Abstract
Serum magnesium (Mg) levels are closely controlled through a variety of Mg transporters and ionic channels during physiological conditions. These levels have been shown to increase during exercise. However, the effect of Mg transporter expression during exercise remains to be determined. The purpose of this study was to examine the gene expression of SLC41A1, a Na+/Mg2+ exchanger, during exercise. In the present study, male C57BL/6JNarl mice (n=16, 8 weeks old) were subjected to 3 h forced exercise on a treadmill. The mice in the control and Mg groups were injected with saline and Mg (MgSO4, 90 mg/kg, intraperitoneal), respectively. Blood samples were obtained at three time points: prior to, following and 24 h after exercise. The gene expression levels of SLC41A1 were significantly downregulated to 23.6±4.6 and 12.6±10.2% following exercise in the control and Mg groups, respectively. The expression levels returned to the basal levels 24 h after exercise in the two groups and there was no significant difference found between the two groups. The downregulated role of SLC41A1 expression and its interaction with the Mg status in exercise requires further investigation.
Keywords: exercise, magnesium, SLC41A1, Na+/Mg2+ exchanger
Introduction
Magnesium (Mg) is an essential mineral and cofactor in >300 enzymatic reactions required for numerous structures and functions in the human body (1). Homeostatic serum or plasma Mg concentrations are closely controlled and maintained, ensuring the appropriate activities for metabolism, neuronal excitability and muscle contraction through a variety of Mg transporters and ionic channels (2). Numerous Mg-sensitive genes, based on molecular and functional characterizations, are responsible for this homeostatic mechanism. In addition, Mg-sensitive gene expression has been demonstrated to markedly fluctuate during physiological and pathological events (2,3). However, the mechanism responsible for regulating plasma Mg concentrations remains to be determined.
Previous studies have demonstrated that Mg deficiency reduces exercise performance and endurance (1,4). Furthermore, Mg supplements have been reported to improve athletic performance, and a short-term, high-intensity exercise has been shown to increase plasma Mg concentrations (4). Numerous studies have been conducted on the beneficial effects of Mg in exercise (4–6), but the associations between exercise and the gene expression of Mg-sensitive genes have not been studied. The solute carrier (SLC) family 41 member A1 (SLC41A1) protein was first identified by Wabakken et al (7), and then validated as a Mg transporter by Goytain and Quamme (8). Findings of previous studies have identified the SLC41A1 protein as a candidate Mg transporter and confirmed that it most likely mediates the Mg efflux as a Na+/Mg2+ exchanger (9–11). Several Mg-sensitive genes have been identified thus far. However, the extent to which transporter genes fluctuate in the blood cells during exercise is unknown. In the present study, the role of SLC41A1 in an animal model was investigated during exercise. The plasma Mg levels are well-known to increase during exercise, but the fluctuation of SLC41A1, a Na+/Mg2+ exchanger, expression during exercise has yet to be established.
The aim of the study was to investigate the expression of SLC41A1 in mice during treadmill exercise. Blood samples were obtained at specific time-points and examined by quantitative polymerase chain reaction (qPCR).
Materials and methods
Animals
Male C57BL/6JNarl mice (n=16, 8 weeks old) were used in the study, and permission was obtained from the Animal Ethics Committee of Taichung Veterans General Hospital (La-98593; Taichung, Taiwan). The blood samples (200 μl) were collected from the mice using retro-orbital sinus blood collection. It is generally accepted that withdrawing a blood volume within 10% of the body weight in an average 25-g mouse is safe. Following the collection of the first blood sample, the mice remained under the same conditions for 7 days. Subsequent to this period, eight mice were intraperitoneally injected with saline and these mice comprised the control group, whereas another eight mice received intraperitoneal injection of magnesium sulfate (MgSO4, 90 mg/kg) and served as the Mg group.
The treadmill was equipped with wire loops and retention sensors at one end of the belt in order for a mild electric shock to be delivered to encourage the animals to run at the speed required for the experiment. Mice were shocked at the lowest level, 0.6 μA, with an inter-pulse interval of <2 sec. Each mouse was forced to exercise for 3 h at a speed of 15 m/min, then allowed to recover for an additional 24 h. Each mouse was allocated a rest period of 1 min and 30 sec every 30 min during the treadmill exercise (10).
RNA
The blood samples were lysed and the RNA was purified immediately following collection to prevent the blood from coagulating and the RNA from undergoing degradation, ensuring accurate experimental data. Total RNA was isolated from the mice blood samples using the PureLink™ RNA Mini kit (Ambion®, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA integrity assessment was performed based on the RNA 6000 Nano LabChip (Agilent 2100 bioanalyzer; Agilent, Santa Clara, CA, USA). RNA (1 μg) was reverse-transcribed using SuperScript® III First-Strand Synthesis SuperMix for qPCR (Invitrogen, Life Technologies).
Semiquantitative determination of the SLC41A1 gene expression
Semiquantitative determination of the SLC41A1 gene expression in blood cells was carried out according to the method described by Kolisek et al (9). The expression levels of SLC41A1 were determined by the SYBR-Green based qRT-PCR (SYBR® Green Real-time PCR Master Mixes; Life Technologies) with specific primers for mouse SLC41A1 (NM_173854; forward, 5′-TTGGACGCTCGCCTTGCCTG-3′; and reverse, 5′-TGGTGTGGAACACCTGCGCC-3′) and normalized to the reference gene, β-actin. A customized oligonucleotide was synthesized by Invitrogen Life Technologies. Amplifications were performed using the StepOne Real-time PCR System (Applied Biosystems, Life Technologies). The PCR conditions for SLC41A1 were: An initial 95°C for 10 min followed by 40 cycles of 95°C for 30 sec and 60°C for 1 min 30 sec, and a melting curve analysis with 95°C for 15 sec and 60°C 1 min, followed by a +0.3°C to 95°C temperature change with 15 sec at 95°C as the final step. The samples were assayed in triplicate and the gene expression levels were determined based on the ΔΔCt method for relative quantification, which includes efficiency correction, inter- and intra-assay validation and enables the use of the reference gene, β-actin (12).
Statistical analysis
Data are presented as means ± SEM. The differences between and within the groups were evaluated using the Mann-Whitney test and the Wilcoxon signed-ranks test, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
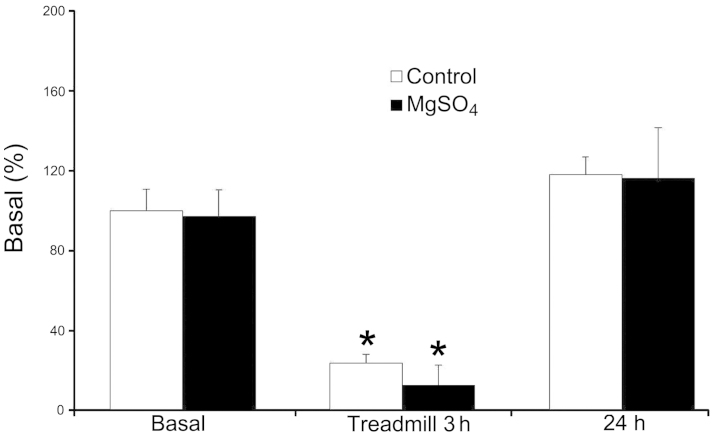
The SLC41A1 gene expression levels were 100.0±10.6 and 96.9±13.7% in the control and Mg groups, respectively (Fig. 1). There was no significant difference between the two groups. After a 3-h treadmill exercise, the mean gene expression levels of SLC41A1 were 23.6±4.6 and 12.6±10.2% in the control and Mg groups, respectively (Fig. 1). There was a significant downregulation of SLC41A1 gene expression following exercise when compared with prior to exercise in the two groups.
Figure 1.
Blood SLC41A1 gene expression prior and subsequent to exercise and 24 h after exercise. The SLC41A1 mRNA expression levels were quantified by qPCR analysis and normalized to the reference gene, β-actin, expression. Data are presented as means + SEM (n=8).*P<0.05 vs. the basal levels within the group (the Wilcoxon signed-ranks test). SLC41A1, solute carrier family 41 member A1; qPCR, quantitative polymerase chain reaction; SEM, standard error of the mean.
There was a 76.4% decrease in the expression levels of SLC41A1 in the control group and a 87.0% decrease in the Mg group as compared to the basal levels, resepectively. However, this difference was not significant. After 24 h, the gene expression levels of SLC41A1 were returned to 118.1±9.0 and 116.1±25.5% in the control and Mg groups, respectively.
Discussion
In the present investigation, the SLC41A1 gene expression levels were significantly downregulated following exercise, decreasing between 76 and 87%, but then returning to the basal levels 24 h after exercise. The mice had much lower SLC41A1 gene expression levels in the Mg group than the control group following exercise, but this was not statistically significant. Thus, the supplementation of Mg may lead to a decreased SLC41A1 gene expression following exercise. By contrast, this may be attributed to small sample sizes or individual differences among the mice in the present study.
The data show that the SLC41A1 gene was downregulated in the blood cells of mice after 3 h of exercise. Thus, SLC41A1 gene expression appears to be involved in the physiological response to exercise. Recently, Nestler et al (13) demonstrated that SLC41A1 gene expression is correlated to blood pressure and Mg homeostasis in pregnant women. A downregulation of SLC41A1 gene expression was interpreted as Mg deficiency in leukocytes during pregnancy. Although the interpretation remains speculative, the data of the present study are corresponds with the hypothesis of Nestler et al (13). The hemodynamic responses of the heart rate and blood pressure to exercise have been extensively studied previously (4). An exaggerated blood pressure response during exercise has been considered to be an indicator of the exercise performance or cardiovascular risks. Therefore, a 70–80% downregulation of SLC41A1 gene expression and highly elevated blood pressure during exercise appears to be parallel and acceptable. A possible explanation for the downregulated expression of the SLC41A1 gene during exercise could be that a decreased SLC41A1 expression in the blood is an obligatory component of the metabolic response to the immediate demands of exercise. The Mg content in leukocytes is higher than that of mononuclear blood cells, which may represent the downregulation of SLC41A1 expression in blood. In our previous studies, plasma Mg concentrations increase in various treadmill exercise modes (5,6). Thus far, it may be concluded that the SLC41A1 gene expression is associated with blood pressure and blood Mg content. However, whether SLC41A1 expression is Mg-dependent remains unclear.
The rapid return to the basal levels within 24 h after exercise implies that the SCL41A1 gene may be a fast-acting gene that is highly responsive to exercise in mice. However, the factors that facilitate Mg transportation also do not rely solely on the SLC41A1 gene, and there are at least nine Mg-sensitive genes (CNNM2, TRPM6, TRPM7, N33, SLC41A1, SLC41A2, SLC41A3, MagT1 and NIPA1) that have been shown to be involved in manipulating intra- and extracellular Mg transportation (2). In the present study, the SLC41A1 gene was downregulated, resulting in an increase and then decrease of Mg levels in the blood during the treadmill exercise. Although just one gene was analyzed in the study, the effects of other Mg transporter genes in response to exercise require further investigation.
Thus, we hypothesize that a downregulated SLC41A1 gene by means of non-exercise or Mg supplementation leads to an increase of Mg in the blood that could confer benefits similar to those observed with exercise, as consumption of various Mg supplements by Mg-deficient subjects is known to have beneficial effects (1–4). Mg is also widely used in the treatment of diseases, including but not limited to, asthma, heart disease, blood clots, hypertension, osteoporosis and hypoglycemia. Determining whether the part-knockdown of the SLC41A1 gene in in vitro or animal models is likely to lead to the similar effects as achieved by consumption of Mg supplements is useful. Future investigations should therefore be conducted to determine whether these similar effects exist.
The findings of the present study suggest that an increased SLC41A1 expression leads to the excess transportation of Mg out of the blood, which subsequently results in Mg deficiency. Mg-deficient mice could be examined in future experiments to determine whether they show higher rates of SLC41A1 expression, and whether the upregulated expression results in decreased plasma Mg levels leading to a poor health status.
There were certain limitations in the present study and several potential confounders. First, subsequent to obtaining an initial 200 μl of blood from the mice, the animals were allowed to rest for a week. However, subsequent to obtaining 200 μl of blood following exercise, the mice were allowed to rest for 24 h prior to obtaining the third blood sample. The total blood volume in a mouse weighing 25 g is <2.0 ml. Relatively large amounts of blood (>400 μl) collected in a short period may have also affected the SLC41A1 gene expression in the blood. In addition, plasma or serum Mg levels were not determined, although results of previous experiments have shown that plasma Mg levels in the blood rise ~20% following exercise (5,6). Furthermore, the SLC41A1 gene expression may vary in different parts of the body. Although the plasma SLC41A1 gene expression was downregulated, the gene expression in the kidney or small intestine may be different, and even within the kidney the SLC41A1 gene expression may vary in the cortex or medulla. Due to the potentially high variability in SLC41A1 gene in different parts of the body, the SLC41A1 gene expression in blood may therefore not be completely reliable without further investigation.
In conclusion, the SLC41A1 gene expression in mice was downregulated following exercise and returned to the basal levels within 24 h. It is known that Mg levels in the blood increase during exercise. Therefore, we hypothesize that the SLC41A1 may play an important role in the interaction of blood Mg levels. However, the exact mechanism of the association between the SLC41A1 gene expression and blood Mg status during exercise remains unclear.
Acknowledgements
This study was supported by Taichung Veterans General Hospital, Taichung, Taiwan, R.O.C (TCVGH-1037313C).
References
- 1.Finstad EW, Newhouse IJ, Lukaski HC, et al. The effects of magnesium supplementation on exercise performance. Med Sci Sports Exerc. 2001;33:493–498. doi: 10.1097/00005768-200103000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Romani AM. Cellular magnesium homeostasis. Arch Biochem Biophys. 2011;512:1–23. doi: 10.1016/j.abb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cinar V, Polat Y, Mogulkoc R, et al. The effect of magnesium supplementation on glucose and insulin levels of tae-kwan-do sportsmen and sedentary subjects. Pak J Pharm Sci. 2008;21:237–240. [PubMed] [Google Scholar]
- 4.Bohl CH, Volpe SL. Magnesium and exercise. Crit Rev Food Sci Nutr. 2002;42:533–563. doi: 10.1080/20024091054247. [DOI] [PubMed] [Google Scholar]
- 5.Chen YJ, Chen HY, Wang MF, et al. Effects of magnesium on exercise performance and plasma glucose and lactate concentrations in rats using a novel blood-sampling technique. Appl Physiol Nutr Metab. 2009;34:1040–1047. doi: 10.1139/H09-105. [DOI] [PubMed] [Google Scholar]
- 6.Cheng SM, Yang LL, Chen SH, et al. Magnesium sulfate enhances exercise performance and manipulates dynamic changes in peripheral glucose utilization. Eur J Appl Physiol. 2010;108:363–369. doi: 10.1007/s00421-009-1235-y. [DOI] [PubMed] [Google Scholar]
- 7.Wabakken T, Rian E, Kveine M, Aasheim HC. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+transporters. Biochem Biophys Res Commun. 2003;306:718–724. doi: 10.1016/s0006-291x(03)01030-1. [DOI] [PubMed] [Google Scholar]
- 8.Goytain A, Quamme GA. Functional characterization of human SLC41A1, a Mg2+transporter with similarity to prokaryotic MgtE Mg2+transporters. Physiol Genomics. 2005;21:337–342. doi: 10.1152/physiolgenomics.00261.2004. [DOI] [PubMed] [Google Scholar]
- 9.Kolisek M, Launay P, Beck A, et al. SLC41A1 is a novel mammalian Mg2+carrier. J Biol Chem. 2008;283:16235–16247. doi: 10.1074/jbc.M707276200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandt T, Song Y, Scharenberg AM, Sahni J. SLC41A1 Mg(2+) transport is regulated via Mg(2+)-dependent endosomal recycling through its N-terminal cytoplasmic domain. Biochem J. 2011;439:129–139. doi: 10.1042/BJ20110807. [DOI] [PubMed] [Google Scholar]
- 11.Kolisek M, Nestler A, Vormann J, Schweigel-Röntgen M. Human gene SLC41A1 encodes for the Na+/Mg(2)+ exchanger. Am J Physiol Cell Physiol. 2012;302:C318–C326. doi: 10.1152/ajpcell.00289.2011. [DOI] [PubMed] [Google Scholar]
- 12.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestler A, Rylander R, Kolisek M, et al. Blood pressure in pregnancy and magnesium sensitive genes. Preg Hyper: An Int J Women’s Card Health. 2014;4:41–45. doi: 10.1016/j.preghy.2013.09.003. [DOI] [PubMed] [Google Scholar]