The structure of GlmU for M. tuberculosis is determined in complex with GlcNAc-1-P, a non-canonical nucleotide substrate – ATP and three Mg2+ ions. ATP is bound at the active site in an unusual conformation.
Keywords: ATP binding, enzyme inhibitor, substrate specificity, sugar nucleotidyltransferase
Abstract
N-Acetylglucosamine-1-phosphate uridyltransferase (GlmU), a bifunctional enzyme exclusive to prokaryotes, belongs to the family of sugar nucleotidyltransferases (SNTs). The enzyme binds GlcNAc-1-P and UTP, and catalyzes a uridyltransfer reaction to synthesize UDP-GlcNAc, an important precursor for cell-wall biosynthesis. As many SNTs are known to utilize a broad range of substrates, substrate specificity in GlmU was probed using biochemical and structural studies. The enzymatic assays reported here demonstrate that GlmU is specific for its natural substrates UTP and GlcNAc-1-P. The crystal structure of GlmU bound to ATP and GlcNAc-1-P provides molecular details for the inability of the enzyme to utilize ATP for the nucleotidyltransfer reaction. ATP binding results in an inactive pre-catalytic enzyme–substrate complex, where it adopts an unusual conformation such that the reaction cannot be catalyzed; here, ATP is shown to be bound together with three Mg2+ ions. Overall, this structure represents the binding of an inhibitory molecule at the active site and can potentially be used to develop new inhibitors of the enzyme. Further, similar to DNA/RNA polymerases, GlmU was recently recognized to utilize two metal ions, MgA 2+ and MgB 2+, to catalyze the uridyltransfer reaction. Interestingly, displacement of MgB 2+ from its usual catalytically competent position, as noted in the crystal structure of RNA polymerase in an inactive state, was considered to be a key factor inhibiting the reaction. Surprisingly, in the current structure of GlmU MgB 2+ is similarly displaced; this raises the possibility that an analogous inhibitory mechanism may be operative in GlmU.
1. Introduction
The family of sugar nucleotidyltransferases (SNTs), also referred to as sugar nucleotide pyrophosphorylases, catalyze the transfer of the NMP (nucleoside monophosphate) moiety of NTP (nucleotide triphosphate) to the phosphate group of a sugar-phosphate molecule to produce sugar nucleotides. A general depiction of the reaction catalyzed is
The product NDP-sugar is then utilized by glycosyltransferases as the activated form of the sugar. In many essential cellular processes such as antigen synthesis, post-translational glycosylation of proteins, cell-wall synthesis and energy metabolism, activated sugars act as donors and are transferred to acceptor molecules such as proteins, lipids, carbohydrates or small organic molecules (Caputto et al., 1950 ▶; Frey, 1996 ▶; Tetteroo et al., 1987 ▶). This explains the prevalence of SNTs in biological systems. These molecules are also precursors of many therapeutically important molecules such as antigens and antibiotics (Luzhetskyy et al., 2008 ▶). Given this important role of SNTs, several studies have been designed to understand their substrate specificities for different nucleotides and sugar phosphates (Mizanur et al., 2004 ▶; Gronwald et al., 2008 ▶; Kotake et al., 2004 ▶; Moretti & Thorson, 2007 ▶; Dickmanns et al., 2011 ▶). This is because SNTs with a broad specificity allow the utilization of different nucleotides and sugar phosphates and such SNTs can easily be employed industrially for the synthesis of diverse sugar nucleotides. Attempts have also been made to enhance the inherent substrate promiscuity by structure-based enzyme engineering (Barton et al., 2002 ▶; Moretti & Thorson, 2007 ▶).
GlmU, an SNT that utilizes UTP and GlcNAc-1-P as its natural substrates, synthesizes UDP-GlcNAc, which forms an important element of the cell wall of several bacteria. Given this important role, GlmU is an established target for antimicrobial drugs, as supported by several investigations (Burton et al., 2006 ▶; Mochalkin et al., 2008 ▶; Pereira et al., 2009 ▶). Previously, we have characterized GlmU from Mycobacterium tuberculosis (GlmUMtb; Parikh et al., 2009 ▶). GlmUMtb is a bifunctional enzyme with independent acetyltransferase and uridyltransferase active sites in the C-terminal and N-terminal domains, respectively (Verma et al., 2009 ▶). In previous studies, we attempted to understand the structure–function relationships that explain the catalytic mechanisms and their regulation in this molecule (Jagtap et al., 2012 ▶, 2013 ▶). As several SNTs possess wide substrate utilization, in this study we set out to characterize the substrate specificity of the uridyltransferase reaction using biochemical and crystallographic studies. These showed that the enzyme is highly specific for its natural substrates UTP and GlcNAc-1-P. Its inability to effectively utilize other nucleotides and sugar phosphates does not make it a suitable candidate for the commercial biosynthesis of diverse sugar nucleotides; it can use CTP, but only marginally. However, based on crystallographic studies, we report an unusual conformation of bound ATP that may provide a platform for the de novo design of an inhibitor against GlmU. In the crystal structure reported here, we find ATP bound along with GlcNAc-1-P and Mg2+ ions at the active site. Unusually, the presence of three instead of two Mg2+ ions and a distorted conformation of the ATP molecule arrests the enzyme in an inactive pre-catalytic state. GlcNAc-1-P, three Mg2+ ions and ATP occupy the active site and render it inactive for catalysis. This molecular complex can thus be used as a ‘lead’ for the development of an inhibitor against the enzyme that interferes with the uridyltransfer activity. Besides this, an analysis of the bound Mg2+ ions suggests an inhibitory mechanism noted for RNA polymerases (Wang et al., 2006 ▶).
2. Materials and methods
2.1. Cloning, mutagenesis and purification of GlmUMtb
Cloning, protein expression and purification of GlmUMtb were performed as described previously (Verma et al., 2009 ▶).
2.2. Uridyltransferase assays
Malachite green assays were employed for uridyltransferase assays (Jagtap et al., 2012 ▶).
Assays were performed in a 10 µl volume containing 25 mM HEPES buffer pH 7.6, 5 mM MgCl2, 1 mM DTT with 1 mM sugar-1-phosphate (GlcNAc-1-P, Glc-1-P and Man-1-P), 2 mM NTP (UTP, ATP, GTP, CTP and TTP), 0.04 U thermostable inorganic pyrophosphatase (TIPP) and subsaturating concentrations of GlmUMtb for 30 min at 30°C. Reactions were terminated by incubation at 65°C for 30 min and the product formed was estimated using a malachite green phosphate assay kit according to the manufacturer’s recommendation.
2.3. Crystal structure of GlmU in complex with ligands of the uridyltransferase reaction
The wild-type GlmUMtb protein was purified and crystallization was carried as described previously (Parikh et al., 2009 ▶). Crystals of GlmU in complex with ligands (ATP and GlcNAc-1-P) and three Mg2+ ions were obtained by soaking apo GlmUMtb crystals in soaking solution consisting of 10% PEG 8000, 100 mM HEPES pH 7.5, 20 mM MgCl2, 4 mM CoCl2 and substrates: 10 mM ATP and 10 mM GlcNAc-1-P at 4°C. The ligand-bound crystals were cryoprotected in a cryosolution consisting of 20% ethylene glycol, 15% PEG 8000, 100 mM HEPES pH 7.5, 20 mM MgCl2, 4 mM CoCl2 prior to X-ray diffraction data collection.
2.4. Data collection, structure determination and refinement
X-ray diffraction data for ligand-bound GlmU crystals were collected using an in-house Rigaku MicroMax-007 HF X-ray source at a wavelength of 1.54 Å (Cu Kα). The data were indexed, integrated and scaled using the XDS program package. The crystals contained one molecule per asymmetric unit and belonged to space group H3 (No. 146). The structure was determined by the molecular-replacement method using Auto-Rickshaw (Panjikar et al., 2005 ▶). The previously determined structure of apo GlmUMtb (in a hexagonal space group; PDB entry 3dj4; Parikh et al., 2009 ▶) was used as a search model. The coordinates for ligands were obtained from the Protein Data Bank (PDB). The library parameters for these ligands were generated using the PRODRG server (Schüttelkopf & van Aalten, 2004 ▶). The initial model was subjected to several rounds of manual building in Coot (Emsley & Cowtan, 2004 ▶), and the initial refinement used REFMAC5 (in CCP4) employing rigid-body refinement followed by several rounds of restrained refinement (Murshudov et al., 2011 ▶). Following the initial structure refinement, ligands were fitted into the electron density and the structure of the complex was further refined using REFMAC5. The quality of the final model was assessed using PROCHECK (Laskowski et al., 1993 ▶). The final refinement statistics are listed in Table 1 ▶. The electron density for Mg2+ ions was differentiated from that of water molecules based on analysis of the coordination geometries (Harding, 2001 ▶). CoCl2 was included in the crystallization experiments as it was necessary for crystal growth. To determine whether any of the metal-binding sites were occupied by Co2+ ions, we examined anomalous dispersion by cobalt, which absorbs X-rays at a wavelength of 1.54 Å. The data processed without merging Bijvoet pairs, i.e. with the anomalous contribution, were combined with the phases of the search model to locate the position of the cobalt ion(s) in the asymmetric unit. The absence of any peaks in the anomalous difference Fourier map suggested that none of the metal-binding sites were occupied by Co2+ ions (data not shown). The atomic coordinates and structure factors for the structure presented here have been deposited in the PDB (http://www.rcsb.org) with accession code 4k6r.
Table 1. Data-collection and refinement statistics for GlmUMtb[GlcNAc-1-P:ATP].
Values in parentheses are for the highest resolution shell.
| Data collection | |
| Space group | H3 |
| Unit-cell parameters (Å, °) | a = b = 79.27, c = 276.73, α = β = 90, γ = 120 |
| Resolution (Å) | 47.82–1.98 (2.10–1.98) |
| R merge (%) | 8.9 (46.2) |
| 〈I/σ(I)〉 | 29.64 (4.04) |
| Completeness (%) | 99.6 (98.7) |
| Refinement | |
| Resolution range (Å) | 47.82–1.98 |
| No. of reflections | 40730 |
| R work | 0.1589 (0.199) |
| R free | 0.2005 (0.265) |
| No. of atoms | |
| Protein | 3445 |
| Ligand/ion | 80 |
| Water | 474 |
| Average B factors (Å2) | |
| Protein | 32.9 |
| Ligand/ion | 40.6 |
| Water | 31.5 |
| R.m.s.d. | |
| Bond lengths (Å) | 0.0266 |
| Bond angles (°) | 2.1569 |
3. Results and discussion
3.1. Biochemical studies to evaluate substrate specificity in GlmU
In order to probe the innate promiscuity of GlmUMtb for the usage of various nucleotides and sugar phosphates, we commenced enzyme kinetics studies in the presence of five commonly used nucleotides and three different sugar phosphates. The nucleotidyltransfer activity of the enzyme towards various nucleotides showed that the enzyme is highly specific for UTP. Of the other nucleotides tested, only marginal activity with CTP was detected, while no activity was found with TTP, ATP and GTP (Fig. 1 ▶ a). Of the three sugar phosphates tested, the enzyme displayed activity only for the substrate GlcNAc-1-P, and was inactive in the assays using glucose 1-phosphate and mannose 1-phosphate (Fig. 1 ▶ b).
Figure 1.
A nucleotidyltransfer assay for GlmUMtb was carried out for different nucleotides and sugar phosphates. (a) The ability of GlmUMtb to utilize different nucleotides (2 mM) is shown. The maximum enzymatic activity was observed for UTP, which is the natural substrate; considering this as 100%, the activities of the other nucleotides are shown as relative percentage activity. Modified malachite green assays were carried out in triplicate and error bars are shown. (b) Similarly, to test for its ability to use different sugars, assays were carried out with UTP as the substrate. Uridyltransfer assays were carried out with three sugar phosphates (indicated) at 2 mM concentration. The maximum activity was observed with the natural substrate GlcNAc-1-P; considering this as 100%, the products formed when glucose-1-P and mannose-1-P were used are shown.
Few SNTs have been found to have the inherently broad substrate specificity that makes them suitable for the commercial synthesis of a wide range of sugar nucleotides (Mizanur et al., 2004 ▶; Gronwald et al., 2008 ▶; Kotake et al., 2004 ▶; Pereira et al., 2009 ▶; Mizanur & Pohl, 2009 ▶). Such enzymes are usually engineered to further broaden their substrate utilization, so that a single enzyme can be employed for the synthesis of a diverse set of molecules for industrial production. One such SNT that has been evaluated for its commercial viability and studied extensively is RmlA (glucose-1-phosphate thymidylyltransferase). It was found to utilize all five of the major nucleotides, i.e. ATP, GTP, TTP, UTP and CTP, and 30 different sugar-phosphate derivatives (Pereira et al., 2009 ▶). The high promiscuity in the substrate utilization of wild-type RmlA renders it ideal for commercial chemoenzymatic synthesis. However, the higher enzymatic activities found with pyrimidine nucleotides compared with purine nucleotides necessitated enzyme engineering in RmlA. Mutations created to alter the pyrimidine/purine bias were also successful (Pereira et al., 2009 ▶). Based on these studies, it appears that only a few of the pyrophosphorylase class of enzymes can utilize a broad range of substrates with varying affinity and reaction rates.
3.2. Crystal structure of GlmU in complex with ATP and GlcNAc-1-phosphate
We have previously determined structures of GlmUMtb bound to its natural substrates and in complex with one of the substrates, GlcNAc-1-P (Jagtap et al., 2013 ▶; PDB entries 4hcq and 4g87). These structures show stabilizing interactions for the nucleotide base similar to those found in the structure of the related SNT RmlA (Moretti et al., 2011 ▶; Blankenfeldt et al., 2000 ▶). RmlA employs dTTP and Glc-1-P as natural substrates. Based on structural and biochemical studies, it was shown that a conserved glutamine (corresponding to Gln83 in GlmUMtb) is responsible for its specificity towards the pyrimidine base, i.e. dTTP (Jagtap et al., 2013 ▶; Blankenfeldt et al., 2000 ▶). However, RmlA was found to utilize all five nucleotides. Intriguingly, despite conserving the identical glutamine, Gln83, at the active site, GlmUMtb shows a completely different nucleotide preference; it can only use UTP (and marginally CTP) in the reaction (Fig. 1 ▶ a). In order to understand the molecular basis of the bias for UTP, we resorted to structure determination using X-ray crystallography in the presence of other nucleotides. For this, we soaked apo crystals of GlmU (as described in §2) in a solution containing purine nucleotides (either ATP or GTP), GlcNAc-1-P and MgCl2.
The crystal structure of GlmUMtb determined from crystals soaked in a solution containing GTP and GlcNAc-1-P showed clear electron density for GlcNAc-1-P but not for GTP (data not shown). This observation is in line with our biochemical studies and is consistent with the inability of the enzyme to perform the nucleotidyltransfer reaction with GTP. Strikingly different results were found after similar soaking experiments carried out with ATP. Clear unbiased electron density could be observed for both GlcNAc-1-P and ATP (Fig. 2 ▶ a) and, except for a slight change in the orientation of two loop regions (Fig. 2 ▶ b), the structure of the active site and the uridyltransferase domain remained unaltered with respect to the structures of the complexes of the enzyme with its natural substrates and with one of the substrates, GlcNAc-1-P (PDB entries 4hcq and 4g87). More remarkably, in the new structure, termed GlmUMtb[GlcNAc-1-P:ATP], an unusual conformation was observed for ATP bound at the active site. This new binding pose was completely different from those of nucleotides observed thus far in available structures of SNTs. This atypical conformation of ATP appears to be stabilized by two Mg2+ ions, MgB′ 2+ and MgC 2+, as shown in Fig. 3 ▶(a). Fig. 3 ▶(b) shows the way that UTP, the natural substrate, is stabilized by MgA 2+ and MgB 2+. The conformation of ATP observed in the present structure positions its Pα 6.15 Å away from the attacking nucleophile of GlcNAc-1-P (Fig. 3 ▶ c). At this distance, the nucleophile would be unable to carry out a nucleophilic attack and hence the nucleotidyltransfer reaction would not occur. Therefore, although ATP binds at the active site, its atypical conformation precludes its participation in the reaction and results in an inactive enzyme–nucleotide complex. With ATP bound at the active site in a different conformation, Gln83 (which interacts with the uracil base in the UTP-bound structure) does not interact with the nucleotide base; instead, it makes a hydrogen-bond interaction with the ribose sugar of the nucleotide (Figs. 3 ▶ d and 3 ▶ e). Apart from this, the nucleotide base occupies a pocket lined by hydrophobic groups. In both the ATP-bound and UTP-bound forms the triphosphate moiety makes similar active-site interactions with the Thr18 and Arg19 residues (Figs. 3 ▶ d and 3 ▶ e). The structural findings and biochemical data taken together indicate that ATP is capable of binding to the active site; however, it is not utilized for product formation because of the catalytically incompetent conformation that it adopts. Thus, the possibility exists that ATP competing with UTP, the canonical substrate, is a mechanism for regulating the activity of the enzyme; further comprehensive studies, including evaluation of the enzymatic activity or its inhibition in the presence of ATP, would be needed to investigate such possibilities.
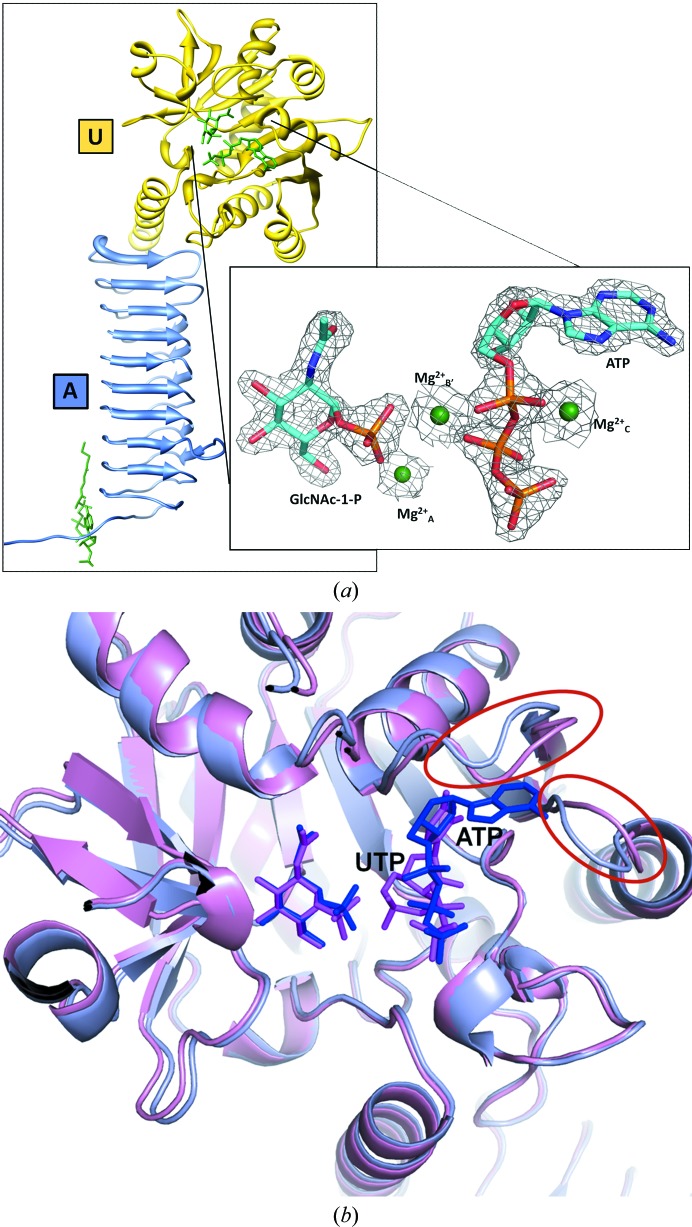
Figure 2.
(a) The structure of GlmUMtb[GlcNAc-1-P:ATP] is shown. The acetyltransferase and uridyltransferase active sites are marked A and U. The inset shows an enlarged view of the uridyltransfer active site, with unbiased electron density for the ligands in an F o − F c map contoured at 2σ. The map was generated using F o − F c coefficients and phases calculated before including the ligands and Mg2+ ions (but after initial refinement of the protein atoms alone). Modelled into the density are ATP, GlcNAc-1-P and the three Mg2+ ions, which are labelled MgA 2+, MgB′ 2+ and MgC 2+. (b) A structural comparison of GlmUMtb[GlcNAc-1-P:ATP] with GlmUMtb[GlcNAc-1-P:UTP] is shown as ribbons coloured purple and pink, respectively. The nucleotides ATP and UTP bound at the active site are shown as sticks coloured blue and magenta, respectively. The loop regions that show a conformational change are indicated.
Figure 3.
The unusual conformation of ATP seen in GlmUMtb[GlcNAc-1-P:ATP]. (a) The regular octahedral hexacoordination goemetry of the three metal ions, MgA 2+, MgB′ 2+ and MgC 2+, is shown. Interactions made by MgB′ 2+ and MgC 2+ appear important in positioning Pα away from the nucleophile. (b) Substrates bound at the active site of GlmUMtb[GlcNAc-1-P:UTP] (reported in Jagtap et al., 2013 ▶), which is the catalytically active form. UTP and GlcNAc-1-P are shown in sticks with C atoms coloured yellow. An asterisk indicates the attacking nucleophile of GlcNAc-1-P. Magnesium ions bound to both structures are shown as green spheres. Coordination interactions of MgB 2+ with the triphosphate of UTP are shown. (c) GlmUMtb[GlcNAc-1-P:ATP] is in a catalytically inactive state. A superposition with the active GlmUMtb[GlcNAc-1-P:UTP] is shown. Nucleotides are marked and shown as sticks with C atoms coloured cyan for ATP and yellow for UTP. In the unusual ATP conformation, Pα (marked Pα-A) is displaced away from the attacking nucleophile of GlcNAc-1-P. The Pα of UTP (marked Pα-U) is in a catalytically competent position optimal for nucleophilic attack. Magnesium ions bound to both structures are shown as spheres; those in dark green correspond to those of GlmUMtb[GlcNAc-1-P:ATP] and those in light green correspond to those of GlmUMtb[GlcNAc-1-P:UTP]. (d) ATP bound in an atypical comformation at the active site is shown as sticks with C atoms coloured cyan. Residues making interactions with ATP are shown in stick representation; those forming the hydrophobic pocket are shown with C atoms coloured green. Residues Thr18 and Arg19 that interact with the γ-phosphate are shown in sticks with their C atoms coloured yellow. The hydrogen-bond interaction made by Gln83 with the ribose sugar is shown as a blue broken line. Ala14, Pro16, His58 and Pro86 forming a hydrophobic pocket around the adenine base are shown in sticks with their C atoms coloured green. (e) With the natural substrate, i.e. UTP, Gln83 interacts with the uracil base. UDP-GlcNAc and pyrophosphate at the active site of GlmUMtb (PDB entry 4g87) are shown as sticks with their C atoms coloured cyan. Hydrogen-bond interactions of Gln83 with the uracil base are shown as blue broken lines. Residues Thr18 and Arg19 that interact with the γ-phosphate are shown in sticks with their C atoms coloured yellow. (f) As (c) but in a different orientation. Here, the magnesium ions are labelled. MgB′ 2+ in GlmUMtb[GlcNAc-1-P:ATP] is displaced by 2.1 Å from the corresponding MgB 2+ in GlmUMtb[GlcNAc-1-P:UTP].
Recently, employing crystal structures, we have explained the catalytic mechanism of uridyl transfer by GlmU (Jagtap et al., 2013 ▶). This mechanism, elucidated based on the GlmUMtb[GlcNAc-1-P:UTP] structure determined with natural substrates, requires two Mg2+ ions, MgA 2+ and MgB 2+, to catalyze the reaction (Fig. 3 ▶ b). MgA 2+ is known to activate the nucleophile and MgB 2+, the second metal ion, makes interactions with the triphosphate moiety of UTP and is likely to stabilize the transition state during the uridyltransfer reaction and also to participate in product release following the reaction (Jagtap et al., 2013 ▶). In this way, the two metal ions efficiently accomplish the nucleotidyltransfer reaction. By contrast, in the current structure, GlmUMtb[GlcNAc-1-P:ATP], three Mg2+ ions were surprisingly found at the active site, which is not in line with the known ‘two-metal-ion’ catalytic mechanism required to catalyze the reaction (Jagtap et al., 2013 ▶). Interestingly, in the new GlmUMtb[GlcNAc-1-P:ATP] structure the position of MgA 2+ is conserved, indicating that if Pα were not displaced then the reaction could occur. A comparison shows that the second metal does not bind at site B but occupies a new site 2.15 Å away; this metal is hence termed MgB′ 2+ (Figs. 3 ▶ a and 3 ▶ f). In this position MgB′ 2+ is not capable of making an interaction with the attacking phosphate group of GlcNAc-1-P. MgB′ 2+ makes one coordination interaction with the Pα of ATP and five more with water molecules to satisfy an octahedral geometry. Such a displacement in the position of MgB 2+ was also observed in the crystal structures of RNA polymerase determined (in complex with various nucleotides) at higher and lower Mg2+ concentrations (Fig. 4 ▶). Together with a displaced Mg2+, the nucleotide was also bound in an alternative conformation in this structure. These studies associated the displacement of Mg2+ and the alternative conformation of UTP with an inactive state of the RNA polymerase. The displacement of Mg2+ from a catalytically competent position to an inactive position was suggested to inhibit the activity of RNA polymerase (Wang et al., 2006 ▶). In GlmUMtb[GlcNAc-1-P:ATP], the third metal ion, MgC 2+, is also stabilized by one coordination interaction with Pα and five coordination interactions with water molecules. Its coordination by Pα results in the stabilization of an inactive conformation of ATP.
Figure 4.

A displaced MgB 2+ is concurrent with the structure of an inactive RNA polymerase. Superposition of the structures of RNA polymerase determined at low (PDB entry 2nvz) and high Mg2+ concentrations (PDB entry 2yu9) in complex with UTP (Wang et al., 2006 ▶). C atoms of UTP are coloured purple in the latter and yellow in the former (also indicated as high and low). Similarly, the corresponding Mg2+ ions are shown as light (low concentration) and dark (high concentration) green spheres. The two metal ions, MgA 2+ and MgB 2+, are indicated and the displacement of MgB 2+ is shown by a dashed line.
Overall, the entire metal–substrate complex renders the enzyme catalytically inactive. This structure could therefore be an initial ‘lead’ which can be further developed into an inhibitor acting at the uridyltransfer active site of the enzyme. Given that GlmU is an actively pursued target for antimicrobial drug discovery, these studies provide an alternate direction to the efforts towards structure-based drug design.
Supplementary Material
PDB reference: GlmU complexed with ATP, 4k6r
Acknowledgments
The authors thank IIT Kanpur for continuous support. NV and VSB acknowledge the Ministry of Human Resource Development (MHRD), India and the Department of Biotechnology (DBT), India, respectively, for financial assistance. This work was supported by grants from the DBT, India.
References
- Barton, W. A., Biggins, J. B., Jiang, J., Thorson, J. S. & Nikolov, D. B. (2002). Proc. Natl Acad. Sci. USA, 99, 13397–13402. [DOI] [PMC free article] [PubMed]
- Blankenfeldt, W., Asuncion, M., Lam, J. S. & Naismith, J. H. (2000). EMBO J. 19, 6652–6663. [DOI] [PMC free article] [PubMed]
- Burton, E., Gawande, P. V., Yakandawala, N., LoVetri, K., Zhanel, G. G., Romeo, T., Friesen, A. D. & Madhyastha, S. (2006). Antimicrob. Agents Chemother. 50, 1835–1840. [DOI] [PMC free article] [PubMed]
- Caputto, R., Leloir, L. F., Cardini, C. E. & Paladini, A. C. (1950). J. Biol. Chem. 184, 333–350. [PubMed]
- Dickmanns, A., Damerow, S., Neumann, P., Schulz, E. C., Lamerz, A. C., Routier, F. H. & Ficner, R. (2011). J. Mol. Biol. 405, 461–478. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Frey, P. A. (1996). FASEB J. 10, 461–470. [PubMed]
- Gronwald, J. W., Miller, S. S. & Vance, C. P. (2008). Plant Physiol. Biochem. 46, 1101–1105. [DOI] [PubMed]
- Harding, M. M. (2001). Acta Cryst. D57, 401–411. [DOI] [PubMed]
- Jagtap, P. K., Soni, V., Vithani, N., Jhingan, G. D., Bais, V. S., Nandicoori, V. K. & Prakash, B. (2012). J. Biol. Chem. 287, 39524–39537. [DOI] [PMC free article] [PubMed]
- Jagtap, P. K., Verma, S. K., Vithani, N., Bais, V. S. & Prakash, B. (2013). J. Mol. Biol. 425, 1745–1759. [DOI] [PubMed]
- Kotake, T., Yamaguchi, D., Ohzono, H., Hojo, S., Kaneko, S., Ishida, H. K. & Tsumuraya, Y. (2004). J. Biol. Chem. 279, 45728–45736. [DOI] [PubMed]
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst. 26, 283–291.
- Luzhetskyy, A., Méndez, C., Salas, J. A. & Bechthold, A. (2008). Curr. Top. Med. Chem. 8, 680–709. [DOI] [PubMed]
- Mizanur, R. M. & Pohl, N. L. (2009). Org. Biomol. Chem. 7, 2135–2139. [DOI] [PubMed]
- Mizanur, R. M., Zea, C. J. & Pohl, N. L. (2004). J. Am. Chem. Soc. 126, 15993–15998. [DOI] [PubMed]
- Mochalkin, I., Lightle, S., Narasimhan, L., Bornemeier, D., Melnick, M., Vanderroest, S. & McDowell, L. (2008). Protein Sci. 17, 577–582. [DOI] [PMC free article] [PubMed]
- Moretti, R., Chang, A., Peltier-Pain, P., Bingman, C. A., Phillips, G. N. Jr & Thorson, J. S. (2011). J. Biol. Chem. 286, 13235–13243. [DOI] [PMC free article] [PubMed]
- Moretti, R. & Thorson, J. S. (2007). J. Biol. Chem. 282, 16942–16947. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Panjikar, S., Parthasarathy, V., Lamzin, V. S., Weiss, M. S. & Tucker, P. A. (2005). Acta Cryst. D61, 449–457. [DOI] [PubMed]
- Parikh, A., Verma, S. K., Khan, S., Prakash, B. & Nandicoori, V. K. (2009). J. Mol. Biol. 386, 451–464. [DOI] [PubMed]
- Pereira, M. P., Blanchard, J. E., Murphy, C., Roderick, S. L. & Brown, E. D. (2009). Antimicrob. Agents Chemother. 53, 2306–2311. [DOI] [PMC free article] [PubMed]
- Schüttelkopf, A. W. & van Aalten, D. M. F. (2004). Acta Cryst. D60, 1355–1363. [DOI] [PubMed]
- Tetteroo, P. A., de Heij, H. T., Van den Eijnden, D. H., Visser, F. J., Schoenmarker, E. & Geurts van Kessel, A. H. (1987). J. Biol. Chem. 262, 15984–15989. [PubMed]
- Verma, S. K., Jaiswal, M., Kumar, N., Parikh, A., Nandicoori, V. K. & Prakash, B. (2009). Acta Cryst. F65, 435–439. [DOI] [PMC free article] [PubMed]
- Wang, D., Bushnell, D. A., Westover, K. D., Kaplan, C. D. & Kornberg, R. D. (2006). Cell, 127, 941–954. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: GlmU complexed with ATP, 4k6r