Abstract
Polyethylene glycol (PEG) is widely utilized in drug delivery and nanotechnology due to its reported “stealth” properties and biocompatibility. It is generally thought that PEGylation allows particulate delivery systems and biomaterials to evade the immune system and thereby prolong circulation lifetimes. However, numerous studies over the past decade have demonstrated that PEGylation causes significant reductions in drug delivery, including enhanced serum protein binding, reduced uptake by target cells, and the elicitation of an immune response that facilitates clearance in vivo. This report reviews some of the extensive literature documenting the detrimental effects of PEGylation, and thereby questions the wisdom behind employing this strategy in drug development.
Keywords: polyethylene glycol, PEG, transfection, DNA, RNA, drug delivery, immunogenicity, stealth
The ultimate goal of pharmaceutical therapy is to administer a therapeutic agent by a route that achieves maximum bioavailability and minimal toxicity. Considering that bioavailability is typically equated with blood levels over time (AUC), it is desirable to evade rapid clearance from the blood, thereby extending circulation times. For relatively low molecule weight drugs (≤ ≈ 40 kDa; e.g., small molecules, oligonucleotides, siRNA) clearance is predominantly through the renal system. Accordingly, conjugation of low molecular weight drugs to albumin or a polymer can be used to increase their effective molecular weight above the threshold for glomerular filtration, which allows the conjugated therapeutic to remain in the circulation for prolonged periods. Large molecular weight (20–50 kDa) polyethylene glycol (PEG) has proven useful for avoiding rapid renal clearance and extending circulation lifetimes [1]. A fundamentally different problem is faced by particulate delivery systems (e.g., nanoparticles) that are too large to be cleared through the kidney. In these cases, the liver is the predominant organ involved in clearance, and scientists have joked that the definition of a nanoparticle is “anything that accumulates in the liver” [2]. It follows that strategies to avoid liver clearance and enhance circulation lifetimes have been pursued, and the attachment of low molecular weight PEG (2–5 kDa) has been demonstrated to extend blood half-life of many nanoparticles [1].
Just like other larger particles, e.g., bacteria, nanoparticles adsorb many different plasma proteins that may interact with the mononuclear phagocyte system (MPS); a process called opsonization. The amount and specificity of these proteins may vary, and depends on the characteristics of the nanoparticle. Opsonization is known to play a major role in clearance of particles as one or more of these proteins may interact with specific receptors on the surface of macrophages and hepatocytes in the liver [3]. It is generally assumed that the mechanism by which PEGylation extends circulation times involves a significant reduction in opsonization, which is responsible for its “stealth” behavior [4–8]. Consistent with this notion, studies have correlated the extent of protein adsorption (as an indicator of opsonization) with half-life in the blood, and measured reduced protein binding to nanoparticles incorporating PEGylated components [8,9]. Indeed, the presence of PEG on the surface of a nanoparticle is thought to mask surface charge (as indicated by a near-neutral zeta potential) and create a hydrophilic barrier that sterically prevents protein adsorption [8,10].
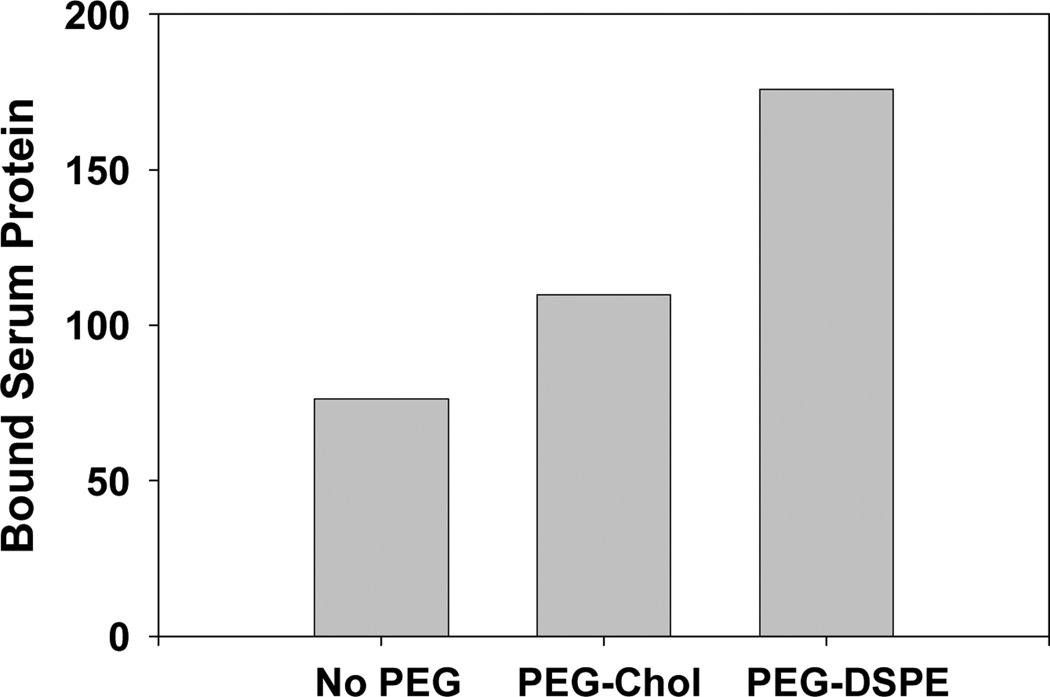
In contrast to the above, some studies have reported that PEGylation does not increase circulation times [1], while other researchers have demonstrated that PEGylation extends circulation half-life but does not reduce protein binding [11,12]. In fact, several studies have reported that PEGylation increases protein adsorption, suggesting that the binding of specific proteins involved in clearance might be preferentially inhibited by PEG [13,14]. Alternatively, it has been suggested that PEG may preferentially bind proteins that function as “dysopsonins”, i.e., proteins that prevent opsonization [11]. Consistent with the studies cited above, Figure 1 shows data from our laboratory indicating that PEGylation of lipoplexes dramatically increases the extent of serum protein binding compared to non-pegylated lipoplexes. Furthermore, our recent publication also demonstrates that small proteins (potentially protein fragments) bind to PEGylated nanoparticles that are not adsorbed to particles lacking PEG [14]. While it has been shown that the density/conformation of PEG molecules on the surface affects protein binding [6,11,15], studies demonstrating enhanced serum protein binding and the adsorption of specific proteins after PEGylation raise significant questions about the ability of PEG to prevent/reduce interactions with serum proteins.
Figure 1.
Serum protein binding to PEGylated (2%) and non-PEGylated lipoplexes. Details can be found in Betker et al. [14].
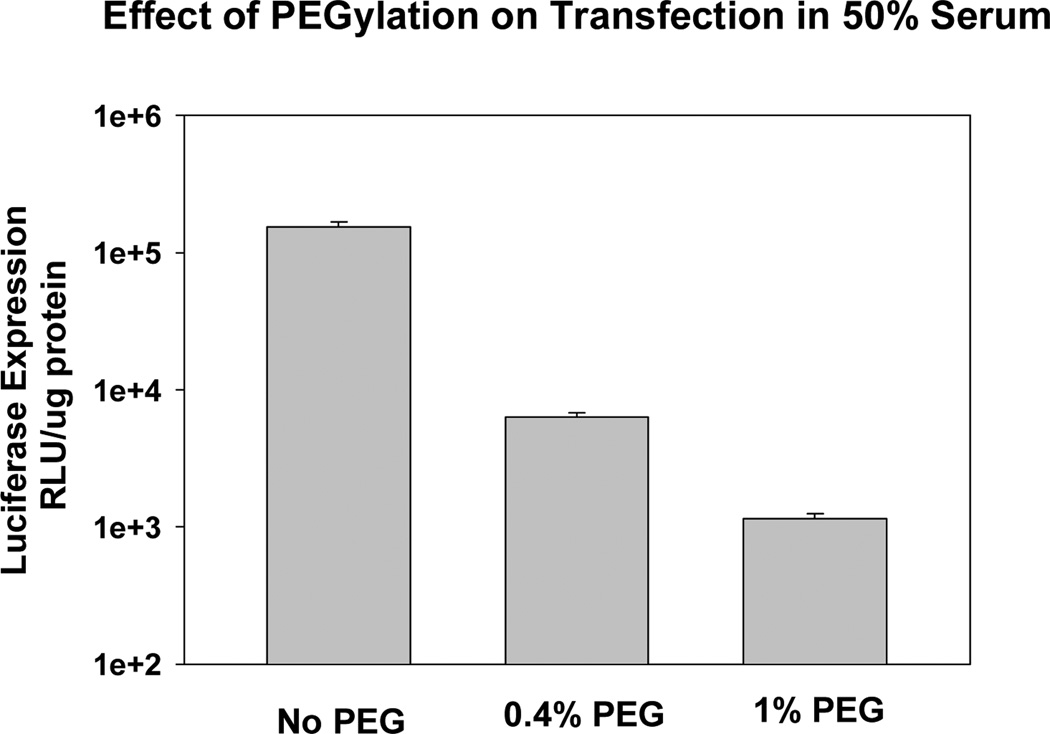
Considering the interaction with MPS and subsequent clearance, many researchers have conducted experiments to determine the extent to which formulation variables alter particle uptake by macrophages in culture. These in vitro experiments have clearly demonstrated that PEGylation can decrease uptake by macrophages, and this effect is thought to be responsible for the ability of PEG to prolong circulation times in vivo [6,16–18]. Enhanced circulation times are also thought to permit greater accumulation in tumors via the Enhanced Permeation and Retention effect (EPR) [19–21]. Intuitively, it makes sense that prolonged circulation times would provide more opportunities for delivery systems to extravasate through “leaky” vasculature. However, as PEGylation reduces interaction with MPS due to its shielding properties, PEGylation would also be expected to inhibit interactions with the tumor cells (e.g., via a receptor) and thereby compromise uptake by the target tissue. Indeed, studies have consistently shown that PEG also inhibits uptake by numerous cell lines [22–25]. While uptake into cells may not be critical for some applications, such as chemotherapeutic agents for which localization to the vicinity of the tumor could be advantageous, therapeutic efficacy of some agents (e.g., RNA, DNA) is dependent on cell uptake and access to intracellular machinery. In this regard, the detrimental effect of PEG is abundantly evident in gene and siRNA delivery studies showing that transfection and silencing rates are progressively decreased with PEGylation [22,26]. In fact, PEGylation levels as low as 0.5% have been shown to significantly reduce transfection [27]. Our experiments are consistent with this observation, and we observe an order of magnitude decrease in transfection at 0.4% PEG, and a > 100-fold decrease at 1% PEG (Fig. 2)! These data demonstrate that therapeutic gene expression in the target tissue may be significantly compromised by PEGylation.
Figure 2.
PEGylation progressively reduces transfection. Details can be found in Xu and Anchordoquy [24].
While it is typically assumed that the observed decrease in transfection is the result of reduced uptake, Harvie et al. [22] showed conclusively that transfection rates were compromised to a greater extent than can be explained by reduced uptake, and suggested that PEG must interfere with intracellular processes that are critical to the transfection process. Subsequent studies have employed various strategies for removing PEG such that it does not compromise delivery to the target tissue. One of the early approaches was to simply shorten the lipid anchor to which the PEG was conjugated, thereby promoting diffusion out of the lipid-based delivery system (“programmed delivery”) [28]. More sophisticated approaches have since been designed whereby the PEG is attached by linkers that are cleaved by specific enzymes relevant to tumors (MMP-sensitive) [29], or by the low pH conditions encountered after cellular uptake [26]. Taken together, these studies indicate that the PEGylated components responsible for prolonged circulation must be efficiently removed to maximize delivery. Clearly, there is a balancing act between employing increased PEG levels in an attempt to enhance deposition via EPR, versus reducing PEG to ensure maximum uptake/delivery to the target tissue. It is also important to recognize that PEG is not biodegradable, and thus the ultimate fate of PEG and potential adverse effects due to its accumulation is unknown [30].
As discussed above, many reports have described results that contradict long-held beliefs with regards to the effects of PEGylation on protein binding and cell uptake. Similarly, it is generally thought that PEG is biologically inert, and therefore it is assumed that PEGylation endows biocompatibility to foreign materials, and suppresses or inhibits the immunogenicity of PEG-conjugates. However, our own findings and those of others stand in stark contrast to this common perception. More specifically, several papers in the last decade reported that an intravenous injection of PEG-conjugates causes a second dose, injected a few days later, to lose its long-circulating characteristics. This phenomenon is referred to as the accelerated blood clearance (ABC) and is observed with PEGylated- proteins, liposomes, micelles, and nanocarriers [31–36]. One of the reasons why the immunogenicity of PEG has not gained much attention is that most studies focus on a potential immune response against the active protein or drug delivery system due to the general belief that PEG is non-immunogenic. Briefly, the ABC phenomenon is thought to result from anti-PEG IgM produced by the spleen in response to a previously-administered dose [37–39]. However, IgM antibodies are unable to directly promote either endocytosis or phagocytosis. It is therefore hypothesized that antibodies generated in response to the initial injection bind to the subsequently-administered dose of PEGylated particles and form a complex with one or more serum components which are then recognized and cleared by MPS due to phagocytosis of Kupffer cells in the liver [38–41].
Because several studies have shown that no memory is established, the immune response seems to be elicited by the innate immune system. The innate immune system is one of the evolutionary older systems able to recognize pathogen specific antigens consisting of repeating structures such as lipopolysaccharides on the cell walls of gram-negative bacteria and can subsequently produce antibodies without help from T-cells [42,43]. Indeed, Li et al. showed that intravenous administration of PEGylated liposomes in beagles only resulted in the ABC phenomenon when the time interval was up to 3 weeks, as no phenomenon was observed when the time interval was prolonged to 4 weeks [44]. This was also confirmed by Isihara et al. who showed that anti-PEG IgM disappeared when there was sufficient time between the first and second injections [45]. Compared to controls, the ABC phenomenon was induced by the first injection and was most apparent at day 7. However, after day 7 the ABC phenomenon attenuated and disappeared at day 28 [45]. Interestingly, the induction of anti-PEG IgM seems to have an inverse relationship with the quantity of the first dose [44,46,47]. In order to induce an antibody response, an optimal amount of TI-2 antigens need to cross-link with B-cells. It is thought that when a high dose (5 µmol/kg) of PEGylated liposomes is administered as a first dose, the density of TI-2 antigens is too high, which causes MZ B-cells to induce immune tolerance or anergy [47,48]. Indeed, Wang et al. found that a low-dose of (0.001 µmol/kg) is the optimal dose to induce the production of anti-PEG IgM [39]. In addition, it has been shown that subsequent receptor signaling is needed to maintain anergy of B-cells. As higher doses lead to prolonged circulation, PEGylated liposomes bind longer to MZ B-cells which might contribute to immune tolerance or anergy as well [47]. It was observed that 5 mol% PEGylated liposomes had the highest clearance rate and hepatic uptake, and both clearance and hepatic uptake were attenuated when the PEG-to-lipid ratio was increased. However, splenic accumulation was similar for all different PEG-to-lipid ratios [46].
In studying the production of antibodies in response to IV administration of PEGylated asparaginase, Armstrong et al. (2007) observed that 46% of patients developed anti-PEG antibodies during therapy which accelerated the clearance of subsequent injections [49]. Even more alarming, the data indicate that 25% of patients had pre-existing anti-PEG antibodies prior to treatment! The authors proposed that the presence of pre-existing anti-PEG antibodies was due to the presence of PEG in many common cosmetic products to which patients had likely been repeatedly exposed [49]. A more recent clinical study has shown that similar responses are elicited upon IM administration [50].
Interestingly, in contrast to PEGylated nanoparticles, soluble “free” PEG does not activate complement in animal models, and studies have shown that PEG must be conjugated to a sufficiently-sized nanoparticle to cause the production of anti-PEG IgM [17,18]. In addition, some studies have shown that methylation of the methoxy group on PEG conjugates to eliminate the anionic group, can dramatically reduce the immune response [51,52]. Furthermore, recent work has demonstrated that accelerated clearance is not observed at high doses of PEGylated liposomes containing cytotoxic drugs due to destruction of the B cells in the spleen responsible for the subsequent antibody response [53,54]. Interestingly, lower doses of the drug exhibited ABC behavior, presumably due to inefficient killing of B cells involved in triggering anti-PEG antibodies.
Although the precise factors that elicit the ABC response have yet to be fully understood, the presence of anti-PEG antibodies in patients would greatly compromise the use of PEGylated delivery systems for therapy. Indeed, the potential for a significant portion (≈ 25%) of patients to have pre-existing anti-PEG antibodies that would stimulate rapid clearance of PEGylated nanoparticles represents a monumental obstacle for the development of stealth delivery systems. While alternative polymers have been investigated as alternatives to PEG, none have proven as useful [1]. As mentioned, the mammalian immune system has evolved to recognize repetitive motifs, therein questioning the potential of polymers to serve as effective materials for systemic drug delivery [55,56]. Clearly, the development of additional strategies for stabilizing delivery systems in blood is greatly needed [2,57–59].
In conclusion, the use of PEGylated components is generally thought to inhibit opsonization and serum protein binding such that nanoparticles and therapeutic proteins exhibit prolonged circulation times. However, some studies have shown minimal, and even negative, effects of PEGylation on both protein binding and circulation half-life. Other published studies have demonstrated that PEGylation reduces uptake and delivery to the target tissues, especially for therapeutic molecules requiring intracellular delivery (e.g., siRNA, genes). Furthermore, it is now well established that the inclusion of PEGylated components on nanoparticles and therapeutic proteins promotes complement activation and the production of anti-PEG antibodies that accelerate clearance upon subsequent injections and compromise therapeutic efficacy. Although a recent review has concluded that the lack of robust methodology for antibody detection does not allow firm conclusions regarding specific antibodies to PEG [60], we feel that the strong reliance of drug delivery technologies on PEGylation is preventing progress toward development and clinical use. In the spirit of several recent reviews questioning the current research trends in drug delivery and nanotechnology [2,61,62], we strongly suggest that the biological interactions with the MPS must be clearly elucidated and alternative strategies to PEGylation must be explored and validated. Furthermore, we feel that the accumulating evidence documenting the detrimental effects of PEG on drug delivery make it imperative that scientists in this field break their dependence on PEGylation, and develop improved approaches for achieving adequate circulation times in order to realize the enormous clinical benefits of targeted drug delivery.
Acknowledgements
Some of the work discussed here was supported by grant #1 RO1EB016378 and #1 RO1GM093287 to TJA.
Footnotes
Conflict of Interest Disclosure
The authors are academic researchers with no financial interest in any of the work described in this manuscript.
References
- 1.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 2.Xu L, Anchordoquy TJ. Drug Delivery Trends in Clinical Trials and Translational Medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. J. Pharm. Sci. 2011;100:38–52. doi: 10.1002/jps.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan X, Scherphof GL, Kamps JAAM. Liposome opsonization. J. Liposome Res. 2005;15:109–139. doi: 10.1081/lpr-64971. [DOI] [PubMed] [Google Scholar]
- 4.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 6.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, et al. ‘Stealth” corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surfaces B: Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 7.Leroux J-C, De Jaeghere F, Anner B, Doelker E, Gurny R. An investigation on the role of plasma and serum opsonins on the internalization of biodegradable poly(D,L-lactic acid) nanoparticles by human monocytes. Life Sci. 1995;57(7):695–703. doi: 10.1016/0024-3205(95)00321-v. [DOI] [PubMed] [Google Scholar]
- 8.Fang C, Shi B, Pei Y-Y, Hong M-H, Wu J, Chen H-Z. In vivo targeting of tumor necrosis factor-α-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur. J. Pharm Sci. 2006;27:27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Semple SC, Chonn A, Cullis PR. Interactions of liposomes and lipid-based carrier systems with blood proteins: relation to clearance behavior in vivo. Adv. Drug Del. Rev. 1998;32:3–17. doi: 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 10.Allen TM. In: Liposomes in the Therapy of Infectious Diseases and Cancer. Lopez-Berestein G, Fidler I, editors. Liss, New York: 1989. pp. 405–415. [Google Scholar]
- 11.Johnstone SA, Masin D, Mayer L, Bally MB. Surface-associated serum proteins inhibit the uptake of phosphatidylserine and poly(ethylene glycol) liposomes by mouse macrophages. Biochim. Biophys. Acta. 2001;1513:25–37. doi: 10.1016/s0005-2736(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 12.Dos Santos N, Allen C, Doppen A-M, Anantha M, Cox KAK, Gallagher RC, et al. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: relating plasma circulation lifetimes to protein binding. Biochim. Biophys. Acta. 2007;1768:1367–1377. doi: 10.1016/j.bbamem.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Sroda K, Rydlewski J, Langner M, Kozubek A, Grzybek M, Sikorski AF. Repeated injections of PEG-PE liposomes generate anti-PEG antibodies. Cell. Mol. Biol. Lett. 2005;10:37–47. [PubMed] [Google Scholar]
- 14.Betker JL, Gomez J, Anchordoquy TJ. The effects of lipoplex formulation variables on the protein corona and comparisons with in vitro transfection efficiency. J. Cont. Rel. 2013 doi: 10.1016/j.jconrel.2013.07.024. in press http://dx.doi.org/10.1016/j.jconrel.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM. Distinct polymer architecture mediates switching of complement-activation pathways at the nanosphere-serum interface: implications for stealth nanoparticle engineering. ACSNano. 2010;4(11):6629–6638. doi: 10.1021/nn101990a. [DOI] [PubMed] [Google Scholar]
- 16.Allen TM, Austin GA, Chonn A, Lin L, Lee KC. Uptake of liposomes by cultured mouse bone marrow macrophages: influence of liposome composition and size. Biochim. Biophys. Acta. 1991;1061:56–64. doi: 10.1016/0005-2736(91)90268-d. [DOI] [PubMed] [Google Scholar]
- 17.Moghimi SM, Andersen AJ, Hashemi SH, Lettiero B, Ahmadvand D, Hunter AC, et al. Complement activation cascade triggered by PEG-PL engineered nanmedicines and carbon nanotubes: the challenges ahead. J. Cont. Rel. 2010;146:175–181. doi: 10.1016/j.jconrel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Molino NM, Bilotkach K, Fraser DA, Ren D, Wang S-W. Complement activation and cell uptake responses toward polymer-functionalized protein nanocapsules. Biomacromolecules. 2012;13:974–981. doi: 10.1021/bm300083e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 20.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advan. Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 21.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Del. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Harvie P, Wong FMP, Bally MB. Use of poly(ethylene glycol)-lipid conjugates to regulate the surface attributes and transfection activity of lipid-DNA particles. J. Pharm. Sci. 2000;89(5):652–663. doi: 10.1002/(SICI)1520-6017(200005)89:5<652::AID-JPS11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Anchordoquy TJ. Effect of cholesterol nanodomains on the targeting of lipid-based gene delivery in cultured cells. Mol. Pharm. 2010;7(4):1311–1317. doi: 10.1021/mp100097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Wempe MF, Anchordoquy TJ. The effect of cholesterol domains on PEGylated liposomal gene delivery in vitro. Ther. Del. 2011;2:451–460. doi: 10.4155/tde.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao Y, Jin Y, Chivukula P, Zhang J, Liu Y, Liu J, et al. Effect of PEGylation on biodistribution and gene silencing of siRNA/lipid nanoparticle complexes. Pharm. Res. 2013;30:342–351. doi: 10.1007/s11095-012-0874-6. [DOI] [PubMed] [Google Scholar]
- 27.Hong K, Zheng W, Baker A, Papahadjopoulos D. Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. FEBS Lett. 1997;400:233–237. doi: 10.1016/s0014-5793(96)01397-x. [DOI] [PubMed] [Google Scholar]
- 28.Ambegia E, Ansell S, Cullis P, Heyes J, Palmer L, MacLachlan I. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim. Biophys. Acta. 2005;1669:155–163. doi: 10.1016/j.bbamem.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Hatakeyama H, Akita J, Kogure K, Oishi M, Nagasaki Y, Kihira Y, et al. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Therapy. 2007;14:68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 30.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lip. Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 31.Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012;9:1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 32.Hamad I, Hunter AC, Rutt KJ, Liu Z, Dai H, Moghimi SM. Complement activation by PEGylated single-walled carbon nanotubes is independent of C1q and alternative pathway turnover. Mol. Immunol. 2008;45:3797–3803. doi: 10.1016/j.molimm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida T, Kiwada H. Anti-polyethyleneglycol antibody response to PEGylated substances. Biol. Pharm. Bull. 2013;36:889–891. doi: 10.1248/bpb.b13-00107. [DOI] [PubMed] [Google Scholar]
- 34.Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: Effect of doxorubicin-encapsulation and high-dose first injection. J. Controlled Release. 2006;115:251–258. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Ishida T, Kashima S, Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J. Controlled Release. 2008;126:162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu T, Ichihara M, Yoshioka Y, Ishida T, Nakagawa S, Kiwada H. Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biol. Pharm. Bull. 2012;35:1336–1342. doi: 10.1248/bpb.b12-00276. [DOI] [PubMed] [Google Scholar]
- 37.Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J. Controlled Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, et al. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int. J. Pharm. 2010;392:218–223. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J. Controlled Release. 2007;119:236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J. Controlled Release. 2006;115:243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, et al. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Cont. Rel. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porto APNA, Lammers AJJ, Bennink RJ, Berge IJM, Speelman P, Hoekstra JBL. Assessment of splenic function. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:1465–1473. doi: 10.1007/s10096-010-1049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, Zhao X, Wang Y, Yang H, Li H, Li H, et al. Prolongation of time interval between doses could eliminate accelerated blood clearance phenomenon induced by pegylated liposomal topotecan. Int. J. Pharm. 2013;443:17–25. doi: 10.1016/j.ijpharm.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Ishihara T, Takeda M, Sakamoto H, Kimoto A, Kobayashi C, Takasaki N, et al. Accelerated Blood Clearance Phenomenon Upon Repeated Injection of PEG-modified PLA-nanoparticles. Pharm. Res. 2009;26:2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- 46.Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: Effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J. Cont. Rel. 2005;105:305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu T, Ishida T, Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 2013;218:725–732. doi: 10.1016/j.imbio.2012.08.274. [DOI] [PubMed] [Google Scholar]
- 48.Laverman P, Brouwers AH, Dams ET, Oyen WJ, Storm G, van Rooijen N, et al. Preclinical and clinical evidence for disappearance of long-circulating characteristics of polyethylene glycol liposomes at low lipid dose. J. Pharmacol. Exp. Ther. 2000;293:996–1001. [PubMed] [Google Scholar]
- 49.Armstrong JK, hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110(1):103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 50.Pidaparti M, Bostrom G. Comparison of allergic reactions to pegasparaginase given intravenously versus intramuscularly. Pediatr. Blood Cancer. 2011 doi: 10.1002/pbc.23380. [DOI] [PubMed] [Google Scholar]
- 51.Moghimi SM, hamad I, Andresen TL, Jorgensen K, Szebeni J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J. 2006;20:E2057–E2067. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- 52.Sherman MR, Williams LD, Sobczyk MA, Michaels SJ, Saifer MGP. Role of methoxy group in immune responses to mPEG-protein conjugates. Bioconj. Chem. 2012;23:485–499. doi: 10.1021/bc200551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki T, Ichihara M, Hyodo K, Yamamoto E, Ishida T, Kiwada H, et al. Accelarated blood clearance of PEGylated liposomes containing doxorubicin upon repeated administration to dogs. Int. J. Pharm. 2012;436:636–643. doi: 10.1016/j.ijpharm.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 54.Abu Lila AS, Eldin NE, Ichihara M, Ishida T, Kiwada H. Multiple adminsistration of PEG-coated liposomal oxaliplatin enhances its therapeutic efficacy: a possible mechanism and the potential for clinical application. Int. J. Pharm. 2012;438:176–183. doi: 10.1016/j.ijpharm.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 55.Hunter AC, Moghimi SM. Therapeutic synthetic polymers: a game of Russian roulette? Drug Discovery Today. 2002;7:998–1001. doi: 10.1016/s1359-6446(02)02444-3. [DOI] [PubMed] [Google Scholar]
- 56.Moghimi SM, Hunter AC, Andresen TL. Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective. Ann. Rev. Pharmacol. Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, Anchordoquy TJ. The role of lipid charge density in the serum stability of cationic lipid/DNA complexes. Biochim. Biophys. Acta Biomembranes. 2004;1663:143–157. doi: 10.1016/j.bbamem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Bradshaw-Pierce EL, DeLille A, Gustafson DL, Anchordoquy TJ. In vivo comparative study of lipid/DNA complexes with different in vitro serum stability: effects on biodistribution and tumor accumulation. J. Pharm. Sci. 2008;97:237–250. doi: 10.1002/jps.21076. [DOI] [PubMed] [Google Scholar]
- 59.Xu L, Anchordoquy TJ. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochim. Biophys.Acta Biomembranes. 2008;1778(10):2177–2181. doi: 10.1016/j.bbamem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Schellekens H, Hennink WE, Brinks V. The immunogenicity of polyethylen glycol: facts and fiction. Pharm. Res. 2013;30:1729–1734. doi: 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- 61.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J. Cont. Rel. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grainger DW. Connecting drug delivery reality to smart materials design. Int. J. Pharm. 2013 doi: 10.1016/j.ijpharm.2013.04.061. 10.1016/j.ijpharm.2013.04.061. [DOI] [PubMed] [Google Scholar]