Abstract
This study was conducted to compare the therapeutic effect and complications of laparoscopic radical gastrectomy (LRG) with those of traditional open surgery in elderly patients with gastric cancer (GC). We conducted a retrospective comparison of therapeutic efficacy and complications between elderly patients with GC (defined as those aged ≥70 years) who received laparoscopic gastrectomy and those who underwent gastrectomy by open surgery. A total of 108 patients who either underwent laparoscopic surgery (n=54) or traditional open surgery (n=54) at the General Hospital of Lanzhou Military Region between June, 2008 and March, 2009 were analyzed. Compared to traditional open surgery, LRG exhibited several advantages, such as being less invasive, with less intraoperative blood loss, shorter bedbound time, less intubation time, low frequency of fever, less time to normal diet, shorter hospital stay and a low overall incidence of complications. No significant difference was observed between laparoscopic and open surgery in terms of operative time and number of lymph nodes dissected. The 3-year cancer recurrence and mortality rates were similar in the two groups. In conclusion, LRG is a safe and effective procedure for the management of GC in elderly patients and was found to be superior to traditional open surgery regarding the short-term curative effect. Therefore, LRG represents a feasible and safe surgical approach for elderly patients with GC.
Keywords: gastric cancer, laparoscopic radical gastrectomy, traditional open surgery, curative effect, prognosis
Introduction
Gastric cancer (GC) is one of the most common cancers worldwide, accounting for ~700,000 deaths annually (1). The incidence of GC among elderly individuals has gradually increased due to the improvement in living conditions, extended life span and aging of the population. As recently reported, the majority of the new cases of GC occurred in elderly individuals (2).
The symptoms of GC in elderly patients are generally atypical and are often masked by concurrent diseases. Elderly patients do not usually tolerate surgery as well and often suffer from multiple postoperative complications, hence the poor prognosis (3). Therefore, gastrectomy may be a challenging surgical procedure for elderly patients with GC.
Laparoscopic radical gastrectomy (LRG) was first introduced in 1994 and has since been considered to be a viable surgical option for GC (4). This procedure is associated with minimal postoperative pain, low degree of trauma, rapid recovery of gastrointestinal function and reduced hospital stay. In addition, this procedure has only a minimal impact on immune function and is thus associated with better survival and improved quality of life. Therefore, LRG has become one of the optimal surgical approaches for the treatment of GC.
Clinical trials in younger patients indicated that LRG is associated with similar or fewer intraoperative and postoperative complications compared to open surgery (5–7). However, the safety profile, feasibility and curative effects of LRG have not been clearly determined in elderly patients. In the present retrospective study, we compared the benefits, complications and patient survival profile between elderly patients who underwent a laparoscopic procedure and those who underwent open surgery in a single centre in China.
Materials and methods
Patients
This study included 108 patients aged >70 years who underwent radical gastrectomy at the General Hospital of Lanzhou Military Region between June, 2008 and March, 2009. All the patients had an American Society of Anesthesiologists score ≥IV (8) and were considered to be suitable for surgery. In all the cases, surgery was performed by the same surgical team and the diagnosis was confirmed by postoperative pathology.
No patients exhibited evidence of remote metastasis on gastroscopy, computed tomography scan or magnetic resonance imaging examination. No patients had received any immunotherapy or had undergone preoperative chemo- and/or radiotherapy within 6 months of surgery. Furthermore, no patients experienced a switch from laparoscopic surgery to an open approach intraoperatively.
Clinical manifestations
All the patients presented with various degrees of abdominal discomfort. The major clinical manifestations included upper quadrant abdominal pain or bloating (n=86, 79.6%), loss of appetite (n=64, 59.3%), fatigue or weight loss (n=82, 75.9%), melena (n=34, 34.5%), nausea and vomiting (n=34, 34.5%) and a choking sensation during eating (n=23, 32.5%). The interval between the onset of symptoms and hospital admission was <1 month in 37 patients (34.4%), 1–3 months in 45 patients (41.7%), 4–6 months in 18 patients (16.7%) and >6 months in 8 patients (7.4%).
The major comorbidities included cardiovascular or respiratory diseases, diabetes mellitus, hypoproteinemia, central nervous system disorders and electrolyte imbalance.
Surgical approach
The patients were randomly divided into a laparoscopic operation group (n=54) and a traditional open surgery group (n=54). All the patients underwent standard lymph node dissection, according to the GC D2 radical procedure (9). In the laparoscopic group, 42 patients underwent radical surgery and 12 received palliative surgery. The type of procedures included total (n=31), proximal (n=14) and distal gastrectomy (n=9). In the open surgery group, 44 patients received radical surgery and 10 received palliative surgery. The type of procedures in this group also included total (n=33), proximal (n=11) and distal gastrectomy (n=10).
Postoperatively, all the patients received 6 cycles of standard FOLFOX4 chemotherapy.
Statistical analysis
The statistical analysis was performed using SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA). Continuous data were analyzed using t-tests and are presented as means ± standard deviation. Categorical data were analyzed using the Chi-square test. The log-rank test was used to evaluate survival data. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The study population included 66 men and 42 women, aged 70–89 years (mean age, 77.6 years). No significant difference between the laparoscopic and open surgical groups was observed in terms of age, gender distribution, pathological type of cancer, TMN status, clinical stage and preoperative comorbidities (Table I).
Table I.
Patient characteristics.
| Parameters | LRG, n=54 (%) | Open surgery, n=54 (%) | χ2 | P-value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 78.6±6.5 | 76.5±7.2 | 0.135 | |
| Male/female | 36/18 | 30/24 | 1.40 | 0.236 |
| Pathological type | 0.89 | 0.828 | ||
| Well differentiated adenocarcinoma | 13 (24.1) | 17 (31.5) | ||
| Moderately differentiated adenocarcinoma | 6 (11.1) | 5 (9.3) | ||
| Poorly differentiated adenocarcinoma | 32 (59.3) | 30 (55.5) | ||
| Signet ring cell carcinoma | 3 (5.5) | 2 (3.7) | ||
| Degree of invasion | 0.38 | 0.83 | ||
| T1 | 5 (9.3) | 4 (7.4) | ||
| T2 | 23 (42.6) | 26 (48.1) | ||
| T3 | 26 (48.1) | 24 (44.5) | ||
| Lymph node metastasis | 0.68 | 0.88 | ||
| N0 | 3 (5.5) | 5 (9.3) | ||
| N1 | 23 (42.6) | 21 (38.9) | ||
| N2 | 21 (38.9) | 20 (37.0) | ||
| N3 | 7 (13.0) | 8 (14.8) | ||
| Clinical staging | 0.34 | 0.56 | ||
| I/II | 24 (44.4) | 21 (38.9) | ||
| III/IV | 30 (55.6) | 33 (61.1) | ||
| Preoperative comorbidities/conditions | 1.21 | 0.94 | ||
| Cardiovascular disorders | 33 (61.1) | 34 (63.0) | ||
| Diabetes mellitus | 12 (22.2) | 16 (29.6) | ||
| Respiratory diseases | 23 (42.6) | 19 (35.2) | ||
| Hypoproteinemia | 13 (24.1) | 13 (24.1) | ||
| Cerebral infarction | 6 (11.1) | 5 (9.3) | ||
| Electrolyte disorders | 11 (20.4) | 13 (24.1) | ||
| Total | 46 (85.2) | 44 (81.5) | 0.27 | 0.61 |
LRG, laparoscopic radical gastrectomy; SD, standard deviation.
Intraoperative and postoperative outcomes
Except for the operative time required and the number of lymph nodes dissected, which did not differ significantly between the two types of surgical procedures (P-values of 0.201 and 0.167, respectively), all other major intra- and postoperative indices were significantly superior with LRG compared to those with open surgery. For example, compared to patients who underwent open surgery, patients who received laparoscopic surgery exhibited a significantly reduced intraoperative blood loss (103.0±34.4 vs. 140.6±44.4 ml, P<0.000), a significantly smaller incision size (5.18±0.7 vs. 17.8±1.0 cm, P<0.000) and a significantly shorter bedbound time (1.0±0.3 vs. 3.2±0.5 days, P=0.000). Further details are shown in Table II.
Table II.
Intraoperative and postoperative data.
| Parameters | LRG, n=54 (mean ± SD) | Open surgery, n=54 | t-value | P-value |
|---|---|---|---|---|
| Intraoperative | ||||
| Operation time (min) | 179.4±22.5 | 173.0±28.8 | 1.288 | 0.201 |
| Blood loss (ml) | 103.0±34.4 | 140.6±44.4 | −4.917 | 0.000 |
| Lymph nodes resected (no.) | 27.8±3.9 | 26.7±4.6 | 1.391 | 0.167 |
| Incision length (cm) | 5.18±0.7 | 17.8±1.0 | −74.258 | 0.000 |
| Postoperative | ||||
| Bedbound time (days) | 1.0±0.3 | 3.2±0.5 | −29.766 | 0.000 |
| Intubation time (days) | 0.8±0.2 | 1.6±0.7 | −7.921 | 0.000 |
| Fever (days) | 2.2±0.8 | 3.2±0.8 | −8.593 | 0.000 |
| Time to normal diet (days) | 3.0±0.4 | 3.8±0.8 | −8.942 | 0.000 |
| Time to bowel open (days) | 3.2±0.8 | 3.6±0.7 | −2.473 | 0.015 |
| Hospital stay (days) | 7.0±1.3 | 9.4±1.5 | −8.987 | 0.000 |
LRG, laparoscopic radical gastrectomy; SD, standard deviation.
Postoperative complications
Postoperative complications were reported in 8 patients (14.8%) who underwent laparoscopic surgery and in 16 (29.6%) who received open surgery (P=0.04). One patient who underwent open surgery succumbed to a lung and abdominal infection and subsequent multiple organ failure. There were no reported deaths in the laparoscopic surgery group. Further details on postoperative complications are listed in Table III.
Table III.
Postoperative complications.
| Complicationsa | LRG, n=54 (%) | Open surgery, n=54 (%) | P-value |
|---|---|---|---|
| Gastrointestinal hypomotility | 2 (3.7) | 3 (5.6) | |
| Anastomotic bleeding | 0 | 0 | |
| Anastomotic leakage | 3 (5.6) | 5 (9.3) | |
| Abdominal infection | 3 (5.6) | 6 (11.1) | |
| Poor healing of incision | 2 (3.7) | 9 (16.7) | |
| Urinary tract infection | 1 (1.9) | 5 (9.3) | |
| Pulmonary infection | 3 (5.6) | 5 (9.3) | |
| Respiratory failure | 0 | 1 (1.9) | |
| Liver/kidney dysfunction | 2 (3.7) | 1 (1.9) | |
| Heart failure | 1 (1.9) | 1 (1.9) | |
| Deep venous thrombosis | 0 | 0 | |
| Intestinal obstruction | 1 (1.9) | 4 (7.4) | |
| Death | 0 | 1 (1.9) | |
| Total | 8 (14.8) | 16 (29.6) | 0.040 |
A total of 11 patients presented with ≥2 complications.
LRG, laparoscopic radical gastrectomy.
Follow-up
The patients were followed up for 3 years. The 6-month, 1- and 3-year survival rates were 92.6, 85.2 and 55.6%, respectively, among patients who underwent laparoscopic surgery and 88.9, 81.5 and 57.4% respectively, among those who underwent open surgery (Table IV). The median survival was 26.8 months (95% confidence interval: 23.6–30.0) in both groups.
Table IV.
Number of patients who survived during the follow-up period.
| Time (years) | LRG, n=54 (%) | Open surgery, n=54 (%) | χ2 | P-value |
|---|---|---|---|---|
| 1/2 | 50 (92.6) | 48 (88.9) | 0.441 | 0.507 |
| 1 | 46 (85.2) | 44 (81.5) | 0.267 | 0.606 |
| 3 | 30 (55.6) | 31 (57.4) | 0.038 | 0.846 |
LRG, laparoscopic radical gastrectomy.
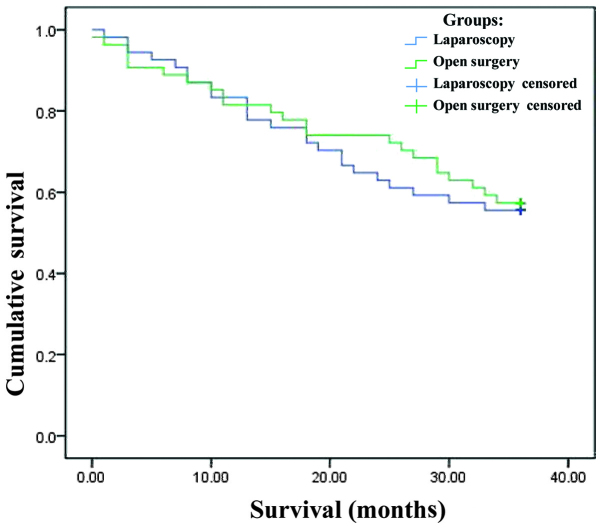
The Kaplan-Meier survival curves for the two groups are shown in Fig. 1. By the log-rank test, there was no significant difference in survival between the two surgical techniques (χ2=0.079; P=0.779).
Figure 1.
Survival functions.
All the patients who underwent palliative surgery succumbed to disease progression during the 3-year follow-up period. The causes of death in the laparoscopic surgery group included cardiovascular accidents (n=10), extensive abdominal and peritoneal metastasis (n=8) and liver metastasis (n=3). Of the patients who underwent palliative treatment through open surgery, 7 died due to cardiovascular accidents, 9 due to extensive abdominal and peritoneal metastasis and 2 due to liver metastasis (data not shown).
Discussion
The symptoms of GC are usually not overt in elderly patients, partly due to an underlying reduction in physiological functionality and partly because the symptoms are often attributed to other comorbidities. Elderly patients with early-stage GC are often either asymptomatic or present with non-specific symptoms, such as dull pain in the upper quadrant, abdominal discomfort, loss of appetite and weight loss (10). Therefore, at the time of diagnosis, the disease has often reached an advanced stage and is difficult to treat. Consequently, in all suspected GC cases, endoscopy and upper gastrointestinal barium meal examination are required for early diagnosis.
Surgery remains the major therapeutic approach for GC. However, elderly patients may not be able to tolerate surgery and are more susceptible to postoperative complications and increased mortality risk as a result of multiple concurrent diseases and declining organ function.
A number of elderly patients exhibit a poor compensatory ability due to a weakened immune system, which may be exacerbated by long-term nutritional insufficiency and digestive tract hemorrhage. The presence of multiple coexisting diseases highlights the significance of a thorough preoperative investigation of heart, lung, liver and kidney function, as well as determination of the coagulation profile and blood glucose levels. Malnutrition and anemia should be corrected prior to surgery and steps should be taken to improve the respiratory function, such as quitting smoking and physiotherapy to improve effective coughing and expectoration. Palliative surgery should be considered for patients with multiple, severe concurrent diseases.
The first laparoscopic partial gastrectomy for early-stage GC was undertaken in 1994 in an attempt to reduce the iatrogenic trauma associated with gastrectomy (11). This was followed 5 years later by LRG for advanced GC, including D2 lymph node dissection (12). A retrospective study of 1,294 patients undergoing gastrectomy at 16 medical centers in Japan reported that laparoscopic and open surgery were of equivalent therapeutic value, feasibility and safety (13). Based on those findings, laparoscopic surgery has been accepted as a novel minimally invasive surgical option for GC patients. However, elderly patients are generally less tolerant to the pneumoperitoneum that forms an essential part of the laparoscopic procedure. The relatively long operative time required for LRG has also limited its application in elderly patients.
Our previous experience with laparoscopic surgery suggests that this type of procedure is able to preserve the integrity of the dissected lymph nodes and lymph ducts, provided that any associated lymphatic and connective tissues are removed en bloc. Laparoscopy facilitates this part of the operative procedure, as the surgical view is amplified, enabling complete removal of all malignant tissue and reducing the risk of recurrence.
Our present study revealed that laparoscopic surgery is advantageous over open surgery in a number of important postoperative endpoints (Table III), which is is partially in line with previously reported data (14). Compared to open surgery, laparoscopic surgery was found to be associated with less intraoperative blood loss, rapid intestinal recovery (i.e., shorter time to bowel function following surgery), less bedbound time, less intubation time and less overall hospital stay. Unlike previous reports suggesting that laparoscopy requires a significantly longer time compared to open gastrectomy in elderly patients, our data demonstrated that the mean operative time was longer by only 6.4 minutes with laparoscopic surgery compared to that with open surgery; this difference was marginal and of no statistic significance. As the laparoscopic procedure is more complicated compared to open surgery, a longer learning curve is required. However, to certain extent, the duration of surgery depends on the experience of the surgeon and the complexity of each case.
In line with previous studies, our analysis indicated that laparoscopic surgery is associated with fewer postoperative complications compared to open surgery. A recent study in GC patients reported a postoperative complication rate of 25.3% with laparoscopic surgery (n=629) and 40.1% with open surgery (n=1,002). In our study, the overall postoperative complication rate was 14.8% with laparoscopic surgery (n=8) and 29.6% with open surgery (n=16). When used for radical and palliative gastrectomy with lymph node dissection, the two procedures had a similar impact on long-term survival, which is consistent with previous studies (15,16).
In summary, LRG appears to be superior to open surgery in improving patient survival and quality of life when combined with appropriate perioperative care in elderly patients with GC.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bai Y, Li ZS. Endoscopic, clinicopathological features and prognosis of very young patients with gastric cancer. J Gastroenterol Hepatol. 2011;26:1626–1629. doi: 10.1111/j.1440-1746.2011.06759.x. [DOI] [PubMed] [Google Scholar]
- 3.Saif MW, Makrilia N, Zalonis A, et al. Gastric cancer in the elderly: an overview. Eur J Surg Oncol. 2010;36:709–717. doi: 10.1016/j.ejso.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 5.Pugliese R, Maggioni D, Sansonna F, et al. Outcomes and survival after laparoscopic gastrectomy for adenocarcinoma. Analysis on 65 patients operated on by conventional or robot-assisted minimal access procedures. Eur J Surg Oncol. 2009;35:281–288. doi: 10.1016/j.ejso.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Hwang SI, Kim HO, Yoo CH, et al. Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc. 2009;23:1252–1258. doi: 10.1007/s00464-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 7.Francescutti V, Choy I, Biertho L, et al. Gastrectomy and esophagogastrectomy for proximal and distal gastric lesions: a comparison of open and laparoscopic procedures. Surg Innov. 2009;16:134–139. doi: 10.1177/1553350609336738. [DOI] [PubMed] [Google Scholar]
- 8.Munish M, Sharma V, Yarussi KM, et al. The use of practice guidelines by the American Society of Anesthesiologists for the identification of surgical patients at high risk of sleep apnea. Chron Respir Dis. 2012;9:221–230. doi: 10.1177/1479972312458680. [DOI] [PubMed] [Google Scholar]
- 9.Uyama I, Sakurai Y, Komori Y, et al. The advances of laparoscopic treatment for gastric cancer. Cancer & Chemotherapy. 2007;34:21–24. (In Japanese) [PubMed] [Google Scholar]
- 10.Ramesh HS, Pope D, Gennari R, et al. Optimising surgical management of elderly cancer patients. World J Surg Oncol. 2005;3:17. doi: 10.1186/1477-7819-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohgami M, Otani Y, Kumai K, et al. Laparoscopic wedge resection of the stomach for early gastric cancer using a lesion-lifting-method: curative and minimally invasive treatment. Zentralbl Chir. 1998;123:465–468. (In German) [PubMed] [Google Scholar]
- 12.Uyama I, Sugioka A, Fujita J, et al. Purely laparoscopic pylorus-preserving gastrectomy with extraperigastric lymphadenectomy for early gastric cancer: a case and technical report. Surg Laparosc Endosc Percutan Tech. 1999;9:418–422. [PubMed] [Google Scholar]
- 13.Kitano S, Shiraishi N, Uyama I, et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunisaki C, Makino H, Takagawa R, et al. Efficacy of laparoscopy-assisted distal gastrectomy for gastric cancer in the elderly. Surg Endosc. 2009;23:377–383. doi: 10.1007/s00464-008-9949-1. [DOI] [PubMed] [Google Scholar]
- 15.Ibanez Aguirre FJ, Azagra JS, Erro Azcarate ML, et al. Laparoscopic gastrectomy for gastric adenocarcinoma. Long-term results. Rev Esp Enferm Dig. 2006;98:491–500. doi: 10.4321/s1130-01082006000700002. (In English, Spanish) [DOI] [PubMed] [Google Scholar]
- 16.Azagra JS, Ibanez-Aguirre JF, Goergen M, et al. Long-term results of laparoscopic extended surgery in advanced gastric cancer: a series of 101 patients. Hepatogastroenterology. 2006;53:304–308. [PubMed] [Google Scholar]