Abstract
Background
Patients with Chagas disease and segmental wall motion abnormality (SWMA) have worse prognosis independent of left ventricular ejection fraction (LVEF). Cardiac magnetic resonance (CMR) is currently the best method to detect SWMA and to assess fibrosis.
Objective
To quantify fibrosis by using late gadolinium enhancement CMR in patients with Chagas disease and preserved or minimally impaired ventricular function (> 45%), and to detect patterns of dependence between fibrosis, SWMA and LVEF in the presence of ventricular arrhythmia.
Methods
Electrocardiogram, treadmill exercise test, Holter and CMR were carried out in 61 patients, who were divided into three groups as follows: (1) normal electrocardiogram and CMR without SWMA; (2) abnormal electrocardiogram and CMR without SWMA; (3) CMR with SWMA independently of electrocardiogram.
Results
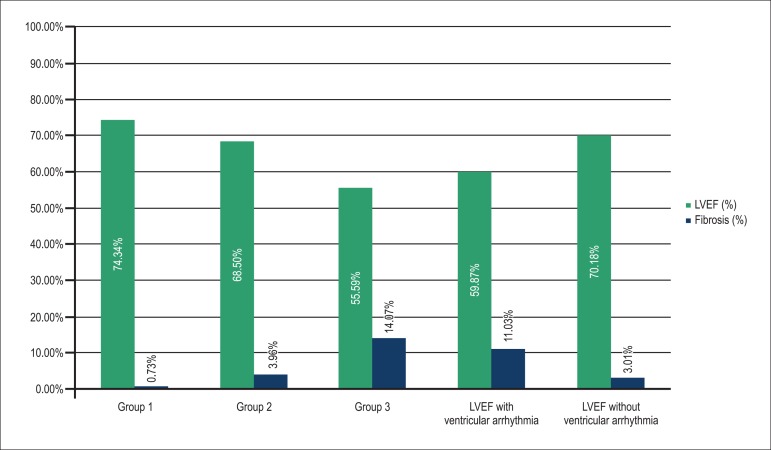
The number of patients with ventricular arrhythmia in relation to the total of patients, the percentage of fibrosis, and the LVEF were, respectively: Group 1, 4/26, 0.74% and 74.34%; Group 2, 4/16, 3.96% and 68.5%; and Group 3, 11/19, 14.07% and 55.59%. Ventricular arrhythmia was found in 31.1% of the patients. Those with and without ventricular arrhythmia had mean LVEF of 59.87% and 70.18%, respectively, and fibrosis percentage of 11.03% and 3.01%, respectively. Of the variables SWMA, groups, age, LVEF and fibrosis, only the latter was significant for the presence of ventricular arrhythmia, with a cutoff point of 11.78% for fibrosis mass (p < 0.001).
Conclusion
Even in patients with Chagas disease and preserved or minimally impaired ventricular function, electrical instability can be present. Regarding the presence of ventricular arrhythmia, fibrosis is the most important variable, its amount being proportional to the complexity of the groups.
Keywords: Arrhythmias, Cardiac, Myocardial Fibrosis, Chagas Heart Disease, Ventricular Dysfunction
Introduction
Chagas disease (CD) remains epidemiologically important1, because of the high number of infected individuals who can develop severe forms of the disease. In Brazil, two to three million people are estimated to be affected, one third of whom have heart disease, of whom, two thirds are minimally impaired2.
The annual death rate is approximately 24/1,000 patients-year, and most patients have preserved or minimally impaired left ventricular (LV) ejection fraction (LVEF)3. Sudden death is common in CD, can occur in any phase of that disease, and 10% result from a first arrhythmic event. Complex cardiac arrhythmias (ventricular extrasystoles > 10/hour and/or ventricular tachycardia) are markers of sudden death in CD4.
Patients with CD and normal electrocardiogram (ECG) are known to have survival rate similar to that of the general population, and initial studies have shown that those with CD, segmental wall motion abnormality and preserved LVEF have worse prognosis5,6.
Cardiac magnetic resonance imaging (CMR) is currently the best method to assess ventricular function7 and to detect segmental wall motion abnormalities8; by adding the delayed enhancement technique9, it can assess myocardial fibrosis. The ability of delayed enhancement CMR to detect abnormalities in chronic Chagas heart disease (CCHD) has already been reported10.
Patients with CCHD and ventricular arrhythmia have a worse prognosis, and there is no study correlating the myocardial fibrosis detected on CMR with the severity of arrhythmias in patients with CD and preserved or minimally impaired ventricular function.
Methods
Patients were recruited between March and December 2010 at the CD Outpatient Clinic at our hospital.
All patients from this study provided written informed consent, and the research protocol was approved by the Ethics Committee of our hospital, in accordance with the Declaration of Helsinki.
The inclusion criteria were as follows: asymptomatic patients older than 21 years or those outside an endemic area of CD for more than 20 years with positive serology for CD, preserved or minimally reduced (> 45%) LVEF on echocardiogram, who had undergone ECG, treadmill exercise test (TET) and 24-hour Holter in the previous 12 months.
Patients with the following characteristics were excluded: renal dysfunction (estimated creatinine clearance < 30 mL/min); previous ablation via electrophysiological study; diabetes or more than two risk factors for coronary artery disease; atrial fibrillation; TET compatible with myocardial ischemia; previous myocardial infarction; any myocardial or peripheral revascularization procedure; contraindication to undergo CMR (permanent pacemaker, implanted cardiac defibrillator, neurosurgical clips, or cochlear implants).
Patients were classified according to ECG and CMR findings, and distributed into three groups as follows: 1) Group 1 - normal ECG and no segmental wall motion abnormality on CMR; 2) Group 2 - altered ECG and no segmental wall motion abnormality on CMR; and 3) Group 3 - segmental wall motion abnormality on CMR regardless of ECG. The ECG was considered altered in the presence of any incomplete or complete bundle-branch block, any type of atrioventricular block, mono- or polymorphic ventricular extrasystole , and nonsustained ventricular tachycardia (NSVT). The CMR was considered altered in the presence of any segmental wall motion abnormality.
On Holter, the following situations were considered electrical instability: presence of ventricular extrasystoles > 30/hour; episodes of monomorphic sustained ventricular tachycardia (defined as ventricular rhythm with heart rate > 100 bpm and duration > 30 seconds); and episodes of NSVT (defined as three or more consecutive beats with duration < 30 seconds).
Regarding the TET, only the tests of patients reaching 7 MET, limit defined as a submaximal exercise level (good correlation between the anaerobic threshold achieved and the 7-MET load achieved), were considered for analysis11. Two observers analyzed and compared the ECGs at rest and during exercise (around 7 MET), identifying in both situations the 30-second period with the greatest number of ventricular arrhythmias and/or NSVT episodes. Exertion-induced arrhythmias were defined as the appearance of ventricular arrhythmias on ECG during exercise as compared to rest, or an increase > 10% in their incidence, and/or presence of NSVT12.
The CMR was performed in a 1.5-Tesla GE HDX scanner (Wakeusha, Wisconsin, USA), and two pulse sequences were acquired: the first was a cine-CMR using Steady-State Free Precession (SSFP) in long- and short-axis projections to measure and calculate mass, volumes, LVEF and right ventricular ejection fraction (RVEF). The most basal slice in the short axis was positioned right after the atrioventricular ring, and all subsequent respiratory pauses at maximum exhalation were acquired with 8-mm slice thickness and 2-mm slice spacing up to the LV apex. The parameters used were: field of view (FOV) of 400 mm; matrix of 224 × 224; 20-24 lines/segment; temporal resolution < 50 ms; repetition time (RT) = 3.9 ms, echo time (ET) = 1.5 ms; flip angle of 50°; and NEX of 1.
Three minutes after injecting 0.3 mmol/kg of gadolinium (Dotarem®, Guerbet), an echo-gradient sequence was performed with inversion recovery (delayed enhancement) in the long- and short-axis projections to investigate myocardial fibrosis with the following parameters: FOV of 360 mm; matrix of 224 × 192; 24 lines/segment; ET = 2.9 ms; flip angle of 20°; 8-mm slice thickness; 2-mm slice spacing; and NEX of 2.
Cardiac magnetic resonance postprocessing
The LV and RV measurements and calculations were performed independently by two researchers blinded to the patient's groups, at a workstation dedicated to CMR, using specific software (Report CARD®, 3.6 version, GE).
Maximum diastole and systole images were chosen on cinematic display at maximum relaxation and maximum contraction, respectively. Ventricular mass was calculated by using manual tracings of endocardial and epicardial borders at end-systole and end-diastole for each slice for both LV and RV. Papillary muscles were excluded from volume measurements and added to ventricular mass calculation. Such areas were multiplied by slice thickness (8 mm + 2 mm of slice spacing) and added to several slices to obtain end-systolic and end-diastolic volumes, respectively. The EF was calculated as follows: end-systolic volume subtracted from end-diastolic volume and divided by end-diastolic volume. Each of the 17 LV wall segments was classified as normokinetic, hypokinetic, dyskinetic or akinetic.
The calculation of fibrosis mass was performed by using a specific applicative of the software for semiquantitative detection of hyperintense areas compatible with fibrosis on short-axis delayed enhancement sequences. The researcher was free to edit the limits of the area of fibrosis.
Statistical analysis
The statistical analysis comprised a nonparametric classification tree and survival curves. The nonparametric classification tree method is based on a decision rule approach, implemented with a theory of conditional inference procedures and selection of variables. The node of the classification tree has a p value that corresponds to the log-rank test. The classification tree is aimed at reducing the impurity degree by finding the point that provides greater homogeneity (higher probability of purity) inside a node and greater heterogeneity between nodes. Then, a log-linear model is used to select the most significant variables and to confirm the results obtained by using a regression tree. The 5% significance level was adopted for the entire study.
The presence of ventricular arrhythmias was considered a categorical variable according to ECG, Holter and/or TET findings.
Interobserver analysis was performed using the survival analytical technique proposed by Luiz et al13 to assess the reliability of the quantitative measures of EF, LV mass and myocardial fibrosis. That method uses Kaplan-Meier curves without data censoring, in which the failures occur in the absolute difference between the values attributed to the observers. Another improved method proposed by Llorca and Delgado-Rodriguez14 was also used. It considers two groups of different real values rather than global differences. The equivalence of functions of the two observers obtained through Llorca's method was evaluated by using Tarone-Ware test, a nonparametric weighted log-rank statistic.
Then the explanation coefficient matrix was performed to measure the predictive capacity of a continuous variable to predict another, and the following were assessed: age; LVEF; RVEF; fibrosis; and LV mass. The R software was used for data analysis.
Results
Sixty-one patients (23 men) participated in the study. Two women did not undergo the post-contrast phase (delayed enhancement) as follows: one due to difficulty in the venous access and another due to history of allergy to gadolinium; they, however, underwent the noncontrast phase (cine-CMR). The patients' mean age was 62.32 ± 10.43 years. The body mass index of the population studied was 26.02. Four patients showed segmental wall motion abnormality with no ECG abnormalities, being then considered Group 3. Table 1 shows the number of patients with fibrosis in the three groups.
Table 1.
General data
| Mean age | 62.32 years |
| BMI | 26.02 kg/m2 |
| Group 1 BMI | 26.56 kg/m2 |
| Group 2 BMI | 25.29 kg/m2 |
| Group 3 BMI | 25.24 kg/m2 |
| Number of patients with fibrosis | 27 (45.8%) |
| Group 1 (normal ECG and no SWMA on CMR) | 26 (42.6%) |
| Group 2 (abnormal ECG and no SWMA on CMR) | 16 (26.2%) |
| Group 3 (SWMA on CMR) | 19 (31.1%) |
| Ventricular arrhythmia (TET or Holter) | 19 (31.1%) |
| Patients with fibrosis in Group 1 | 5 (19.2%) |
| Patients with fibrosis in Group 2 | 7 (43.7%) |
| Patients with fibrosis in Group 3 | 15 (78.9%) |
| Patients with fibrosis and ventricular arrhythmia | 13 (68.4%) |
BMI: body mass index; ECG: electrocardiogram; CMR: cardiac magnetic resonance imaging; SWMA: segmental wall motion abnormality; TET treadmill exercise test.
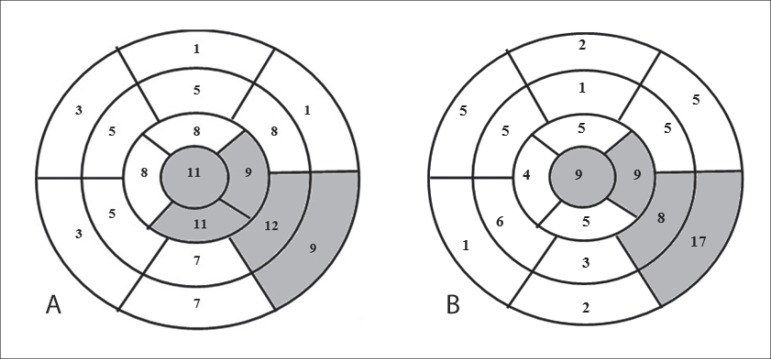
Figure 1 shows the number of patients with segmental wall motion abnormality and fibrosis in each LV wall segment according to the CMR findings. Segmental wall motion abnormality was detected in 19 patients (31.1%). Abnormality was identified in 113 wall segments (10.89% of the 1,037 possible segments), and the segments most frequently affected were: infero-apical (9.7%); infero-lateral-medial (10.6%); basal (7.9%); and LV apex (9.7%).
Figure 1.
Representation of the 17 left ventricular wall segments. (A) The figure in each segment indicates the number of patients with segmental wall motion abnormality. The grey color represents the segments most frequently affected by segmental wall motion abnormality. (B) Number of patients with fibrosis in each left ventricular wall segment. The grey color represents the segments most frequently affected by fibrosis.
On CMR, fibrosis was detected in 27 patients (45.8%) (Figure 1), the mean amount of fibrosis being 15.02 g. Detectable myocardial fibrosis was observed in 87 segments (8.67% of the 1,003 possible segments), the infero-lateral-basal LV wall segment being the most often affected (19.5%).
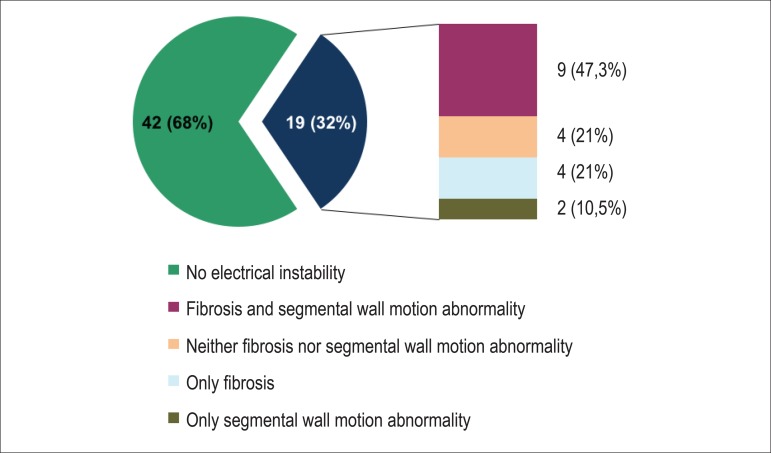
Electrical instability was detected on Holter or TET in 19 patients (32%), and Graph 1 shows the presence of fibrosis or segmental wall motion abnormality in that group of patients. Of the patients with electrical instability, 78.9% had segmental wall motion abnormality and/or fibrosis on CMR. Of the 42 patients without electrical instability, only 14 patients had myocardial fibrosis and 8 had segmental wall motion abnormality.
Graph 1.
Ventricular arrhythmia on Holter or treadmill exercise test and presence of fibrosis.
Graph 2 shows the amount of fibrosis and the LVEF in each group.
Graph 2.
Ejection fraction and amount of fibrosis in the different groups. LVEF: left ventricular ejection fraction.
The presence of ventricular arrhythmia in the groups was as follows: four patients in Group 1; four patients in Group 2; and 11 patients in Group 3.
Regarding interobserver agreement to detect ventricular arrhythmias at rest and during exertion, a kappa of 0.87 was obtained [95% confidence interval (95% CI): 0.72-0.92). Kappa for intraobserver agreement was 0.93 (95% CI: 0.74-0.99).
The interobserver disagreement by using Llorca's method showed that the variables LVEF, RVEF and mass were not significant (0.4 and 0.09 respectively; p = 0.5). Only the variable 'percentage of fibrosis' showed significance (p = 0.007) up to 6% of the absolute value of the difference of the result, after which there was no difference.
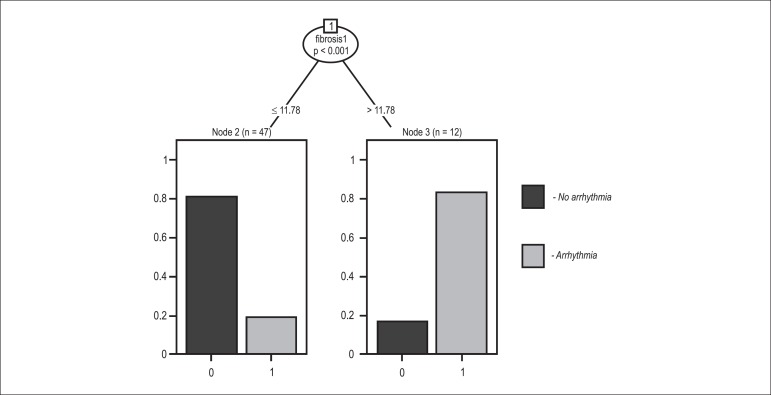
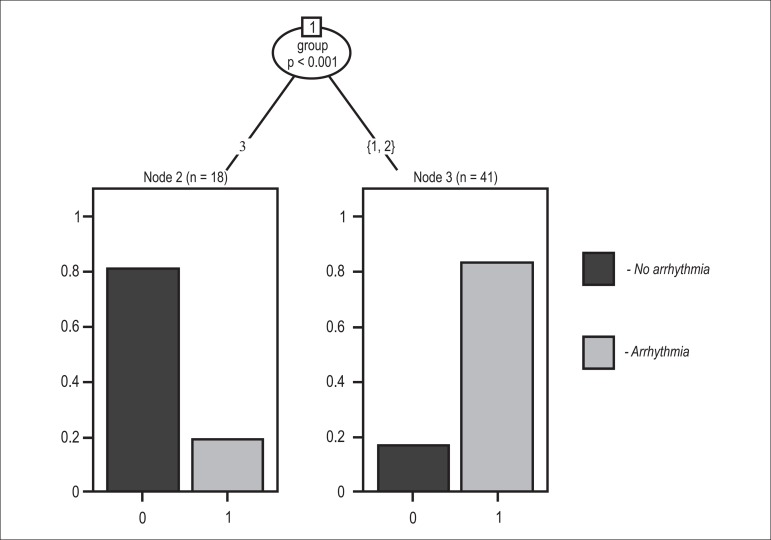
The categorical analyses of the presence of segmental wall motion abnormality and detection of fibrosis had an interobserver kappa of 0.96. The variables used in the first classification tree (Figure 2) were: segmental wall motion abnormality on CMR; groups (1, 2 and 3); age; LVEF; ventricular arrhythmia; and myocardial fibrosis. Myocardial fibrosis was the only significant variable in the classification tree for the presence of ventricular arrhythmia, with a cutoff point of 11.78% for fibrosis mass (p < 0.001). In addition to segmental wall motion abnormality on CMR and myocardial fibrosis, the second classification tree considered the interactions of those variables with the groups (1, 2 and 3). Group 3 gathered the majority of patients with ventricular arrhythmia (p < 0.001) (Figure 3).
Figure 2.
Classification tree showing that fibrosis is the most significant variable for the presence of ventricular arrhythmia, with a cutoff point of 11.78%.
Figure 3.
Classification tree per group, showing that the group with segmental wall motion abnormality (Group 3) had the majority of patients with ventricular arrhythmia.
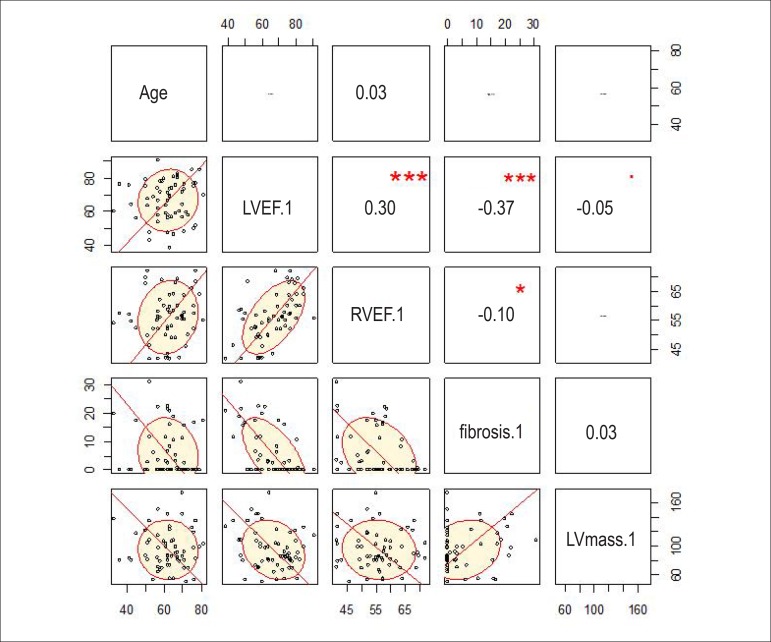
The explanation coefficient matrix was built (Figure 4), enabling R2calculation of the variables analyzed without using a response variable (ventricular arrhythmia, in the case), and showing that LVEF was inversely proportional to fibrosis (R2 = -0.37), while LVEF and RVEF were proportional to each other (R2 = 0.30).
Figure 4.
Correlation matrix (values as R2). The more oval the tracing, the better the correlation. LV: left ventricular; LVEF: left ventricular ejection fraction; RVEF: right ventricular ejection fraction.
Discussion
This study shows objectively that, even in patients with CD and preserved or minimally impaired LV function, electrical instability can occur. It was demonstrated by the presence of exertion-induced or spontaneous ventricular arrhythmias in one third of the patients (32%). In addition, a good inverse correlation between LVEF and fibrosis (R2 = -0.37) was identified, because of the 19 patients with electrical instability, 15 (79%) had segmental wall motion abnormality. Moreover, by using logistic regression and tree classification, myocardial fibrosis was identified as the most significant variable for the presence of arrhythmia ventricular. The advantage of the classification tree is that, in addition to identifying the most relevant variable, it calculates the cutoff point (11.78% for fibrosis mass). In the group with fibrosis > 11.78%, only two patients had no ventricular arrhythmia (p < 0.001), which might mean that patients with a greater percentage of fibrosis have an increased risk for frequent ventricular arrhythmia.
According to Myerburg et al15, three factors are required for electrical sudden death: arrhythmogenic substrate; triggering events; and functional changes. Fibrosis plays a relevant role in the first factor. In patients with CD, those three factors can be clearly identified: the arrhythmogenic substrate is represented by the myocardial fibrosis and inflammation; the triggering events, by the frequent ventricular extrasystoles; and the functional changes, by the autonomous nervous system physiological changes.
Ventricular arrhythmia in CD is associated with ventricular dysfunction, and patients with ventricular dysfunction and complex ventricular arrhythmias have worse prognosis. The presence of ventricular electrical instability in patients with preserved LVEF and segmental wall motion abnormality is little studied.
It is possible that the 19.2% of Group 1 patients who had myocardial fibrosis detectable on CMR could be in an initial phase of CCHD, especially because four of them had ventricular arrhythmia. According to pathological studies16,17, the initial process is myocarditis with fibrosis, which can progress to segmental wall motion abnormality and ventricular dysfunction.
Our population is considered of low risk according to any prognostic score. Nevertheless, 45.7% of them have myocardial fibrosis detectable on CMR. The presence and amount of fibrosis were higher in Group 3 patients. That group also had more patients with ventricular arrhythmia, which could indirectly mean a higher arrhythmogenic potential.
Prospective studies on CCHD using CMR are scarce. Recently, Regueiro et al18, in a descriptive study, have shown the association of ECG changes (with segmental wall motion abnormality) with the increase in fibrosis mass and right and left ventricular dysfunctions. Mello et al19, assessing patients with CCHD on CMR, have shown that transmural fibrosis in more than two LV myocardial segments was more often associated with ventricular tachycardia.
In the present study, the segments most often affected by fibrosis were also those with more segmental wall motion abnormalities. This suggests a topographical relationship between sympathetic denervation, fibrosis and hypokinesia. The temporal relationship between those variables can help to understand the pathophysiological process and should be the object of future studies.
It is worth noting the reliability of the imaging test. Echocardiography is examiner-dependent and its interobserver variability is a well-known limitation. The CMR is known for its high reproducibility. In this study, the interobserver variability was < 5% for LVEF and RVEF, thus, not significant (p = 0.5 and 0.4, respectively). The percentage of fibrosis mass showed a statistical difference of 6%; however, a variability of such magnitude, regarding the small mean fibrosis amount found (15.02 g), can be of little clinical and scientific relevance, because, considering the standard deviation, it would be 15.02g ± 0.9 g. In addition, if regarded as categorical variable, it would have an excellent correlation (kappa = 0.96).
Study limitations
The patients in this study have not undergone coronary cineangiography to exclude coronary arterial disease as the cause of myocardial fibrosis. However, patients with diabetes and two or more risk factors for coronary arterial disease were excluded, thus reducing that probability. In addition, no patients with abnormalities suggestive of coronary arterial disease on TET or CMR were identified. Recently, Carvalho et al20 have shown a small prevalence of coronary arterial disease in patients with CD.
Most episodes of sudden death occur between the ages of 20 and 50 years in men. This differs from our population, mainly comprised by women with a mean age of 63 years, a fact that can underestimate prognostic events. Nevertheless, in that low-risk group, there was significant substrate for the presence of arrhythmias (segmental wall motion abnormality and presence of myocardial fibrosis).
The definition of ventricular arrhythmia includes the presence of frequent ventricular extrasystoles > 30/h, which can be considered of little clinical relevance. However, this study was aimed at assessing the triggering mechanism of early ventricular instability (fibrosis and segmental wall motion abnormality), rather than the immediate clinical impact.
Conclusions
On CMR, both myocardial fibrosis and segmental wall motion abnormality were associated with ventricular arrhythmia in patients with CCHD.
Even in patients with CCHD and preserved or minimally impaired ventricular function, the arrhythmogenic substrate can be present. Myocardial fibrosis detected on CMR is the most important variable associated with ventricular arrhythmia.
Acknowledgements
We thank Dr. Marco Lauzi, from Guerbet, for providing the gadolinium (Dotarem®) used in this study.
Footnotes
Author contributions
Conception and design of the research: Tassi EM, Pereira BB, Pedrosa RC; Acquisition of data: Tassi EM, Continentino MA; Analysis and interpretation of the data: Tassi EM, Continentino MA, Nascimento EM, Pereira BB, Pedrosa RC; Statistical analysis: Nascimento EM, Pereira BB; Writing of the manuscript: Tassi EM, Continentino MA, Pedrosa RC; Critical revision of the manuscript for intellectual content: Tassi EM, Pedrosa RC.
Sources of Funding
This study was partially funded by FAPESP.
Study Association
This article is part of the thesis of master submitted by Eduardo Marinho Tassi from Faculdade de Medicina da Universidade Federal do Rio de Janeiro.
References
- 1.Dias JC. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):41–45. doi: 10.1590/s0074-02762009000900007. [DOI] [PubMed] [Google Scholar]
- 2.Dias JC, Prata A, Correia D. Problems and perspectives for Chagas disease control: in search of a realistic analysis. Rev Soc Bras Med Trop. 2008;41(2):193–196. doi: 10.1590/s0037-86822008000200012. [DOI] [PubMed] [Google Scholar]
- 3.Rassi A, Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, et al. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355(8):799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 4.Rassi A, Jr, Rassi JG, Rassi A. Sudden death in Chagas' disease. Arq Bras Cardiol. 2001;76(1):75–96. doi: 10.1590/s0066-782x2001000100008. [DOI] [PubMed] [Google Scholar]
- 5.Pazin-Filho A, Romano MM, Almeida OC, Filho, Furuta MS, Viviani LF, Schimidt A, et al. Minor segmental wall motion abnormalities detected in patients with Chagas' disease have adverse prognostic implications. Braz J Med Biol Res. 2006;39(4):483–487. doi: 10.1590/s0100-879x2006000400008. [DOI] [PubMed] [Google Scholar]
- 6.Terzi FV, Siqueira AG, Filho, Nascimento EM, Pereira Bde B, Pedrosa RC. Regional left ventricular dysfunction and its association with complex ventricular arrhythmia, in chagasic patients with normal or borderline electrocardiogram. Rev Soc Bras Med Trop. 2010;43(5):557–561. doi: 10.1590/s0037-86822010000500017. [DOI] [PubMed] [Google Scholar]
- 7.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 8.Peshock RM, Rokey R, Malloy GM, McNamee P, Biya LM, Parkey RW, et al. Assessment of myocardial systolic wall thickening using nuclear magnetic resonance imaging. J Am Coll Cardiol. 1989;14(3):653–659. doi: 10.1016/0735-1097(89)90106-x. [DOI] [PubMed] [Google Scholar]
- 9.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu F, Bundy JM, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 10.Rochitte CE, Oliveira PF, Andrade JM, Ianni BM, Parga JR, Avila LF, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with Chagas' disease: a marker of disease severity. J Am Coll Cardiol. 2005;46(8):1553–1558. doi: 10.1016/j.jacc.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira FP, Pedrosa RC, Giannella-Neto A. Gas exchange during exercise in different evolutional stages of chronic Chagas' heart disease. Arq Bras Cardiol. 2000;75(6):481–498. doi: 10.1590/s0066-782x2000001200003. [DOI] [PubMed] [Google Scholar]
- 12.Pedrosa RC, Salles JH, Magnanini MM, Bezerra DC, Bloch KV. Prognostic value of exercise-induced ventricular arrhythmia in Chagas' heart disease. Pacing Clin Electrophysiol. 2011;34(11):1492–1497. doi: 10.1111/j.1540-8159.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- 13.Luiz RR, Costa AJ, Kale PL, Werneck BL. Assessment of agreement of a quantitative variable: a new graphical approach. J Clin Epidemiol. 2003;56(10):963–967. doi: 10.1016/s0895-4356(03)00164-1. [DOI] [PubMed] [Google Scholar]
- 14.Llorca J, Delgado-Rodriguez M. Survival analytical techniques were used to assess agreement of a quantitative variable. J Clin Epidemiol. 2005;58(3):314–315. doi: 10.1016/j.jclinepi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Myerburg RJ, Kessler KM, Bassett AL, Castellanos A. A biological approach to sudden cardiac death: structure, function and cause. Am J Cardiol. 1989;63(20):1512–1516. doi: 10.1016/0002-9149(89)90017-9. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi ML, Fukasawa S, de Brito T, Parzianello LC, Bellotti G, Ramires JA. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas' disease: a three dimensional confocal microscopy study. Heart. 1999;82(3):279–285. doi: 10.1136/hrt.82.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mady C, Ianni BM, Arteaga E, Montes GS, Caldini EG, Andrade G, et al. Relation between interstitial myocardial collagen and the degree of clinical impairment in Chagas' disease. Am J Cardiol. 1999;84(3):354–356. doi: 10.1016/s0002-9149(99)00295-7. Erratum in Am J Cardiol. 1999;84(12):1456. [DOI] [PubMed] [Google Scholar]
- 18.Regueiro A, Garcia-Alvarez A, Sitges M, Ortoz-Perez JT, De Coralt MT, Pinazo MJ, et al. Myocardial involvement in Chagas disease: insights from cardiac magnetic resonance. Int J Cardiol. 2011;165(1):107–112. doi: 10.1016/j.ijcard.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 19.Mello RP, Szarf G, Schvartzman PR, Nakano FM, Espinosa MM, Szenfeld D, et al. Delayed enhancement cardiac magnetic resonance imaging can identify the risk for ventricular tachycardia in chronic Chagas' heart disease. Arq Bras Cardiol. 2012;98(5):421–430. doi: 10.1590/s0066-782x2012005000031. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho G, Rassi S, Bastos JM, Camara SS. Asymptomatic coronary artery disease in chagasic patients. Arq Bras Cardiol. 2011;97(5):408–412. doi: 10.1590/s0066-782x2011005000103. [DOI] [PubMed] [Google Scholar]