Summary
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a major negative regulatory molecule for T cell activation with a complex biology and function. CTLA-4 is known to regulate homeostatic lymphoproliferation as well as tolerance induction and has been proposed to be an important effector molecule by which regulatory T cells suppress immunity. The immunoregulatory properties of CTLA-4 are primarily mediated by competition with the co-stimulator CD28 for ligand-binding but also by delivering negative signals to T cells through its cytoplasmic tail. In this study, we addressed the effect of directly mutating the amino acid residue, Tyrosine 201 (Tyr201), of the intracellular domain of CTLA-4 in situ and its implications in T cell function in the context of autoimmunity. Therefore a novel CTLA-4 knock-in mouse (Y201V KI) was generated, in which Tyr201 was replaced by a valine that could not be phosphorylated. Mice expressing the CTLA-4 mutant molecule were generally healthy and did not show signs of disruption of T cell homeostasis under steady state conditions seen in CTLA-4 deficient mice. However, T cells isolated from Y201V KI mice expressed higher levels of CTLA-4 on the cell surface and displayed a Th2 biased phenotype following TCR stimulation. Furthermore, Y201V KI mice developed exacerbated disease as compared to wild-type upon antigen-specific T cell activation in an in vivo model of experimental autoimmune encephalomyelitis (EAE). Importantly, the Y201V mutation resulted in impaired suppressive activity of T regulatory cells (Treg) while T effector function remained intact. These data suggest that effects associated with and mediated through Tyr201 of CTLA-4s intracellular domain are critical for Treg function.
Keywords: CTLA-4, T regulatory cells, autoimmunity, EAE/MS
Introduction
Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) was the first negative checkpoint regulatory molecule identified on T cells [1–3]. The expression of CTLA-4 on the cell surface is essential for T cell homeostasis in neonates, as genetic disruption leads to massive lymphoproliferative disease (LPD) and death within 3–4 weeks after birth [4;5]. Moreover, both functional and genetic data suggest that CTLA-4 plays a fundamental role in peripheral T cell tolerance in the autoimmune setting [6]. The molecule is induced on all activated T cells and constitutively expressed on regulatory T cells (Tregs). CTLA-4 shares significant structural homology with the major co-stimulatory receptor CD28 [7–9] including sharing the same ligands B7-1 and B7-2. CTLA-4 binds the B7 molecules with a substantially higher avidity than CD28 [10–12], which has led to the suggestion that CTLA-4 functions as a checkpoint regulator by selectively competing with CD28 thus inhibiting co-stimulation. In fact, in the case of Tregs, several groups have suggested that the major function of CTLA-4 on Tregs is to downregulate or strip the CD28/CTLA-4 ligands from the cell surface of antigen presenting cells leading to suppression of immune reactivity [13;14]. However, in addition to the indirect role of CTLA-4 on T cell activation, we and others have documented a direct role for CTLA-4 in modifying T cell signaling through the T cell receptor interactions with phosphatases bound to the intracellular domain of the co-receptor. The intracellular portion of the CTLA-4 molecule contains a lysine- and a proline-rich motif, as well as 2 tyrosine phosphorylation sites at position 201 and 218 [15;16] that can function biochemically to directly regulate the activation of pathogenic T-cells by modulating T-cell receptor signaling at a very proximal stage [17]. Moreover, sub-cellular localization of the CTLA-4 molecule is mediated by binding of the adaptor proteins AP-1 and AP-2 to the unphosphorylated Y201VKM motif in the cytoplasmic tail of CTLA-4 [18–20].
Upon T cell activation, CTLA-4 is rapidly translocated to the site of TCR engagement and surface expression is stabilized by phosphorylation of the Y201VKM motif [7;21;22], therefore abolishing AP-2 binding and internalization [7;23]. In the past, the generation of CD2-driven CTLA-4 transgenes which either lack the entire intracellular domain or carry a point-mutation within the Y201VKM motif [24;25], are more effective in reaching the cell surface and localizing to appropriate immune synapses/lipid-rafts. The investigators observed a very limited direct effect on CTLA-4 function. The transgenic mice were largely normal suggesting that the intracellular functions of the molecule were secondary to their critical indirect role in blocking B7 engagement and subsequent CD28-mediated signaling. These results, however, might reflect the use of a constitutive promoter driven transgene resulting in non-physiologic expression and high levels of constitutively–expressed CTLA-4 that could alter the relevance of the tail in a natural setting [26;27].
The aim of the present study was to explore the in vivo contribution of Tyrosine 201 (Tyr201), of the intracellular domain of CTLA-4 in the context of T cell homeostasis and upon an immune response in the in situ setting at physiological levels. To achieve this goal, we generated a CTLA-4 knock-in mouse, (Y201V KI), in which the tyrosine residue at position 201 in the intracellular YVKM motif was replaced with a non-functional amino acid. This strategy assures the expression of the CTLA-4 Y201V mutant molecule at physiological levels. We observed that the Y201V mutation resulted in increased surface expression of CTLA-4 on T effector/memory cells as well as on activated T effector and T regulatory cells but had no effect on the overall T cell phenotype in mutant mice under homeostatic conditions. However, mice expressing the Y201V mutant molecule develop exacerbated disease in a model of experimental autoimmune encephalomyelitis (EAE) due to impaired Treg function rather than accelerated T effector function. Thus, these results demonstrate the importance of CTLA-4s intracellular domain in Treg biology.
Results
Generation of Y201V KI mice
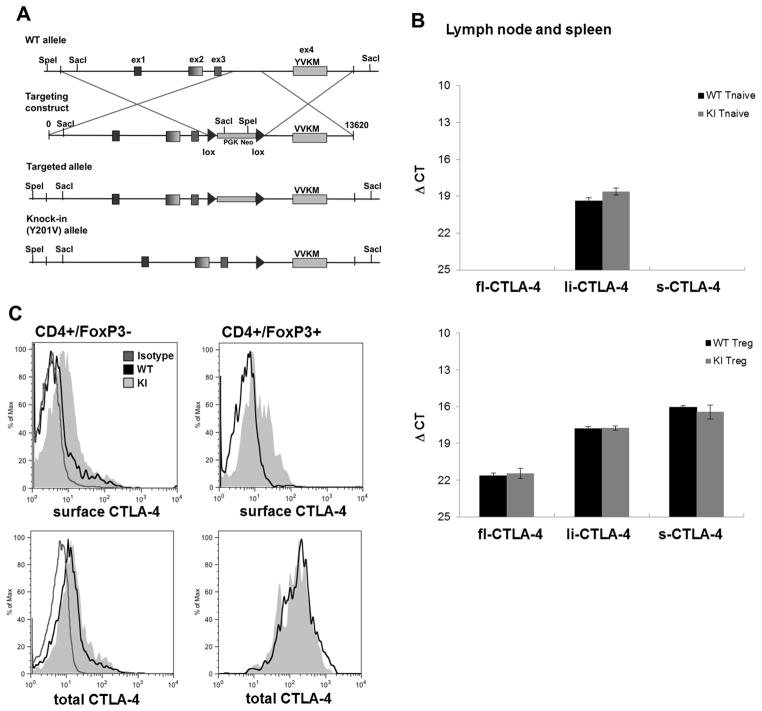
A genomic fragment containing the entire mouse CTLA-4 locus from a bacterial artificial chromosome (clone RP23-146J17: BACPAC) was obtained and the nucleotide sequence was modified to introduce an amino acid change from tyrosine (Y) to valine (V) at position 201 within Ex4 (Fig 1A). This modified construct was used to target a B6 ES-cell line and selected clones were injected into BALB/c embryos. The chimeric mice were screened for germline transmission, and backcrossed onto the B6 background. The KI mice expressed the mutant form of the CTLA-4 protein, based on nucleotide sequence analysis (data not shown). Moreover, the Y201V KI CTLA-4 molecule was at least partially functional as it rescued the CTLA-4 KO lethal phenotype.
Figure 1.
(A) A 13.6 kilobase genomic fragment containing the entire mouse CTLA-4 locus was retrieved from a bacterial artificial chromosome (clone RP23-146J17: BACPAC). The nucleotide sequence was modified resulting in an amino acid change from Tyrosine (Y) to Valine (V) at position 201 within Exon4. Further, a LoxP-flanked PGK/em7-promotor driven neo cassette was inserted to allow selection of successful targeted B6 ES cell clones. Deletion of the NEO-cassette was achieved by breeding founder mice with OX40-Cre transgenic mice. (B) T naïve and T regulatory cells of CTLA-4 WT and Y201V knock-in mice were FACS sorted and mRNA expression levels of the different CTLA-4 isoforms were measured by quantitative real-time PCR. The ΔCt (threshold cycle) value was determined using the following formula: . Data are displayed as mean ΔCt ± SD. (C) Surface (upper panel) and total (lower panel) CTLA-4 expression in primary CD4+/FoxP3− T conventional and CD4+ FoxP3+ T regulatory cells from WT and Y201V knock-in mice was assessed by flow cytometry.
Similar expression levels of CTLA-4 isoforms but increased CTLA-4 surface expression in Y201V KI mice
Beside the full-length CTLA-4 molecule, two other splice variant isoforms of CTLA-4 have been described, including a ligand non-binding (liCTLA-4) as well as a soluble, secreted variant (sCTLA-4) [28;29]. Importantly, polymorphisms in the CTLA-4 gene, resulting in differential expression of the splice variants, have been associated with the susceptibility to multiple autoimmune diseases, including type 1 diabetes (T1D), multiple sclerosis, rheumatoid arthritis, Grave’s disease, hypothyroidism, and systemic lupus erythematosus [29–31]. To examine whether the Y201V mutation altered overall CTLA-4 transcription, we examined mRNA levels of the full-length, ligand-independent and soluble CTLA-4 isoforms in T naive and Treg cells isolated from lymph node and spleen of 8-week old littermates. Consistent with previous observations, naive T cells only expressed the li-CTLA-4 form but Treg cells constitutively express all three isoforms. Of note, there were no differences in expression levels of any of the CTLA-4 isoforms when comparing WT and Y201V KI mice. These results demonstrated that the Y201V mutation did not affect relative CTLA-4 isoform expression patterns or mRNA levels (Fig 1B). Next, we examined the protein expression of the full-length CTLA-4, both cell surface and intracellular staining. Surface protein expression of full-length CTLA-4 was significantly elevated on T conventional as well as T regulatory cells in Y201V KI mice (Fig 1C, upper panel and Suppl. Fig 1A), whereas total CTLA-4 expression was unaltered (Fig 1C, lower panel and Suppl. Fig 1B). This result is most likely a consequence of abolished adaptor protein (AP)-2 binding to the mutated Y201VKM motif, which regulates internalization of the receptor from the surface [7;23]. It is important to note that there were no differences in CTLA-4 cell surface expression levels, lymph node cellularity and T cell phenotype, even after activation between heterozygote and wild type mice at 2–3 months of age.
Expression of the CTLA-4 Y201V mutant molecule alters the cytokine profile of activated T cells
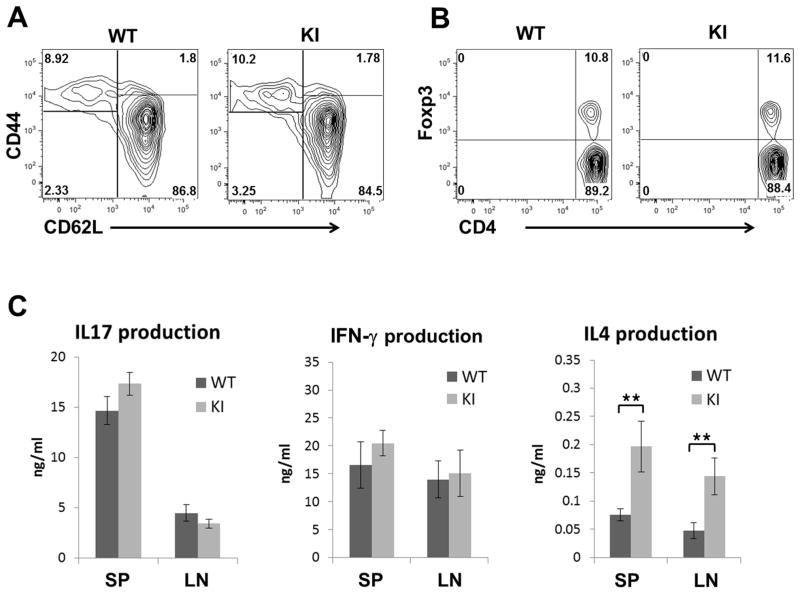
Although KI mice did not appear to have gross defects in CTLA-4 function based on survival data, we examined whether the Y201V mutation might affect the activation state of T cells. There were comparable frequencies of various T cell subsets CD4+/CD44+/CD62L− T effector, resting CD4+/CD44−/CD62Lhi naive T cells (Fig 2A) and CD4+/FoxP3+ Treg cells (Fig 2B) harvested from lymph nodes of 8 week old Y201V KI mice, and the total cellularity of axillary lymph nodes (axLN), mesenteric lymph node (mesLN) and spleen, was unaltered as compared to wild type animals (Suppl. Fig 2A). In addition, there were no detectable differences in absolute numbers of CD4+ T conventional, as well as effector/memory and Treg cells (Suppl. Fig 2B) in young mice. However, the Y201V KI mice developed a mild form of lymphadenopathy by the age of 3 months. This phenotype of lymphoproliferation did not result in premature lethality but was accompanied by increased lymph node cellularity and an accumulation of CD44+/CD69+ activated T cells (Suppl. Fig 2C and D).
Figure 2.
Representative flow plots, showing the frequencies of activated CD4+/CD44+/CD62L− T effector and resting CD4+/CD44−/CD62Lhi naïve T cells (A) as well as CD4+/Foxp3+ regulatory T cells (B) in lymph node cells from 8 week old WT and CTLA-4 Y201V knock-in mice. (C) CD4+/CD44−/CD62Lhi naïve T cells from lymph nodes of 8 week old WT and CTLA-4Y201V knock-in mice were stimulated with soluble anti-CD3 (1ug/ml) and anti-CD28 (1ug/ml) in the presence of 200U/ml IL2. At day 3, culture supernatants were assayed by ELISA for detection of IFN-γ, IL17 and IL4. Cytokine production displayed as mean ± SD is representative for three independent experiments. Statistical analysis was performed using an unpaired Student’s t test (**, P < 0.01).
Importantly, there were significant changes in the cytokine production of mutant Y201V versus wild type CTLA-4-expressing T cells. The proliferative response of the naive T cells was comparable in WT and Y201V T cells (Suppl. Fig 3), however, there was a significant increase in IL-4 production by activated Y201V T cells. IFN-γ and IL-17 production was unchanged (Fig 2C). Thus, replacement of a single amino acid, in the Y201V CTLA-4 mutant results in a Th2 biased phenotype upon T cell activation. The observed Th2 bias was consistent with previous studies demonstrating an altered T helper subset differentiation in mice with altered CTLA-4 expression [24;25;32].
More severe EAE in Y201V KI mice is a consequence of impaired Treg function
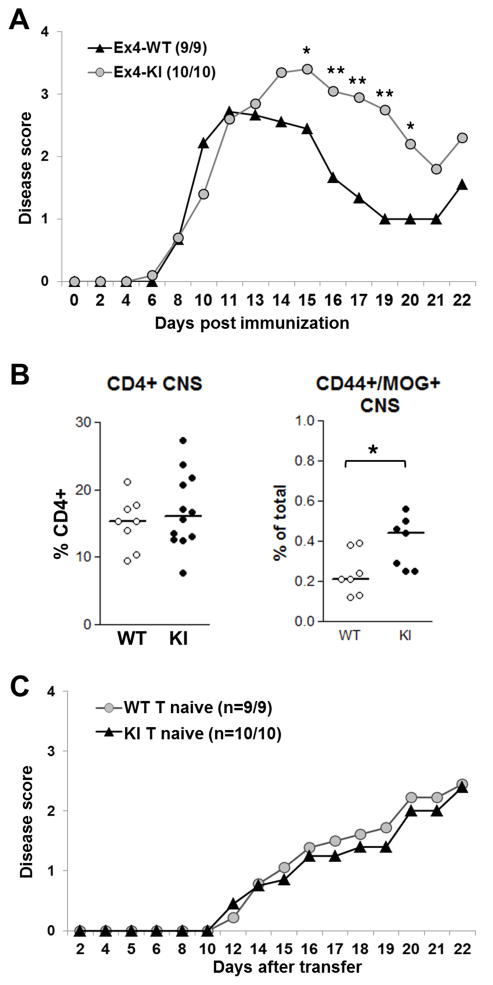
Next, we examined the effect of the mutation in the autoimmune setting of experimental autoimmune encephalomyelitis (EAE) which has been shown to be effected by T cell cytokine balance. Y201V KI mice and wild-type littermate controls (5–7 weeks old) were immunized with MOG35-55 peptide emulsified in CFA and injected along with pertussis toxin to induce this rapid and profound central nervous system (CNS) autoimmune disease leading to paralysis. Y201V KI mice presented with exacerbated EAE as compared to wild-type (Fig 3A). At initial stages of disease onset, clinical symptoms were similar to those observed in controls but were more severe at peak of disease and clinical scores remained high throughout remission phase. This was associated with an increase of MOG35-55 antigen-specific but not total CD4+ T cells infiltrating the CNS at peak disease (Fig 3B). Overall, total cellularities of spleen and CNS as well as absolute numbers of CD4+ T cells were unchanged comparing wild type and Y201V mutant mice (Suppl. Fig 4A and B). Nevertheless, CD4+ conventional T cells as well as polyclonal and antigen-specific Tregs isolated from the site of inflammation, the CNS, at peak disease displayed significantly higher CTLA-4 surface expression (Suppl. Fig 4C). Thus, the observation of exacerbated disease in Y201V KI mice was surprising, given that higher surface expression levels of CTLA-4 have been shown to sequester B7 ligands, thus diminishing CD28 co-stimulation [14]. Moreover, multiple studies report that a Th2-biased phenotype and or increased IL4 expression ameliorates EAE [33–35]. Thus, we hypothesized that the mutant CTLA-4 KI molecule was not altering the development of effector T cells. To confirm this, CD4+/CD62L high/CD25− naive T cells were adoptively transferred into Rag-KO recipients followed by MOG35-55/CFA immunization. The development of disease and its progression was comparable in mice receiving either Y201V KI or wild-type naive T cells (Fig 3C), suggesting that the Y201V mutation does not promote accelerated T effector function and the Y201V KI did not affect the generation of effector Th1 or Th17 cells confirming the in vitro data.
Figure 3.
(A) 5–7 week old WT and CTLA-4Y201V knock-in mice were immunized with 200ug MOG35-55 emulsified in CFA and injected with Pertussis toxin. Development of clinical symptoms of EAE was assessed over time. Data are displayed as mean clinical scores and statistical analysis were performed using an unpaired Student’s t test (*, P < 0.05; **, P < 0.01). (B) Lymphoid cells were isolated from CNS at peak disease and the overall percentage of infiltrating CD4+ (left panel) and CD4+/CD44+/MOG+ antigen-specific T cells (right panel) was assessed by flow cytometry. (C) 3.5×105 purified CD4+/CD25−/CD62Lhi naive T cells from WT and CTLA-4-Y201V knock-in mice were adoptively transferred into 5 week old B6.Rag-KO’s. Recipients were immunized with 200ug MOG35-55 emulsified in CFA on day 2 post-transfer. Pertussis toxin was injected on day 2 and 4, respectively. Development of EAE was evaluated over time.
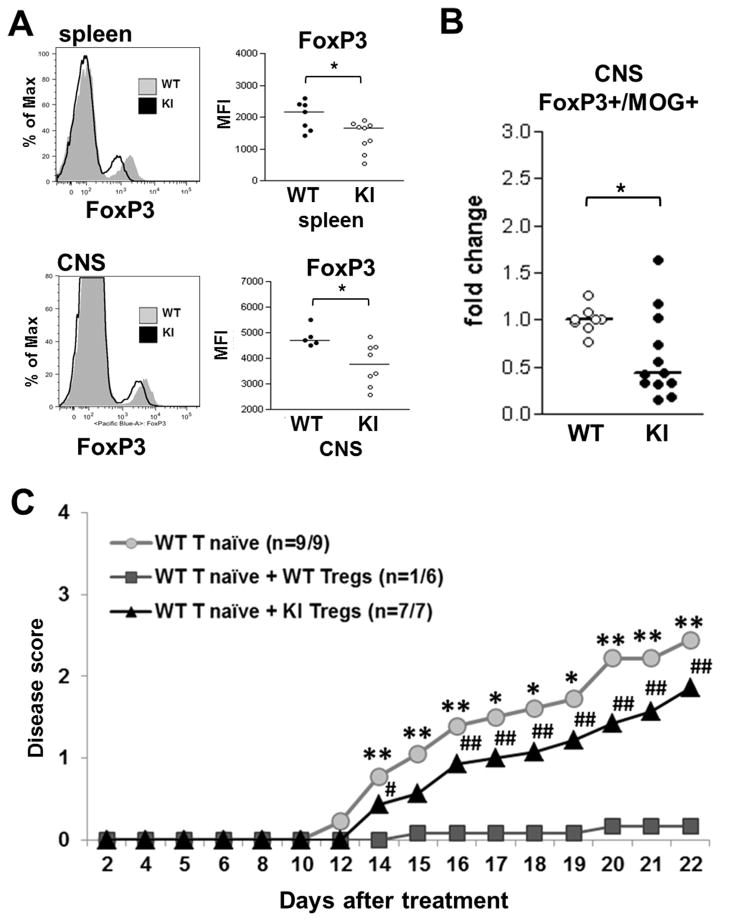
Next, we tested whether the Y201V mutation might affect the suppressive activity of Treg cells. As seen in Figure 4A, Tregs cells from Y201V KI mice expressed significantly less FoxP3 on a per cell basis as compared to wild-type Tregs. The reduction in FoxP3 protein was most prominent in Tregs isolated from spleen and CNS at peak disease (Fig 4A). More importantly, we observed a significant decrease in antigen-specific Treg cells in the CNS of immunized Y201V KI mice as compared to wild-type mice (Fig 4B) suggesting that the loss of FoxP3 expression might have led to the reduced number and function of Tregs in this setting. Of note, Treg numbers in the thymus and periphery as well as FoxP3 expression in Tregs in the thymus are unchanged under steady state condition in Y201V mice (Suppl. Fig 2B and 5), suggesting that this phenotype is a result of T cell activation in the autoimmune setting and not based on impaired Treg development in the thymus.
Figure 4.
EAE was induced in 5–7 week old WT and CTLA-4Y201V knock-in littermates with 200ug MOG35-55 emulsified in CFA and injection with Pertussis toxin. Lymphoid cells from spleen and CNS were isolated at peak disease and assessed by flow cytometry. (A) Representative flow plots (left panel) and quantifications (right panel) displayed as mean fluorescent intensity (MFI) of FoxP3 protein expression on CD4+ T regulatory cells. (B) The overall percentage of antigen-specific CD4+/FoxP3+/MOG+ Tregs in the CNS, shown as fold change compared to WT. Statistical analysis was performed using an unpaired Student’s t test (*, P < 0.05). (C) 3.5×105 WT naive T cells were either transferred alone or together with 1.75×105 Tregs from WT or CTLA-4Y201V knock-in mice into 5 week old B6.Rag-KO’s Recipients were immunized with 200ug MOG35-55 emulsified in CFA on day 2 post-transfer. Pertussis toxin was injected on day 2 and 4, respectively. Development of EAE was evaluated over time. Data are displayed as mean clinical scores and statistical analyses were performed using an unpaired Student’s t test. *,# Mean disease score significantly higher than WT T naïve + WT Tregs (*, P < 0.05; **, P < 0.01). There were no statistical differences between WT T naïve only and WT T naïve + KI Tregs.
To further test if the CTLA-4 Y201V mutation directly affects suppressive activity of Treg cells, we performed adoptive transfer experiments. Naive WT T cells alone or in combination with either WT or Y201V Tregs were transferred into RAG-KO recipients at a ratio of 2:1 and mice were immunized to induce EAE as described above. As shown in Figure 4C, WT Tregs efficiently suppress disease development in immunized recipients. Only one out of six mice developed clinical symptoms. In contrast, Tregs derived from the Y201V KI mice were not able to control T effector functions, as all recipients presented with EAE and clinical scores were similar to those observed in mice not receiving any Treg cells. Taken together, the results suggest that Tyr201 in the intracellular domain of CTLA-4 is indispensable in the context of Treg cell function.
Discussion
In the present study, we examined the importance of Tyr201 within the YVKM motif for CTLA-4 on its intrinsic function in vivo. Consistent with previous in vivo studies in mice expressing a CD2-driven CTLA-4 Y201V-transgene in the CTLA-4 KO background [24;25], we documented increased CTLA-4 surface expression (Fig 1C) and a Th2 biased T cell phenotype (Fig 2C) in Y201V KI mice. Moreover, we observed that the Y201V mutation does not affect T cell homeostasis in young mice up to 8 weeks of age (Fig 2A and suppl. Fig 1A–B) but results in a mild form of lymphadenopathy and an increase in activated T effector/memory cells as the mice grow older (Suppl. Fig 1 C–D). Finally, we observed that Y201V KI mice immunized with MOG35-55 peptide to induce EAE developed more severe disease compared to wild-type littermate controls (Fig 3A). This outcome was unexpected; since we speculated that higher CTLA-4 surface expression and increased IL-4 production might confer a protective function, thus resulting in disease amelioration. The results were a consequence of defective Treg cell function due to the Y201V mutation in the CTLA-4 gene (Fig 4C). The single amino acid mutation led to a significant decrease of Treg cells in the CNS at peak disease and reduced FoxP3 expression within the antigen-specific cells. We cannot exclude that the reduction of Tregs in the CNS is based on defects in cell survival, expansion and or trafficking. However, we could not observe differences in the frequency of Tregs in the thymus or the periphery under steady state conditions. Thus, the Treg phenotype could as well be a direct consequence of impaired signal transduction, as it appears unlikely that the Y201V mutation affects Treg development or homeostatic Treg survival or expansion. Moreover, a recent study from our laboratory demonstrated that the loss of FoxP3 expression in a subset of Tregs results in an exFoxP3 population, which acquires effector function [36]. It will be interesting to determine whether the altered CTLA-4 function not only increases the number of exFoxP3 cells but that those cells take on effector function that plays a role in the exacerbated disease observed in this setting.
Previous studies have shown that ERK1/2, CD3-ζ and AKT phosphorylation is altered in Tregs, in some instances as a direct result of CTLA-4 [37–40]. We hypothesize that the intracellular domain of CTLA-4 plays a role in controlling these TCR-mediated biochemical signals which has been shown to play a critical role for Treg cell development, homeostasis and function. In this regard, the biochemical basis for cell intrinsic CTLA-4 function in T effector cells was described to be dependent on the association of the Y201VKM motif of the cytoplasmic domain with a variety of signaling molecules including the phosphatases, SHP-2 [41;42] and PP2A [7;16]. We, and others, have shown that this biochemical interaction promotes dephosphorylation of the TCRζ chain as well as other TCR complex components like LAT and ZAP70 [42–44]. Moreover, crosslinking of CTLA-4 in conjunction with co-stimulation, has been reported to inhibit ERK phosphorylation/activation as well as c-JNK and therefore differentially regulates members of the MAPK family [45]. In addition, multiple signaling pathways initiated by TCR/CD3, IL-2R/STAT, the PI3K/Akt/mTOR as well as the TGF-β/Smad and Notch signaling pathways have been implicated in FoxP3 transcriptional regulation [46;47]. Specifically, recent work by Sauer et al. demonstrated that TCR signal deprivation as well as inhibition of PI3K-signaling promotes Treg cell development and FoxP3 expression [48]. Although our study was not designed to address the specific signaling events downstream of Tyr201, we speculate that the above described studies together with our results and the fact that the Y201VKM motif of the CTLA-4 intracellular domain alters TCR and PI3K-signaling in T effector cells [17;49] might provide a mechanism of action how the Y201V mutation could affect FoxP3 expression in Tregs. In addition, Singer and colleagues reported that TCR-hyposignaling in Tregs indeed requires the intracellular domain of CTLA-4 to control CD3-ζ phosphorylation as well as calcium mobilization suggests a potential link between the YVKM motif and Treg development and function [50]. Interestingly, unlike our work, recent studies observed that the cytoplasmic domain of CTLA-4 was dispensable for Treg function in vitro [51] and in a model of inflammatory bowel disease (IBD) [50]. This discrepancy could be based simply on the different disease models as there is a relatively higher ratio of Treg to Teff cells used in the IBD adoptive transfer experiments, compared to our study. Moreover, the critical function of the Tregs to control EAE versus IBD, including the relative differences in the role of IL-10 in EAE regulation versus TGF-β and IL-10 as well as induced Tregs in IBD may be significant. In this regard, it should be noted that the fundamental role of CTLA-4 on Tregs appears to be different in different settings [13;52] (Bluestone J.A. and Tang Q., unpublished observation). Further, one could speculate that Tregs lacking a significant portion of the cytoplasmic domain as compared to the Y201V Tregs that only carry a single point mutation may also be affecting T effector cells. Finally, mutating Tyr201 of the intracellular tail of CTLA-4 prevents AP-2 binding and consequently results in increased surface expression and decreased internalization of the molecule. This could negatively affect trans-endocytosis of CTLA-4’s ligands B7-1 and B7-2 [14] leading to altered co-stimulation through CD28, which has been reported to be critical for induction of peripheral Tregs from naïve T cells [53;54]. In addition, increased CTLA-4 surface expression could modulate the TCR repertoire in the thymus [55], thus alter Treg cell generation. Of note, we did not observe differences in the frequency of Treg cells in the thymus nor the periphery under steady state conditions, suggesting that CTLA-4 surface overexpression did not interfere with Treg development or homeostasis.
Taken together, our data indicate that the intracellular domain and especially the Y201VKM motif of CTLA-4 influences Treg biology, given the significant reduction of FoxP3 expression and the severely impaired suppressive activity of Y201V KI Tregs in vivo.
Materials and Methods
Generation of B6.CTLA-4 Y201V knock-in mice
Targeting strategy to generate CTLA-4 Y201V knock-in mice. A 13.6 kilobase genomic fragment containing the entire mouse CTLA-4 locus was recovered from a bacterial artificial chromosome (clone RP23-146J17: BACPAC http://bacpac.chori.org). The fragment was cloned into the pBluescript II SK(−) vector (Stratagene, Santa Clara, CA USA) using the RED-ET recombineering strategy [56] followed by insertion of a LoxP-flanked PGK/em7-promotor driven neo cassette. The nucleotide sequence was further modified, resulting in an amino acid change from Tyrosine (Y) to Valine (V) at position 201 within Ex4. This mutant construct was used to target B6-PRX mouse embryonic stem cells and selected clones were injected into BALB/c embryos. The chimeric mice were screened for germline transmission, and further crossed with OX40-Cre transgenic mice [57] to delete the selection cassette. Mice were housed in a specific pathogen-free facility at the University of California at San Francisco. All experiments complied with the Animal Welfare Act and the National Institutes of Health guidelines for the ethical care and use of animals in biomedical research and were approved by the Institutional Animal Care and Use Committee of the University of California at San Francisco.
Induction and clinical evaluation of EAE
Mice 5–7 weeks old were immunized s.c. with an emulsion of Complete Freund’s Adjuvant (containing 4mg/ml heat inactivated and dried Mycobacterium tuberculosis H37 Ra; DIFCO laboratories, Franklin Lakes, NJ USA) and 4 mg/ml MOG35-55 peptide (Genemed Synthesis, INC; San Antonio, TX USA). 100 μl of the emulsion were distributed over three spots on the flank. In addition, 200 ng of Pertussis toxin (List biological laboratories; Campbell, CA USA) in 200 μl saline solution was injected i.p. on days 0 and 2 after immunization. Mice were evaluated daily and clinical development was assessed and rated according to the following criteria: 0= no signs of disease, 1= limp tail, 2= limp tail and hind limb weakness, 3= partial hind limb paralysis, 4= total hind limb paralysis.
Adoptive Transfer experiments
CD4+/CD62Lhi/CD25− naive T cells and CD4+/CD62Lhi/CD25+ Treg cells from 6–8 week old WT and Y201V KI mice were purified by FACS-sorting. Either 3.5 ×105 naive T cells or 3.5×105 naive T cells together with 1.75×105 Treg cells were adoptively transferred into B6-RagKO mice. Mice were immunized with MOG35-55 on day 2 post-transfer as described above. Pertussis toxin was injected on day 2 and 4 after transfer. Clinical evaluation of disease onset and progression was performed as stated above.
Isolation of lymphoid cells from the CNS and immunofluorescence
Spinal cord, cerebellum and brain stem were collected in HBSS buffer after perfusing the mouse with 50 ml cold PBS. Organs were minced and digested with 300U/ml Collagenase D in DMEM for 30 mins at 37°C. Digestion was terminated by adding 10% FCS. Cells were filtered using a 40μm nytex filter and washed with Ca+/Mg+ free HBSS, containing 2% FCS. Lymphocytes were separated from myelin using a percoll gradient. Cells were resuspended in 30% percoll (in PBS), then gently under-laid with 70% percoll (in PBS) and spun for 30 mins at 2400 rpm at room temperature without braking. Mononuclear cells were collected from the interface and washed twice with Ca+/Mg+ free HBSS, containing 2% FCS. Centrifugation step in between washes was performed at 1400 rpm for 10 mins. Cells were resuspended at 5 × 107/ml in tetramer staining buffer (DMEM, 25mM Hepes, 2% FCS) supplemented with 5% rat serum (heat inactivated) and Fc Block (1:100 dilution) and incubated on ice for 10 mins. Cells were washed and stained with APC-conjugated MOG35-55-tetramer (NIH tetramer core facility, Atlanta, GA USA) at a final dilution of 1:100 for 3h in the dark at room temperature, followed by staining for surface and intracellular markers.
Flow cytometry and cell sorting
Labeled antibodies specific for CD4 (RM4-5), CD44 (IM7), CD25 (PC61), CD62L (MEL14), CTLA-4/CD152 (UC10-4F10), FoxP3 (FJK-16s) and Hamster IgG1,κ (A19-3) were purchased from BD Biosciences or eBioscience. Isolated cells from lymph nodes and spleen were stained with commercially available antibodies, listed above. Intracellular staining for FoxP3 and CTLA-4 (CD152) was performed using a FoxP3 staining buffer set, according to the manufacturers’ instructions (eBioscience, San Diego, CA USA). Stained single-cell suspensions were analyzed using a BD LSRII flow cytometer running FACSDiva software (BD Biosciences, San Jose, CA USA). Cell sorting experiments were performed with a MoFlo cytometer high-speed cell sorter (Dako) or a FACSAria (BD Biosciences).
T cell proliferation and cytokine assay
FACS-sorted CD4+/CD62Lhi/CD25− naive T cells from spleen and lymph node were resuspended in PBS at a concentration of 1×108 cells/ml and labeled by adding an equal volume of 5μM CFSE in PBS (Molecular probes, Grand Island, NY USA) for 5 min at room temperature. Labeling was terminated by adding an equal volume of FBS for 1 min at room temperature. Cells were washed with PBS, resuspended at a concentration of 2×106/ml in complete culture media (DMEM/10% FCS supplemented with 50 μM 2-ME, 1 mM sodium pyruvate, nonessential amino acids, L-glutamine, and 100U of penicillin/100 μg of streptomycin/ml). Cells were stimulated with soluble anti-CD3 (1μg/ml) and anti-CD28 (1μg/ml) in the presence of 200U/ml IL-2 and CFSE dilution upon T cell proliferation was assessed by flow cytometry, at the indicated time-points. To measure cytokine production, FACS-sorted CD4+/CD62Lhi/CD25− T naive cells from lymph nodes were stimulated with soluble anti-CD3 (1μg/ml) and anti-CD28 (1μg/ml) in the presence of 200U/ml IL2. At day 3, culture supernatants were collected and analyzed for IL-4, INF-γ, and IL-17 using standard ELISA methods (BD Biosciences, San Jose, CA USA).
Real-time PCR analysis
RNA was isolated from sorted CD4+/CD62Lhi/CD25− naive T cells and CD4+/CD62Lhi/CD25+ Treg cells from total lymph nodes, using the RNeasy Kit (Qiagen, Valencia, CA USA). Reverse transcription into cDNA was performed using the Superscript kit (Invitrogen). The quantities of full-length CTLA-4 (fl-CTLA-4), ligand-independent CTLA-4 (li-CTLA-4) and soluble CTLA-4 (s-CTLA-4) cDNA were measured using quantitative real-time PCR analysis (GeneAmp 7900; Applied Biosystems, Carlsbad, CA USA) and were normalized to 18S expression (Eukaryotic 18S rRNA). The TaqMan primer-probe for 18S was purchased from Applied Biosystems (ID: 4333760F). Primers and probes for fl-CTLA-4, li-CTLA-4 and sCTLA-4, according to Kissler et al., 2011 [58], were purchased from Integrated DNA Technologies.
Statistical analysis
Statistical analyses were performed using either an unpaired two-tailed Student’s t-test or an unpaired two-tailed Mann-Whitney U test. Values of P ≤ 0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Nigel Killeen from the Mutagenesis Core Facility at UCSF for providing the OX40-Cre mice as well as performing the ES cell injections to generate the Y201V knock-in mice. We also thank Mike Lee for technical help with cell sorting. This work was supported by National Institutes of Health Grant P01 AI35297, U19 AI056388, P30DK63720-06A1.
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 3.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 5.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 6.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang E, Fisher TS, Morgan RW, Robbins MD, Duerr JM, Vander Heiden MG, Gardner JP, Hambor JE, Neveu MJ, Thompson CB. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 8.Metzler WJ, Bajorath J, Fenderson W, Shaw SY, Constantine KL, Naemura J, Leytze G, Peach RJ, Lavoie TB, Mueller L, Linsley PS. Solution structure of human CTLA-4 and delineation of a CD80/CD86 binding site conserved in CD28. Nat Struct Biol. 1997;4:527–531. doi: 10.1038/nsb0797-527. [DOI] [PubMed] [Google Scholar]
- 9.Peach RJ, Bajorath J, Brady W, Leytze G, Greene J, Naemura J, Linsley PS. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J Exp Med. 1994;180:2049–2058. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, Emswiler J, Greene J, Turk LA, Bajorath J, Townsend R, Hagerty D, Linsley PS, Peach RJ. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 11.Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 12.Morton PA, Fu XT, Stewart JA, Giacoletto KS, White SL, Leysath CE, Evans RJ, Shieh JJ, Karr RW. Differential effects of CTLA-4 substitutions on the binding of human CD80 (B7-1) and CD86 (B7-2) J Immunol. 1996;156:1047–1054. [PubMed] [Google Scholar]
- 13.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baroja ML, Luxenberg D, Chau T, Ling V, Strathdee CA, Carreno BM, Madrenas J. The inhibitory function of CTLA-4 does not require its tyrosine phosphorylation. J Immunol. 2000;164:49–55. doi: 10.4049/jimmunol.164.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Baroja ML, Vijayakrishnan L, Bettelli E, Darlington PJ, Chau TA, Ling V, Collins M, Carreno BM, Madrenas J, Kuchroo VK. Inhibition of CTLA-4 function by the regulatory subunit of serine/threonine phosphatase 2A. J Immunol. 2002;168:5070–5078. doi: 10.4049/jimmunol.168.10.5070. [DOI] [PubMed] [Google Scholar]
- 17.Chikuma S, Bluestone JA. CTLA-4 and tolerance: the biochemical point of view. Immunol Res. 2003;28:241–253. doi: 10.1385/IR:28:3:241. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Allison JP. Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein. Proc Natl Acad Sci USA. 1997;94:9273–9278. doi: 10.1073/pnas.94.17.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang E, Alegre ML, Duckett CS, Noel PJ, Vander Heiden MG, Thompson CB. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J Immunol. 1997;159:144–151. [PubMed] [Google Scholar]
- 20.Schneider H, Martin M, Agarraberes FA, Yin L, Rapoport I, Kirchhausen T, Rudd CE. Cytolytic T lymphocyte-associated antigen-4 and the TCR zeta/CD3 complex, but not CD28, interact with clathrin adaptor complexes AP-1 and AP-2. J Immunol. 1999;163:1868–1879. [PubMed] [Google Scholar]
- 21.Schneider H, Prasad KV, Shoelson SE, Rudd CE. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J Exp Med. 1995;181:351–355. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider H, Schwartzberg PL, Rudd CE. Resting lymphocyte kinase (Rlk/Txk) phosphorylates the YVKM motif and regulates PI 3-kinase binding to T-cell antigen CTLA-4. Biochem Biophys Res Commun. 1998;252:14–19. doi: 10.1006/bbrc.1998.9559. [DOI] [PubMed] [Google Scholar]
- 23.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 24.Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 25.Yi LA, Hajialiasgar S, Chuang E. Tyrosine-mediated inhibitory signals contribute to CTLA-4 function in vivo. Int Immunol. 2004;16:539–547. doi: 10.1093/intimm/dxh055. [DOI] [PubMed] [Google Scholar]
- 26.Perkins D, Wang Z, Donovan C, He H, Mark D, Guan G, Wang Y, Walunas T, Bluestone J, Listman J, Finn PW. Regulation of CTLA-4 expression during T cell activation. J Immunol. 1996;156:4154–4159. [PubMed] [Google Scholar]
- 27.Vijayakrishnan L, Slavik JM, Illes Z, Rainbow D, Peterson LB, Sharpe AS, Wicker LS, Kuchroo VK. An autoimmune disease-associated CTLA4 splice variant lacking the B7 binding domain signals negatively in T cells. Novartis Found Symp. 2005;267:200–212. doi: 10.1002/047002139x.ch13. [DOI] [PubMed] [Google Scholar]
- 28.Oaks MK, Hallett KM. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol. 2000;164:5015–5018. doi: 10.4049/jimmunol.164.10.5015. [DOI] [PubMed] [Google Scholar]
- 29.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di GG, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 30.Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol Rev. 2005;204:102–115. doi: 10.1111/j.0105-2896.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 31.Holmberg D, Cilio CM, Lundholm M, Motta V. CTLA-4 (CD152) and its involvement in autoimmune disease. Autoimmunity. 2005;38:225–233. doi: 10.1080/08916930500050210. [DOI] [PubMed] [Google Scholar]
- 32.Bour-Jordan H, Grogan JL, Tang Q, Auger JA, Locksley RM, Bluestone JA. CTLA-4 regulates the requirement for cytokine-induced signals in T(H)2 lineage commitment. Nat Immunol. 2003;4:182–188. doi: 10.1038/ni884. [DOI] [PubMed] [Google Scholar]
- 33.Pal E, Yamamura T, Tabira T. Autonomic regulation of experimental autoimmune encephalomyelitis in IL-4 knockout mice. J Neuroimmunol. 1999;100:149–155. doi: 10.1016/s0165-5728(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 34.Kohm AP, Carpentier PA, Miller SD. Regulation of experimental autoimmune encephalomyelitis (EAE) by CD4+CD25+ regulatory T cells. Novartis Found Symp. 2003;252:45–52. [PubMed] [Google Scholar]
- 35.Butti E, Bergami A, Recchia A, Brambilla E, Del CU, Amadio S, Cattalini A, Esposito M, Stornaiuolo A, Comi G, Pluchino S, Mavilio F, Martino G, Furlan R. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther. 2008;15:504–515. doi: 10.1038/gt.2008.10. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 38.Tsang JY, Camara NO, Eren E, Schneider H, Rudd C, Lombardi G, Lechler R. Altered proximal T cell receptor (TCR) signaling in human CD4+CD25+ regulatory T cells. J Leukoc Biol. 2006;80:145–151. doi: 10.1189/jlb.0605344. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, Boussiotis VA. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068–3073. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and Ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J Immunol. 2006;177:2186–2194. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- 41.Schneider H, Rudd CE. Tyrosine phosphatase SHP-2 binding to CTLA-4: absence of direct YVKM/YFIP motif recognition. Biochem Biophys Res Commun. 2000;269:279–283. doi: 10.1006/bbrc.2000.2234. [DOI] [PubMed] [Google Scholar]
- 42.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 43.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med. 2001;194:1675–1681. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guntermann C, Alexander DR. CTLA-4 suppresses proximal TCR signaling in resting human CD4(+) T cells by inhibiting ZAP-70 Tyr(319) phosphorylation: a potential role for tyrosine phosphatases. J Immunol. 2002;168:4420–4429. doi: 10.4049/jimmunol.168.9.4420. [DOI] [PubMed] [Google Scholar]
- 45.Calvo CR, Amsen D, Kruisbeek AM. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and Jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor zeta and ZAP70. J Exp Med. 1997;186:1645–1653. doi: 10.1084/jem.186.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Z, Chen L, Hao F, Wu J. Transcriptional regulation of Foxp3 gene: multiple signal pathways on the road. Med Res Rev. 2009;29:742–766. doi: 10.1002/med.20152. [DOI] [PubMed] [Google Scholar]
- 47.Haiqi H, Yong Z, Yi L. Transcriptional regulation of Foxp3 in regulatory T cells. Immunobiology. 2011;216:678–685. doi: 10.1016/j.imbio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tai X, Van LF, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood. 2012 doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi T, Kishi A, Osaki M, Morikawa H, Prieto-Martin P, Wing K, Saito T, Sakaguchi S. Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proc Natl Acad Sci USA. 2013;110:E2116–E2125. doi: 10.1073/pnas.1307185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 53.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 54.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verhagen J, Genolet R, Britton GJ, Stevenson BJ, Sabatos-Peyton CA, Dyson J, Luescher IF, Wraith DC. CTLA-4 controls the thymic development of both conventional and regulatory T cells through modulation of the TCR repertoire. Proc Natl Acad Sci USA. 2013;110:E221–E230. doi: 10.1073/pnas.1208573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Copeland NG, Jenkins NA, Court DL. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet. 2001;2:769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- 57.Klinger M, Kim JK, Chmura SA, Barczak A, Erle DJ, Killeen N. Thymic OX40 expression discriminates cells undergoing strong responses to selection ligands. J Immunol. 2009;182:4581–4589. doi: 10.4049/jimmunol.0900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerold KD, Zheng P, Rainbow DB, Zernecke A, Wicker LS, Kissler S. The soluble CTLA-4 splice variant protects from type 1 diabetes and potentiates regulatory T-cell function. Diabetes. 2011;60:1955–1963. doi: 10.2337/db11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.