein thrombosis; PE, pulmonary embolism.
Histopathology and Immunohistochemistry
Hematoxylin and eosin stains were obtained in all specimens. Reticulin stains were obtained in some specimens where hematoxylin and eosin stains were less clear for an adenoma. Immunohistochemistry was introduced as a routine part of tissue examination in 1983. Thus, the first 19 patients with Cushing disease were operated on without immunohistochemical confirmation of ACTH staining. Since then, each specimen has been stained for hormonal markers. No frozen sections were obtained from tissue specimens considered by the surgeon as representative of adenoma tissue. The adenoma tissue was submitted for permanent sections. In patients with microadenomas, frozen sections were obtained from tissue surrounding the tumor bed. If frozen section of the tumor bed tissue revealed adenoma remnants, an additional sliver of tissue was removed. If no clear adenoma was identified at surgery, all removed pituitary tissue was submitted to the neuropathologist to be sectioned with thin-cut technique and stained with hematoxylin and eosin, reticulin as indicated, and for ACTH.
RESULTS
Combined IPBR in 108 microadenomas with available IPPC data and 13 macroadenomas occurred in 101 patients (83.4 %). With the exception of 2 patients with immediate postoperative plasma cortisol levels of 5 and 5.3 μg/dL, respectively, all other patients with IPBR had IPPC levels below 5 μg/dL. All 101 patients with IPBR developed symptoms of adrenal cortical insufficiency and required glucocorticoid replacement. Patients who did not undergo IPBR continued having the preoperative high plasma cortisol levels and did not develop symptoms of adrenal-cortical insufficiency. Thus, these patients did not require glucocorticoid replacement.
Microadenomas
There were 123 patients with microadenomas: 97 females and 26 males.
Immediate Postoperative Results
Postoperatively, patients were initially not given hydro-cortisone replacement. Plasma cortisol levels were drawn postoperatively at 6-hour intervals. In patients with no remission, drawings the 6-hour intervals were discontinued after 24 hours. In patients with remission, plasma cortisol drawings at 6-hour intervals were discontinued once the patient was started on glucocorticoid replacement. The timing of glucocorticoid replacement varied in accordance with the managing endocrinologist’s tolerance level relative to the severity of symptoms of adrenal cortical insufficiency that all patients with IPBR experienced, albeit to various degrees.
IPBR was achieved when, commensurately with the drop in plasma cortisol level to <0.5 to 5.3, the patient became symptomatic because of adrenal cortical insufficiency with symptoms such as headache, nausea, general ill feeling, and vital sign changes, including low-grade fever, requiring glucocorticoid replacement therapy. Thus, all patients with IPBR had symptoms of adrenal cortical insufficiency. The interval from the operation to the time the patient became symptomatic, requiring gluco-corticoid replacement, has varied from 8 to 72 hours, with most patients experiencing significant symptoms at 18 to 24 hours postoperatively. Following discharge, the patients were evaluated at their respective referring centers, and the IPBR was confirmed.
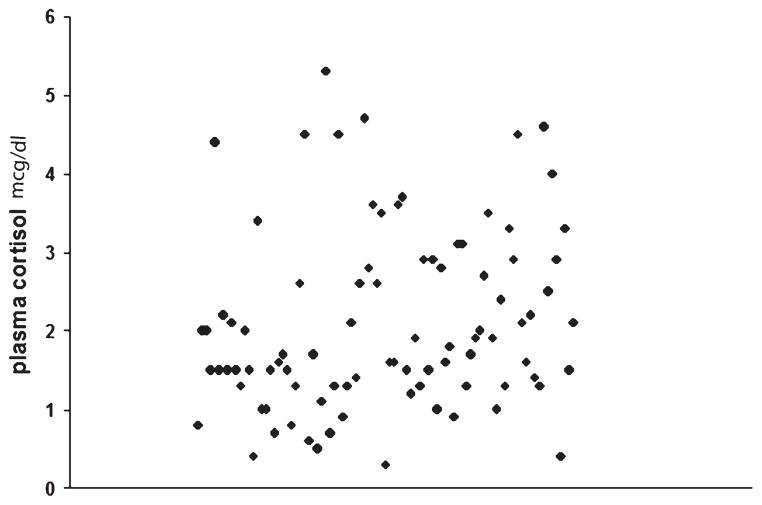
IPPC data were available in 108 patients. IPBR was accomplished in 97 (89.8%) patients. The mean IPPC level in this group of patients was 2.1 μg/dL (range, <0.5–5.3 μg/dL). Individual IPPC values in patients with IPBR are shown as a scatter plot in Figure 2. Fifty-three patients had an immediate IPPC of less than 2 μg/dL, 1 patient had an IPPC value of greater than 5 μg/dL (5.3), and the remaining 43 patients had an IPPC from 2 to 5 μg/dL. In 15 patients, the information relative to the IPPC was not available. These patients were excluded from statistical analysis of IPBR. Ten of these patients, however, required immediate postoperative glucocorticoid replacement therapy, suggesting biochemical remission. In 11 (11%) patients, the IPPC remained significantly elevated and they did not develop symptoms of adrenal cortical insufficiency. Accordingly, these patients did not require postoperative glucocorticoid replacement. These patients were classified as not having IPBR. Two of these patients were lost to long-term follow-up. There were no late remissions in the remaining 9 patients: 5 underwent adrenalectomy, 3 had stereotactic radiosurgery, and 1 was treated with o,p′-dichlorodiphenyldichloroethane.
FIGURE 2.
Scatter plot of individual immediately postoperative plasma cortisol levels.
We were interested in the relationship between the imaging data and the surgeon’s ability to find a microadenoma at surgery, and between the imaging data and the IPBR. We were further interested in the relationship between the IPSS test and IPBR, as well as in the influence of the surgical technique on IPBR.
CT Data
Of 16 patients with available data, the CT scan was positive for a discreet microadenoma in 11 patients (68%). In this group, a microadenoma was found at surgery and confirmed with histopathology in 9 patients (82%). Eight of these patients underwent selective microadenomectomy and 1 underwent a partial hypophysectomy. In 1 additional patient with a positive CT scan, the lesion could not be verified surgically, but was found on histopathology of the hypophysectomy specimen. Immediate postoperative plasma cortisol data in this group were available in 3 patients, all having a biochemical remission (mean plasma cortisol, 2.4 μg/dL), with 3 additional patients having long-term follow-ups without clinical recurrence, suggesting IPBR as well, for a total of 6 patients (54%) achieving remission. Two patients had no remission, and the data were insufficient to make a determination in this regard in the remaining 3 patients.
In the 5 patients with a negative CT scan for a discreet lesion, a microadenoma was found at surgery and confirmed with histopathology in 3 patients, with only one having a documented IPBR. Two patients with a negative CT, in whom the removed specimens proved negative for a definite microadenoma on histopathology, were dependant postoperatively on glucocorticoid replacement, suggesting IPBR.
Thus, in this early group of patients with clinical and biochemical evidence for Cushing disease, studied with CT scan technique, a documented IPBR was seen in 4 patients (25%). However, a total of 9 patients (56%), were dependant postoperatively on glucocorticoid replacement, suggesting IPBR, and had long-term clinical remission.
MRI Data
In 105 patients with available MRI data, the MRI was unequivocally positive for a discreet microadenoma in 82 patients (78%). In this group of 82 patients, a discreet microadenoma was found at surgery in 78 patients (95%) and confirmed with histopathology and immunohistochemistry in 76 patients (93%). In the same group of 82 patients, a documented IPBR occurred in 69 patients (84%). Five patients with a positive MRI in whom a microadenoma was found at surgery and confirmed with histopathology and immunostaining, but in whom the immediate postoperative plasma cortisol data were not available, also required postoperative glucocorticoid replacement and had a documented long-term clinical remission, suggesting IPBR. Three patients with a positive MRI, in whom a microadenoma was not found at surgery, underwent hypophysectomy (complete in 2, partial in 1) with a positive histopathology and immunohistochemistry confirmation in 2, but with immediate documented biochemical remission in all 3 patients. One patient with a positive MRI for a supradiaphragmatic microadenoma underwent extended transsphenoidal approach with removal of the lesion, without, however, histopathologic or immunohistochemical confirmation. Nevertheless, this patient had a documented IPBR (plasma cortisol 3.5 μg/dL) requiring glucocorticoid replacement and remains free of Cushing disease after a 24-month follow-up.
In 23 patients with a negative MRI (22%), a discreet microadenoma was found at surgery and confirmed with histopathology and immunohistochemistry in 12 (52%) patients. Nine of the 12 patients in this group were studied additionally with the IPSS test, with a positive petrosal to peripheral ACTH gradient in all 9 patients. IPBR in this group was achieved in all 12 patients: 11 underwent selective microadenomectomy and 1 underwent a partial hypophysectomy. One patient in this group in whom a microadenoma was found at surgery, but could not be confirmed with histopathology and immunohistochemistry, also underwent documented IPBR.
In the 10 (43%) patients with a negative MRI, in whom a microadenoma was not found at surgery, a complete hypophysectomy was performed in 8 patients. Seven of these patients had IPSS test, and all were positive for a central to peripheral ACTH gradient. In 6 of the 8 patients who underwent hypophysectomy, a discrete microadenoma was confirmed with histopathology and with immunostaining, and all 6 had documented IPBR. Two patients in this group, one each with a positive and negative histopathology, failed to undergo biochemical remission despite a complete hypophysectomy. Both patients had IPSS test that was negative for a central to peripheral ACTH gradient. Two patients with a negative MRI underwent a mere negative exploration of their pituitary. IPSS test was obtained in 1 of these 2 patients and was also negative for an ACTH gradient.
In 23 patients with a negative MRI, the IPSS test was thus performed in 19 patients and was positive for a significant central to peripheral ACTH gradient in 16 patients (84%). Nine of these 16 patients underwent selective microadenomectomy and 7 underwent hypophysectomy, resulting in IPBR in 15 of the 16 patients (93%). In contrast, the 3 patients with a negative MRI, in whom the IPSS test was negative for an ACTH gradient, did not have IPBR. These 3 patients underwent surgery based on circumstantial evidence of endogenous hypercortisolism supported by screening differential diagnosis with the dexamethasone suppression tests. Three additional patients with a positive MRI were studied with the IPSS test for a total of 22 patients who had this test. Positive correlation as to the lateralization between the IPSS test and the location of tumor at surgery or on histopathology of the hypophysectomy specimens was found in 18 of the 22 patients (81.8%).
With these raw data at hand, the following questions were raised and analyzed statistically: whether imaging findings influenced the odds of finding a microadenoma at surgery, whether imaging findings influenced the IPBR, whether in patients with a negative MRI there was a difference in IPBR if microadenoma was found at surgery and removed selectively vs not found and the patient underwent hypophysectomy, and whether in patients with a negative MRI there was a difference in IPBR if the performed IPSS test was positive vs negative.
The statistical analysis of the above-described raw data revealed the following: (1) Compared with the CT scan, MRI was more sensitive in detecting a microadenoma. (2) Compared with a negative MRI, a positive MRI was associated with 18 times greater odds of finding the microadenoma at surgery (P < .001) (Table 3). (3) Compared with a negative MRI, a positive MRI for microadenoma had 4 times greater odds of being associated with IPBR (P = .07) (Table 4). (4) There was no statistical difference in IPBR in patients with a negative MRI, whether a microadenoma was found at surgery vs not found and the patient underwent hypophysectomy (P = .15) (Table 5). This, despite the fact that raw data showed that 2 of 8 patients who underwent hypophysectomy did not have IPBR. The reason for this may be the relatively small sample. (5) Finally, in patients with a negative MRI, a positive IPSS test was associated with significantly greater incidence of IPBR in comparison with patients in whom this test was negative for a central to peripheral ACTH gradient (P = .004) (Table 6).
TABLE 3.
MIA Surgeon’s Ability to Find MIA at Surgery Relative to Preoperative MRI Data in 105 Patients With Available Dataa
| MRI | MIA Found at Surgery/Pathology | Total | |
|---|---|---|---|
| No | Yes | ||
| Negative | 11 (47.83) | 12 (52.17) | 23 |
| Positiveb | 4 (4.88) | 78 (95.12) | 82 |
| Total | 15 | 90 | 105 |
MIA, microadenoma.
Compared with a negative MRI, a positive MRI was associated with statistically significant greater chance (odds ratio, 17.88) of finding a microadenoma at surgery, P < .001; 95% confidence interval, 4.89 to 65.31.
TABLE 4.
Microadenoma IPBR Relative to Preoperative MRI Data in 105 Patients With Available Dataa
| MRI | IPBR | Total | |
|---|---|---|---|
| No | Yes | ||
| Negative | 4 (17.39) | 19 (82.61) | 23 |
| Positiveb | 4 (4.85) | 78 (95.12) | 82 |
| Total | 8 | 97 | 105 |
IPBR, immediate postoperative biochemical remission.
Compared with a negative MRI, a positive MRI had marginally greater odds of being associated with IPBR, P = .07; odds ratio, 4.11; 95% confidence interval, 0.94 to 17.92.
TABLE 5.
| Group | IPBR + | IPBR − | Total |
|---|---|---|---|
| MIA found at surgery | 12 (100) | 0 (0) | 12 |
| MIA not found at surgery/HYPO | 6 (75) | 2 (25) | 8 |
| Total | 18 | 2 | 20 |
IPBR, immediate postoperative biochemical remission; MIA, microadenoma; HYPO, hypophysectomy.
There was no statistically significant difference in the incidence of IPBR in patients with a negative MRI whether a microadenoma was found at surgery or not found and the patient underwent hypophysectomy, P = .15.
TABLE 6.
MRI Negative for MIA Comparison in IPBR in Patients in Whom IPSS Test Was Done and Was Positive vs Negativea,b
| MRI Negative | IPBR | Total | |
|---|---|---|---|
| No | Yes | ||
| IPSS positive | 1 (6.25) | 15 (93.75) | 16 |
| IPSS negative | 3 (100) | 0 (0) | 3 |
| Total | 4 | 15 | 19 |
IPBR, immediate postoperative biochemical remission; IPSS, inferior petrosal sinus sampling; MIA, microadenoma.
Patients with a negative MRI had a significantly greater incidence of IPBR when the IPSS test was positive, P = .004.
Follow-up Results
A recurrence of Cushing disease in this study was defined when a patient with initial biochemical and clinical evidence for remission developed symptomatic recurrence of Cushing disease with biochemical reconfirmation of pituitary-dependent endogenous hypercortisolism.
Five patients were lost to follow-up (0 month follow-up). Eighteen patients had a follow-up of less than 6 months. Five of these patients had no immediate postoperative remission; one of these patients was operated on for a recurrence following an initial operation elsewhere. There were no recurrences in the remaining 13 patients. In the group of 100 patients with a follow-up of 6 months or longer, 7 additional patients did not have immediate postoperative remission; one of these patients was operated for a recurrence. The follow-up periods for the remaining 93 patients ranged from 6 to 396 months with a mean follow-up of 68.4 months or 5.7 years. In the group of 93 patients followed for 6 months or longer, there were 9 clinical and biochemically confirmed recurrences (9.67%). For these 9 patients, the actual times of recurrence were 176, 144, 120, 108, 108, 25, 18, 18, and 12 months, respectively, with a mean follow-up until recurrence of 80.3 months or 6.6 years. Table 7 shows the number of recurrences as they relate to the duration of follow-ups. The mean immediate postoperative plasma cortisol in 9 patients with recurrences was 2.7 μg/dL (range, 0.5–4.6). Conversely, the mean follow-up period in 84 patients who did not develop a recurrence was 68.9 months (range, 6–396 months) or 5.7 years. The mean immediate postoperative plasma cortisol in this group was somewhat lower at 1.8 μg/dL (range, 0.5–5.3).
TABLE 7.
Duration of Follow-up and Recurrences in Patients With Initial Biochemical Remission
| Recurrence | Follow-up in Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6~<12 | 12~<24 | 24~<36 | 36~<60 | 60~<120 | 120~<240 | 249 | 279 | 396 | Total | |
| No | 9 | 12 | 11 | 24 | 11 | 14 | 1 | 1 | 1 | 84 |
| Yes | 0 | 3 | 1 | 0 | 2 | 3 | 0 | 0 | 0 | 9 |
| Total | 9 | 15 | 12 | 24 | 13 | 17 | 1 | 1 | 1 | 93 |
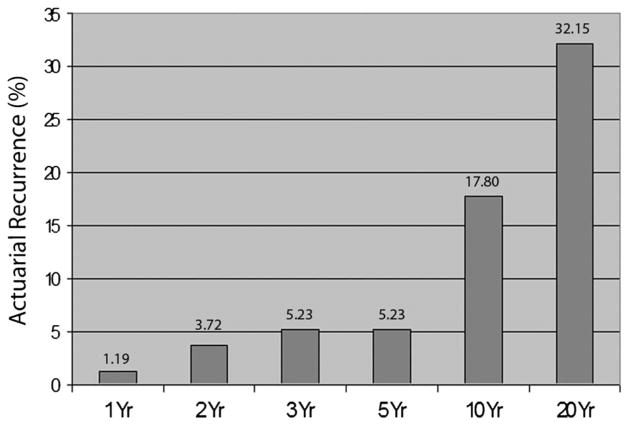
The statistical analysis of the above-described raw data revealed that there was no statistically significant difference in the duration of follow-up between patients who developed a recurrence and patients who did not have a recurrence (P = .48) (Table 8) The overall anticipated incidence of recurrences of the initial cohort of patients in remission increases with longer follow-ups. The actuarial estimated recurrence rates in patients with initial IPBR at 1, 2, 3, 5, 10, and 20 years were 1.19%, 3.72%, 5.23%, 5.23%, 17.80%, and 32.15%, respectively (Figure 3).
TABLE 8.
Microadenoma Duration of Follow-up in 93 Patients With Available Dataa
| Recurrence | N | Mean | Std Dev | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| No | 84 | 68.94 | 73.87 | 41 | 6 | 396 |
| Yes | 9 | 80.33 | 62.77 | 102 | 12 | 176 |
There was no statistically significant difference in the duration (months) of follow-up between the patients with recurrences and patients who did not develop a recurrence, P = .48. Analysis used logistic regression.
FIGURE 3.
Bar graph shows estimated actuarial rate of recurrences with passage of time.
We also evaluated statistically the influence of IPPC on recurrences. When the IPPC was collapsed to <2, 2 to 3, and >3 μg/dL, the incidence of recurrences associated with an IPPC of less than 2 μg/dL was 3.77%. There was a 3 times greater incidence of recurrences associated with an IPPC between 2 and 3 μg/dL (12%) and 4 times greater incidence of recurrences associated with an IPPC of greater than 3 μg/dL (15.79%) (Table 9). This difference in recurrences, however, was statistically not significant (P = .08). A similar result was obtained (P = .08) when patients with initial IPBR who were followed for less than 6 months were excluded from the analysis. There was no association between the level of the IPPC and recurrences when the IPPC was collapsed to <2, 2 to 5, and >5 μg/dL (P = .15). Because only 1 patient had an IPPC of greater than 5 μg/dL (5.3) and had no recurrence, we lacked statistical power to determine whether IPPC of greater than 5 μg/dL was associated with recurrence.
TABLE 9.
Microadenoma Recurrences Relative to Immediate Postoperative Plasma Cortisol in 97 Patients With Available Dataa
| Plasma Cortisol | Recurrence | Total | |
|---|---|---|---|
| No | Yes | ||
| <2 | 51 (96.23) | 2 (3.77) | 53 |
| 2–3 | 22 (88.00) | 3 (12.00) | 25 |
| >3 | 16 (84.21) | 3 (15.79) | 19 |
| Total | 89 | 8b | 97 |
There was a greater incidence of recurrence associated with immediate postoperative plasma cortisol of >2 μg/dL, without statistical significance. Mantel-Haenszel χ2 statistic = 3.2535; df = 1, P = .08.
One patient with recurrence, for whom there were no plasma cortisol data, required glucocorticoid replacement postoperatively.
Finally, data regarding glucocorticoid replacement were available in 86 of the 93 patients who had initial IPBR and were followed for 6 months or longer. Fifty-one (59.3%) patients were weaned off glucocorticoid replacement at a mean time of 12.8 months (range, 6–36 months). Of the remaining 35 patients who required glucocorticoid replacement for the duration of follow-up, 30 (34.8%) were followed for 12 months or longer (mean, 68.7 months; range, 12–236 months). Considering the retrospective and multicenter nature of this study, there was no specific, unified algorithm for withdrawal of glucocorticoids. Tapering and withdrawal of glucocorticoids was performed by the referring endocrinologist based on outpatient testing of the pituitary corticotroph–adrenal axis.
Macroadenomas
There were 11 women and 2 men with ACTH-secreting macroadenomas, ranging in age from 22 to 80 years, with the mean of 47 years.
Pathology and Immunohistochemistry
All 13 patients had histopathologic and immunohistochemical confirmation of an ACTH-producing adenoma.
RESULTS
In 13 patients with Cushing disease with macroadenomas, IPBR was achieved in only 4 patients (30%). None of these patients had invasive tumors, and all tumors were 2.0 cm or less in diameter. One of these patients was lost to follow-up. The remaining 3 patients, including the patient who was operated on in the second trimester of pregnancy, had a sustained biochemical remission with a mean follow-up of 67 months (5.6 years).
DISCUSSION
Diagnosis of Cushing disease remains a challenge despite a streamlined diagnostic process that includes late-night salivary cortisol determination in the detection of endogenous hypercortisolism, and the introduction of the overnight dexamethasone suppression test, not only as a diagnostic tool in Cushing disease, but also as a method to evaluate immediate postoperative results and predict recurrences.27,28 Additionally, the introduction of the IPSS test as a definitive study confirming pituitary-dependent ACTH hypersecretion has become an important method in the diagnosis of Cushing disease. Similarly, the imaging detection of ACTH-secreting microadenomas has been refined with the modern MRI technology and protocols detecting upward from 80% of microadenomas.8,23,29,30 Nevertheless, understanding of the complex biochemical processes in Cushing disease remains the bedrock of diagnosis.
Numerous reports have been published in the neurosurgical and endocrine literature attesting to the safety of transsphenoidal surgery.3,22,30–34 However, transsphenoidal surgery for removal of ACTH-secreting microadenomas can be challenging in that these adenomas are not always well demarcated, and they can be multilobular.30 In addition, it has also been our experience that patients harboring ACTH-producing microadenomas frequently have a pronounced anterior circular venous sinus that in some patients occupies the entire anterior sella dura.7,30 This anatomic variant can be the cause of persistent intraoperative bleeding that requires frequent and patient attention to hemostasis, preferably with the liberal temporary use of gel-foam or similar agents. Bipolar coagulation can make the situation worse in that it can shrink the outer layer of the opened circular sinus, resulting in more profuse venous bleeding. We have not resorted to exploration of the cavernous sinus in patients with suspected invasion of the medial cavernous sinus wall, as reported by Hofmann and coworkers.30
Immediate Postoperative Results
There is a uniform agreement among endocrinologists and pituitary surgeons that transsphenoidal surgery is the first treatment choice in patients with Cushing disease. Immediate postoperative remissions for microadenomas were reported variously in the literature as ranging from 46% to 98%,23,29–33,35–40 and from 17% to 68% for macroadenomas.27,30,33,40,41
Our immediate postoperative results in microadenomas approximate the higher end of this range. We believe that the primary reason for this was our surgical strategy, used prospectively in each patient undergoing selective microadenomectomy, of removing the pseudocapsule or, in the absence of a clearly identifiable pseudocapsule, of removing circumferentially (as per geometry of the tumor bed) a layer, usually measuring 1 to 2 mm in thickness, of residual pituitary tissue surrounding the tumor bed. It is also possible, however, that this surgical strategy may have been responsible for 35% of our patients requiring long-term glucocorticoid replacement. We desisted from using the term “cure” inasmuch as such a term implies both a life-long freedom from Cushing disease as well as a return to endocrine normalcy which, as indicated above, is not always the case, because in a number of patients, especially in those undergoing hypophysectomy, there is no recovery of the physiological pituitary—adrenal axis. Consequently, we prefer to use the term IPBR. In our series of patients, IPBR occurred when the immediate postoperative plasma cortisol dropped into the <0.5 to 5.3 μg/dL range (mean, 2.1) in conjunction with symptomatic adrenal cortical insufficiency, requiring glucocorticoid replacement. We view this relatively wide range in the postoperative plasma cortisol levels from patient to patient in our series as a trend, rather than as an absolute value, that was interrupted by the managing endocrinologist by starting gluco-corticoid replacement therapy in accordance with his or her tolerance level relative to the severity of symptoms of adrenal-cortical insufficiency that all patients with IPBR experienced, albeit to various degrees. Thus, we can not answer the question as to the precise relationship between the level of the immediate postoperative plasma cortisol and the development of symptoms of adrenal cortical insufficiency, other than for the fact that all patients who became symptomatic had a postoperative plasma cortisol value of 5.3 μg or less. This, and the issue of when to intervene with glucocorticoid replacement in the postoperative period of a patient in remission, may require a prospective study. In his editorial on Cushing disease, Oldfield,25 and in separate reports Utz et al39 and Rees et al,40 reviewed criteria published in the literature that have been suggested by different authors to define postoperative remission. These definitions, constructed in part with an eye on prediction of recurrences, included such disparate immediate postoperative results as a normal urinary free cortisol, continued need for glucocorticoid replacement,37 plasma cortisol levels as strict as less than 2 μg/dL,8,37,42 to a somewhat higher 3.6 μg/dL,38 to equal to or less than 5 μg/dL,28,40 to less than 20.5 μg/dL,31 and rather liberal definitions of remission such as “clinical remission and low or normal urinary free cortisol”35 and plasma cortisol designations of “low or normal.”7,36 Other authors advocated more complex criteria, including a low-dose dexamethasone suppression test as reported by Chen et al27 and Hammer et al.28 Oldfield recommends that patients whose immediate postoperative plasma cortisol is 2 to 3 μg/dL or less should be additionally studied with the CRH stimulation test to enhance the capacity to detect residual tumor cells. He further recommends that patients with an immediate postoperative plasma cortisol that is higher than 2 to 3 μg/dL should undergo an overnight dexamethasone suppression test to distinguish cortisol derived from residual tumor cells as opposed to normal corticotrophs.25
Previous reports in the literature are somewhat contradictory as to whether positive preoperative imaging for a microadenoma, as detected with CT or MRI, influences remissions, with some reports indicating that there is a direct correlation40,43 and some reporting no correlation.23,30 Our data indicate that a positive MRI for a microadenoma is associated not only with a statistically significantly greater chance that the surgeon will localize the microadenoma at operation, but also that a positive MRI portends a better prognosis for an IPBR. Our data further suggest that in patients with a negative MRI, in whom a microadenoma is identified and selectively removed at surgery, the incidence of IPBR is as high as in patients with a positive MRI. Even though patients with a negative MRI in whom a microadenoma was identified and selectively removed at surgery seemed to have a higher incidence of IPBR, compared with patients in whom a microadenoma was not found at surgery and the patient underwent hypophysectomy, this difference was statistically not significant. The reason for this may be the small sample. Nevertheless, it should probably be emphasized during preoperative discussion with the patient who presents with clinical and biochemical incontrovertible evidence for Cushing disease, including a positive IPSS test, but whose MRI is negative for a distinct microadenoma, that should the surgical exploration and histopathology fail to identify a microadenoma, there is a fair chance, possibly as high as 1 in 4, that the patient will not have IPBR despite a proposed hypophysectomy. The literature is supportive of this conclusion.43,44
Ever since the introduction of the IPSS test in the differential diagnosis of Cushing disease by Findling and colleagues in 1981,17,18 various authors have reported on the usefulness of this test not only in establishing the diagnosis of a pituitary-dependent ACTH hypersecretion, but also in terms of its influence on the IPBR, with some authors finding a direct correlation between a positive IPSS test and the incidence of IPBR,20,27,46 whereas others had some reservations in this regard.30,47 One author concluded that, although the incidence of remissions was similar in patients with a positive IPSS test compared with patients who did not undergo this study, patients in whom a differential lateralization was found had a statistically significant greater incidence of IPBR compared with patients in whom no lateralization could be found.43 The statistical analysis of our data suggests that, in patients with a negative MRI, the incidence of IPBR is significantly higher when the patients had a positive IPSS test compared with patients in whom this test was negative (P = .004). However, we recognize the limitation of statistical analysis of a relatively small number of patients studied with the IPSS test, especially the small number of patients who were operated on despite a negative IPSS test. Consequently, a positive IPSS test can not be considered as a statistical predictor of surgical success in patients with Cushing disease. In addition, false-negative IPSS tests can occur in 2% to 6% of cases. One of the reasons for this can be the integrity of the venous sample obtained. Concurrent measurement of the prolactin during the IPSS test can validate the integrity of venous sampling by correcting the pituitary ACTH gradient to the prolactin gradient.18 Nevertheless, based on our experience, we recommend that the IPSS test be obtained in patients with suspected Cushing disease whose MRI is negative for a distinct microadenoma and that surgical indications should perhaps be re-reviewed in case a technically well-executed IPSS test is negative. In short, we feel that the presence of a positive IPSS test, in face of a negative MRI, strengthens the surgical indication, gives the surgeon a greater degree of confidence to recommend surgery, and the patient a peace of mind when consenting to surgery.
Long-term Results
The incidence of recurrences as reported in the literature has varied widely from 0% to 100%.29 This impressive difference in the reported incidence of recurrences depended on a variety of factors. These included the following: (1) Consideration of the immediate postoperative cortisol secretion, as most commonly determined by plasma cortisol, with or without the overnight dexamethasone suppression test, or less frequently by measurement of 24-hour urinary free cortisol; (2) in some centers, by determining ACTH response to exogenous CRH stimulation; (3) by the absence or presence of adrenal cortical insufficiency and the need for lengthy glucocorticoid replacement; and (4) the length of follow-up.25,27,35,37,38,43,48 In an impressive series of 174 patients followed for more than 5 years by Chen and coworkers,27 there was a 7% incidence of recurrence when the immediate postoperative plasma cortisol was <3 μg/dL and 100% when the postoperative plasma cortisol was 3 to 8 μg/dL, especially when the latter levels were obtained following an early postoperative overnight 1-mg dexamethasone suppression test. In the extensive experience of Patil and coworkers,37 patients with immediate postoperative plasma cortisol values of greater than 2 μg/dL had a statistically significant 2.5 times greater chance of developing a recurrence compared with patients whose immediate postoperative plasma cortisol was less than 2 μg/dL. The same authors have also shown that the actuarial incidence of recurrences increases with longer follow-ups.37 Similarly, Hofmann and coworkers,30 analyzing the Fahlbusch series, have also confirmed, both from their own data and from the review of the literature, that the incidence of recurrence rises with longer follow-ups. Bochicchio and coworkers have shown that lengthy periods of postoperative adrenal cortical insufficiency, requiring glucocorticoid replacement, are associated with a lower incidence of recurrence.43 Given similar criteria for immediate postoperative remission that included low postoperative plasma cortisol of less than 2 to 5 μg/dL, postoperative adrenal cortical insufficiency requiring glucocorticoid replacement therapy and similar duration of follow-ups, most authors report a recurrence rate ranging between 5% and 20%.28,30,33,37,40,43,48,49
Our long-term results, specifically the incidence of recurrences, are similar to those reported in the literature. The results of this study have shown that, following initial remission, the estimated actuarial incidence of recurrences increases with the passage of time from 1.19% at 1 year to 32.15% at 20 years. Our data also lend marginal support to the previously reported relationship between the postoperative plasma cortisol and recurrences that an immediate postoperative plasma cortisol of higher than 2 μg/dL is associated with greater odds for a recurrence.8,28,37,38,40 It is perhaps of interest that, in our patients who did not develop a recurrence, the mean immediate postoperative plasma cortisol (1.8 μg/dL) approximated the recommended immediate postoperative plasma cortisol value as reported by Arnaldi and coworkers42 in their multicenter consensus statement on criteria for biochemical assessment of remission or “cure” of Cushing disease.
As previously reported in the literature by Hoffman et al,30 Oyesiku et al,33 and DeTomasi et al41 on the remission rate following transsphenoidal surgery in patients with Cushing disease harboring macroadenomas, the immediate postoperative results in our patients with macroadenomas were also less favorable, with only a third of patients having IPBR. However, when IPBR was accomplished, these patients had a favorable long-term prognosis.
CONCLUSION
Transsphenoidal microsurgery is a largely effective and safe first-treatment choice in patients with Cushing disease.
In patients with ACTH-producing microadenomas a positive MRI portends excellent prognosis, not only in terms of localizing the adenoma at surgery and thus making it easier for the surgeon to selectively remove the microadenoma, but also, compared with a negative MRI, a 4 times greater chance of achieving IPBR. We also conclude that removal of a rim of anterior pituitary tissue surrounding the tumor bed is conducive to achieving IPBR, although this surgical maneuver may be executed at the expense of recovery of the physiological pituitary–adrenal axis.
Our data suggest that patients with a negative MRI had a more favorable prognosis for IPBR when they had a positive IPSS test, compared with patients in whom this test was negative. However, in view of a relatively small number of patients who were operated on despite a negative IPSS test, a positive IPSS test, in face of a negative MRI, can not be considered as a predictor of surgical success in patients with Cushing disease. Nevertheless, we believe that the IPSS test is recommended for patients with suspected Cushing disease if the MRI is negative for an unequivocal microadenoma. Even though there was no statistically significant correlation in this regard, our data further suggest that, in patients with Cushing disease, whose MRI is negative for a microadenoma, a failure to identify the microadenoma at surgery is associated with a fair chance, perhaps as high as 1 in 4, that the patient will not have an IPBR even if a complete hypophysectomy is performed.
Long-term follow-up results indicate that the incidence of recurrences increases with the passage of time and that immediate postoperative plasma cortisol of less than 2 μg/dL appears to portend a better long-term prognosis, although without statistical significance.
IPBR is more difficult to accomplish in ACTH-producing macroadenomas.
ABBREVIATIONS
- ACTH
adrenocorticotropin
- CRH
corticotrophin-releasing hormone
- IPBR
immediate postoperative biochemical remission
- IPPC
immediate postoperative plasma cortisol
- IPSS
inferior petrosal sinus sampling
Footnotes
Disclosure
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism) Bull Johns Hopkins Hosp. 1932;50:137–195. [Google Scholar]
- 2.Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13(5):597–614. doi: 10.1016/0002-9343(52)90027-2. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg. 1969;16:185–217. doi: 10.1093/neurosurgery/16.cn_suppl_1.185. [DOI] [PubMed] [Google Scholar]
- 4.Salassa RM, Laws ER, Jr, Carpenter PC, Northcutt RC. Transsphenoidal removal of pituitary microadenoma in Cushing’s disease. Mayo Clin Proc. 1978;53(1):24–28. [PubMed] [Google Scholar]
- 5.Bigos ST, Somma M, Rasio E, et al. Cushing’s disease: management by transsphenoidal pituitary microsurgery. J Clin Endocrinol Metab. 1980;50(2):348–354. doi: 10.1210/jcem-50-2-348. [DOI] [PubMed] [Google Scholar]
- 6.Guthrie FW, Jr, Ciric I, Hayashida S, Kerr WD, Murphy ED. Pituitary Cushing’s syndrome and Nelson’s syndrome: diagnostic criteria, surgical therapy, and results. Surg Neurol. 1981;16(5):316–323. doi: 10.1016/0090-3019(81)90262-7. [DOI] [PubMed] [Google Scholar]
- 7.Boggan JE, Tyrrell JB, Wilson CB. Transsphenoidal microsurgical management of Cushing’s disease. Report of 100 cases. J Neurosurg. 1983;98(2):967–973. doi: 10.3171/jns.1983.59.2.0195. [DOI] [PubMed] [Google Scholar]
- 8.Fahlbusch R, Buchfelder M, Müller OA. Transsphenoidal surgery for Cushing’s disease. J R Soc Med. 1986;79(5):262–269. doi: 10.1177/014107688607900504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekkers OM, Biermasz NR, Pereira AM, et al. Mortality in patients treated for Cushing’s disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2007;92(3):976–981. doi: 10.1210/jc.2006-2112. [DOI] [PubMed] [Google Scholar]
- 10.Swearingen B, Biller BM, Barker FG, Jr, et al. Long-term mortality after transsphenoidal surgery for Cushing disease. Ann Intern Med. 1999;130(10):821–824. doi: 10.7326/0003-4819-130-10-199905180-00015. [DOI] [PubMed] [Google Scholar]
- 11.Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83(8):2681–2686. doi: 10.1210/jcem.83.8.4936. [DOI] [PubMed] [Google Scholar]
- 12.Brown RL, Weiss RE. An approach to the evaluation and treatment of Cushing’s disease. Expert Rev Anticancer Ther. 2006;9(Suppl 9):837–846. doi: 10.1586/14737140.6.9s.S37. [DOI] [PubMed] [Google Scholar]
- 13.Orth DN. Cushing’s syndrome. N Engl J Med. 1995;332 (12):791–803. doi: 10.1056/NEJM199503233321207. [DOI] [PubMed] [Google Scholar]
- 14.Liddle GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1960;20(12):1539–1560. doi: 10.1210/jcem-20-12-1539. [DOI] [PubMed] [Google Scholar]
- 15.Nugent CA, Nichols T, Tyler FH. Diagnosis of Cushing’s syndrome; single dose dexamethasone suppression test. Arch Intern Med. 1965;116(2):172–176. doi: 10.1001/archinte.1965.03870020012006. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham JM, Buxton OM, Weiss RE. Circadian variation in Cushing’s disease and pseudo-Cushing states by analysis of F and ACTH pulsatility. J Endocrinol Invest. 2002;25(9):791–799. doi: 10.1007/BF03345514. [DOI] [PubMed] [Google Scholar]
- 17.Findling JW, Aron DC, Tyrell JB, et al. Selective venous sampling for ACTH in Cushing’s syndrome: differentiation between Cushing disease and the ectopic ACTH syndrome. Ann Intern Med. 1981;94(5):647–652. doi: 10.7326/0003-4819-94-5-647. [DOI] [PubMed] [Google Scholar]
- 18.Findling JW, Kehoe ME, Shaker JL, Raff H. Routine inferior petrosal sinus sampling in the differential diagnosis of adrenocorticotropin (ACTH)-dependent Cushing’s syndrome: early recognition of the occult ectopic ACTH syndrome. J Clin Endodocrinol Metab. 1991;73(2):408–413. doi: 10.1210/jcem-73-2-408. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield EH, Chrousos GP, Schulte HM, et al. Preoperative lateralization of ACTH-secreting pituitary microadenomas by bilateral and simultaneous inferior petrosal venous sinus sampling. N Engl J Med. 1985;312(2):100–103. doi: 10.1056/NEJM198501103120207. [DOI] [PubMed] [Google Scholar]
- 20.Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325(13):897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 21.Yanovski JA, Cutlre GB, Jr, Chrousos GP, Nieman LK. Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. A new test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. JAMA. 1993;269(17):2232–2238. [PubMed] [Google Scholar]
- 22.Ciric I, Rosenblatt S, Zhao JC. Transphenoidal microsurgery. Neurosurgery. 2002;51(1):161–169. doi: 10.1097/00006123-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Jagannathan J, Smith R, De Vroom HL, et al. Outcome of using the histological pseudocapsule as a surgical capsule in Cushing disease. J Neurosurg. 2009;111(3):531–539. doi: 10.3171/2008.8.JNS08339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laws ER., Jr Editorial. Pituitary pseudocapsule. J Neurosurg. 2006;104(1):1–3. doi: 10.3171/jns.2006.104.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Oldfield EH. Cushing’s disease. J Neurosurg. 2003;98(5):948–951. doi: 10.3171/jns.2003.98.5.0948. [DOI] [PubMed] [Google Scholar]
- 26.Oldfield EH, Vortmeyer AO. Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg. 2006;104(1):7–19. doi: 10.3171/jns.2006.104.1.7. [DOI] [PubMed] [Google Scholar]
- 27.Chen JC, Amar AP, Choi S, Singer P, Couldwell WT, Weiss MH. Trans-sphenoidal microsurgical treatment of Cushing disease: postoperative assessment of surgical efficacy by application of an overnight low-dose dexamethasone suppression test. J Neurosurg. 2003;98(5):967–973. doi: 10.3171/jns.2003.98.5.0967. [DOI] [PubMed] [Google Scholar]
- 28.Hammer GD, Tyrell JB, Lamborn KR, et al. Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. J Clin Endocrinol Metab. 2004;89(12):6348–6357. doi: 10.1210/jc.2003-032180. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann BM, Fahlbusch R. Treatment of Cushing’s disease: a retrospective clinical study of the latest 100 cases. Front Horm Res. 2006;34:158–184. doi: 10.1159/000091580. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Mueller OA, Fahlbusch R. Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg. 2008;108(1):9–18. doi: 10.3171/JNS/2008/108/01/0009. [DOI] [PubMed] [Google Scholar]
- 31.Chandler WF, Schteingart DE, Lloyd RV, McKeever PE, Ibarra-Perez G. Surgical treatment of Cushing’s disease. J Neurosurg. 1987;66(2):204–212. doi: 10.3171/jns.1987.66.2.0204. [DOI] [PubMed] [Google Scholar]
- 32.Laws ER, Jr, Reitmeyer M, Tapar K, Vance ML. Cushing’s disease resulting from pituitary corticotrophic microadenoma. Treatment results from transsphenoidal microsurgery and gamma knife radiosurgery. Neurochirurgie. 2002;48(2–3 pt 2):294–299. [PubMed] [Google Scholar]
- 33.Oyesiku NM, Tindall GT, Blevins LS. Transsphenoidal surgery for Cushing’s disease: results in 120 cases over a 23 year period: 731. Neurosurg. 1997;41(3):727–731. [Google Scholar]
- 34.Reitmeyer M, Vance ML, Laws ER., Jr The neurosurgical management of Cushing’s disease. Mol Cell Endocrinol. 2002;197(1–2):73–79. doi: 10.1016/s0303-7207(02)00281-2. [DOI] [PubMed] [Google Scholar]
- 35.Arnott RD, Pestell RG, McKelvie PA, Henderson JK, Mc Neill PM, Alford FP. A critical evaluation of transsphenoidal pituitary surgery in the treatment of Cushing’s disease: prediction of outcome. Acta Enodocrinol (Copenh) 1990;123(123):423–430. doi: 10.1530/acta.0.1230423. [DOI] [PubMed] [Google Scholar]
- 36.Barbetta L, Dall’Asta C, Tomei G, Locatelli M, Giovanelli M, Ambrosi B. Assessment of cure and recurrence after pituitary surgery for Cushing’s disease. Acta Neurochir (Wien) 2001;143(5):477–481. doi: 10.1007/s007010170077. [DOI] [PubMed] [Google Scholar]
- 37.Patil CG, Prevedello DM, Lad SP, et al. Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab. 2008;93(2):358–362. doi: 10.1210/jc.2007-2013. [DOI] [PubMed] [Google Scholar]
- 38.Pieters GF, Hermus AR, Meijer E, Smals AG, Kloppenborg PW. Predictive factors for initial cure and relapse rate after pituitary surgery for Cushing’s disease. J Clin Endocinol Metab. 1989;69(6):1122–1126. doi: 10.1210/jcem-69-6-1122. [DOI] [PubMed] [Google Scholar]
- 39.Utz AL, Swearingen B, Biller BM. Pituitary surgery and postoperative management in Cushing’s disease. Endocrinol Metab Clin North Am. 2005;34(2):459–478. doi: 10.1016/j.ecl.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF. Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf) 2002;56(4):541–551. doi: 10.1046/j.1365-2265.2002.01511.x. [DOI] [PubMed] [Google Scholar]
- 41.DeTomassi C, Vance ML, Okonkwo DO, Diallo A, Laws ER., Jr Surgical management of adrenocorticotropic hormone-secreting macroadenomas: outcome and challenges in patients with Cushing’s disease or Nelson’s syndrome. J Neurosurg. 2005;103(5):825–830. doi: 10.3171/jns.2005.103.5.0825. [DOI] [PubMed] [Google Scholar]
- 42.Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593–5602. doi: 10.1210/jc.2003-030871. [DOI] [PubMed] [Google Scholar]
- 43.Bochicchio D, Losa M, Buchfelder M. Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J Clin Endocrinol Metab. 1995;80(11):3114–3120. doi: 10.1210/jcem.80.11.7593411. [DOI] [PubMed] [Google Scholar]
- 44.Pouratian N, Prevedello DM, Jagannathan J, Lopes MB, Vance ML, Laws ER., Jr Outcomes and management of patients with Cushing’s disease without pathological confirmation of tumor resection after transsphenoidal surgery. J Clin Endocrinol Metab. 2007;92(9):3383–3388. doi: 10.1210/jc.2007-0208. [DOI] [PubMed] [Google Scholar]
- 45.Sheehan JM, Lopes MB, Shehan JP, Ellegala D, Webb KM, Laws ER., Jr Results of transsphenoidal surgery for Cushing’s disease in patients with no histologically confirmed tumor. Neurosurgery. 2000;47(1):33–36. doi: 10.1097/00006123-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Zovickian J, Oldefield EH, Doppman JL. Usefulness of inferior petrosal sinus venous endocrine markers in Cushing’s disease. J Neurosurg. 1988;68(2):205–210. doi: 10.3171/jns.1988.68.2.0205. [DOI] [PubMed] [Google Scholar]
- 47.Swearingen B, Katznelson L, Miller K. Diagnostic errors after inferior petrosal sinus sampling. J Clin Endocrinol Metab. 2004;89(8):3752–3763. doi: 10.1210/jc.2003-032249. [DOI] [PubMed] [Google Scholar]
- 48.Averginos PC, Chrousos GP, Nieman LK. The corticotropin-releasing hormone test in the postoperative evaluation of patients with Cushing’s syndrome. J Clin Endocrinol Metab. 1987;65(5):906–913. doi: 10.1210/jcem-65-5-906. [DOI] [PubMed] [Google Scholar]
- 49.Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B. Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin Endocrinology (Oxf) 2005;63(5):549–559. doi: 10.1111/j.1365-2265.2005.02380.x. [DOI] [PubMed] [Google Scholar]