Abstract
This study was conducted to test a new substaging system in a population of patients with stage T1 bladder cancer (BC) at diagnosis and assess its prognostic role in terms of disease progression and disease-specific survival (DSS). Patients with primary stage T1G3 urothelial carcinoma of the bladder were stratified according to the following models: i) T1a [the tumour does not infiltrate the muscularis mucosae-vascular plexus, (MM-VP)]; T1b (the tumour partially infiltrates the MM-VP); and T1c (the tumour infiltrates and invades the MM-VP). ii) T1m (diameter of tumour infiltrating the lamina propria ≤0.5 mm under a high-resolution microscope; and T1e (diameter of tumour infiltrating the lamina propria >0.5 mm). Age, gender, tumour size and multifocality were not found to be of statistical significance. Using the T1a/T1b/T1c system, patients with stage T1a disease exhibited a 5- and 10-year progression rate of 13.3 and 20%, respectively, without reaching statistical significance. Moreover, patients with stage T1a disease exhibited a 5- and 10-year DSS of 93.3 and 73.3%, respectively, which was higher compared to T1b and T1c but not statistically significant. Using the T1m/T1e system, patients with stage T1m disease exhibited a disease progression rate of 8.3 and 16.7% at 5 and 10 years, respectively, which was not statistically significant. Moreover, patients in group T1m presented with DSS rates of 91.7 and 83.3% at 5 and 10 years, respectively, which were higher compared to those in the T1e group (71.4 and 60.7%), although not reaching statistical significance. In conclusion, in our study, neither of the two substaging systems of stage T1 BC reached the prognostic conventional significance level for tumour progression or DSS.
Keywords: bladder carcinoma, substaging, prognosis, transitional cell carcinoma
Introduction
Bladder cancer (BC) is the most common malignancy of the urinary tract. At diagnosis, ~75–85% of BCs are confined to the mucosa [stage Ta and carcinoma in situ (CIS)] or submucosa (stage T1) (1). These categories are grouped as non-muscle invasive bladder cancer (NMIBC).
BC cannot be considered as a single nosological entity, but rather as a chronic, heterogeneous and polychronotopic neoplastic diathesis, spatially and temporally multicentric. Due to these characteristics, BC has a largely unpredictable natural history.
This tumour is, in fact, characterized by various types of spreading; it may be circumscribed or locally invasive, multicentered, often with areas of CIS, aggressive, it may grow rapidly, be invasive and develop early distant metastases.
The unpredictable biological behaviour of BC raises a number of questions, amongst which why some superficial tumours remain localized, whereas others, with identical characteristics, become invasive. The six most significant clinicopathological factors that determine a prognostic score for BC regarding recurrence and disease progression are as follows: number of tumours, size, previous recurrence rate, T stage, presence of concomitant CIS and tumour grade (2).
With regard to prognosis and treatment, stage T1 BC requires careful consideration, as a number of these tumours have a high ratio of undifferentiated cells, with a consequently high risk of progression and poor prognosis.
The treatment options for patients with high-grade T1 stage NMIBC are complete resection of the lesion, followed by a cycle of adjuvant intravesical Bacillus Calmette-Guérin (BCG) instillation, which was shown to achieve a reduction of the recurrence rate and, to a lesser extent, reduce or at least delay the progression of the disease. An alternative treatment option is radical cystectomy with urinary diversion (3–6).
With conservative therapy, 20–40% of the patients will develop progression of the disease within 5 years (7). Patients undergoing cystectomy for muscle invasive tumour progression exhibit a lower disease-free survival rate compared to those undergoing radical cystectomy for muscle invasive BC at diagnosis (28 vs. 55%) (8).
We should consider that radical cystectomy as a primary option may be an overtreatment for ~50% of the patients (9).
Due to the high risk of progression, the uncertainty of long-term outcome and the weak efficacy of BCG treatment on disease progression, we must identify T1 tumours with aggressive behaviour in order to plan the most appropriate treatment, as they are prone to recurrence and progression.
Therefore, several authors attempted to subclassify stage T1 tumours into subgroups, using different anatomical landmarks with different results (10–27).
A stratification of the T1 stage based on the infiltration (T1b) or not (T1a) of the muscularis mucosae or its invasion (T1c), has provided satisfactory results and is possibly the most accurate evaluation of tumour depth (as regards the chorion). However, this substaging system may be used in 58–100% of the cases, due to the absence of the muscularis mucosae in different histological preparations (10–23,27). A method which evaluates the depth of invasion (< or >1.5 mm) of the lamina propria may be more easily achievable, although less precise (24,28–29). A number of these substaging systems were found to exhibit a significant correlation with disease progression (11–12,14–15,19–25,27); however, few exhibited a significant correlation with overall survival (OS) (16,23) as well as disease-specific survival (DSS) (10,23,25,27).
The pathologist should always provide an indication of the depth of invasion into the subepithelial stroma and should always report the presence in the sample of muscle bundles definitely free from invasion and belonging to the detrusor, if present.
Due to the possibility of a pathological mismatch regarding the identification of the muscularis mucosae-vascular plexus (MM-VP), in the attempt to overcome this discrepancy, van der Aa et al (24) proposed a new and reliable subclassification system, with the primary aim of predicting the clinical behaviour of these tumours. This system does not require the identification of the MM-VP, but applies the concept of microinvasion (T1m) in the case of single invasion of the lamina propria with length of <0.5 mm at high magnification and extensive invasion (T1e) in the presence of multifocal areas of infiltration or with a length of >0.5 mm (24).
Recently, van Rhijn et al (27), evaluated the impact of the method suggested by van der Aa et al (24) on clinical outcome and reported a significant correlation of this substaging system not only with disease progression, but also with cancer-specific survival. The aim of this study was to test this new substaging system in our population of patients with stage T1 NMIBC at diagnosis and assess its prognostic role in terms of disease progression and DSS.
Material and methods
Patients
All patients with primary stage T1G3 urothelial carcinoma of the urinary bladder who were referred to our institution between January, 2000 and December, 2006 were included in this study. Patients with a history of BC prior to the occurrence of a T1 tumour were not considered. The pathological specimens were reviewed in order to confirm the diagnosis of T1 and the substaging.
We retrospectively identified 40 patients (8 women and 32 men) with a mean age ± standard deviation (SD) at diagnosis of 69.9±10.5 years. All the patients had undergone a transurethral resection of the bladder tumour (TUR-BT). A second TUR-BT within 6 weeks was performed in cases of macroscopic incomplete TUR-BT or absence of muscularis mucosae after the first endoscopic resection. All the patients were initially managed conservatively with an induction course of BCG. Random biopsies, a standard repeat TUR and a single instillation of chemotherapy following TUR were not performed (26). The surveillance of the patients included cystoscopy and cytology every 3–4 months in the first 2 years and at a lower frequency thereafter (6–12 months) if no recurrence was detected. Upper urinary tract imaging was performed every 2 years or when indicated by clinical suspicion (26).
Disease progression was defined as detection of a muscle-invasive BC in case of recurrence or the presence of local or distant metastases. The patients were followed up until death or until their last outpatient follow-up.
Staging
The original slides of 40 primary T1 bladder tumours were reviewed by one pathologist (M.B.) to determine stage and grade. The pathologist was blinded to the clinical outcome but was aware of the original T1 diagnosis. The World Health Organization 1973 and 2004 classifications were used to review the grade. After confirming stage T1, the pathologist further stratified the patients according to the following models: i) T1a (the tumour does not infiltrate the MM-VP), T1b (the tumour partially infiltrates the MM-VP) and T1c (the tumour infiltrates and invades the MM-VP). If the MM-VP was not present at the invasion front, the case was assigned to T1a or T1c, based on the extent of invasion into the lamina propria by examining the MM-VP in tumour-free areas in the same or other TUR chips. ii) T1m (maximum diameter of the tumour infiltrating the lamina propria ≤0.5 mm under a high-resolution microscope) and T1e (maximum diameter of the tumour infiltrating the lamina propria >0.5 mm).
Statistical analysis
Statistical analysis was performed using SPSS software, version 20.0 (IBM, Armonk, NY, USA). Continuous variables are reported as means ± SD (in cases of normal distribution) or median and interquartile range (IQR) (in cases of non-normal distribution). The t-test, Mann-Whitney U test and Pearson’s Chi-square test were used to compare categorical and continuous variables. The clinical outcomes, such as disease progression and DSS, were analyzed using univariate statistical analysis according to Kaplan-Meier and multivariate analysis using Cox regression, in order to identify independent prognostic factors among age, gender, tumour size, tumour multifocality and substaging. A two-sided P<0.05 was considered to indicate a statistically significant difference.
Results
Patient and tumour characteristics, patient distribution and outcomes
Table I summarizes the demographic characteristics of patients and tumours following pathological review. The distribution of patients with regard to the two different models is presented in Table II. The MM-VP was not present at the tumour invasion front in 3 tumours (7.5%). These cases were all classified as T1a, as indicated in ‘Patients and methods’. The median follow-up was 9.5 years (IQR, 3–11.25 years). No patients experienced disease recurrence at the level of the upper urinary tract. No patients underwent radical cystectomy for stage T1 disease at diagnosis.
Table I.
Patients and tumour characteristics after pathological review.
| Variables | No. (%) |
|---|---|
| No. of patients | 40 (100) |
| Gender | |
| Male | 32 (80) |
| Female | 8 (20) |
| No. of tumours | |
| Single | 38 (91) |
| Multiple | 2 (9) |
| Tumour size (cm) | |
| <3 | 39 (97.5) |
| >3 | 1 (2.5) |
| Concurrent carcinoma in situ | |
| Yes | 0 (0) |
| No | 40 (100) |
| Endovesical therapy | |
| Bacillus Calmette-Guérin | 21 (52.5) |
| No | 19 (47.5) |
Table II.
Distribution of patients in accordance with the two modes of T1 substaging.
| T1 substaging | No. (%) |
|---|---|
| According to MM-VP invasion | |
| T1a | 15 (37.5) |
| T1b | 25 (62.5) |
| T1c | 0 (0) |
| According to tumour diameter invading the lamina propria | |
| T1m | 12 (30) |
| T1e | 28 (70) |
MM-VP, muscularis mucosae-vascular plexus.
No patients underwent intravesical chemotherapy after TUR-BT; 21 patients (52.5%) were subjected to intravesical immunotherapy with BCG. Age, gender, tumour size and multifocality were not found to be of statistical significance.
The DSS at 5 and 10 years was 80 and 67.5%, respectively. A total of 13 patients (32.5%) developed disease progression at 5 years and eventually succumbed to BC.
DSS and disease progression according to substage
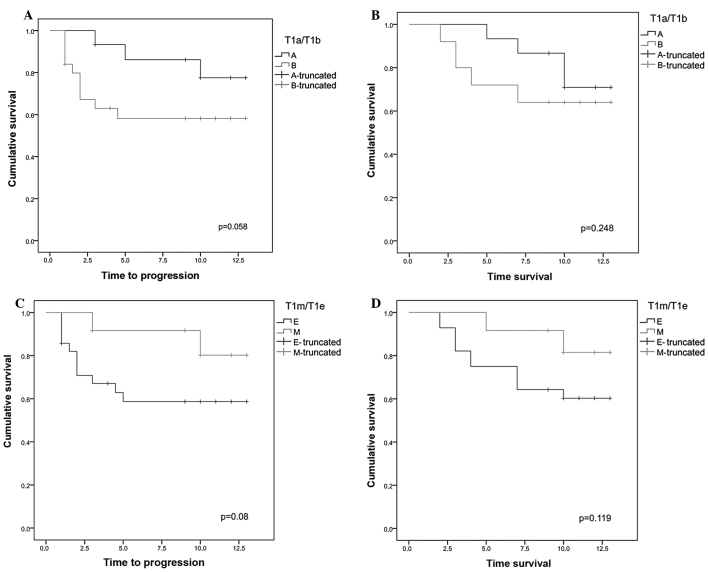
The Kaplan-Meier curves regarding DSS and disease progression according to the two types of substaging are shown in Fig. 1. With regard to disease progression, patients with stage T1a disease exhibited a 5- and 10-year progression rate of 13.3 and 20%, respectively, which was lower compared to that in patients with stage T1b disease, without reaching full statistical significance (P=0.058).
Figure 1.
(A) Kaplan-Meier curves regarding disease progression in groups T1a and T1b. The stage T1a group exhibited a disease progression rate of 13.3 and 20% at 5 and 10 years, respectively; in the T1b stage group, the disease progression at 5–10 years was 40% (P=0.058). (B) Kaplan-Meier curves regarding disease-specific survival (DSS) in groups T1a and T1b. In the stage T1a group the DSS rate at 5 and 10 years was 93.3 and 73.3%, respectively, whereas the T1b stage group exhibited 5- and 10-year DSS rate of 72 and 64%, respectively (P=0.248). (C) Kaplan-Meier curves regarding disease progression in groups T1e and T1m. In the stage T1m group the percentage of patients free of disease progression at 5 and 10 years was 91.7 and 83.3%, respectively, whereas in the stage T1a group it was 60.7% at 5 and 10 years (P=0.08). (D) Kaplan-Meier curves regarding DSS in groups T1m and T1e. In the stage T1m group the DSS rate at 5 and 10 years was 91.7 and 83.3%, respectively, whereas in the stage T1e group it was 71.4 and 60.7% at 5 and 10 years, respectively (P=0.119).
With regard to DSS, patients with stage T1a disease exhibited a 5- and 10-year DSS of 93.3 and 73.3%, respectively, which was higher but not statistically significant compared to that of the T1b group (72 and 64%, P=0.248).
Using the T1m/T1e substaging system, the group of patients with stage T1m disease exhibited a disease progression rate of 8.3 and 16.7% at 5 and 10 years, respectively, which was lower but not statistically significant compared to that in the group with stage T1e disease (39.3%, P=0.08).
With regard to DSS, the patients in the T1m group presented with DSS rates of 91.7 and 83.3% at 5 and 10 years, respectively, which was higher compared to those in the T1e group (71.4 and 60.7%), although not reaching statistical significance (P=0.119).
Discussion
The treatment of stage T1 urothelial bladder tumours represents a therapeutic challenge. Conservative treatment may lead to disease progression and death; however, a radical intervention, such as radical cystectomy, may represent an overtreatment of patients who may not ultimately develop disease progression. Therefore, it is important to establish a substaging system which is simple and useful in clinical practice and able to provide prognostic information, in order to guide the urologist through the decision-making process for the management of such patients.
Several studies investigated the usefulness of the MM-VP layer in stratifying stage T1 urothelial carcinoma of the bladder into T1a, T1b and T1c substages (10–23,27). The main problem of this system lies with the lack of consensus regarding the identification of the MM-VP at the level of the invasive front of the tumour (28,29). Consequently, the percentages of substaging were highly variable, between 58 and 100% (10–23,27). This high variability may be further explained by the different quality of TUR-BT and by the possible damage to the lamina propria due to TUR-BT itself and by the intravesical treatment. In our study, we obtained an overall substaging rate of 92.5%. However, it is not always possible to obtain a satisfactory excisional biopsy specimen comprising an adequate sample of muscle layer, making it difficult to perform this type of substaging.
For this reason, Cheng et al (25) proposed a new staging system based on the depth of invasion expressed in μm. The authors demonstrated that millimeter measurement of the depth of invasion in TUR-BT specimens was possible in 100% of the 83 TUR-BT samples analyzed and proposed a 1.5-mm cut-off (25). The possible limitations of this cut-off lie with the variability of the thickness of the lamina propria.
In order to overcome this limitation, van der Aa et al (24) modified the concept of Cheng et al (25), recommending a depth of 0.5 mm to distinguish microinvasion (T1m) from ‘extended’ invasion (T1e). This new substaging tool is easy to apply, reproducible, useful in 100% of cases and more reliable compared to other substaging systems (28).
Recently, van Rhijn et al (27) analyzed the T1a/T1b/T1c and T1m/T1e substaging system, demonstrating a higher prognostic value in terms of disease progression as well as DSS. In fact, in this study, the prognostic value of the T1a/T1b/T1c system was statistically significant only for disease progression and only at the univariate analysis. However, this substaging was only possible in 63% of the samples; the number of patients was not sufficient to corroborate the result. In our study, it was not possible to identify any prognostic significance for this system, neither for disease progression nor for DSS, although substaging was possible in 92.5% of the cases. However, the sample size of our study was insufficient for a reliable statistical evaluation.
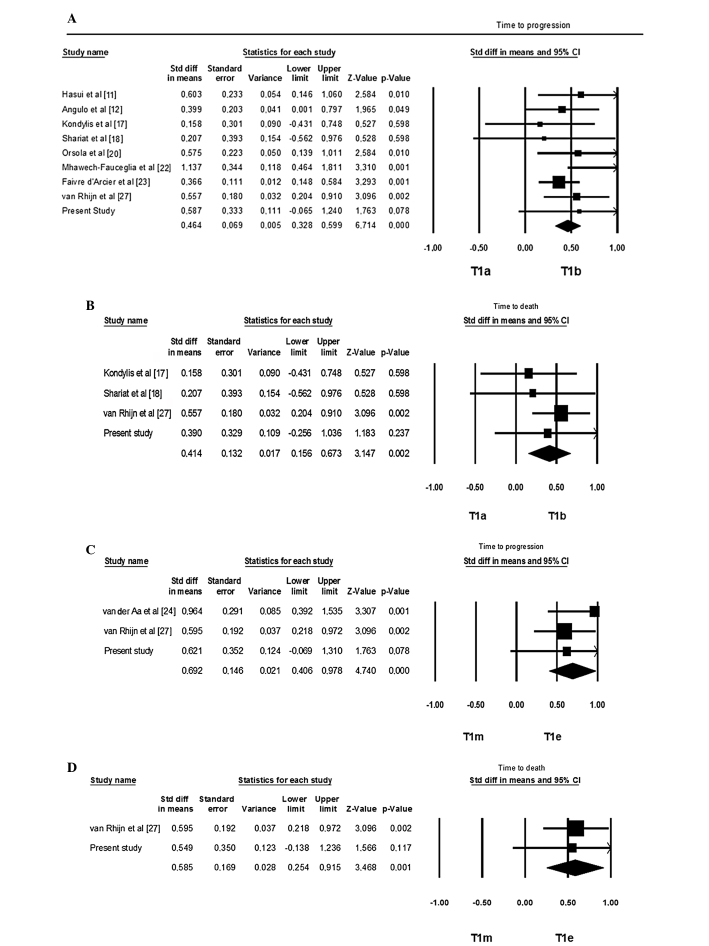
Faivre d’Arcier et al (23), analyzing a population 3 times more numerous and with a substaging percentage of 100%, reported a significant prognostic value for such substaging, not only with regard to the disease progression, but also with regard to the cancer-specific survival and OS. When performing a pooled analysis of data in the literature (including our own), despite the considerable heterogeneity among the studies, a significant prognostic role for T1a/T1b/T1c substaging emerged, with regard to disease progression and DSS (Fig. 2).
Figure 2.
(A) Pooled analysis of prognostic value of T1a/T1b/T1c substaging in relation to disease progression. (B) Pooled analysis of prognostic value of T1a/T1b/T1c substaging in relation to the disease-specific survival (DSS) in favour of T1a. (C) Pooled analysis of prognostic value of T1m/T1e substaging in relation to disease progression. (D) Cumulative analysis of the prognostic value of T1m/T1e substaging in relation to DSS in favour of T1m.
With regard to the T1m/T1e substaging, two studies investigated its prognostic value on disease progression (24,27) and only one on the DSS (27); those studies identified a significance at the multivariate analysis, which was not fully confirmed, however, by our study.
However, at the cumulative analysis of the literature data (including our own study), this substaging system was of significant prognostic value on disease progression and DSS, although with the same limitations regarding heterogeneity as in the other substaging system (Fig. 2).
All studies available in the literature, including ours, present with several limitations, such as the retrospective design, the small sample size and the lack of a standard TUR-BT. The advantage of the T1m/T1e substaging system is represented by its greater reproducibility and feasibility, regardless of the presence of the MM-VP.
In conclusion, although in our study the two substaging systems of T1 carcinoma of the bladder were not proven to be fully prognostic regarding tumour progression or DSS, the cumulative analysis of the literature indicated that both systems, namely the T1a vs. T1b/T1c and the T1m vs. T1 appear to be predictive of the behaviour of BC.
The T1m/T1e system may be more applicable and reproducible, being independent from the presence of MM-VP on the sample and independent from the quality of the initial TUR-BT. Therefore, this system may be a valid and useful prognostic tool for guiding the decision-making process for the treatment of patients with stage T1 BC.
However, further prospective, properly designed and well conducted cohort studies with adequate sample size are required in order to determine the optimal prognostic substaging model for T1 BC.
References
- 1.Anastasiadis A, de Reijke TM. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther Adv Urol. 2012;4:13–32. doi: 10.1177/1756287211431976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–477. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 3.van Rhijn BW, Burger M, Lotan Y, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009;56:430–442. doi: 10.1016/j.eururo.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Herr HW. Is maintenance Bacillus Calmette-Guérin really necessary? Eur Urol. 2008;54:971–973. doi: 10.1016/j.eururo.2008.06.062. [DOI] [PubMed] [Google Scholar]
- 7.Stein JP. Indications for early cystectomy. Urology. 2003;62:591–595. doi: 10.1016/s0090-4295(03)00584-3. [DOI] [PubMed] [Google Scholar]
- 8.Schrier BP, Hollander MP, van Rhijn BW, Kiemeney LA, Witjes JA. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol. 2004;45:292–296. doi: 10.1016/j.eururo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Stein JP, Penson DF. Invasive T1 bladder cancer: indications and rationale for radical cystectomy. BJU Int. 2008;102:270–275. doi: 10.1111/j.1464-410X.2008.07743.x. [DOI] [PubMed] [Google Scholar]
- 10.Younes M, Sussman J, True LD. The usefulness of the level of the muscularis mucosae in the staging of invasive transitional cell carcinoma of the urinary bladder. Cancer. 1990;66:543–548. doi: 10.1002/1097-0142(19900801)66:3<543::aid-cncr2820660323>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Hasui Y, Osada Y, Kitada S, et al. Significance of invasion to the muscularis mucosae on the progression of superficial bladder cancer. Urology. 1994;43:782–786. doi: 10.1016/0090-4295(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 12.Angulo JC, Lopez JI, Grignon DJ, et al. Muscularis mucosa differentiates two populations with different prognosis in stage T1 bladder cancer. Urology. 1995;45:47–53. doi: 10.1016/s0090-4295(95)96490-8. [DOI] [PubMed] [Google Scholar]
- 13.Platz CE, Cohen MB, Jones MP, et al. Is microstaging of early invasive cancer of the urinary bladder possible or useful? Mod Pathol. 1996;9:1035–1039. [PubMed] [Google Scholar]
- 14.Holmang S, Hedelin H, Anderstrom C, et al. The importance of the depth of invasion in stage T1 bladder carcinoma: a prospective cohort study. J Urol. 1997;157:800–804. doi: 10.1016/s0022-5347(01)65044-4. [DOI] [PubMed] [Google Scholar]
- 15.Smits G, Schaafsma E, Kiemeney L, et al. Microstaging of pT1 transitional cell carcinoma of the bladder: identification of subgroups with distinct risks of progression. Urology. 1998;52:1009–1014. doi: 10.1016/s0090-4295(98)00374-4. [DOI] [PubMed] [Google Scholar]
- 16.Hermann GG, Horn T, Steven K. The influence of the level of lamina propria invasion and the prevalence of p53 nuclear accumulation on survival in stage T1 transitional cell bladder cancer. J Urol. 1998;159:91–94. doi: 10.1016/s0022-5347(01)64021-7. [DOI] [PubMed] [Google Scholar]
- 17.Kondylis FI, Demirci S, Ladaga L, et al. Outcomes after intravesical bacillus Calmette-Guerin are not affected by substaging of high grade T1 transitional cell carcinoma. J Urol. 2000;163:1120–1123. [PubMed] [Google Scholar]
- 18.Shariat SF, Weizer AZ, Green A, et al. Prognostic value of p53 nuclear accumulation and histopathologic features in T1 transitional cell carcinoma of the urinary bladder. Urology. 2000;56:735–740. doi: 10.1016/s0090-4295(00)00756-1. [DOI] [PubMed] [Google Scholar]
- 19.Bernardini S, Billerey C, Martin M, et al. The predictive value of muscularis mucosae invasion and p53 over expression on progression of stage T1 bladder carcinoma. J Urol. 2001;165:42–46. doi: 10.1097/00005392-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Orsola A, Trias I, Raventos CX, et al. Initial high-grade T1 urothelial cell carcinoma: feasibility and prognostic significance of lamina propria invasion microstaging (T1a/b/c) in BCG-treated and BCG-non-treated patients. Eur Urol. 2005;48:231–238. doi: 10.1016/j.eururo.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Andius P, Johansson SL, Holmang S. Prognostic factors in stage T1 bladder cancer: tumor pattern (solid or papillary) and vascular invasion more important than depth of invasion. Urology. 2007;70:758–762. doi: 10.1016/j.urology.2007.06.638. [DOI] [PubMed] [Google Scholar]
- 22.Mhawech-Fauceglia P, Fischer G, Alvarez V, Jr, et al. Predicting outcome in minimally invasive (T1a and T1b) urothelial bladder carcinoma using a panel of biomarkers: a high throughput tissue microarray analysis. BJU Int. 2007;100:1182–1187. doi: 10.1111/j.1464-410X.2007.07090.x. [DOI] [PubMed] [Google Scholar]
- 23.Faivre d’Arcier B, Celhay O, Safsaf A, et al. T1 bladder carcinoma: prognostic value of the muscularis mucosae invasion (T1a/T1b). A multicenter study by the French Urological Association (CCAFU) Prog Urol. 2010;20:440–449. doi: 10.1016/j.purol.2010.02.002. (In French) [DOI] [PubMed] [Google Scholar]
- 24.van der Aa MN, van Leenders GJ, Steyerberg EW, et al. A new system for substaging pT1 papillary bladder cancer: a prognostic evaluation. Hum Pathol. 2005;36:981–986. doi: 10.1016/j.humpath.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Neumann RM, Weaver AL, et al. Predicting cancer progression in patients with stage T1 bladder carcinoma. J Clin Oncol. 1999;17:3182–3187. doi: 10.1200/JCO.1999.17.10.3182. [DOI] [PubMed] [Google Scholar]
- 26.van Rhijn BW, van der Kwast TH, Kakiashvili D, et al. Pathological stage review is indicated in primary pT1 bladder cancer. BJU Int. 2010;106:206–211. doi: 10.1111/j.1464-410X.2009.09100.x. [DOI] [PubMed] [Google Scholar]
- 27.van Rhijn BW, van der Kwast TH, Alkhateeb SS, et al. A new and higly prognostic system to discern T1 bladder cancer substage. Eur Urol. 2012;61:378–384. doi: 10.1016/j.eururo.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Nieder AM, Brausi M, Lamm D, et al. Management of stage T1 tumors of the bladder: International Consensus Panel. Urology. 2005;66(Suppl 1):108–125. doi: 10.1016/j.urology.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 29.Eble J, Sauter G, Epstein JI, Sesterhenn IA, editors. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press; Lyon: 2004. [Google Scholar]