Abstract
Background
Walking and yoga have been independently evaluated for weight control; however, there are very few studies comparing the 2 with randomization.
Material/Methods
The present study compared the effects of 90 minutes/day for 15 days of supervised yoga or supervised walking on: (i) related biochemistry, (ii) anthropometric variables, (iii) body composition, (iv) postural stability, and (v) bilateral hand grip strength in overweight and obese persons. Sixty-eight participants, of whom 5 were overweight (BMI ≥25 kg/m2) and 63 were obese (BMI ≥30 kg/m2; group mean age ±S.D., 36.4±11.2 years; 35 females), were randomized as 2 groups – (i) a yoga group and (ii) a walking group – given the same diet.
Results
All differences were pre-post changes within each group. Both groups showed a significant (p<0.05; repeated measures ANOVA, post-hoc analyses) decrease in: BMI, waist circumference, hip circumference, lean mass, body water, and total cholesterol. The yoga group increased serum leptin (p<0.01) and decreased LDL cholesterol (p<0.05). The walking group decreased serum adiponectin (p<0.05) and triglycerides (p<0.05).
Conclusions
Both yoga and walking improved anthropometric variables and serum lipid profile in overweight and obese persons. The possible implications are discussed.
Keywords: Yoga, Walking, Obesity, Adipokines, Lipid Profile, Anthropometry
Background
The prevalence of obesity and metabolic syndrome are increasing in India and other South Asian countries [1]. Weight reduction can be achieved by several methods of varying degrees of usefulness [2], particularly high levels of physical activity [3,4] and a change in the attitude towards food choices [5,6]. Two methods compared in the present study are walking and yoga, due to their increasing popularity and relative safety when supervised [7,8]. The effects of these 2 methods individually are described below.
Participation in an organized walking program for 2 months significantly improved anthropometric variables, BMI, body weight, percent body fat, fat mass, lean mass, and lipid profile in participants with bulimia nervosa [9] and in those with cardiac disease [10].
The effects of walking have also been studied in obese persons of different age groups. Twenty-seven obese middle school girls, allocated to a walking group and a control group, showed significant differences in the waist circumference, triglycerides, body weight, fat mass, and percent body fat after 12 weeks [11]. The effects of walking on energy requirements were assessed in adult women, showing an overall increase in daily energy needs at the end of the program [12].
Walking influenced the lipid profile and other biochemical factors. Altered adipokine concentration is an early sign of adipose tissue dysfunction [13]. Obese persons showed a decrease in serum leptin and serum chemerin [13]. A separate study reported no change in serum adiponectin [14]. The study also reported a decrease in body mass index, systolic blood pressure, triglycerides, and TNF-alpha after 50 days.
The studies cited above have shown that walking favorably influences anthropometric measures, body composition, energy expenditure, the lipid profile, and certain adipokines in obese persons.
Yoga is a practice which origins in ancient India. The practice includes physical postures (asanas), voluntarily regulated breathing techniques (pranayamas), meditation, and certain philosophical principles [15].
Yoga practice has been used in the management of obesity associated with other disorders. Practicing yoga resulted in a reduction in BMI, glycemic control, malondialdehyde and increase in glutathione, and vitamin C in persons with type 2 diabetes mellitus [16].
Other benefits followed yoga practice in persons with coronary artery disease [17]. There was a decrease in percent body fat, total cholesterol, triglycerides, and low-density lipoprotein, as well as systolic blood pressure, diastolic blood pressure, and heart rate.
Yoga practice has also been shown to influence adipokine levels [18]. After yoga practice, healthy post-menopausal women with more than 36% body fat showed increased adiponectin, improved serum lipid profile, and beneficial changes in metabolic syndrome risk factors. Also, a short-term, intensive yoga program caused a decrease in serum leptin levels, along with favorable changes in the body mass index, waist-hip circumference, total cholesterol, better postural stability, and increased hand grip strength [19].
Hence, like walking, yoga also improved the BMI, anthropometric measurements, body composition, and relevant biochemical measurements in persons with obesity.
As described above, both walking and yoga have individually shown benefits in persons with obesity. There have also been studies comparing the effects of yoga with those of walking in obese persons. Walking and yoga interventions improved sub-domains of self-esteem for both walking and yoga [20]. Apart from this, there are few other studies comparing the effects of yoga with those of walking assessing the adipokine levels, lipid profile, anthropometric variables, and body composition.
The hypothesis of the present study was that 15 days of supervised walking or supervised yoga would have comparable effects in overweight and obese persons, when both interventions were separately evaluated in a residential setting where the diet was the same.
Material and Methods
Participants
Sixty-eight participants, of whom 63 were obese (group mean BMI ±S.D., 37.9±5.6 kg/m2) and 5 were overweight (group mean BMI ±S.D., 28.5±0.6 kg/m2), with ages between 20 and 55 years (group average age ±S.D., 36.4±11.2 years; 35 females), were enrolled in the study. For the yoga group the sample size was calculated based on an earlier study [19]. A required sample size (n=32) was obtained by applying Cohen’s formula for an Effect size of 0.30 (medium) and an alpha of 0.05, powered at 0.90 using G power program [21]. The effect size was calculated from mean and S.D. of waist circumference, which was changed significantly in the study on 47 subjects. For the walking group a required sample size (n=31) was obtained for an Effect size of 0.34 (medium) and an alpha of 0.05, powered at 0.95 using G power program [21]. The effect size was calculated from mean and S.D. of triglycerides, which was changed significantly in the study [14] on 22 subjects. The post hoc analyses showed that for the present study, with a sample size as 23 in each group, and with the Cohen’s d of 0.74 (large) for the yoga group and 0.51 (large) for the walking group, obtained from data on total cholesterol, which changed significantly within each group, the power was 0.9999 and 0.9966, respectively [21]. Recruitment was done by an advertisement on a television channel. The study was conducted in a residential yoga center located in northern India, with no charge to the participants. Participants of both sexes were included if: (i) their BMI was ≥25 kg/m2 and (ii) they were able to perform mild to moderate physical activity. Participants were excluded from the trial if they had: (i) endocrine disorders (e.g., hypothyroidism, polycystic ovarian syndrome), (ii) drug-induced changes in the fat distribution (e.g., following the chronic intake of corticosteroids), and (iii) any other disease, some of which are complications of obesity (e.g., uncontrolled hypertension, hypercholesterolemia, and type 2 diabetes mellitus). The baseline characteristics of the participants are given in Table 1. The approval for the study was obtained from the ethics committee of Patanjali Research Foundation. Signed informed consent from all the participants was obtained. The study was registered with the Clinical Trials Registry of India (CTRI/2012/12/003210).
Table 1.
Baseline characteristics of the yoga and walking groups.
| Yoga | Walking | |
|---|---|---|
| n (males/females) | 34 (16/18) | 34 (17/17) |
| Age (years) Mean ±S.D. |
36.0±10.3 | 36.8±12.1 |
| Total cholesterol (mg/dl) Mean ±S.D. |
169.6±25.3 | 167.4±45.0 |
| LDL cholesterol (mg/dl) Mean ±S.D. |
109.5±23.6 | 105.7±31.2 |
| HDL cholesterol (mg/dl) Mean ±S.D. |
44.0±10.2 | 42.6±10.4 |
| Triglycerides (mg/dl) Mean ±S.D. |
163.3±64.5 | 183.6±127.7 |
Design
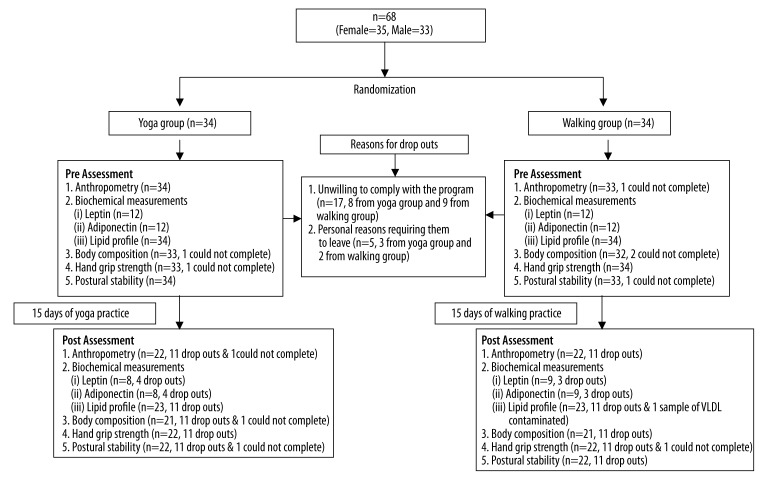
The trial was a comparative study with randomization between 2 interventions known to help in weight regulation. Participants were assigned random numbers from a standard table [22] and then blindly allocated to the 2 groups. The participants were recruited in July 2011 and post-intervention assessment was done in August 2011. The final number in each group is shown in the Trial Profile (Figure 1).
Figure 1.
Trial profile for the RCT.
Assessments
The primary outcome variables were (i) biochemical measures, (ii) anthropometric measurements, and (iii) body composition. The secondary outcome variables were (i) postural stability and (ii) bilateral hand grip strength.
Biochemical measures
Blood samples were taken from the antecubital vein following an overnight 12-hour fast. Fasting serum leptin and serum adiponectin were estimated using ELISA. Estimation of total cholesterol was done using the Cholesterol Oxidase Peroxidase method. Triglycerides were estimated using the Glycerol-3-Phosphate Oxidase and Peroxidase method. High-density lipoprotein, and low-density lipoprotein were estimated using the Direct Enzyme Clearance method.
Anthropometric measurements
(i) Body weight, (ii) BMI, (iii) waist circumference, (iv) hip circumference, (v) waist-hip ratio, and (vi) mid-arm circumference were assessed using standard methods [19].
Body composition
Body composition was determined by the bio-electrical impedance analysis method using a body composition analyzer (BF-907, Maltron, U.K.). A standard method for recording, with participants resting supine, with their legs and arms slightly apart, was used. All relevant parameters such as height, weight, age, sex, and ethnicity were entered in the recording unit. The duration for recording was approximately 5 minutes.
Postural stability
Postural stability was measured using a stability platform (Lafayette Instrument, Model 16030, U.S.A.). In the present study, a standard method was used [19] with 3 test times of 20, 40, and 60 seconds. The time spent tilted towards the left, the right, or at the center was displayed on the screen.
Hand grip strength
Handgrip strength was measured using a hand grip dynamometer (Lafayette Instrument, Model 7498-05, U.S.A.). Participants held the dynamometer with their arm at right angles to the trunk of the body and their elbow touching the side of the body. The participants were instructed to squeeze the dynamometer with maximum isometric strength. Each hand was tested in 3 trials alternately, spaced 10 seconds apart. All participants were right hand dominant based on a standard handedness inventory [23]. For each hand the best value obtained of 3 trials was used for analysis.
Intervention
Yoga
The participants of the yoga group practiced yoga for 45 minutes twice a day between (i) 05:00 hours and 05: 45 hours and (ii) 17:00 hours and 17:45 hours, for 15 days. Two sessions of yoga were selected as being fairly feasible to carry out at home. An instructor, who had been studying yoga for 5 years leading to a university degree, with a total of 17 years of study including practice, supervised the participants throughout the yoga session. Each 45-minute yoga session included breathing techniques for 33 minutes. The remaining time was spent in specific yoga postures. Participants repeated postures as many times as was possible within the time. The details of the yoga intervention are given in Table 2.
Table 2.
Details about the yoga program which was repeated throughout the fifteen days.
| SI. no. | Name of the yoga practice | Duration |
|---|---|---|
| I | Start: Sukhasana (easy posture) + Prathana mantra (universal prayer) | 1 min |
|
| ||
| II | Pranayama series: | |
| 1. Bhastrika (bellows breathing) | 36–50 strokes/3 min | |
| 2. Kapalabhati (high frequency yoga breathing) | 750–900 strokes/18 min* | |
| 3. Anulom-vilom (alternate nostril yoga breathing) | 20–25 rounds/6 min | |
| 4. Bhramri (bumble bee breathing) | 8–10 rounds/3 min | |
| 5. Udgeeth (OM chanting) | 6–8 rounds/3 min | |
|
| ||
| III | Standing postures: | |
| 1. Trikonasana (triangular pose) | 1 min | |
| 2. Konasana (angle pose) | 1 min | |
| 3. Padahastasana (hand to foot pose) | 1 min | |
|
| ||
| IV | Supine postures: | |
| 1. Uttanpadasana (straight leg raised pose) | 2 min | |
| 2. Markatasana (monkey pose) | 1 min | |
|
| ||
| V | Relaxation technique: Savasana/Yoga nidra (corpse pose with guided relaxation) | 5 min |
|
| ||
| Total time of intervention | 45 minutes | |
After each five minutes of practice of kapalabhati (high frequency yoga breathing) there was one minute without practice.
Walking
At the same times of the day, (i.e., 05:00–05:45 hours and 17:00–17:45 hours), there were two sessions of walking. The participants walked at their own paces. The walking track was cemented and rectangular. The total perimeter of the track was approximately 1200 meters. An instructor, who had been studying science for 5 years leading to a university degree, with a total of 17 years of study, supervised the participants throughout the walking session. The instructors for the 2 interventions were different. On average, the participants walked approximately 2600 meter/session at the average speed of 3.5 km/hr.
Common activities
Between the 2 interventions in the morning and evening participants carried out routine activities such as laundry, reading, watching the television, and so on. None of them practiced yoga or walking on their own at other times, because they were instructed to avoid this. On questioning, all of them compiled with the instructions. All individuals went to sleep at 21:30 and woke up at 04:45, which was regulated by the experimenter.
Diet (common to both groups)
Participants of both groups were given a plant-based diet regulated at approximately 1650 kcal/day. This calorie restricted diet was part of the weight loss program. The energy intake of nutritive values of Indian foods was determined from a database available at http://www.medindia.net/calories-in-indian-food/.
Data analysis
Repeated measures analyses of variance (ANOVA) followed by post hoc analyses with Bonferroni adjustment (corrected Bonferroni value of 0.025) were done to compare data after the 2 interventions with pre-intervention data using PASW Version 18.0. There was 1 Within subjects factor (States with 2 levels (pre and post)) and 1 Between subjects factor (Groups with 2 levels [yoga and walking]). Missing value analyses were used for leptin and adiponectin data when performing the Repeated Measures Analyses of Variance. Statistical significance was set at α=0.05.
Results
None of the participants reported adverse events during the intervention, which were specifically checked after each session. The group mean values ±S.D. for the different variables are given in Tables 3–6.
Table 3.
Adipokines and lipid profile. Values are group mean ±S.D.
| Variables | n | Yoga (n=23) | Walking (n=23) | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Cohen’s d | Pre | Post | Cohen’s d | ||
| Serum leptin (ng/ml) | 12 | 9.66±5.59 | 15.39±9.44** | 0.76 | 7.94±6.14 | 9.28±6.48 | 0.21 |
| Serum adiponectin (μg/ml) | 12 | 7.92±5.30 | 6.92±2.27 | 0.26 | 10.92±8.13 | 6.43±1.42* | 0.94 |
| Total cholesterol (mg/dl) | 23 | 174.00±22.72 | 154.04±31.25* | 0.74 | 170.74±48.37 | 151.17±28.37* | 0.51 |
| Triglycerides (mg/dl) | 23 | 165.35±56.33 | 135.09±42.43 | 0.61 | 187.26±145.19 | 127.26±44.17* | 0.64 |
| LDL cholesterol (mg/dl) | 23 | 111.78±22.66 | 96.61±24.02* | 0.66 | 107.83±33.77 | 93.61±22.82 | 0.51 |
| HDL cholesterol (mg/dl) | 23 | 45.13±11.12 | 43.09±6.89 | 0.21 | 42.52±11.37 | 43.09±8.36 | 0.08 |
| VLDL (mg/dl) | 22 | 33.50±11.34 | 27.05±8.69 | 0.67 | 32.04±13.29 | 25.64±9.00 | 0.60 |
p<0.05;
p<0.01, post-hoc analyses with Bonferroni adjustment compared with pre.
Table 6.
Values (group mean ±S.D.) for the postural stability and bilateral hand grip strength.
| Variables | Yoga (n=22) | Walking (n=22) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Cohen’s d | Pre | Post | Cohen’s d | |
| Center 20 sec (s) | 6.21±3.18 | 6.88±4.54 | 0.17 | 5.43±3.85 | 7.54±4.21* | 0.52 |
| Center 40 sec (s) | 13.32±7.18 | 15.97±10.04 | 0.31 | 12.19±7.43 | 17.23±8.75** | 0.62 |
| Center 60 sec (s) | 20.94±11.93 | 27.61±14.38** | 0.51 | 20.01±12.15 | 24.30±12.82 | 0.34 |
| Right hand grip strength (kg) | 29.45±9.93 | 32.64±9.96* | 0.32 | 36.23±15.96 | 39.05±16.55* | 0.17 |
| Left hand grip strength (kg) | 28.86±9.34 | 31.36±9.89 | 0.26 | 33.09±12.68 | 37.86±16.08*** | 0.33 |
p<0.05,
p<0.01,
p<0.001, post-hoc analyses with Bonferroni adjustment compared with pre.
Repeated measures analysis of variance (ANOVA)
The ANOVA values for the Within-Subjects factors (States), Between-Subjects factors (Groups), and interaction between the 2 for the different variables for (i) biochemical measurements, (ii) anthropometric variables, (iii) body composition, and (iv) postural stability with bilateral hand grip strength are given in Tables 7–10, respectively. A significant interaction between Groups and States for any variable suggests that 2 are interdependent.
Table 7.
ANOVA table for variables of the biochemical measurements.
| SI. no. | Factors | Variable | F | df | Huynh-Feldt ɛ | p value |
|---|---|---|---|---|---|---|
| I | Within subjects (states) | Serum leptin | 9.784 | 1,22 | 1 | 0.005 |
| Serum adiponectin | 3.283 | 1,22 | 1 | 0.084 | ||
| Total cholesterol | 8.811 | 1,44 | 1 | 0.005 | ||
| Triglycerides | 6.280 | 1,44 | 1 | 0.016 | ||
| LDL cholesterol | 7.701 | 1,44 | 1 | 0.008 | ||
| HDL cholesterol | 0.158 | 1,44 | 1 | 0.693 | ||
| VLDL | 7.394 | 1,42 | 1 | 0.009 | ||
| II | Between subjects (groups) | Serum leptin | 2.171 | 1,22 | 1 | 0.155 |
| Serum adiponectin | 0.819 | 1,22 | 1 | 0.375 | ||
| Total cholesterol | 0.166 | 1,44 | – | 0.685 | ||
| Triglycerides | 0.174 | 1,44 | – | 0.678 | ||
| LDL cholesterol | 0.381 | 1,44 | – | 0.540 | ||
| HDL cholesterol | 0.370 | 1,44 | – | 0.546 | ||
| VLDL | 0.423 | 1,42 | – | 0.519 | ||
| III | States × groups | Serum leptin | 3.793 | 1,22 (states) × 22 (groups) | – | 0.064 |
| Serum adiponectin | 1.325 | 1,22 (states) × 22 (groups) | – | 0.262 | ||
| Total cholesterol | 0.001 | 1,44 (states) × 44 (groups) | – | 0.977 | ||
| Triglycerides | 0.682 | 1,44 (states) × 44 (groups) | – | 0.413 | ||
| LDL cholesterol | 0.008 | 1,44 (states) × 44 (groups) | – | 0.928 | ||
| HDL cholesterol | 0.493 | 1,44 (states) × 44 (groups) | – | 0.486 | ||
| VLDL | 0.000 | 1,42 (states) × 42 (groups) | – | 0.992 |
Table 10.
ANOVA table for the postural stability and bilateral hand grip strength.
| SI. no. | Factors | Variable | F | df | Huynh-Feldt ɛ | p value |
|---|---|---|---|---|---|---|
| I | Within subjects | Center (20 s) | 4.478 | 1,42 | 1 | 0.040 |
| Center (40 s) | 9.905 | 1,42 | 1 | 0.003 | ||
| Center (60 s) | 11.223 | 1,42 | 1 | 0.002 | ||
| Right hand grip | 10.004 | 1,42 | 1 | 0.003 | ||
| Left hand grip | 14.924 | 1,42 | 1 | 0.000 | ||
| II | Between subjects | Center (20 s) | 0.004 | 1,42 | 1 | 0.952 |
| Center (40 s) | 0.001 | 1,42 | 1 | 0.976 | ||
| Center (60 s) | 0.365 | 1,42 | 1 | 0.549 | ||
| Right hand grip | 2.783 | 1,42 | 1 | 0.103 | ||
| Left hand grip | 2.240 | 1,42 | 1 | 0.142 | ||
| III | States × groups | Center (20 s) | 1.198 | 1,42 (States) × 42 (Groups) | 1 | 0.280 |
| Center (40 s) | 0.956 | 1,42 (States) × 42 (Groups) | 1 | 0.334 | ||
| Center (60 s) | 0.532 | 1,42 (States) × 42 (Groups) | 1 | 0.470 | ||
| Right hand grip | 0.037 | 1,42 (States) × 42 (Groups) | 1 | 0.849 | ||
| Left hand grip | 1.457 | 1,42 (States) × 42 (Groups) | 1 | 0.234 |
For the variables mentioned above, there were no significant differences between Groups and no significant interaction between States and Groups.
Post-hoc analyses
All comparisons were made with respective ‘pre’ states within a group, hence changes reported are pre-post, not between groups.
Biochemical measurements
The yoga group alone showed a significant increase in the leptin levels (p<0.01) with 95% Confidence Interval (CI) of [−2.420, −9.046], while the walking group alone showed a significant reduction in adiponectin levels (p<0.05) with 95% CI of [8.922, 0.045]. There was a significant reduction in total cholesterol (p<0.05) with 95% CI of [38.931, 0.982] for the yoga group; (p<0.05) with 95% CI of [38.540, 0.591] for the walking group. Triglycerides levels were decreased significantly in the walking group alone (p<0.05) with 95% CI of [111.328, 8.672], while LDL cholesterol was reduced significantly in the yoga group alone (p<0.05) with 95% CI of [30.267, 0.081].
Anthropometric measurements
There was a significant decrease in: (i) BMI (p<0.001) with 95% CI of [1.910, 1.335] for the yoga group, (p<0.001) with 95% CI of [2.198, 1.621] for the walking group; (ii) hip circumference (p<0.001) with 95% CI of [3.899, 1.765] for the yoga group, (p<0.001) with 95% CI of [5.190, 3.056] for the walking group; and (iii) in waist circumference (p<0.05) with 95% CI of [4.563, 0.482] for the yoga group,(p<0.01) with 95% CI of [4.858, 0.778] for the walking group. Mid-arm circumference was reduced significantly in the walking group alone (p<0.05) with 95% CI of [2.025, 0.257].
Body composition
There was a significant reduction in (i) lean mass (p<0.001) with 95% CI of [4.792, 2.198] for the yoga group; (p<0.001) with 95% CI of [5.845, 3.250] for the walking group and (ii) in body water (p<0.001) with 95% CI of [3.644, 1.727] for the yoga group; (p<0.001) with 95% CI of [4.378, 2.460] for the walking group.
Postural stability
The walking group showed a significant increase in postural stability when the stability platform was centered in the: (i) 20-second trial (p<0.05) with 95% CI of [−0.234, −3.989], and (ii) 40-second trial (p<0.01) with 95% CI of [−1.553, −8.526]. The yoga group showed a significant increase in postural stability when the stability platform was centered in the 60-second trial (p<0.01) with 95% CI of [−2.005, −11.349].
Hand grip strength
The walking group showed a significant increase in right hand grip strength (p<0.05) with 95% CI of [−0.111, −5.525] and in left hand grip strength (p<0.001) with 95% CI of [−2.086, −7.459]. The yoga group showed a significant increase in the right hand grip strength alone (p<0.05) with 95% CI of [−0.475, −5.8889].
Discussion
The present comparative trial demonstrated comparable changes in: (i) total cholesterol, (ii) anthropometric variables, (iii) body composition, (iv) postural stability, and (v) hand grip strength following 15 days of yoga and walking.
The most obvious change in the yoga and walking groups were in the levels of serum leptin and serum adiponectin. The participants of the yoga group showed a significant increase in fasting serum leptin levels. In contrast, in a previous study [19] with comparable baseline average age, sex, BMI, and fat mass as the present study, 6 days of yoga and diet change resulted in a significant reduction in fasting serum leptin levels. The difference in results could be related to the baseline levels of leptin. In the earlier 6-day yoga program [19] the baseline fasting serum leptin levels were 53.71±34.61 ng/ml, whereas in the present study the group average baseline levels were considerably lower – 9.66±5.59 ng/ml (yoga) and 7.94±6.14 ng/ml (walking). Excessively high blood leptin levels observed in certain obese persons are associated with development of leptin resistance [24], which could have explained the high leptin levels in an earlier study [19]. In such cases decreased leptin levels are suggestive of improvement, with increased sensitivity to appetite-suppressing factors [25]. In contrast, the increased leptin levels in the present study, where baseline levels were close to normal, could suggest a decrease in self-perceived appetite, because the 2 are inversely related [26]. The reason the baseline leptin levels differed in the 2 studies was not related to their body composition or other variables associated with obesity, and remains to be explained.
In the present study the walking group showed a significant reduction in the serum adiponectin levels. Numao et al., in 2011 in a Japanese population, reported a significant reduction in adiponectin levels in middle-aged centrally obese men, immediately after the cessation of high-intensity aerobic exercise [27]. In contrast, another study reported a significant increase in plasma adiponectin levels in inactive, centrally obese Caucasian men after acute and short-term aerobic exercise [28]. The exact reasons for these contrasting findings are not clear. One of the aspects in which the 2 groups differed is their ethnicity. The group that showed a decrease in adiponectin levels was of Asian origin, and although there are considerable differences between South Asia (the Indian population of the present study) and East Asia (Japan), there may be ethnic factors involved in the decrease in adiponectin. Adiponectin circulates in isoforms of different molecular weight [29] and the relative proportions of these isoforms vary with ethnicity [30,31]. The decrease in adiponectin levels in the walking group suggests an increased predisposition to obesity-related disorders such as coronary heart disease [32], which is difficult to associate with a walking program. These contradictory findings could be due to the different isoforms of adiponectin differing in their association with chronic disease [32], lack of separate evaluation, and the small sample size.
The decrease in total cholesterol, LDL cholesterol, and triglycerides suggest that both walking and yoga may reduce the risk of cardiovascular diseases associated with dyslipidemia [33]. These results are similar to those reported earlier [34].
In both groups, the decrease in BMI could be partly attributed to the increased energy expenditure as well as to the diet, which was a low-fat, plant-based diet of around 1650 kcal/day. A low-fat, vegan diet adopted for 14 weeks was associated with significant weight loss in overweight post-menopausal women, despite the fact that there were no limits on portion size or energy intake [35]. Also, a 1-week program that used a low-fat, low-energy, lacto-ovo-vegetarian diet and exercise in a stress-free environment reduced BMI, serum cholesterol, and other risk factors for cardiovascular disease in 1349 volunteers [36].
Both yoga and walking resulted in a significant decrease in the waist circumference and in the hip circumference but no change in the waist/hip ratio. These results suggest that there was no difference between the reductions in fat stored centrally inside the abdomen (waist circumference) and in fat stored peripherally (hip circumference) with the 2 interventions.
In the walking group, the reduced mid-arm circumference could be due to a decrease in the mid-arm muscle or in the circumferential skin-fold layer, as the mid-arm muscle circumference was not calculated separately.
The changes in body composition (ie, a decrease in the lean mass and in the body water) are of concern. In an earlier study [19] with a single group who practiced yoga for 6 days and who had a lacto-vegetarian diet without regulation of the portions or fluid intake, there was also a decrease in lean mass (4.4%) and body water (4.4%). In the present study, the decrease in lean mass was 6.3% (yoga) and 7.9% (walking). Body water decreased by 6.7% (yoga) and 8.1% (walking). In the earlier study, the decrease in lean muscle mass was believed to be due to calorie restriction, which enhances a catabolic response with a decrease in lean muscle mass [37]. The findings of the present study could be explained by the participants receiving around 1650 kcal/day, which is a calorie-restricted diet. It is possible that the plant-based diet was the basis for the change in lean mass, because a study that compared the body composition in lacto-ovo-vegetarians and those who were omnivorous did not report negative alterations in body composition [38], while the effects of a purely plant-based diet have not been reported. The decrease in lean mass in both groups is possibly related to a reduced intake of protein in the plant-based diet without eggs or milk and no compensatory increase in plant sources of protein. With regard to the body water, every attempt was made to ensure that the participants were adequately hydrated by including this advice in their daily interaction with the doctor. Also they drank approximately 1.0 liter/day while under supervision. Evidently this was not adequate. The decrease in body water could have contributed to the decrease in BMI.
In the study cited earlier, as in the present study, obese participants were able to remain posturally stable or balanced for a longer time after yoga [19]. It has also been shown that in normal individuals [39] and in older persons [40] the practice of yoga improved the ability to balance. Walking has also proved beneficial for community-dwelling stroke patients, improving their balance and helping in rehabilitation [41]. It is well recognized that a decrease in balance and stability is strongly correlated with an increase in body weight, and is an important risk factor for falls in obese persons [42]. Hence, the increase in balance following yoga and walking would probably facilitate mobility in obese persons, and compliance with physical activity programs.
The participants in the walking group showed an increase in bilateral hand grip strength, while the yoga group increased right hand grip strength. The importance of an increase in hand grip strength in obese persons is that it is an indicator of musculoskeletal fitness, which is considered a significant predictor of weight gain [43]. The yoga group improved right hand grip strength alone, possibly related to certain structural and functional differences between the right and left hands [44].
Yoga practice has been found to favorably influence the body composition and lipid profile in obese children [45], adults of both sexes [17], and in females of different age groups [18]. The programs included yoga postures either alone or in combination with yoga breathing exercises and meditation. Walking was also found to favorably influence the body composition, as well as anthropometric and biochemical measures of persons of different age groups and of both sexes [2,9–11]. The present study results showed that a 15-day program of supervised yoga or of supervised walking had comparable beneficial effects in the variables assessed.
Limitations and future directions
The present study has demonstrated several benefits of both supervised yoga and supervised walking for obese persons. However, the findings are limited by the following factors: (i) The adipokines leptin and adiponectin were evaluated in a small sample based on the funds allocated by the funding agency and due to the high cost per test (approximately USD $180 for leptin/person and USD $300 for adiponectin/person). This was particularly difficult to finance because the program was free. (ii) The absence of a control group limits attributing changes seen to either yoga or walking. (iii) The high drop-out rate (approximately 33%), which was due to factors such as lack of compliance with the program (participants who practiced yoga/walking in between the sessions were excluded), and personal reasons requiring them to leave before the 15 days were complete. (iv) The age range for both groups was similar, but wide (21–54 years in the yoga group and 20–55 years in the walking group). Hence, the groups included participants whose metabolism would be influenced by different physiological factors. (v) The interventions were 15 days in duration and it was not possible to determine whether the effects extended beyond that period or not. The participants were contacted 1 month after the program to find out how many of them were continuing yoga/walking, revealing that 78.3% continued yoga and 69.5% continued walking. However, there was no attempt to note their weight. These limitations suggest possible directions for future study in this area of considerable medical concern.
Conclusions
Supervised yoga and supervised walking favorably and similarly influence multiple outcomes in overweight and obese adults. However, yoga increased serum leptin levels and walking reduced serum adiponectin levels. The findings suggest the usefulness of these interventions in obesity and also suggest directions for further investigation.
Table 4.
Values (group mean ±S.D.) for the anthropometric variables.
| Variables | Yoga (n=22) | Walking (n=22) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Cohen’s d | Pre | Post | Cohen’s d | |
| BMI (kg/m2) | 38.23±6.81 | 36.61±6.54*** | 0.24 | 35.65±6.35 | 33.80±5.76*** | 0.31 |
| Mid-arm circumference (cm) | 35.69±4.27 | 35.32±5.05 | 0.08 | 34.05±2.50 | 32.91±3.25* | 0.40 |
| Hip circumference (cm) | 123.55±12.79 | 120.71±12.47*** | 0.22 | 119.85±11.37 | 115.73±11.53*** | 0.36 |
| Waist circumference (cm) | 112.35±12.72 | 109.83±13.28* | 0.19 | 111.00±15.50 | 108.18±14.41** | 0.19 |
| Waist/hip ratio | 0.91±0.05 | 0.91±0.05 | 0.0 | 0.93±0.08 | 0.93±0.08 | 0.0 |
p<0.05;
p<0.01;
p<0.001, post-hoc analyses with Bonferroni adjustment compared with pre.
Table 5.
Values (group mean ±S.D.) for body composition.
| Variables | Yoga (n=21) | Walking (n=21) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Cohen’s d | Pre | Post | Cohen’s d | |
| Body fat (kg) | 40.72±13.39 | 40.08±11.92 | 0.05 | 39.01±12.15 | 38.47±12.54 | 0.04 |
| Lean mass (kg) | 55.05±13.57 | 51.56±13.65*** | 0.26 | 57.70±17.08 | 53.15±15.59*** | 0.28 |
| Body water (l) | 40.30±9.92 | 37.61±10.14*** | 0.27 | 42.33±12.42 | 38.91±11.42*** | 0.29 |
p<0.001, post-hoc analyses with Bonferroni adjustment compared with pre.
Table 8.
ANOVA table for the anthropometric variables.
| SI. no. | Factors | Variable | F | df | Huynh-Feldt ɛ | p value |
|---|---|---|---|---|---|---|
| I | Within subjects | BMI | 296.780 | 1,42 | 1 | 0.000 |
| Mid-arm circumference | 5.933 | 1,42 | 1 | 0.019 | ||
| Hip circumference | 86.472 | 1,42 | 1 | 0.000 | ||
| Waist circumference | 13.955 | 1,42 | 1 | 0.001 | ||
| Waist/hip ratio | 0.517 | 1,42 | 1 | 0.476 | ||
| II | Between subjects | BMI | 1.973 | 1,42 | 1 | 0.167 |
| Mid-arm circumference | 3.184 | 1,42 | 1 | 0.082 | ||
| Hip circumference | 1.441 | 1,42 | 1 | 0.237 | ||
| Waist circumference | 0.129 | 1,42 | 1 | 0.721 | ||
| Waist/hip ratio | 1.007 | 1,42 | 1 | 0.321 | ||
| III | States × groups | BMI | 1.271 | 1,42 (states) × 42 (groups) | 1 | 0.266 |
| Mid-arm circumference | 1.556 | 1,42 (states) × 42 (groups) | 1 | 0.219 | ||
| Hip circumference | 2.979 | 1,42 (states) × 42 (groups) | 1 | 0.092 | ||
| Waist circumference | 0.043 | 1,42 (states) × 42 (groups | 1 | 0.837 | ||
| Waist/hip ratio | 0.647 | 1,42 (states) × 42 (groups) | 1 | 0.426 |
Table 9.
ANOVA table for the variables of body composition.
| SI. no. | Factors | Variable | F | df | Huynh-Feldt å | p value |
|---|---|---|---|---|---|---|
| I | Within subjects | Body fat | 1.864 | 1,40 | 1 | 0.180 |
| Lean mass | 78.517 | 1,40 | 1 | 0.000 | ||
| Body water | 82.836 | 1,40 | 1 | 0.000 | ||
| II | Between subjects | Body fat | 0.187 | 1,40 | 1 | 0.668 |
| Lean mass | 0.210 | 1,40 | 1 | 0.649 | ||
| Body water | 0.244 | 1,40 | 1 | 0.624 | ||
| III | States × groups | Body fat | 0.012 | 1,40 (states) × 40 (groups) | 1 | 0.913 |
| Lean mass | 1.344 | 1,40 (states) × 40 (groups | 1 | 0.253 | ||
| Body water | 1.195 | 1,40 (states) × 40 (groups) | 1 | 0.281 |
Acknowledgements
The authors gratefully acknowledge the staff of PRF, Haridwar, India for help in data collection.
Footnotes
Trial registration
The trial was registered with the Clinical Trials Registry of India (CTRI/2012/12/003210).
Conflict of interest
None declared.
Source of support: The research was funded by the Central Council for Research in Yoga and Naturopathy (CCRYN), Dept. of AYUSH, Government of India, New Delhi
References
- 1.Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for metabolic syndrome in Asian Indians: A community study from urban Eastern India. J Cardiovasc Dis Res. 2012;3:204–11. doi: 10.4103/0975-3583.98895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fogelholm M, Kukkonen-Harjula K, Nenonen A, Pasanen M. Effects of walking training on weight maintenance after a very-low-energy diet in premenopausal obese women: a randomized controlled trial. Arch Intern Med. 2000;160:2177–84. doi: 10.1001/archinte.160.14.2177. [DOI] [PubMed] [Google Scholar]
- 3.Kayman S, Bruvold W, Stern JS. Maintenance and relapse after weight loss in women: behavioral aspects. Am J Clin Nutr. 1990;52:800–7. doi: 10.1093/ajcn/52.5.800. [DOI] [PubMed] [Google Scholar]
- 4.Svendsen OL, Hassager C, Christiansen C. Six months’ follow-up on exercise added to a short-term diet in overweight postmenopausal women – effects on body composition, resting metabolic rate, cardiovascular risk factors and bone. Int J Obes Relat Metab Disord. 1994;18:692–98. [PubMed] [Google Scholar]
- 5.Blackburn GL, Kanders BS, Lavin PT. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr. 1997;65:409–18. doi: 10.1093/ajcn/65.2.409. [DOI] [PubMed] [Google Scholar]
- 6.Westerterp-Plantenga MS, Kempen KP, Saris WH. Determinants of weight maintenance in women after diet-induced weight reduction. Int J Obes Relat Metab Disord. 1998;22:1–6. doi: 10.1038/sj.ijo.0800536. [DOI] [PubMed] [Google Scholar]
- 7.The Times of India Group. Available from: http://articles.timesofindia.indiatimes.com/2013-10-19/fitness/33286632_1_health-benefits-breast-cancer-bone-loss.
- 8.The Times of India Group. Available from: http://articles.timesofindia.indiatimes.com/2013-11-15/fitness/36615323_1_weight-loss-power-yoga-yoga-mat.
- 9.Habibzadeh N, Daneshmandi H. The Effects of Exercise in Obese Women with Bulimia Nervosa. Asian J Sports Med. 2010;1:209–13. doi: 10.5812/asjsm.34829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mertens DJ, Kavanagh T, Campbell RB, Shephard RJ. Exercise without dietary restriction as a means to long-term fat loss in the obese cardiac patient. J Sports Med Phys Fitness. 1998;38:310–16. [PubMed] [Google Scholar]
- 11.Kim YH, Yang YO. Effects of walking exercise on metabolic syndrome risk factors and body composition in obese middle school girls. Taehan Kanho Hakhoe Chi. 2005;35:858–67. doi: 10.4040/jkan.2005.35.5.858. [DOI] [PubMed] [Google Scholar]
- 12.Moore JM, Oddou WE, Leklem JE. Energy need in childhood and adult-onset obese women before and after a nine-month nutrition education and walking program. Int J Obes. 1991;15:337–44. [PubMed] [Google Scholar]
- 13.Venojärvi M, Wasenius N, Manderoos S, et al. Nordic walking decreased circulating chemerin and leptin concentrations in middle-aged men with impaired glucose regulation. Ann Med. 2013;45:162–70. doi: 10.3109/07853890.2012.727020. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi J, Murase Y, Asano A, et al. Effect of walking with a pedometer on serum lipid and adiponectin levels in Japanese middle-aged men. J Atheroscler Thromb. 2006;13:197–201. doi: 10.5551/jat.13.197. [DOI] [PubMed] [Google Scholar]
- 15.Taimini IK. The Science of Yoga. Madras: The Theosophical Publishing House; 1986. [Google Scholar]
- 16.Hegde SV, Adhikari P, Kotian S, et al. Effect of 3-month yoga on oxidative stress in type 2 diabetes with or without complications: a controlled clinical trial. Diabetes Care. 2011;34:2208–10. doi: 10.2337/dc10-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal A, Srivastava N, Tiwari S, et al. Effect of yogic practices on lipid profile and body fat composition in patients of coronary artery disease. Complement Ther Med. 2011;19:122–27. doi: 10.1016/j.ctim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Lee JA, Kim JW, Kim DY. Effects of yoga exercise on serum adiponectin and metabolic syndrome factors in obese postmenopausal women. Menopause. 2012;19:296–301. doi: 10.1097/gme.0b013e31822d59a2. [DOI] [PubMed] [Google Scholar]
- 19.Telles S, Naveen KV, Balkrishna A, Kumar S. Short term health impact of a yoga and diet change program on obesity. Med Sci Monit. 2010;16(1):CR35–40. [PubMed] [Google Scholar]
- 20.Elavsky S, McAuley E. Exercise and self-esteem in menopausal women: a randomized controlled trial involving walking and yoga. Am J Health Promot. 2007;22:83–92. doi: 10.4278/0890-1171-22.2.83. [DOI] [PubMed] [Google Scholar]
- 21.Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. [Google Scholar]
- 22.Zar JH. Biostatistical Analysis. UK: Pearson Education Publishers; 1999. [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–13. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013;7:51. doi: 10.3389/fnins.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enriori PJ, Evans AE, Sinnayah P, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–94. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Mars M, de Graaf C, de Groot CP, et al. Fasting leptin and appetite responses induced by a 4-day 65%-energy-restricted diet. Int J Obes (Lond) 2006;30:122–28. doi: 10.1038/sj.ijo.0803070. [DOI] [PubMed] [Google Scholar]
- 27.Numao S, Katayama Y, Hayashi Y, et al. Influence of acute aerobic exercise on adiponectin oligomer concentrations in middle-age abdominally obese men. Metabolism. 2011;60:186–94. doi: 10.1016/j.metabol.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Saunders TJ, Palombella A, McGuire KA, et al. Acute exercise increases adiponectin levels in abdominally obese men. J Nutr Metab. 2012;2012:148729. doi: 10.1155/2012/148729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 30.Lara-Castro C, Doud EC, Tapia PC, et al. Adiponectin multimers and metabolic syndrome traits: relative adiponectin resistance in African Americans. Obesity (Silver Spring) 2008;16:2616–23. doi: 10.1038/oby.2008.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Retnakaran R, Hanley AJ, Connelly PW, et al. Low serum levels of high-molecular weight adiponectin in Indo-Asian women during pregnancy: evidence of ethnic variation in adiponectin isoform distribution. Diabetes Care. 2006;29:1377–79. doi: 10.2337/dc06-0413. [DOI] [PubMed] [Google Scholar]
- 32.von Eynatten M, Hamann A, Twardella D, et al. Atherogenic dyslipidaemia but not total- and high-molecular weight adiponectin are associated with the prognostic outcome in patients with coronary heart disease. Eur Heart J. 2008;29:1307–15. doi: 10.1093/eurheartj/ehn135. [DOI] [PubMed] [Google Scholar]
- 33.Ebong IA, Goff DC, Jr, Rodriguez CJ, et al. Association of lipids with incident heart failure among adults with and without diabetes mellitus: Multiethnic study of atherosclerosis. Circ Heart Fail. 2013;6:371–78. doi: 10.1161/CIRCHEARTFAILURE.112.000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gokal R, Shillito L, Maharaj SR. Positive impact of yoga and pranayam on obesity, hypertension, blood sugar, and cholesterol: a pilot assessment. J Altern Complement Med. 2007;13:1056–57. doi: 10.1089/acm.2007.0679. [DOI] [PubMed] [Google Scholar]
- 35.Barnard ND, Scialli AR, Turner-McGrievy G, et al. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am J Med. 2005;118:991–97. doi: 10.1016/j.amjmed.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 36.Slavícek J, Kittnar O, Fraser GE, et al. Lifestyle decreases risk factors for cardiovascular diseases. Cent Eur J Public Health. 2008;16:161–64. doi: 10.21101/cejph.a3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biolo G, Ciocchi B, Stulle M, et al. Calorie restriction accelerates the catabolism of lean body mass during 2 wk of bed rest. Am J Clin Nutr. 2007;86:366–72. doi: 10.1093/ajcn/86.2.366. [DOI] [PubMed] [Google Scholar]
- 38.Siani V, Mohamed EI, Maiolo C, et al. Body composition analysis for healthy Italian vegetarians. Acta Diabetol. 2003;10:S297–98. doi: 10.1007/s00592-003-0091-1. [DOI] [PubMed] [Google Scholar]
- 39.Dhume RR, Dhume RA. A comparative study of the driving effects of dextroamphetamine and yogic meditation on muscle control for the performance of balance on balance board. Indian J Physiol Pharmacol. 1991;35:191–94. [PubMed] [Google Scholar]
- 40.Yang Y, Verkuilen JV, Rosengren KS, et al. Effect of combined Taiji and Qigong training on balance mechanisms: a randomized controlled trial of older adults. Med Sci Monit. 2007;13(8):CR339–48. [PubMed] [Google Scholar]
- 41.Batcho CS, Stoquart G, Thonnard JL. Brisk walking can promote functional recovery in chronic stroke patients. J Rehabil Med. 2013;45:854–59. doi: 10.2340/16501977-1211. [DOI] [PubMed] [Google Scholar]
- 42.Hue O, Simoneau M, Marcotte J, et al. Body weight is a strong predictor of postural stability. Gait Posture. 2007;26:32–38. doi: 10.1016/j.gaitpost.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Mason C, Brien SE, Craig CL, et al. Musculoskeletal fitness and weight gain in Canada. Med Sci Sports Exerc. 2007;39:38–43. doi: 10.1249/01.mss.0000240325.46523.cf. [DOI] [PubMed] [Google Scholar]
- 44.Nathan PW, Smith MC, Deacon P. The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain. 1990;113:303–24. doi: 10.1093/brain/113.2.303. [DOI] [PubMed] [Google Scholar]
- 45.Seo DY, Lee S, Figueroa A, et al. Yoga training improves metabolic parameters in obese boys. Korean J Physiol Pharmacol. 2012;16:175–80. doi: 10.4196/kjpp.2012.16.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]