Abstract
OBJECTIVE
To investigate the relationship between multiple cryoprobes was investigated to determine whether they work in an additive or synergistic fashion in an in vivo animal model because 1.47 mm (17-gauge) cryoprobes have been introduced to the armamentarium for renal cryotherapy.
METHODS
Laparoscopic-guided percutaneous cryoablation was performed in both renal poles of 3 pigs using 3 IceRod cryoprobes. These 12 cryolesions were compared with 12 cryolesions using a single IceRod cryoprobe. Each cycle consisted of two 10-minute freeze cycles separated by a 5-minute thaw. The iceball volume was measured using intraoperative ultrasonography. The kidneys were harvested, and cryolesion surface area was calculated. The lesions were fixed and excised to obtain a volume measurement. Statistical analysis was used to compare the single probe results multiplied by 3 to the multiple probe group for iceball volume, cryolesion surface area, and cryolesion volume.
RESULTS
The iceball volume for the first freeze cycle for the single cryoprobe multiplied by 3 was 8.55 cm3 compared with 9.79 cm3 for the multiple cryoprobe group (P = .44) and 10.01 cm3 versus 16.58 cm3 for the second freeze (P =.03). The cryolesion volume for the single cryoprobe multiplied by 3 was 11.29 cm3 versus 14.75 cm3 for the multiple cyroprobe group (P =.06). The gross cryolesion surface area for the single cryoprobe multiplied by 3 was 13.14 cm2 versus 13.89 cm2 for the multiple probe group (P =.52).
CONCLUSION
The cryolesion created by 3 simultaneously activated 1.47-mm probes appears to be larger than that of an additive effect. The lesions were significantly larger as measured by ultrasonography and nearly so (P =.06) as measured by the gross cryolesion volume.
The frequent use of abdominal imaging has increased the diagnosis of asymptomatic renal masses and caused stage migration.1 As the U.S. population ages, more small renal masses (cT1, <4 cm) will be found in elderly patients who tend to have higher American Society of Anesthesiologists scores, which relates to a greater incidence of perioperative complications and transfusions.2
The traditional standard of care for clinically localized renal cell cancer is surgical resection by way of radical or partial nephrectomy.3,4 Nephron-sparing surgery and ablative therapy have been developed with the goal of eradicating malignancy and preserving nephron mass. This is particularly important in patients with poor renal function or risk factors for nephron loss, because s chronic kidney disease increases the risk of cardiovascular risk and mortality.5,6 In 2009, the American Urological Association guidelines7 expanded the treatment options to include thermal (ie, radiofrequency heat and cryoprobe therapy) ablation8,9 and, in select cases, active surveillance.10,11
Because thermal ablation is a relatively new modality of treatment, few standards have been developed for its most efficacious use. The miniaturization of cryoprobes, with probe downsizing from 3.4 to 1.47 mm (17-gauge) is thought to be beneficial by allowing the use of multiple smaller probes rather than a single probe to treat a larger area, with, possibly, a lower risk of parenchymal cracking and subsequent hemorrhage. The interaction between multiple probes is largely unknown. In the present study, we investigated whether multiple probes work in an additive or synergistic fashion in an in vivo animal model.
MATERIAL AND METHODS
Three female Yorkshire farm pigs weighing 39–42 kg were used in the present study, which was approved by the institutional animal care and use committee. After premedication with intramuscular 0.05 mg/kg atropine, anesthesia was induced with intramuscular 6 mg/kg telazol and 2 mg/kg xylazine. An ear vein cannula was placed and the pig intubated. Anesthesia was induced with 4%–5% isoflurane and maintained at 1%–2%. The pigs were placed in the left lateral decubitus position and insufflated with a Veress needle to 15 mm Hg. A 12-mm laparoscopic port was placed at the umbilicus for the camera, and 2 additional 12-mm ports were placed along the rectus edge as working ports. The kidney was exposed and mobilized using blunt and sharp dissection. A BK Pro Focus ultrasound 5–12-MHz laparoscopic probe (Copenhagen, Denmark) was placed in the midline port and checked to ensure that it could easily slide beneath the upper and lower pole.
A paper pattern with markers for 3 IceRods and 3 multipoint Thermal Sensors (both 1.47 mm in diameter, 17 gauge, Galil Medical, Arden Hill, MN) was placed in the abdomen and centered on the upper or lower pole. The IceRods, which create an elliptic-shaped iceball, were placed percutaneously under direct vision, in a triangular configuration 1 cm apart from each other, each with a multipoint Thermal Sensor 1 cm peripherally. The multipoint Thermal Sensor probes have thermal sensors at 5, 15, 25, and 35 mm from the tip. The cryoprobes and temperature sensors were all placed 1 cm into the parenchyma, which traversed about one half the thickness of the porcine kidney. The temperatures were measured at a depth of 5 mm from the kidney surface.
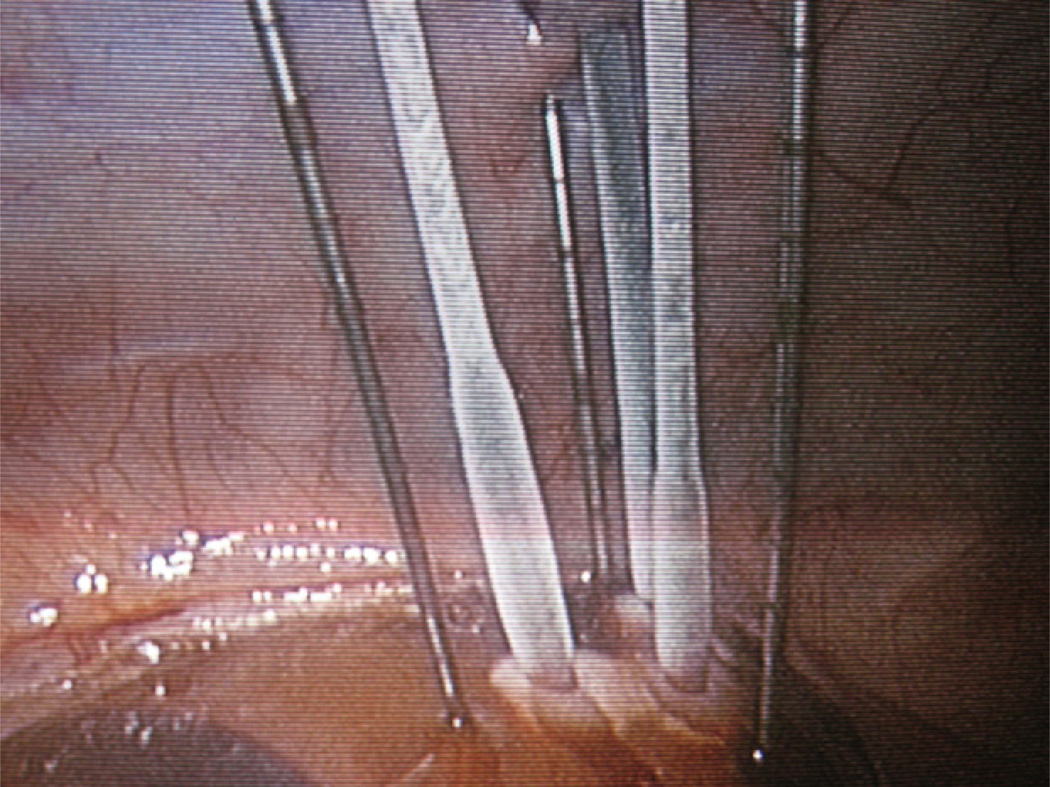
Two 10-minute freeze cycles were administered with a PresIce cryoablation unit (Galil Medical) using pressurized argon gas at 3500 psi, separated by a 5-minute active thaw with pressurized helium gas at 2200 psi (Fig. 1). Iceball progression was monitored with intraoperative laparoscopic ultrasonography, and the iceball volume was calculated at the conclusion of each freeze cycle.
Figure 1.
Freeze cycle 1 in progress, with 3 IceRods in triangular configuration 1 cm apart from each other and 1 Multipoint Thermal Sensor 1 cm peripheral to each cryoprobe.
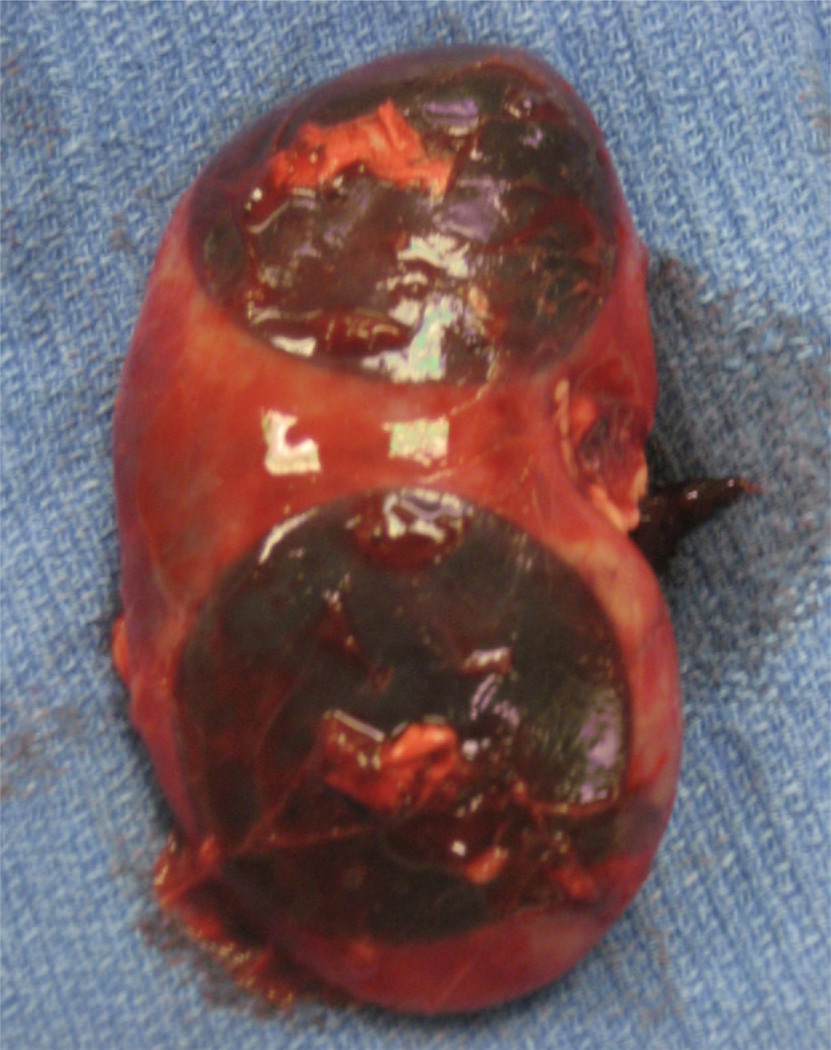
The procedure was repeated on the opposite pole. The pigs were then placed in the right lateral decubitus position, and the same procedure was performed on the left kidney. The pigs were killed with 0.1 mL/kg pentobarbital sodium (Eutha-6) and the kidneys immediately harvested through a midline incision. The length and width of each cryolesion was measured on the kidney surface at necroscopy. These values were averaged and used to calculate the surface area (Fig. 2).
Figure 2.
Necroscopy of porcine kidney after cryoablation with 3 probes. Cryolesion surface area measured and calculated.
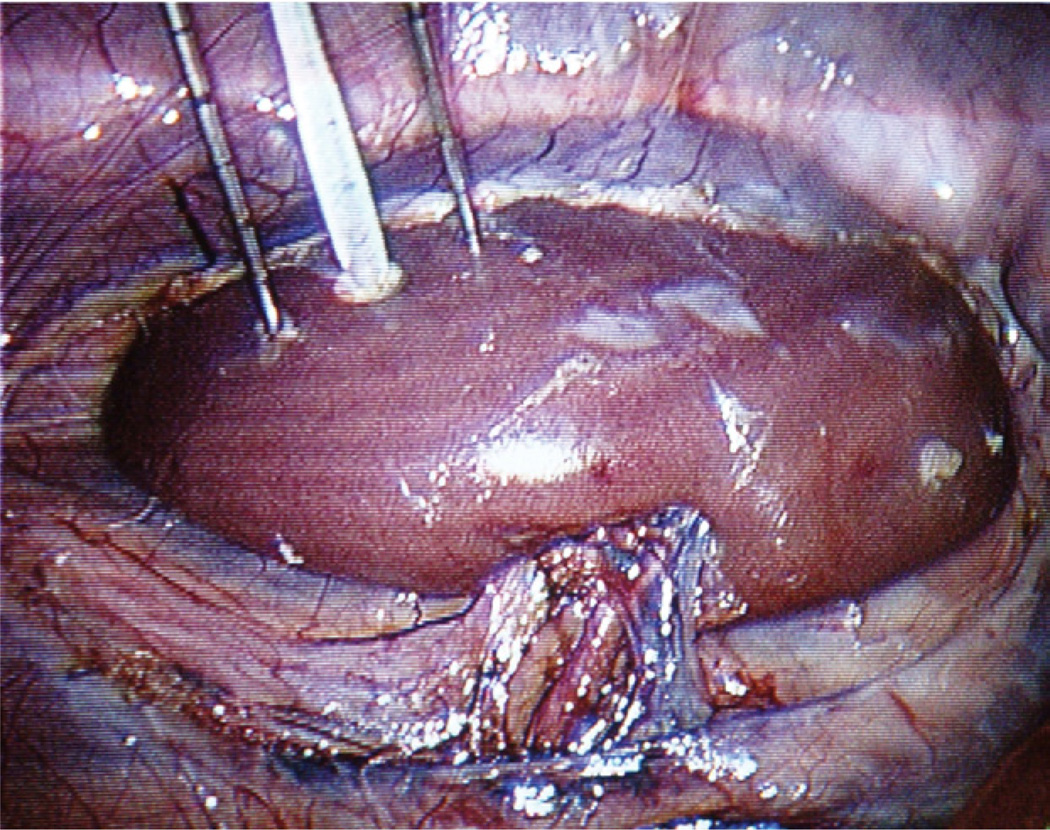
The lesions were then excised and fixed in 10% neutral buffered formalin. Once fixed, the lesions were dissected out with a 1-mm margin of normal tissue, and the volume was obtained by fluid displacement in a graduated cylinder (Fig. 3).
Figure 3.
Cryolesion volume obtained by fluid displacement in graduated cylinder.
Identical measurements were taken from a previous study using a single IceRod with the pneumoperitoneum also at 15 mm Hg (Fig. 4).12 The same laboratory, personnel, statistician, surgical technique, PresIce cryoablation unit, BK Pro Focus ultrasound 5–12-MHz laparoscopic probe, pig farm, and animal housing and food were used. The same gross surface measurements were taken, and the same method was followed for the volume measurements. These measurements were multiplied by 3 and compared with the measurements from the 3 cryoprobes.
Figure 4.
Freeze cycle initiated with single IceRod, with 1 temperature sensor placed medially and 1 peripherally.
Statistical analysis was performed by a statistician (K.E.O.). The mean values for the cryolesion area and volume were compared between the single and multiprobe groups using 2-sided 2-group t tests, with a 5% significance level. t Tests were used to compare the mean temperatures between the single and multiple probe groups.
RESULTS
Cryolesions were created in the upper and lower pole of both kidneys in all 3 pigs. No intraoperative complications or deaths occurred. The mean operating time was 3.6 hours for the single probe group and 3.3 hours for the multiple probe group. Each single probe trial was multiplied by 3 to compare the results of a single probe multiplied by 3 (additive arm) with the results for the 3 probes activated simultaneously (synergistic arm). In the single probe group multiplied by 3, the average temperature 1 cm from the probe at the end of freeze cycle 2 was 2.8°C (range 26° to −16°C) for the peripheral sensor and 6.3°C (range 28° to −13°C) for the medial sensor. For the multiple probe group, the average temperature 1 cm peripheral to each probe at the end of freeze cycle 2 was −15.0°C (range 0° to −32°C), −14.3°C (range 13° to −45°C) and −15.3°C (range 3° to −43°C). After the second freeze cycle, there was a 19 degree temperature difference between the multiple probe group and the single probe group (P = 0.0001).
The mean iceball volume as determined by ultrasonography for the first freeze cycle for the single cryoprobe multiplied by 3 was 8.55 cm3 (range 1.86–14.82) compared with 9.79 cm3 (range 4.32–15.50) for the multiple probe group (P = .44) and 10.02 cm3 (range 5.70–17.82) compared with 16.58 cm3 (range 6.01–38.70) for the second freeze cycle (P = .03). The mean cryolesion volume was 11.29 cm3 (range 7.20–17.40) for the single probe multiplied by 3 versus 14.75 cm3 (range 7.0–26.0) for the multiprobe group (P = .06). The mean gross cryolesion surface area for the single probe multiplied by 3 was 13.14 cm2 (range 9.35–18.47) compared with 13.89 cm2 (range 8.68–19.80) for the multiple probe group (P = .52).
In the single probe group multiplied by 3, the mean iceball volume as measured by ultrasonography for the first freeze was 8.07 cm3 (range 4.02–12.45) for the first ablation site and 12.00 cm3 (range 1.86–14.82) for the second ablation site. The mean iceball volume as measured using ultrasonography for the second freeze cycle was 9.15 cm3 (range 5.70–17.82) for the first ablation site and 10.89 cm3 (range 4.92–15.06) for the second ablation site. The mean cryolesion volume as measured using fluid displacement was 12.0 cm3 (range 8.40–17.40) for the first ablation site and 10.8 cm3 (range 7.20–14.10) for the second ablation site. The mean gross cryolesion surface area was 13.5 cm2 (range 10.80–18.48) for the first ablation site and 12.78 cm2 (range 9.36–16.02) for the second ablation site.
In the multiple probe group, the mean iceball volume as measured using ultrasonography for the first freeze cycle was 8.27 cm3 (range 4.32–15.5) for the first ablation site and 11.31 cm3 (range 9.60–14.05) for the second ablation site. The mean iceball volume using ultrasonography for the second freeze cycle was 16.23 cm3 (range 8.22–38.70) for the first ablation site and 16.94 cm3 (range 6.01–26.90) for the second ablation site. The mean cryolesion volume as measured using fluid displacement was 12.8 cm3 (range 7.0–19.0) for the first ablation site and 16.7 cm3 (range 11.0–26.0) for the second ablation site. The mean gross cryolesion surface area was 12.50 cm2 (range 8.68–16.67) for the first ablation site and 15.28 cm2 (range 13.03–19.80) for the second ablation site.
COMMENT
Three-dimensional numeric simulations of multiple 1.47 mm (17-gauge) cryoprobes were matched with experimental data in a phase-changing gel by Magalov et al.13 They found additional cryoprobes resulted in an increased expansion of the isotherm in all 3 directions and cited this as clear evidence of the synergistic effect of multiple cryoprobes. They found the effect of different placement decreased as the density of the probes increased. They cautioned against placing the probes too close together to take advantage of the synergy created with multiple probes.
Single and multiple 1.47-mm cryoprobes were tested in a porcine kidney using laparoscopic-guided percutaneous approach by Ames et al.14 When analyzed 2 weeks after treatment, no significant difference was found in the mean temperature 1 cm from the center of the iceball or the size of the cryolesion for a single cryoprobe with an elliptic-shaped iceball, 3 standard probes with a sphereshaped iceball, or a single 3.4-mm probe. However, a trend was noted toward a larger lesion with the 3 probes (P = .14). The treatment in that chronic study was two 8-minute freezes with an unspecified thaw interval.
The mean temperature 1 cm from the center of the iceball was −41.5°C, much colder than the 2.8°C and 6.3°C temperatures in the present study. The mean temperature 1 cm from the 3 standard cryoprobes was −39.2°C, colder than the −15.0°C, −14.3°C, and −15.3°C temperatures we obtained. The average gross cryolesion size for the elliptic probe was 2.3 cm, yielding a surface area of 4.15 cm2, smaller than the 4.38 cm2 obtained in the present study. The average gross lesion size for the 3 standard, sphere-shaped, probes was 3.1 cm, yielding a surface area of 7.55 cm2, smaller than the 13.89 cm2 obtained in our results.
Weld et al15 investigated 1, 3, and 4 IceRods 2-cm apart and 3-cm deep in a porcine kidney using a laparoscopic approach. After a double 10-minute freeze cycle, separated by a 3-minute thaw, they found a zone of complete ablation 1 cm from the tip of the rod 2.0, 4.4, and 4.9 cm in diameter, respectively. The average iceball size produced by the single rod was 2.2 cm, yielding a volume of 5.58 cm3, larger than the 3.34 cm3 found in the present study. Confluent central necrosis in the quadratic configuration in which the diagonal rods were 2.8 cm apart, suggested a synergistic relationship using multiple probes.
Thermal maps around 2 simultaneously activated 2.4-mm cryoprobes, 2-cm apart, 2-cm deep in a porcine kidney using a midline laparotomy were created by Permpongkosol et al.16 After a 12- and 8-minute freeze cycle, they noted a complete coagulative zone of 4.0 cm. Citing previous work in which a single 2.4-mm cryoprobe created a 2.1-cm zone of necrosis, they hypothesized that the cryoprobes were additive rather than synergistic.
Single and a template of 3 IceRods were investigated in a porcine kidney using a laparoscopic-assisted percutaneous approach by Breda et al.17 A double 10-minute freeze separated by a 5-minute thaw was used. The gross surface area was measured, and volume was obtained by histologic reconstruction from 3-mm cuts of the area of necrosis. The temperature 1 cm from the single probe was −29°C to −30°C, colder than the 2.8°C and 6.3°C found in our study. The temperature 1 cm from the 3 elliptic probes placed 1 cm apart in a triangular configuration was −68°C to −78°C, also colder than the −14.3°C to −15.3°C we found.
The single elliptic probe resulted in a necrotic lesion 2.5 cm in diameter, yielding a surface area of 4.91 cm2, larger than the 4.38 cm2 obtained in the present study. The volume by histologic reconstruction was 7.1 cm3. Multiplied by 3, this would yield a volume of 21.3 cm3, larger than the 11.29 cm3 found in the present study. The template of 3 elliptic probes produced a 4.5-cm lesion, yielding a surface area of 15.90 cm2, larger than the 13.89 cm2 obtained in our study. The volume by histologic reconstruction was 41 cm3, much larger than the 14.75 cm3 found on the multiprobe group in our study. This is of interest because we determined the volume of necrosis grossly using fluid displacement instead of using histologic cuts.
The same group evaluated the cryolesion size when 3 elliptic probes were placed 1, 1.5, and 2 cm apart. They concluded that placing the probes 1.5 cm apart led to the largest area of necrosis with the safest margin. They did not, however, investigate whether the interaction between the probes was additive or synergistic. The depth of probe placement was not cited in that study, and 3 experiments were run in each kidney, raising the possibility of the overlap of the cryolesions.
Tsakiris et al18 evaluated the in vivo factors influencing the freezing cycle during cryoablation of small renal masses in 67 patients. They found a consistently lower freezing rate in the periphery of the benign masses. This might be explained by the fact that renal cell carcinoma secretes more vascular endothelial growth factor than benign growths. Malignant growths stimulate the inflow of warm circulating blood by way of angiogenesis and might cause a slower freezing rate than a benign tumor with fewer vessels.
After multivariate analysis, they found diabetes and tumor location in the lower pole to correlate with a faster freezing rate. They cited the changes in renal microvasculature inherent to diabetes as a possible etiology of the faster freeze. That the polar arteries are more frequently encountered in the upper pole was discussed as an explanation for the faster freeze in the lower pole. Another explanation could be that the lower pole might be more accessible, allowing more accurate cryoneedle placement for a faster freeze.
Beemster et al19 found the performance of 17-gauge cryoprobes was less variable in a multiple probe configuration than a single cryoprobe when the temperature was measured 3 mm from the cryoprobe in agar and gel. In that study, the average temperature variation was 3.8°C greater in the multiple probe group. This might have been because our testing was in vivo instead of in gel or agar. Additionally, our temperature sensors were 1 cm from the cryoprobe instead of 3 mm.
Wright et al20 studied the effect of microwave ablation in a porcine liver and found that sequential activation of 3 equidistant probes yielded an additive effect with twice the area of a single freeze and simultaneous activation had a synergistic effect with 6 times the volume of a single freeze. Berger and Poledna21 reported placing cryoprobes outside small liver tumors to take advantage of the synergy between the probes to cool rapidly and avoid damage to the surrounding healthy liver.
Littrup et al22 studied temperature and ice formation as measured by computed tomography temperature using thermocouples that were 5, 10, and 15 mm from one to four 1.7- or 2.4-mm diameter cryoprobes in agar phantoms at a baseline temperature of 6°C, 24°C, or 39°C. The found probe synergy between the multiple cryoprobes allowed the lethal ice diameter of single cryoprobes to increase from approximately 35% to >70% for ≥3 cryoprobes, while maintaining a <1-cm nonlethal margin from the leading edge of visible ice (0°C).22
The present study is novel in the use of fixing and then excising lesions to obtain a volumetric measurement using Archimedes’ principle of fluid displacement. The limitations included the use of normal porcine tissue rather than malignant tissue. Temperatures within a malignancy might be warmer than in normal healthy tissue because of the increased vascularity; alternatively, the malignant tissue might freeze more rapidly than normal tissue if necrosis is present within the tumor. Additionally, the present study only examined the effect of simultaneous activation of the cryoprobes. The end result could be different if the probes were activated in a delayed sequence in series. Finally, the area of necrosis was determined grossly in the present study, without histologic evaluation. However, the visible area of necrosis has been shown to correlate closely with the histologic area of necrosis.14
Variations in the temperature, iceball size, and cryolesion size were present within our study and comparing our study with previous work. This might have resulted from the variability in renal vascularity, probe placement depth, point of probe activation/thaw time, and intraand interobserver variability in the ultrasound technique. The intraobserver variance for ultrasonography of a pediatric kidney has been reported to be 4.6 mm, with an interobserver variance of 7.8 mm.23 This equates to a 30%–40% variation in the ultrasound estimation of renal volume between 2 observers or 2–3 years of growth in the pediatric kidney.24 This variability with ultrasound techniques causes us to believe that it is important to use temperature sensors to ensure that the target tissue reaches a lethal temperature. The multipoint Thermal Sensors have temperature sensors 5, 15, 25, and 35 mm from the probe tip. This allows one to attain 4 critical data points with the placement of one 17-gauge probe in the kidney.
Because it is well known that the kidney (and even the contralateral kidney) respond with changes in blood flow to an insult to the parenchyma, another limitation of the present study might be that both poles were tested in each kidney. The measurements were larger for the second ablation site for all parameters in the multiple probe group. This limits the applicability to treatment of a single lesion in the clinical setting. It is of interest, however, if >1 lesion is being treated in 1 kidney.
A future direction for the use of multiple cryoprobes will be the clinical application of mathematical models previously described25 to optimize their interaction and reduce the subjectivity of cryotherapy treatment planning.
CONCLUSIONS
The cryolesion created by 3 simultaneously activated 1.47-mm probes appears to be synergistic rather than additive. The lesions created by 3 simultaneously activated cryoprobes 1-cm apart were significantly larger as measured by ultrasonography. Moreover, the gross cryolesion volume showed a trend for being larger (P = .06).
Acknowledgments
Funding Support: Galil Medical, Inc. provided financial support and the use of a PresIce cryotherapy machine and IceRods for the study.
Footnotes
Financial Disclosure: BK Medical provided the use of an ultrasound machine and laparoscopic ultrasound probe for the study. Department of Urology, University of California, Irvine, provided the use of the Atellas Education Center as a study site, including laparoscopic surgical equipment, preoperative animal housing, and animal disposal.
References
- 1.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 2.Han KR, Kim HL, Pantuck AJ, et al. Use of American Society of Anesthesiologists physical status classification to assess perioperative risk in patients undergoing radical nephrectomy for renal cell carcinoma. Urology. 2004;63:841–846. doi: 10.1016/j.urology.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 3.Frank I, Blute ML, Leibovich BC, et al. Independent validation of the 2002 American Joint Committee on Cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol. 2005;173:1889–1892. doi: 10.1097/01.ju.0000158043.94525.d6. [DOI] [PubMed] [Google Scholar]
- 4.Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol. 1999;162:1930–1933. doi: 10.1016/S0022-5347(05)68071-8. [DOI] [PubMed] [Google Scholar]
- 5.McKiernan J, Simmons R, Katz J, et al. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59:816–820. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared to partial nephrectomy. J Urol. 2008;179:468–471. doi: 10.1016/j.juro.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 7.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol. 2008;179:1227–1233. doi: 10.1016/j.juro.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 9.Gill IS, Remer EM, Hasan WA, et al. Renal cryoablation: outcome at 3 years. J Urol. 2005;173:1903–1907. doi: 10.1097/01.ju.0000158154.28845.c9. [DOI] [PubMed] [Google Scholar]
- 10.Oda T, Miyao N, Takahashi A, et al. Growth rates of primary and metastatic lesions of renal cell carcinoma. Int J Urol. 2001;8:473–477. doi: 10.1046/j.1442-2042.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- 11.Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world. J Urol. 2006;175:425–431. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 12.Young JL, Louie MK, Ortiz-Vanderdys CG, et al. Impact of pneumoperitoneum on renal cryotherapy. J Endourol. 2009;23:1451–1455. doi: 10.1089/end.2009.0396. [DOI] [PubMed] [Google Scholar]
- 13.Magalov Z, Shitzer A, Degani D. Isothermal volume contours generated in a freezing gel by embedded cryo-needles with applications to cryo-surgery. Cryobiology. 2007;55:127–137. doi: 10.1016/j.cryobiol.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Ames CD, Vanlangendonck R, Venkatesh R, et al. Enhanced renal parenchymal cryoablation with novel 17-gauge cryoprobes. Urology. 2004;64:173–175. doi: 10.1016/j.urology.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Weld KJ, Hruby G, Humphrey PA, et al. Precise characterization of renal parenchymal response to single and multiple cryoablation probes. J Urol. 2006;176:784–786. doi: 10.1016/j.juro.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 16.Permpongkosol S, Nicol TL, Khurana H, et al. Thermal maps around two adjacent cryoprobes creating overlapping ablations in porcine liver, lung, and kidney. J Vasc Interv Radiol. 2007;18:283–287. doi: 10.1016/j.jvir.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Breda A, Lam JS, Riggs S, et al. In vivo efficacy of laparoscopic assisted percutaneous renal cryotherapy: evidence based guidelines for the practicing urologist. J Urol. 2008;179:333–337. doi: 10.1016/j.juro.2007.08.089. [DOI] [PubMed] [Google Scholar]
- 18.Tsakiris P, Beemster P, Wijkstra H, et al. In vivo factors influencing the freezing cycle during cryoablation of small renal masses. J Endourol. 2009;23:545–549. doi: 10.1089/end.2008.0226. [DOI] [PubMed] [Google Scholar]
- 19.Beemster PWT, Lagerveld BW, Witte LPW, et al. The performance of 17-gauge cryoprobes in vitro. Technol Cancer Res Treat. 2008;7:321–327. doi: 10.1177/153303460800700406. [DOI] [PubMed] [Google Scholar]
- 20.Wright AS, Lee FT, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- 21.Berger WK, Poledna J. New strategies for the placement of cryoprobes in malignant tumors of the liver for reducing the probability of recurrences after hepatic cryosurgery. Int J Colorectal Dis. 2001;16:331–339. doi: 10.1007/s003840100317. [DOI] [PubMed] [Google Scholar]
- 22.Littrup, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20:1343–1351. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sargent MA, Wilson BP. Observer variability in the sonographic measurement of renal length in childhood. Clin Radiol. 1992;46:344–347. doi: 10.1016/s0009-9260(05)80382-4. [DOI] [PubMed] [Google Scholar]
- 24.Sargent MA, Long G, Karmali M, et al. Interobserver variation in the sonographic estimation of renal volume in children. Pediatr Radiol. 1997;27:663–666. doi: 10.1007/s002470050207. [DOI] [PubMed] [Google Scholar]
- 25.Baissalov R, Sandison GA, Reynolds D, et al. Simultaneous optimization of cryoprobe placement and thermal protocol for cryosurgery. Phys Med Biol. 2001;46:1799–1814. doi: 10.1088/0031-9155/46/7/305. [DOI] [PubMed] [Google Scholar]