Abstract
The aim of this study was to investigate the possible mechanisms of action of Tripterygium glycosides (TG) in the treatment of ankylosing spondylitis (AS). In total, 20 patients with active AS received treatment with 20 mg TG tablet (TGT) 3 times per day for 6 weeks. In addition, 20 healthy age- and gender-matched individuals were recruited as the control group. The efficacy measures included the Bath AS disease activity index (BASDAI), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels. The serum interleukin (IL)-17 levels were measured using ELISA. The expression of CD4+CD25+CD127low regulatory T cells (Tregs) in the peripheral blood was evaluated by flow cytometry. A bivariate correlation analysis was used to determine the association of IL-17 levels with BASDAI, ESR, CRP and CD4+CD25+CD127low Tregs in AS patients. Prior to treatment, the BASDAI, ESR and CRP levels in AS patients were found to be elevated compared to those in healthy controls and were significantly reduced following TGT treatment (P<0.05, P<0.05 and P<0.05, respectively). Prior to treatment, the AS patients exhibited significantly higher IL-17 levels compared to those in healthy controls (P<0.05). Following TGT treatment, the IL-17 levels were significantly reduced in AS patients (P<0.01) but were not significantly different in the control subjects (P>0.05). In addition, prior to treatment, the ratio of CD4+CD25+CD127low Tregs in AS patients was significantly lower compared to that in healthy controls (P<0.05) and it was significantly increased following TGT treatment (P<0.05). The correlation analysis between the BASDAI, ESR or CRP levels and IL-17 revealed a positive linear correlation (P<0.001, P<0.001 and P<0.01, respectively), whereas CD4+CD25+CD127low Tregs were found to be negatively correlated with IL-17 (P<0.01). In conclusion, TGT is efficient for the treatment of AS patients and its mechanism of action may be correlated with the upregulation of CD4+CD25+CD127low Tregs and the downregulation of IL-17 in the peripheral blood.
Keywords: Tripterygium glycosides, ankylosing spondylitis, interleukin 17, CD4+CD25+CD127low regulatory T cells
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease that primarily affects the axial skeleton, peripheral joints, attachments of ligaments and entheses. The main clinical characteristic of AS is inflammatory lower back pain and, over time, certain patients develop spinal immobility and ankylosis (1,2). AS exhibits a male predominance (3).
Despite our longstanding knowledge of the familial associations of AS, the underlying pathogenetic mechanism has not been fully elucidated. A number of cytokines were shown to play a critical role in the pathogenesis of AS. Interleukin (IL)-17 and IL-23 are cytokines associated with inflammation, autoimmunity and defense against bacteria. The elevated levels of IL-17 and IL-23 in AS patients reflect their critical roles in the pathogenesis of AS (4,5). It was previously demonstrated that the carriers of single-nucleotide polymorphisms of the IL-23 receptor in the Chinese Han population are susceptible to AS (6), whereas other studies reported conflicting results (7,8). The pathogenetic mechanism underlying the development of AS has not been fully elucidated. Genetic findings may provide novel insight into the etiology of AS. However, broader validation in different populations and further investigation of the underlying mechanisms are required.
AS is significantly associated with the human leukocyte antigen (HLA)-B27 gene (9); however, HLA-B27 accounts for only 16% of the genetic variability in AS (10). Compared to healthy subjects, AS patients exhibit increased expression of circulating CD4+ and CD8+ T cells (11,12); therefore, the abnormal expression of these T cells may also be associated with AS. Furthermore, the pathogenesis of AS may be correlated with CD4+CD25+CD127low regulatory T cells (Tregs).
The Tripterygium glycosides tablet (TGT) comprises wilforlide A (C30H46O3), extracted from Tripterygium wilfordii, which is a traditional medicinal plant that has been used in China for several years for the long-term treatment of inflammatory conditions, such as rheumatoid arthritis, various skin disorders, chronic nephritis and AS. In the present study, 20 patients with active AS were selected (AS group) and treated with TGT (20 mg, 3 times/day) for 6 weeks. A total of 20 healthy age- and gender-matched volunteers were recruited as the control group. To investigate the possible mechanisms of action of TGT in the treatment of AS, IL-17 and CD4+CD25+CD127low Tregs were measured in the peripheral blood of patients with AS. Our results demonstrated that TGT was effective in improving the signs and symptoms of patients with AS, possibly through the upregulation of CD4+CD25+CD127low Treg and the downregulation of IL-17 in the peripheral blood.
Materials and methods
Ethics and study populations
Ethical approval for this study was obtained from the Human Research Ethics Committee of the Affiliated Hospital of Nanjing University of Traditional Chinese Medicine (no. 2010NL_085_02) and the participants provided written informed consent. TGT was purchased from Deeng Pharmaceutical Company (product no. 0802102; Zhejiang DND Pharmaceutical Co., Ltd, Shaoxing, China).
Between January, 2010 and January, 2013, a total of 20 AS patients and 20 healthy age- and gender-matched controls were recruited in our hospital. All the patients and controls were Han Chinese. The AS patients were treated with 20 mg TGT 3 times per day for 6 weeks, without any additional treatment. The diagnosis of AS was confirmed by experienced rheumatologists, according to the modified New York criteria (13). Subjects with rheumatoid arthritis, inflammatory bowel disease, psoriasis or other autoimmune diseases, were excluded from the study. Subjects under treatment with non-steroidal anti-inflammatory drugs (NSAIDs) or any other medication were also excluded from the study. The patients were followed up for 6 weeks. Peripheral blood samples (2 ml) were obtained from the patients prior to and after TGT treatment for 6 weeks. The samples were collected in heparin-containing tubes and immediately stored at −70°C until further processing.
Basic data acquisition
The Bath AS disease activity index (BASDAI), the most widely used tool for the assessment of the AS functional status and disease activity, was calculated for all the AS patients using questionnaires (13). Serum assays for erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were performed on AS patients prior to and after TGT treatment (normal range of ESR, 0–15 mm/h; normal range of CRP, 0–10 mg/l).
Serum IL-17 activity
The activity of serum IL-17 was measured using a commercial human IL-17 ELISA kit (Bender Med Systems, San Diego, CA, USA) according to the manufacturer’s instructions. The IL-17 levels in the serum of the AS patients prior to and after TGT treatment were compared to those of the control group.
Determination of CD4+CD25+CD127low Treg ratio by flow cytometry
A total of 10 μl mouse anti-human CD4-fluorescein isothiocyanate, 20 μl mouse anti-human phycoerythrin-labeled CD25 and 10 μl phycoerythrin and cyanine-5-labeled CD127 monoclonal antibodies (all from Beckman Coulter, Miami, FL, USA) were added to 100 μl of peripheral blood. Following incubation at room temperature in the dark for 15–30 min, 1 ml erythrocyte lysing solution was added to the samples and incubated under the same conditions for 15–20 min. The cells were washed with 2 ml phosphate-buffered saline (PBS) and resuspended in 400 μl PBS. The cells were then analyzed using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Statistical analyses were performed using the statistical SPSS software, version 14.0 (SPSS Inc., Chicago, IL, USA). The results are expressed as means ± SD. P<0.05 was considered to indicate statistically significant differences. A bivariate correlation analysis was used to determine the association of IL-17 levels with BASDAI, ESR, CRP and CD4+CD25+CD127low Treg in AS patients.
Results
Clinical characteristics
BASDAI, ESR and CRP levels for the AS patients prior to and after TGT treatment are presented in Table I. Prior to treatment, the BASDAI in 17 of the patients was >50 mm, with an even higher average score in all these AS patients (51.94±11.57) compared to the normal range. After 6 weeks of TGT treatment, the BASDAI score was significantly decreased (24.47±13.01).
Table I.
BASDAI, ESR and CRP levels in AS patients prior to and after TGT treatment (n=20).
| Efficacy measures | Prior to treatment (mean ± SD) | After treatment (mean ± SD) |
|---|---|---|
| BASDAI | 51.94±11.57 | 24.47±13.01a |
| ESR (mm/h) | 31.18±15.63 | 15.87±14.65a |
| CRP (μg/ml) | 16.30±11.28 | 6.10±6.88a |
P<0.05 compared to the AS group prior to TGT treatment.
BASDAI, Bath AS disease activity index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AS, ankylosing spondylitis; TGT, Tripterygium glycosides tablet; SD, standard deviation.
Prior to treatment, the ESR and CRP levels were higher compared to those in healthy controls. After 6 weeks of TGT treatment, the levels of ESR and CRP were significantly decreased to within the normal range.
IL level
The IL-17 level in the healthy control group was within the normal range (1.82±0.81 pg/ml). Prior to TGT treatment, the IL-17 level in patients with active AS was significantly higher compared to that in healthy controls (P<0.05). The IL-17 level in 18 patients with active AS was higher than the normal range, and in 5 of these patients, the level was significantly higher (>10 times the normal range). After 6 weeks of TGT treatment, the IL-17 level in 16 of the AS patients was decreased and in 5 of these patients it returned within the normal range. The decrease in the IL-17 level in AS patients after TGT treatment was found to be statistically significant (P<0.01). There was no significant difference in the IL-17 level between the AS patients after TGT treatment and that in healthy controls (P>0.05; Fig. 1).
Figure 1.
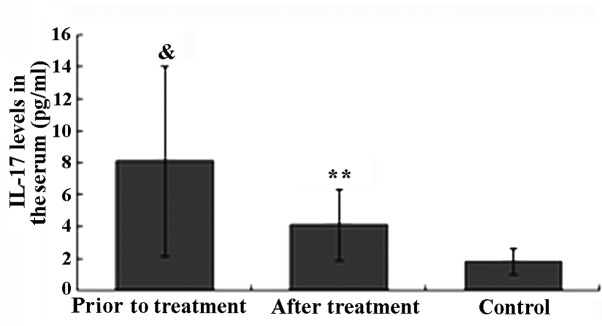
Interleukin (IL)-17 levels prior to and after Tripterygium glycosides tablet (TGT) treatment in ankylosing spondylitis (AS) patients and healthy individuals (mean ± SD). &P<0.05 compared to the control; **P<0.05 compared to the AS group prior to TGT treatment. Treg, regulatory T cell.
Ratio of CD4+CD25+CD127low Tregs
As shown in Fig. 2, the ratio of CD4+CD25+CD127low Tregs in the healthy control group was within the normal range (58.6±10.2%). Prior to treatment, the ratio of CD4+CD25+CD127low Tregs in patients with active AS was significantly lower compared to that in healthy controls (P<0.05). After TGT treatment, the ratio of CD4+CD25+CD127low Tregs in the serum of AS patients was significantly increased from 40.1±17.5 to 49.6±16.0% (P<0.05).
Figure 2.
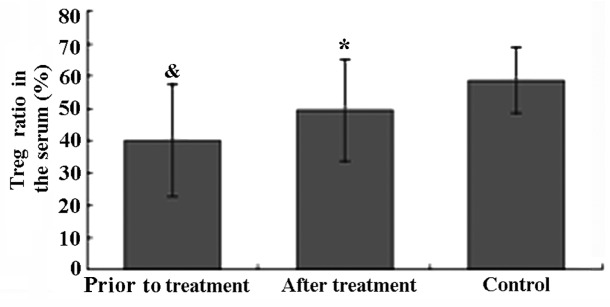
Ratio of CD4+CD25+CD127low regulatory T cells (Tregs) in the serum of ankylosing spondylitis (AS) patients prior to and after Tripterygium glycosides tablet (TGT) treatment. &P<0.05 compared to the control; *P<0.05 compared to the AS group prior to TGT treatment.
Association of IL-17 level with BASDAI, ESR, CRP and CD4+CD25+CD127low Tregs in AS patients
Prior to TGT treatment, the BASDAI and IL-17 levels were higher compared to those in healthy individuals and were decreased after 6 weeks of TGT treatment. The correlation analysis revealed that 16 patients exhibiting a decrease in the IL-17 levels also exhibited a significantly decreased BASDAI score (P<0.01). By computing the Pearson’s correlation coefficient, we observed a significantly positive correlation between BASDAI and IL-17 (r=0.960 and P<0.001).
Prior to TGT treatment, the ESR and CRP levels in AS patients were higher compared to those in healthy controls and were decreased after 6 weeks of TGT treatment. The Pearson’s correlation coefficient demonstrated that ESR and CRP were significantly correlated with IL-17 (r=0.826 and P<0.001; and r=0.754 and P<0.01, respectively).
The ratio of CD4+CD25+CD127low Tregs was significantly increased following TGT treatment in AS patients and the Pearson’s correlation coefficient demonstrated that CD4+CD25+CD127low Tregs were significantly negatively correlated with IL-17 (r=−0.739 and P<0.01).
Discussion
AS is a chronic inflammation of the sacroiliac joints, spine and peripheral joints. NSAIDs are currently the first-line treatment for the pain and stiffness associated with AS. However, there are significant side effects associated with NSAIDs, such as injury to the gastrointestinal tract. When treatment with NSAIDs is not sufficient, second-line medications, occasionally refered to as disease-modifying antirheumatic drugs, including sulfasalazine, methotrexate and corticosteroids, may be used. Tumour necrosis factor (TNF) blockers, including Remicade, Enbrel and Humira, recently emerged as promising medications for the treatment of AS. However, TNF blockers are also associated with severe side effects, including reactivation of latent tuberculosis and neurological problems (14).
TGT, a product derived from traditional Chinese medicinal plants, has been used to treat inflammatory conditions, such as rheumatoid arthritis, various skin disorders, chronic nephritis and AS in China. Over the last few years, TGT has been used for the treatment of patients with active AS, with good results. In the present study, the calculation of BASDAI and blood tests for CRP and ESR in AS patients demonstrated the high efficiency of TGT in the treatment of AS. However, the mechanism of action of TGT has not been clearly determined. To elucidate the mechanisms underlying the action of TGT in the treatment of AS, IL-17 and CD4+CD25+CD127low Tregs were measured in the peripheral blood of AS patients prior to and after TGT treatment.
IL-17, as an inflammatory cytokine, is closely correlated with the development of autoimmune diseases (15). Wang et al (16) reported significantly higher IL-17 levels in patients with active AS compared to those in healthy individuals and a significantly positive association between IL-17 and BASDAI, Bath ankylosing spondylitis metrology index, Bath ankylosing spondylitis functional index, ESR or CRP. We also observed that the high expression of IL-17 in patients with active AS is correlated with disease activity and the level of IL-17 in the peripheral blood is closely correlated with ESR and CRP levels. The level of IL-17 in AS patients was significantly decreased after TGT treatment for 6 weeks (P<0.01). These results demonstrated that TGT significantly inhibited IL-17 expression in patients with active AS and was associated with an improvement in other laboratory parameters.
Tregs, as major suppressors of the immune system, exhibit a decreased prevalence in the blood of AS patients, indicating that their absence may contribute to the pathogenesis of AS (17). In the present study, we also investigated the prevalence of CD4+CD25+CD127low Tregs in the peripheral blood of AS patients and found that their number was significantly lower compared to that in healthy individuals, whereas it was significantly increased after TGT treatment, demonstrating the alteration of the body’s immune response through T-cell activation. Furthermore, the enhanced immune inhibition was correlated with the decrease in IL-17 levels.
Overall, these results suggest that TGT treatment effectively improves the signs and symptoms of patients with AS, possibly through the upregulation of CD4+CD25+CD127low Tregs and the downregulation of IL-17 in the peripheral blood. However, the mechanism of action of TGT in AS patients has not been clearly determined and requires further investigations. Our findings also demonstrated that IL-17 may be a useful index for assessing the AS activity and the treatment efficacy, which requires validation by further clinical studies.
Acknowledgements
This study was supported by the Fund for the Talents in Traditional Chinese Medicine of Jiangsu province (grant no. LJ200907) and the Jiangsu Province Administration of Traditional Chinese Medicine (grant no. LZ11014).
References
- 1.Rolle AS, Zimmermann B, Poon SH. Etanercept-induced Henoch-Schönlein purpura in a patient with ankylosing spondylitis. J Clin Rheumatol. 2013;19:90–93. doi: 10.1097/RHU.0b013e3182863027. [DOI] [PubMed] [Google Scholar]
- 2.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 3.Calin A, Brophy S, Blake D. Impact of sex on inheritance of ankylosing spondylitis: a cohort study. Lancet. 1999;354:1687–1690. doi: 10.1016/S0140-6736(99)03219-5. [DOI] [PubMed] [Google Scholar]
- 4.Wendling D, Cedoz JP, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304–305. doi: 10.1016/j.jbspin.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Mei Y, Pan F, Gao J, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–273. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Huang J, Lin Z, et al. Single-nucleotide polymorphisms and expression of IL23R in Chinese ankylosing spondylitis patients. Rheumatol Int. 2010;30:955–959. doi: 10.1007/s00296-009-1085-2. [DOI] [PubMed] [Google Scholar]
- 7.Davidson SI, Wu X, Liu Y, et al. Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 2009;60:3263–3268. doi: 10.1002/art.24933. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Zhang X, Li J, Wang Y. Associations of IL-23R polymorphisms with ankylosing spondylitis in East Asian population: a new case-control study and a meta-analysis. Int J Immunogenet. 2012;39:126–130. doi: 10.1111/j.1744-313X.2011.01067.x. [DOI] [PubMed] [Google Scholar]
- 9.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 10.Khan MA, Ball EJ. Genetic aspects of ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2002;16:675–690. [PubMed] [Google Scholar]
- 11.Duftner C, Goldberger C, Falkenbach A, et al. Prevalence, clinical relevance and characterization of circulating cytotoxic CD4+CD28−T cells in ankylosing spondylitis. Arthritis Res Ther. 2003;5:R292–R300. doi: 10.1186/ar793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schirmer M, Goldberger C, Würzner R, et al. Circulating cytotoxic CD8+CD28−T cells in ankylosing spondylitis. Arthritis Res. 2002;4:71–76. doi: 10.1186/ar386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 14.Murdaca G, Colombo BM, Puppo F. Anti-TNF-alpha inhibitors: a new therapeutic approach for inflammatory immune-mediated diseases: an update upon efficacy and adverse events. Int J Immunopathol Pharmacol. 2009;22:557–565. doi: 10.1177/039463200902200301. [DOI] [PubMed] [Google Scholar]
- 15.Zhu S, Qian Y. IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci (Lond) 2012;122:487–511. doi: 10.1042/CS20110496. [DOI] [PubMed] [Google Scholar]
- 16.Wang XW, Lin ZM, Liao ZT, Wei QJ, Jiang YJ, Gu JR. The study on expression of IL-23 and IL-17 in ankylosing spondylitis. Chin J Immunol. 2009;25:266–270. (In Chinese) [Google Scholar]
- 17.Wu Y, Ren M, Yang R, et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res Ther. 2011;13:R29. doi: 10.1186/ar3257. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


