Abstract
Radiation-induced fibrosis is one of the late complications of radiotherapy (RT) for nasopharyngeal carcinoma (NPC). The aim of this study was to investigate the association between X-ray repair cross-complementing protein 1 and 3 (XRCC1 and XRCC3, respectively) gene haplotypes and radiation-induced fibrosis in NPC patients. Genomic DNA was extracted from blood samples of 120 NPC patients previously treated with RT. In total, 12 tag single-nucleotide polymorphisms (SNPs) were selected from the XRCC1 and XRCC3 genes and were genotyped using restriction fragment length polymorphism analysis or unlabeled probe melting analysis. Single-marker and haplotype analyses were performed using multivariate logistic regression analysis. The functional variant rs861539 of XRCC3 may be associated with radiation-induced fibrosis [asymptotic P-value (Pasym)<0.05]. No significant association was observed between radiation-induced fibrosis and any of the tag SNPs of XRCC1 and XRCC3 in either single-marker or haplotype analysis after 10,000 permutations [empirical P-value (Pemp)>0.05]. Our preliminary results indicated that the rs861539 variant of XRCC3 may be associated with an increased risk of radiation-induced fibrosis; however, a large-scale study is required to confirm this result.
Keywords: nasopharyngeal carcinoma, chronic fibrosis, radiation toxicities, X-ray repair cross-complementing protein 1, X-ray repair cross-complementing protein 3
Introduction
Nasopharyngeal carcinoma (NPC) is a common type of cancer in Southern China, whereas its incidence in Western countries is relatively low (1). Due to its deep-seated anatomic location and relative radiosensitivity, radiotherapy (RT) is the treatment of choice for primary NPC. Advanced RT techniques, such as intensity-modulated RT (IMRT) have achieved improvements the locoregional control of the tumour and reduced the incidence of complications (2). However, radiation-induced fibrosis remains one of the major late complications, regardless of the treatment method (2,3). Since different patients may present with different degrees of skin fibrosis despite receiving identical treatment, it was hypothesized that the severity of such complications may be genetically determined. Previous studies investigated the association of single-nucleotide polymorphisms (SNPs) in genes involved in DNA repair, such as X-ray repair cross-complementing protein 1 (XRCC1) rs25487 (c.1196A>G, p.Gln399Arg) and X-ray repair cross-complementing protein 3 (XRCC3) rs861539 (c.722C>T, p.Thr241Met) with late complications in various types of cancer (4–18).
The identification of particular functional variants in cancer patients prior to the initiation of any treatment is likely to improve the prediction of the severity of radiation-induced complications in individual patients, leading to improved patient care and customization of treatment protocols. Since the majority of studies were conducted in Caucasian populations and allelic frequencies vary between ethnic groups, little is known regarding the association between genetic variants and post-RT complications in Chinese patients. The previously published studies mainly focused on particular functional variants of candidate genes; however, common genetic variants were overlooked. Therefore, this preliminary study aimed to evaluate the association of radiation-induced fibrosis with XRCC1 and XRCC3 functional variants and possible haplotypes in Chinese NPC patients.
Materials and methods
Subject recruitment
A total of 120 Chinese NPC patients, including 70.8% men and 29.2% women, aged ≥18 years, without distant metastasis, who were treated with conventional RT (CRT) or IMRT, were recruited during their follow-up between 2010 and 2011 at the Department of Clinical Oncology, Queen Mary Hospital, Hong Kong (Table I). RT was administered at 2 Gy per fraction, 5 fractions per week for 6–7 weeks. The patients who received CRT and IMRT generally received a total dose of 66–68 Gy and 70–76 Gy to the tumour, respectively. The patients were classified as ‘cases’ if they presented with persistent ≥grade 1 fibrosis at the time of the follow-up, according to the radiation morbidity scoring criteria published by the Radiation Therapy Oncology Group. Patients without significant fibrotic changes (grade 0) for ≥2-years post-RT were classified as ‘controls’. All the patients signed a written informed consent form prior to enrolment.
Table I.
Summary clinical characteristics of recruited patients.
| Clinical variables | Controls (n=91) | Cases (n=29) | P-value |
|---|---|---|---|
| Age, years [mean (SD)] | 52.60 (10.46) | 55.10 (9.05) | NS |
| Follow-up, years [mean (SD)] | 8.13 (5.57) | 12.38 (5.15) | <0.01 |
| Gender, n | <0.05 | ||
| Male | 69 | 16 | |
| Female | 22 | 13 | |
| TNM stage, n | NS | ||
| I | 16 | 0 | |
| II | 26 | 6 | |
| III | 29 | 15 | |
| IV | 16 | 4 | |
| Unavailable | 4 | 4 | |
| Radiotherapy, n | <0.01 | ||
| CRT | 26 | 19 | |
| IMRT | 65 | 10 | |
| Chemotherapy, n | 46 | 15 | NS |
NS, not significant; CRT, conventional radiotherapy; IMRT, intensity-modulated radiotherapy; TNM, tumor node metastasis.
Tag SNP selection and genotyping
The selection of the tag SNPs of XRCC1 and XRCC3 was performed with Tagger software (Cambridge, MA, USA) using the International HapMap Project data for Han Chinese subjects (release 27, phase II+III, Feb 9) (19,20). Tag SNPs were selected using a pairwise tagging algorithm, r2=0.8 and a minor allele frequency (MAF) of ≥0.2. Previously reported SNPs were also included in the analysis. In order to capture any upstream and downstream regulatory elements of the candidate gene, tag SNPs were selected from 3 kb at the start and the end of the candidate region. A total of 12 tag SNPs were selected and were genotyped using restriction fragment length polymorphism analysis (RFLP) or unlabeled probe melting analysis.
Genotyping method
For the DNA analysis of each patient, 6 ml venous blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes. DNA was extracted using a FlexiGene DNA kit (Qiagen, Hilden, Germany). The concentration of DNA was measured using NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, MA, USA). The DNA concentration was adjusted to 10 ng/μl using Tris-EDTA buffer for polymerase chain reaction (PCR). A total of 10 μl reaction mixture was used for both RFLP and unlabeled probe melting analysis. Each reaction mixture contained 10 ng of genomic DNA, 0.2 mM of each deoxynucleotide triphosphate, 0.3 U of HotStarTaq Plus DNA polymerase with 1X PCR buffer [containing Tris-Cl, KCl, (NH4)2SO4 and 15 mM MgCl2, pH 8.7] (Qiagen) and specific optimized primers and MgCl2 concentrations.
Statistical analysis
Genotyped data were analyzed using the PLINK statistical package (http://pngu.mgh.harvard.edu/~purcell/plink), version 1.07 (21). The genotypes in the control and case groups were tested for deviation from the Hardy-Weinberg equilibrium (HWE) using the Fisher’s exact test and the odds ratio (OR) for each genotype was calculated. Analysis of single markers was performed using the PLINK toolset. Gender, age, treatment regimen and other clinical factors were assessed by multivariate logistic regression analysis. Analyses of all the possible haplotypes using the sliding window approach were also performed using PLINK. Empirical P-values (Pemp) for single markers, as well as haplotype analysis, were generated by multiple comparisons based on 10,000 permutations. Genotype data from published studies were extracted to perform combined genotype analysis. Combined genotype analysis was performed with the Meta package, version 2.5.1 in R version 2.15.1 for Windows (http://cran.r-project.org/web/packages/meta/index.html) (22).
Results
Group comparison
Of the 120 patients, 45 received CRT and 75 received IMRT, whereas 61 patients were treated with chemotherapy. A total of 29 patients suffered from ≥grade 1 neck fibrosis that persisted for ≥2 years. There were significantly more patients who received CRT rather than IMRT in the case group compared to those in the control group (P<0.01). There were also significant differences in the mean follow-up duration (P<0.01) and in the number of men and women (P<0.05) between the control and case groups. There were no significant differences in the number of patients who received chemotherapy, tumor stage and mean age between the two groups (P>0.05). All the abovementioned clinical factors that may affect the severity of radiation-induced fibrosis were used as covariates in the logistic regression analysis.
Statistical analysis
All the tag SNPs were successfully genotyped and were in HWE (P>0.05), except the control group of tag SNPs rs861544 (P=0.003). This tag SNP was included in the analysis, since ~1 significant result could be obtained due to random chance with 12 comparisons when α=0.05.
Single-marker multivariate logistic regression analysis revealed that only the T allele of rs861539 was associated with increased risk of fibrosis [asymptotic P-value (Pasym)=0.0116; OR=3.88]. However, a significance level was lost after multiple comparisons (Pemp=0.0632). There was no significant association between fibrosis and the remaining tag SNPs in the single-marker multivariate logistic regression analysis (Table II).
Table II.
Summary statistics of selected tag SNPs in the XRCC1 and XRCC3 genes and results of single-marker analysis.
| Genotype counts 11/12/22 | MAF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Gene | SNPa | A1 | A2 | Control | Case | Control | Case | ORb | L95 | U95 | Pasym | Pemp |
| XRCC1 | rs3213282 (XRCC1.S1) | C | G | 4/35/52 | 2/11/16 | 0.2363 | 0.2586 | 1.19 | 0.53 | 2.65 | 0.6975 | 0.9899 |
| rs12611088 (XRCC1.S2) | T | C | 7/33/51 | 1/11/17 | 0.2582 | 0.2241 | 0.59 | 0.25 | 1.36 | 0.2345 | 0.6137 | |
| rs1001581 (XRCC1.S3) | A | G | 11/46/34 | 4/13/12 | 0.3736 | 0.3621 | 0.67 | 0.32 | 1.39 | 0.2900 | 0.7165 | |
| rs3213344 (XRCC1.S4) | G | C | 8/32/51 | 2/11/16 | 0.2637 | 0.2586 | 1.34 | 0.64 | 2.82 | 0.4430 | 0.8889 | |
| rs1799782 (XRCC1.S5) | T | C | 8/31/52 | 2/11/16 | 0.2582 | 0.2586 | 1.40 | 0.67 | 2.94 | 0.3833 | 0.8289 | |
| rs25487 (XRCC1.S6) | A | G | 6/35/50 | 1/13/15 | 0.2582 | 0.2586 | 0.80 | 0.36 | 1.79 | 0.6099 | 0.9705 | |
| XRCC3 | rs1799794 (XRCC3.S1) | G | A | 17/50/24 | 6/10/13 | 0.4615 | 0.3793 | 0.65 | 0.32 | 1.31 | 0.2408 | 0.5839 |
| rs861530 (XRCC3.S2) | G | A | 19/52/20 | 8/13/8 | 0.4945 | 0.5000 | 1.08 | 0.55 | 2.13 | 0.8371 | 0.9992 | |
| rs3212090 (XRCC3.S3) | A | G | 15/44/32 | 2/16/11 | 0.4066 | 0.3448 | 0.68 | 0.33 | 1.38 | 0.2959 | 0.6733 | |
| rs12432907 (XRCC3.S4) | A | G | 18/47/26 | 7/11/11 | 0.4560 | 0.4310 | 0.82 | 0.42 | 1.62 | 0.5821 | 0.9502 | |
| rs861539 (XRCC3.S5) | T | C | 1/8/82 | 0/8/21 | 0.0550 | 0.1379 | 3.88 | 1.25 | 12.07 | 0.0116c | 0.0632 | |
| rs861544 (XRCC3.S6) | T | C | 10/59/22 | 3/18/8 | 0.4341 | 0.4138 | 0.94 | 0.43 | 2.05 | 0.8795 | 0.9999 | |
Tag SNPs were listed down the column according to their position on the target gene from the 5′ to 3′ end of the sense strand.
OR was calculated using A2 as a reference.
SNP, single nucleotide polymorphism; A1, minor allele; A2, major allele; MAF, minor allele frequency; OR, odds ratio; L95, lower boundary of the 95% confidence interval; U95, upper boundary of the 95% confidence interval; Pasym, asymptotic P-value; Pemp, empirical P-value. XRCC1, X-ray repair cross-complementing protein-1; XRCC3, X-ray repair cross-complementing protein-3.
A total of 42 sliding windows were generated for XRCC1 and XRCC3, with 21 sliding windows created for each gene. No association was found in any of the sliding windows following multiple comparisons (omnibus test Pemp>0.05) (Table III).
Table III.
Summary of sliding window haplotype analysis of all the possible sizes based on omnibus test of the XRCC1 and XRCC3 genes.
| Most significant omnibus test | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Gene | NSNP | First SW | Last SW | SW | Pasym | Pemp |
| XRCC1 | 2 | XRCC1.S1..XRCC1.S2 | XRCC1.S5..XRCC1.S6 | XRCC1.S4..XRCC1.S5 | 0.4500 | 0.9159 |
| 3 | XRCC1.S1..XRCC1.S3 | XRCC1.S4..XRCC1.S6 | XRCC1.S2..XRCC1.S4 | 0.2924 | 0.8450 | |
| 4 | XRCC1.S1..XRCC1.S4 | XRCC1.S3..XRCC1.S6 | XRCC1.S2..XRCC1.S5 | 0.2925 | 0.8450 | |
| 5 | XRCC1.S1..XRCC1.S5 | XRCC1.S2..XRCC1.S6 | XRCC1.S2..XRCC1.S6 | 0.0877 | 0.5960 | |
| 6 | XRCC1.S1..XRCC1.S6 | XRCC1.S1..XRCC1.S6 | XRCC1.S1..XRCC1.S6 | 0.2633 | 0.8891 | |
| XRCC3 | 2 | XRCC3.S1..XRCC3.S2 | XRCC3.S5..XRCC3.S6 | XRCC3.S5..XRCC3.S6 | 0.0147 | 0.1944 |
| 3 | XRCC3.S1..XRCC3.S3 | XRCC3.S4..XRCC3.S6 | XRCC3.S1..XRCC3.S3 | 0.0561 | 0.3531 | |
| 4 | XRCC3.S1..XRCC3.S4 | XRCC3.S3..XRCC3.S6 | XRCC3.S1..XRCC3.S4 | 0.0301 | 0.3231 | |
| 5 | XRCC3.S1..XRCC3.S5 | XRCC3.S2..XRCC3.S6 | XRCC3.S2..XRCC3.S6 | 0.1132 | 0.6833 | |
| 6 | XRCC3.S1..XRCC3.S6 | XRCC3.S1..XRCC3.S6 | XRCC3.S1..XRCC3.S6 | 0.0339 | 0.4571 | |
NSNP, number of single-nucleotide polymorphisms in this haplotype; SW, sliding window; Pasym, asymptotic P-value; Pemp, empirical P-value. XRCC1, X-ray repair cross-complementing protein-1; XRCC3, X-ray repair cross-complementing protein-3.
The forest plots of rs25487 from XRCC1 and rs861539 from XRCC3 in NPC patients are shown in Figs. 1 and 2. The percentage of between-study variation due to heterogeneity is presented as I2 (23). There was no significant difference for the two SNPs analyzed by the random effects model (P>0.05). However, a significant difference was observed when the fixed effects model was used for rs25487 (P=0.0415).
Figure 1.
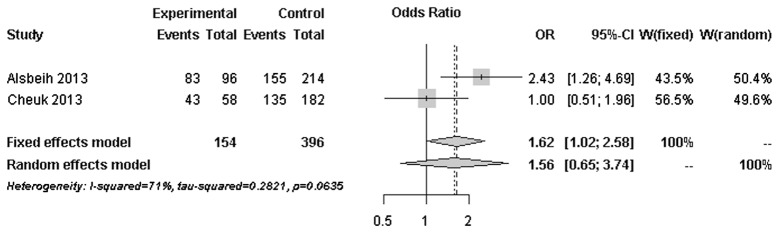
Forest plot of rs25487 (G vs. A) from the XRCC1 gene. Odd ratios (ORs) of the major allele (G) were obtained. The allele is protective when OR<1. CI, confidence interval; XRCC1, X-ray repair cross-complementing protein-1.
Figure 2.
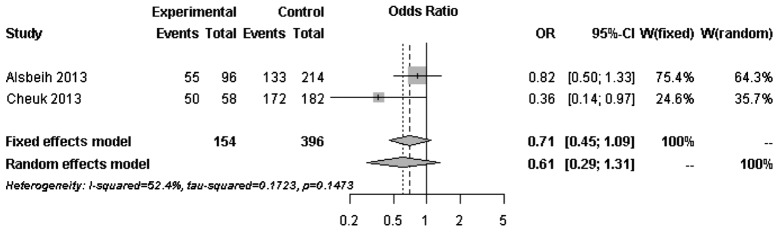
Forest plot of rs861539 (C vs. T) from the XRCC3 gene. Odd ratios (ORs) of the major allele (C) were obtained. The allele is protective when OR<1. CI, confidence interval; XRCC3, X-ray repair cross-complementing protein-3.
Discussion
In this retrospective study, the association of DNA repair genes with the development of post-RT fibrosis in Chinese NPC patients was investigated. Selected tag SNPs, as well as previously reported functional SNPs that were associated with late complications, were determined to obtain comprehensive information on common genetic variants of the XRCC1 and XRCC3 genes and their association with radiation-induced fibrosis. Additional haplotype analysis was performed in order to investigate the effects of all the possible haplotypes on radiation-induced fibrosis. No significant association was found in the SNPs in either single-marker or haplotype analysis following multiple comparisons.
Radiation-induced fibrosis, unlike normal wound healing, is a complex process that its endpoint is the result of accumulated chronic inflammatory reactions (24). Since DNA is the critical target of ionizing radiation damage, it is hypothesized that functional variants in DNA repair genes may affect the DNA repair capacity and lead to variations in radiation sensitivity. XRCC1 and XRCC3 are two of the genes that are involved in DNA repair. The association of functional variants of these genes with radiation-induced late complications was previously investigated with inconsistent results (25). The majority of the published studies included breast and prostate cancer patients in Caucasian populations. No association with rs25487 of XRCC1 and rs861539 of XRCC3 was identified. Our results were in line with findings reported by other studies with relatively large sample sizes (16,17). However, our results were in contrast to those reported by 3 studies performed in Saudi Arabian NPC patients by Alsbeih et al (4,5,18). Our results indicated a trend of possible association between rs861539 of XRCC3 and radiation-induced fibrosis in Chinese NPC patients, but not rs25487 of XRCC1. In order to investigate the heterogeneity between studies with a similar study design, forest plots of rs25487 and rs861539 were constructed based on genotyped data from our study and the most recent study by Alsbeih et al (18).
Moderate to significant heterogeneity was observed for rs25487 and rs861539. The discrepancies between studies may be due to variations in the study design, length of follow-up period and allele frequencies in different ethnic groups. The MAFs of rs25487 obtained by Alsbeih et al (18) and the present study were 0.232 and 0.258, respectively. The MAFs of rs861539 obtained by Alsbeih et al (18) and the present study were 0.394 and 0.075, respectively. While the deviation of allele frequencies between the two studies was small for rs25487, an increased risk of developing fibrosis was not identified by the present study. The median follow-up time was 8 years in the present study and 40 months in the study by Alsbeih et al (18). Since late toxicities may progress over time several years after post-RT, the length of the follow-up is significantly associated with the development of fibrosis.
Normal tissue radiosensitivity is a complex phenomenon. Unlike truncated proteins, such as ataxia telangiectasia mutated, particular functional variants in selected genes may not be sufficient to cause severe radiosensitivity. Apart from specific functional variants, the role of other common variants of candidate genes and their effect have not been clearly determined. This study aimed to investigate the association of common and functional variants of DNA repair genes with fibrosis in NPC patients. We captured certain common variants and constructed haplotype structures of all possible sizes using tag SNPs, in order to obtain more information on the effects of DNA repair genes. One of the major limitations of our study was the relatively small sample size. A small sample size may not have enough power to detect SNPs with small and modest effects. Genotyped SNPs with small and modest effects were possibly not the causal SNPs of this phenomenon, but served as surrogates of other SNPs in high linkage disequilibrium located in distant regions. In addition, the effect sizes of common variants are likely to be small, in contrast to the large effect caused by rare variants (26,27). Therefore, individual common variants may not be ideal universal biomarkers for identification of radiosensitivity.
Two large cohort studies focusing on radiation-induced toxicities developed in breast and prostate cancers were recently published (26,28). One study by Barnett et al (26), involving 1,613 breast and prostate cancer patients, captured common variants using tag SNPs with MAF<0.05 and functional SNPs in 92 genes and found no association in any of the SNPs. Furthermore, a replication study by Talbot et al (28), involving 2,036 patients from three cohorts, captured 43 candidate SNPs in 35 genes and found that TNF-α may be associated with increased risk of radiation toxicities in breast cancer patients. No previously reported associations were found to be significant in those studies. The negative findings of the studies indicated that previously published SNPs may not exert clinical effects individually and significant SNPs may have small effect sizes.
The first genome-wide association analysis focused on the association of African-American prostate cancer patients with the risk of radiation-induced erectile dysfunction suggested that cancer type and ethnicity may be genetic predictors (29). Based on our findings, the low-frequency variant rs861539 may be an ethnic group-specific marker in predicting radiation-induced toxicities in Chinese NPC patients. The amount of radiation dose received by NPC patients is generally different from that received by breast and prostate cancers patients, regardless of the different RT methods. Non-irradiated cells were shown to exhibit similar inflammatory responses as irradiated cells (30). In addition to genetic factors, epigenetic factors may also affect individual radiosensitivity. Large-scale studies should also be conducted in combined ethnic groups with all the types of cancer that require RT as standard treatment, in order to investigate the effects of common and functional variants. With the advancements in technology, the genetic profiles of individual patients may be obtained to assess the risk effect or perform a combined analysis. In addition, meta-analyses may be performed to assess the risk of radiation-induced toxicities in NPC patients and obtain more cancer type-specific information. The Standardized Total Average Toxicity score can be used in future studies to enhance data pooling (31). Since normal tissue radiosensitivity may be the result of the combined effects of genes involved in different cellular pathways, functional study focus on the performance of DNA repair proteins following irradiation with respect to genotypes and epigenetic changes may be conducted. Casual common or functional variants may be used as pre-treatment biomarkers for identifying highly radiosensitive cancer patients, leading to the customization of treatment protocols and improved treatment outcomes in the future.
References
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 2.Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440–1450. doi: 10.1016/j.ijrobp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, Law SC, Ng SH, et al. Retrospective analysis of nasopharyngeal carcinoma treated during 1976–1985 late complications following megavoltage irradiation. Br J Radiol. 1992;65:918–928. doi: 10.1259/0007-1285-65-778-918. [DOI] [PubMed] [Google Scholar]
- 4.Alsbeih G, Al-Harbi N, Al-Hadyan K, El-Sebaie M, Al-Rajhi N. Association between normal tissue complications after radiotherapy and polymorphic variations in TGFB1 and XRCC1 genes. Radiat Res. 2010;173:505–511. doi: 10.1667/RR1769.1. [DOI] [PubMed] [Google Scholar]
- 5.Alsbeih GA, El-Sebaie MM, Al-Rajhi NM, et al. Association between XRCC1 G399A polymorphism and late complications to radiotherapy in Saudi head and neck cancer patients. J Egypt Natl Canc Inst. 2008;20:302–308. [PubMed] [Google Scholar]
- 6.Andreassen CN, Alsner J, Overgaard J, et al. TGFB1 polymorphisms are associated with risk of late normal tissue complications in the breast after radiotherapy for early breast cancer. Radiother Oncol. 2005;75:18–21. doi: 10.1016/j.radonc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Andreassen CN, Alsner J, Overgaard M, Overgaard J. Prediction of normal tissue radiosensitivity from polymorphisms in candidate genes. Radiother Oncol. 2003;69:127–135. doi: 10.1016/j.radonc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Azria D, Ozsahin M, Kramar A, et al. Single nucleotide polymorphisms, apoptosis, and the development of severe late adverse effects after radiotherapy. Clin Cancer Res. 2008;14:6284–6288. doi: 10.1158/1078-0432.CCR-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brem R, Cox DG, Chapot B, et al. The XRCC1 -77T->C variant: haplotypes, breast cancer risk, response to radiotherapy and the cellular response to DNA damage. Carcinogenesis. 2006;27:2469–2474. doi: 10.1093/carcin/bgl114. [DOI] [PubMed] [Google Scholar]
- 10.Burri RJ, Stock RG, Cesaretti JA, et al. Association of single nucleotide polymorphisms in SOD2, XRCC1 and XRCC3 with susceptibility for the development of adverse effects resulting from radiotherapy for prostate cancer. Radiat Res. 2008;170:49–59. doi: 10.1667/RR1219.1. [DOI] [PubMed] [Google Scholar]
- 11.Damaraju S, Murray D, Dufour J, et al. Association of DNA repair and steroid metabolism gene polymorphisms with clinical late toxicity in patients treated with conformal radiotherapy for prostate cancer. Clin Cancer Res. 2006;12:2545–2554. doi: 10.1158/1078-0432.CCR-05-2703. [DOI] [PubMed] [Google Scholar]
- 12.Giotopoulos G, Symonds RP, Foweraker K, et al. The late radiotherapy normal tissue injury phenotypes of telangiectasia, fibrosis and atrophy in breast cancer patients have distinct genotype-dependent causes. Br J Cancer. 2007;96:1001–1007. doi: 10.1038/sj.bjc.6603637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moullan N, Cox DG, Angèle S, Romestaing P, Gerard JP, Hall J. Polymorphisms in the DNA repair gene XRCC1, breast cancer risk, and response to radiotherapy. Cancer Epidemiol Biomarkers Prev. 2003;12:1168–1174. [PubMed] [Google Scholar]
- 14.Suga T, Iwakawa M, Tsuji H, et al. Influence of multiple genetic polymorphisms on genitourinary morbidity after carbon ion radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:808–813. doi: 10.1016/j.ijrobp.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Zschenker O, Raabe A, Boeckelmann IK, et al. Association of single nucleotide polymorphisms in ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with clinical and cellular radiosensitivity. Radiother Oncol. 2010;97:26–32. doi: 10.1016/j.radonc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Chang-Claude J, Ambrosone CB, Lilla C, et al. Genetic polymorphisms in DNA repair and damage response genes and late normal tissue complications of radiotherapy for breast cancer. Br J Cancer. 2009;100:1680–1686. doi: 10.1038/sj.bjc.6605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popanda O, Marquardt JU, Chang-Claude J, Schmezer P. Genetic variation in normal tissue toxicity induced by ionizing radiation. Mutat Res. 2009;667:58–69. doi: 10.1016/j.mrfmmm.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Alsbeih G, El-Sebaie M, Al-Harbi N, Al-Hadyan K, Shoukri M, Al-Rajhi N. SNPs in genes implicated in radiation response are associated with radiotoxicity and evoke roles as predictive and prognostic biomarkers. Radiat Oncol. 2013;8:125. doi: 10.1186/1748-717X-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 20.International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 21.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. [Accessed, July 19, 2013];Am J Hum Genet. 2007 81:559–575. doi: 10.1086/519795. http://pngu.mgh.harvard.edu/~purcell/plink. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarzer G. Meta: An R package for meta-analysis. [Accessed, September 8, 2013];R News. 2007 7:40–45. http://cran.r-project.org/web/packages/meta/index.html. [Google Scholar]
- 23.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, version 5.10. The Cochrane Collaboration; 2011. [Accessed July 27, 2013]. http://handbook.cochrane.org. [Google Scholar]
- 24.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 25.Andreassen CN, Alsner J. Genetic variants and normal tissue toxicity after radiotherapy: a systematic review. Radiother Oncol. 2009;92:299–309. doi: 10.1016/j.radonc.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Barnett GC, Coles CE, Elliott RM, et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: a prospective analysis study. Lancet Oncol. 2012;13:65–77. doi: 10.1016/S1470-2045(11)70302-3. [DOI] [PubMed] [Google Scholar]
- 27.West CM, Dunning AM, Rosenstein BS. Genome-wide association studies and prediction of normal tissue toxicity. Semin Radiat Oncol. 2012;22:91–99. doi: 10.1016/j.semradonc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Talbot CJ, Tanteles GA, Barnett GC, et al. A replicated association between polymorphisms near TNFα and risk for adverse reactions to radiotherapy. Br J Cancer. 2012;107:748–753. doi: 10.1038/bjc.2012.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerns SL, Ostrer H, Stock R, et al. Genome-wide association study to identify single nucleotide polymorphisms (SNPs) associated with the development of erectile dysfunction in African-American men after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:1292–1300. doi: 10.1016/j.ijrobp.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorimore SA, Coates PJ, Wright EG. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. 2003;22:7058–7069. doi: 10.1038/sj.onc.1207044. [DOI] [PubMed] [Google Scholar]
- 31.Barnett GC, West CM, Coles CE, et al. Standardized total average toxicity score: a scale- and grade-independent measure of late radiotherapy toxicity to facilitate pooling of data from different studies. Int J Radiat Oncol Biol Phys. 2012;82:1065–1074. doi: 10.1016/j.ijrobp.2011.03.015. [DOI] [PubMed] [Google Scholar]


