Abstract
Primary central nervous system lymphoma (PCNSL) is an aggressive form of non-Hodgkin lymphoma with a poor prognosis. [18F] 2-fluoro-2-deoxy-D-glucose (FDG) and L-(methyl-11C)-methionine (MET) are the most widely used tracers in oncological positron emission tomography studies for PCNSL and commonly identify hypermetabolic lesions through increased uptake of FDG and MET. However, the mechanisms underlying the uptake of FDG and MET in PCNSL have not been clearly determined. The present study aimed to investigate the mRNA expression levels of glucose transporter (GLUT)1, GLUT3 and L-type amino acid transporter 1 (LAT1) in resected PCNSL specimens, in order to identify whether these transporters are associated with the increased uptake of FDG and MET. A total of 7 patients diagnosed with PCNSL were investigated. The uptake of FDG and MET by the tumors was evaluated based on the maximum standardized uptake value (SUVmax). The quantity of GLUT1, GLUT3 and LAT1 mRNA in the PCNSL specimens was measured to determine whether GLUT1, GLUT3 and/or LAT1 are involved in the increased uptake of FDG and MET in PCNSL. Furthermore, microvessel density (MVD) and cell density (CD) were measured in all the cases. Our results indicated that the expression of GLUT3, but not GLUT1, was significantly correlated with FDG SUVmax and the expression of LAT1 was significantly correlated with MET SUVmax. However, neither MVD nor CD were found to be significantly associated with the uptake of FDG and MET. GLUT3 was identified as a key determinant of FDG accumulation, whereas LAT1 was a key determinant of MET accumulation in PCNSL. Therefore, GLUT3 and LAT1 may represent potential targets for the future development of novel therapeutic agents for PCNSL.
Keywords: primary central nervous lymphoma, glucose transporter 3, L-type amino acid transporter 1, fluorodeoxyglucose, methionine, positron emission tomography
Introduction
Primary central nervous system lymphoma (PCNSL) is an aggressive form of non-Hodgkin lymphoma and its incidence has significantly increased among immunocompetent patients over the last few years (1). It was previously reported that positron emission tomography (PET) with either [18F] 2-fluoro-2-deoxy-D-glucose (FDG) (2,3) or L-(methyl-11C)-methionine (MET) (4) commonly reveals a significant accumulation of tracers in PCNSL tissues and may be useful for the diagnosis and assessment of therapeutic response in PCNSL cases. However, the mechanisms underlying the uptake of these tracers in PCNSL have not been clearly determined. Warburg demonstrated that cancer cells exhibit higher rates of glycolysis compared to normal cells, which has been applied to FDG-PET imaging (5). Significantly elevated glucose transporter (GLUT)1 and GLUT3 expression levels are considered to be responsible for the accumulation of FDG in malignant tumors (6–9). Furthermore, hexokinases are involved in glucose metabolism and the expression of these proteins may be correlated with FDG uptake and is useful for PET scanning. System L is a major transport system involed in the cellular uptake of neutral amino acids. Among system L transporters, L-type amino acid transporter 1 (LAT1) is a cancer cell-type transporter, which is highly upregulated in malignant tumors and its expression is associated with a poor prognosis (10,11). The present study aimed to investigate the mRNA levels of GLUT1, GLUT3 and LAT1 in resected PCNSL specimens, in order to identify whether these transporters are associated with the increased uptake of FDG and MET observed in PET studies.
Patients and methods
Patients
Between December, 2008 and December, 2012, 13 patients were newly diagnosed with PCNSL (diffuse large B-cell type) at the Hokuto Hospital (Obihiro, Japan). Among these, 7 patients (5 men and 2 women) whose resected tumor samples were adequate for RNA extraction were included in this retrospective study (Table I). The laboratory examinations were negative for acquired immunodeficiency syndromes in all the patients. FDG-PET and MET-PET were performed on the same day prior to resection. None of the patients received steroid therapy prior to resection.
Table I.
Characteristics of patients with primary central nervous system lymphoma.
| Cases | Age, years | Gender | MET SUVmax | FDG SUVmax | Volume, ml | Survival, months |
|---|---|---|---|---|---|---|
| 1 | 60 | Male | 2.2 | 13.9 | 6.3 | 48 (Alive) |
| 2 | 79 | Male | 2.5 | 13.0 | 7.9 | 9 (Deceased) |
| 3 | 70 | Female | 2.7 | 12.4 | 1.5 | 2 (Deceased) |
| 4 | 80 | Male | 5.5 | 19.9 | 21.8 | 24 (Alive) |
| 5 | 78 | Female | 6.9 | 22.4 | 6.5 | 3 (Deceased) |
| 6 | 83 | Male | 6.6 | 17.0 | 6.1 | 9 (Alive) |
| 7 | 78 | Male | 4.9 | 13.7 | 13.7 | 1 (Deceased) |
MET, L-(methyl-11C)-methionine; FDG, [18F] 2-fluoro-2-deoxy-D-glucose; SUVmax, maximum standardized uptake value.
Methods
PET imaging
PET was performed using the Discovery ST Elite (GE Healthcare Japan Co. Ltd., Tokyo, Japan). This imaging system enabled simultaneous acquisition of 47 transverse slices per field of view (FOV), with an intersection spacing of 3.27 mm, for a total axial FOV of 15.7 cm. The in-plane (transverse) reconstruction resolution was 5.2 mm full-width at half-maximum in the brain FOV. Each patient received an intravenous injection of FDG (mean dose, 84.7±5 MBq; range, 172–200 MBq) and MET (mean dose, 416±25 MBq; range, 375–459 MBq). The serum glucose levels were measured prior to the injection and were found to be within normal limits. The patients remained in the supine position in a resting room with low ambient light and room noise and were instructed not to move or speak. After 45–60 min of FDG-PET or 20 min of MET-PET, the transmission images of the brain underwent computed tomography (CT) attenuation correction (120 kV, 30 mA, 0.8-sec scan) and regional emission images were then obtained for 7 min. All the images were acquired with the patients in the supine position, resting, with their eyes closed. The uptake of FDG and MET into brain lesions was semiquantitatively assessed by determining the standardized uptake value (SUV; activity concentration/injected dose/body weight). A region of interest was set manually by an observer around the hottest area of each lesion or its center located by magnetic resonance imaging (MRI). The maximal value of SUV (SUVmax) was considered to be the representative value for each tumor.
RNA extraction, reverse transcription-polymerase chain reaction (PCR) and quantitative (q)PCR
Formalin-fixed paraffin-embedded sections were deparaffinized. For the preparation of total RNA from tumors, the MasterPure™ RNA Purification kit (Epicentre, Madison, WI, USA) was used according to the manufacturer’s instructions. cDNA was synthesized from total RNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). The cDNA (50–60 ng) was used for qPCR analyses via TaqMan Gene Expression assays for GLUT1 (Hs00892681_m1), GLUT3 (Hs00359840_m1) and LAT1 (Hs00185826_m1). The PCRs were conducted using the Applied Biosystems 7900HT Fast Real-Time PCR system. Ribosomal RNA (Hs99999901_s1) served as the reference gene. The relative quantification (RQ) method was applied to determine gene expression levels. Values of RQ within the range (RQ±2SD) of the corresponding reference group were accepted as normal. Threshold cycle (Ct) values were automatically calculated for each replicate and used to determine the expression of the gene of interest relative to that of the reference gene for treated and untreated samples using the 2−ΔΔCt method.
Immunohistochemistry for CD34 and evaluation of microvessel density (MVD)
The formalin-fixed paraffin-embedded tissue samples were serially sliced into 3-μm sections. Antigen epitopes were heat-retrieved in ImmunoSaver™ (Nissin EM Ltd., Tokyo, Japan) at 90°C for 45 min. The samples were then incubated with CD34 monoclonal antibody (dilution, 1:100; Nichirei Bioscience Inc., Tokyo, Japan) for 1 h at room temperature. The primary antibody was diluted using 0.05% Tween-TBS. Incubation with the peroxidase-conjugated anti-mouse IgG secondary antibody was performed for 1 h at room temperature using Histofine® Simple Stain MAX PO (Nissin EM Ltd.). The peroxidase activity was visualized using a Histofine DAB substrate kit (Nichirei Bioscience Inc.). The sections were counterstained with Mayer’s hematoxylin, then dehydrated and mounted onto slides.
MVD was determined using a modification of the method described by Zhen et al (12). Individual microvessels were counted in areas exhibiting the most intense neovascularization marked by CD34 expression in the vascular endothelium (magnification, ×400). MVD was expressed as the absolute number of microvessels per field (magnification, ×400) for each case.
Evaluation of cell density (CD)
To determine CD, hematoxylin and eosin-stained specimens were viewed under a light microscope model Olympus BX52 (Olympus, Japan, Tokyo) at a magnification of ×1,000. Three FOVs from each specimen were selected and the cells within these FOVs were counted. The mean of the 3 middle densities was then calculated.
Volumetric analysis of tumor size
Tumor size was evaluated by volumetric assessment using SuperPACS™ architecture (Carestream, Rochester, NY, USA). The enhanced area of the tumor in each slice image was measured by manual tracing of the tumor boundaries and the sum of the enhanced areas was then multiplied by the slice interval of the MRI series.
Statistical analysis
The correlation between two parameters was analyzed using Pearson’s correlation test. P<0.05 was considered to indicate a statistically significant difference. All the statistical analyses were performed using StatView 5.0 software (SAS Institute Inc., Cary, NC, USA).
Results
Correlation of FDG SUVmax with GLUT1 and GLUT3 mRNA expression
The mean FDG SUVmax of all 7 patients with PCNSL was 16.043 (95% CI: 12.485–19.601). The FDG SUVmax did not exhibit a statistically significant association with GLUT1 mRNA expression (r=0.522, P=0.2473) (Fig. 1). However, there was a strong positive correlation between FDG SUVmax and GLUT3 mRNA expression (r=0.927, P=0.0010) (Fig. 2).
Figure 1.
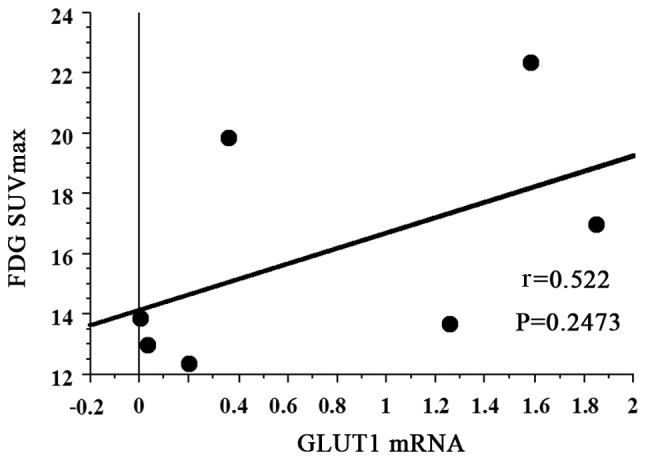
Correlation of FDG-PET maximum standardized uptake value (SUVmax) with glucose transporter (GLUT)1 mRNA expression. FDG SUVmax exhibited a negative correlation with GLUT1 mRNA (r=0.522, P=0.2473). FDG, [18F] 2-fluoro-2-deoxy-D-glucose; PET, positron emission tomography.
Figure 2.
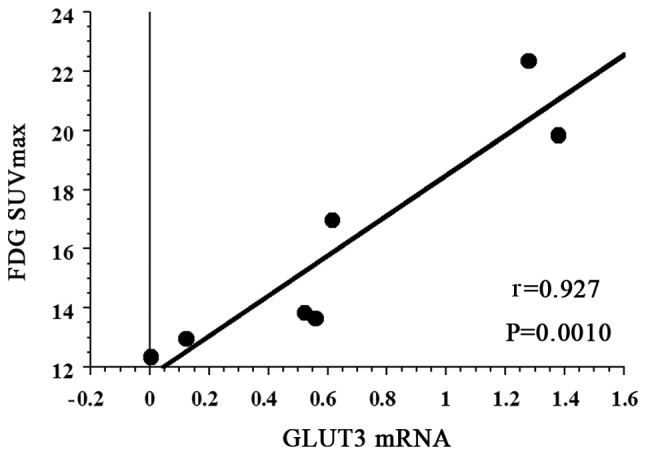
Correlation of FDG-PET maximum standardized uptake value (SUVmax) with glucose transporter (GLUT)3 mRNA expression. FDG SUVmax exhibited a strong positive correlation with GLUT3 mRNA (r=0.927, P=0.0010). FDG, [18F] 2-fluoro-2-deoxy-D-glucose; PET, positron emission tomography.
Correlation between MET SUVmax and LAT1 mRNA expression
The mean MET SUVmax of all 7 patients with PCNSL was 4.471 (95% CI: 2.627–6.315). A strong positive correlation was observed between MET SUVmax and LAT1 mRNA expression (r=0.931, P=0.0009) (Fig. 3).
Figure 3.
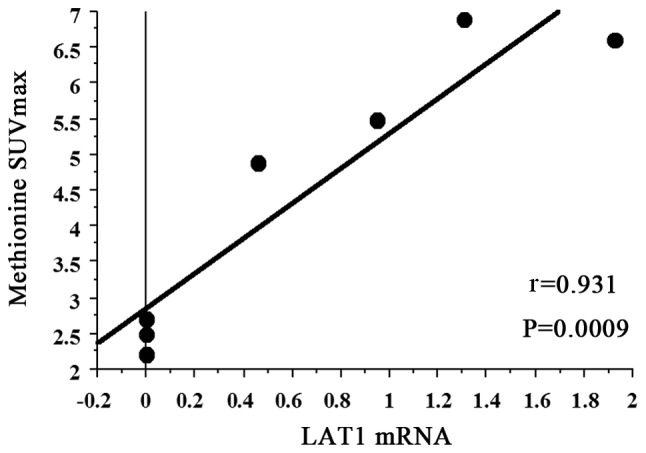
Correlation of MET-PET maximum standardized uptake value (SUVmax) with L-type amino acid transporter 1 (LAT1) mRNA expression. MET SUVmax exhibited a strong positive correlation with glucose transporter LAT1 mRNA (r=0.931, P=0.0009). MET, L-[methyl-11C]-methionine; PET, positron emission tomography.
Correlation of PET SUVmax with MVD and CD
The FDG SUVmax did not exhibit a statistically significant association with MVD (r=−0.061, P=0.9028). In addition, the MET SUVmax was not significantly correlated with MVD (r=−0.026, P=0.9583). Furthermore, FDG SUVmax and MET SUVmax were not statistically significantly associated with CD (r=−0.410, P=0.3833; and r=−0.472, P=0.3050, respectively) (data not shown).
Correlation of PET SUVmax with tumor volume
Neither FDG SUVmax nor MET SUVmax exhibited a statistically significant association with tumor volume (r=0.366, P=0.6049; and r=0.308, P=0.7050, respectively) (data not shown).
Discussion
To the best of our knowledge, this is the first retrospective study to investigate the correlation between GLUT1 and GLUT3 mRNA expression and FDG uptake in PCNSL. A positive correlation between LAT1 mRNA expression and MET uptake in PCNSL tissues was demonstrated.
Malignant tumor cells require a steady and sufficient glucose and amino acid supply in order to maintain the high levels of energy metabolism and protein synthesis required for rapid growth and continuous proliferation. This supply is supported by the upregulation of transporters specialized for these nutrients (13).
Energy-independent glucose uptake into malignant and non-malignant cells is regulated via the expression of GLUT proteins (6–9). GLUT1 and GLUT3, which are members of the SLC2A group, have a high affinity for glucose (14). There are several published reports on the association of GLUT1 and GLUT3 expression with FDG-PET SUV in other types of tumors (15,16); however, the expression of these markers and their association with FDG uptake in PCNSL have not been clearly determined. Therefore, we quantified GLUT1 and GLUT3 mRNA expression in PCNSL specimens. Tumor metabolism imaging with PET has become an established technique for the diagnostic and therapeutic assessment of PCNSL. FDG-PET commonly identifies hypermetabolic lesions through increased uptake of FDG in PCNSL (2,3). Furthermore, FDG-PET was found to be suitable for evaluating therapeutic response (17). Our results suggested that FDG uptake was dependent upon GLUT3 rather than GLUT1 in PCNSL. However, the lack of significance may be due to the small patient sample. Therefore, future studies should include a larger sample size. The addition of high-dose methotrexate (MTX) to radiation therapy (RT) was found to improve the overall survival of patients with PCNSL to 30–40 months, compared to the 12–18 months reported with RT alone (18,19). However, over half of the patients eventually relapse and uncontrolled PCNSL remains the primary cause of death. Kawai et al (20) reported that the overall survival time of patients with PCNSL with low-to-moderate FDG uptake was significantly longer compared to that of patients with a high FDG uptake. Accordingly, GLUT3 may be a molecular target for PCNSL treatment in future studies.
Transporters for essential amino acids are particularly important, as they are indispensable for the protein synthesis required to maintain cell integrity and cell cycle progression. Among several amino acid transporters, LAT1 is a Na+ ion-independent neutral amino acid transport agent, which is essential for the transport of large neutral amino acids through the plasma membrane (21). LAT1 was cloned from rat glioma C6 cells (21) and exhibits a high affinity for several essential amino acids, including methionine. MET-PET revealed high uptakes in PCNSL (4) at sites corresponding to the enhanced portion of the tumor on CT/MRI and the area of increased uptake is often larger than the enhanced lesions (3). This larger area of MET uptake reflects tumor infiltration beyond the enhanced portion observed on MRI and CT (3). The size and degree of MET accumulation in the tumor tissue were decreased following RT (3). Thus, MET-PET may provide a more accurate delineation of tumor volume for evaluating the effects of RT, as well as for the detection of residual or recurrent tumors following treatment (3). A previous study demonstrated a significant correlation between tumor grade and MET uptake in gliomas (22). Although an increased rate of protein synthesis was hypothesized to be the mechanism underlying increased MET uptake in gliomas, transport mechanisms play a major role in this uptake process. There are currently no reports investigating the LAT1 expression in PCNSL. Therefore, we investigated LAT1 mRNA expression in PCNSL and our results indicated that MET uptake is dependent on LAT1.
The uptake of FDG and MET was found to be dependent on proliferative activity, as well as on other factors, such as MVD (23,24) and CD (25). In oligodendrocytic tumors, abundant microvessels and/or high CD may occasionally cause increased uptake of FDG and MET (23). We next investigated MVD and CD in our tumor samples. FDG SUVmax did not exhibit a statistically significant association with MVD. MET SUVmax was not found to be significantly correlated with MVD. In addition, FDG SUVmax and MET SUVmax were not significantly correlated with CD. Thus, in PCNSL, FDG and MET uptake depends mainly on transport systems, rather than MVD or CD. Similarly, tumor volume did not affect FDG or MET uptake in PCNSL. Our results indicated that FDG and MET uptake may be dependent on the transporters investigated in this study.
In conclusion, GLUT3 and LAT1 mRNA expression is significantly correlated with FDG and MET uptake, respectively, in PCNSL. Furthermore, FDG and MET uptake were not found to be correlated with increased MVD or CD. Although high-dose MTX chemotherapy followed by RT was shown to improve overall survival in patients with PCNSL, the survival duration remains unsatisfactory. Although additional studies are required to draw definitive conclusions, GLUT3 and LAT1 may represent potential novel therapeutic targets for PCNSL.
References
- 1.Corn BW, Marcus SM, Topham A, Hauck W, Curran WJ., Jr Will primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000? Cancer. 1997;79:2409–2413. [PubMed] [Google Scholar]
- 2.Kawai N, Nishiyama Y, Miyake K, Tamiya T, Nagao S. Evaluation of tumor FDG transport and metabolism in primary central nervous system lymphoma using [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) kinetic analysis. Ann Nucl Med. 2005;19:685–690. doi: 10.1007/BF02985117. [DOI] [PubMed] [Google Scholar]
- 3.Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Kawai N, Satoh K, Ohkawa M. Diagnostic value of kinetic analysis using dynamic FDG PET in immunocompetent patients with primary CNS lymphoma. Eur J Nucl Med Mol Imaging. 2007;34:78–86. doi: 10.1007/s00259-006-0153-z. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa T, Kanno I, Hatazawa J, et al. Methionine PET for follow-up of radiation therapy of primary lymphoma of the brain. Radiographics. 1994;14:101–110. doi: 10.1148/radiographics.14.1.8128041. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 6.Reske SN, Grillenberger KG, Glatting G, Port M, Hildebrandt M, Gansauge F, Beger HG. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med. 1997;38:1344–1348. [PubMed] [Google Scholar]
- 7.Brown RS, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993;72:2979–2985. doi: 10.1002/1097-0142(19931115)72:10<2979::aid-cncr2820721020>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80:1046–1051. doi: 10.1002/(sici)1097-0142(19970915)80:6<1046::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Iwazaki A, Katagiri H, Oka Y, Redpath JL, Stanbridge EJ, Kitagawa T. Enhanced expression of glucose transporter GLUT3 in tumorigenic HeLa cell hybrids associated with tumor suppressor dysfunction. Eur J Biochem. 1999;262:534–540. doi: 10.1046/j.1432-1327.1999.00421.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaira K, Oriuchi N, Shimizu K, Imai H, Tominaga H, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Kanai Y, et al. Comparison of L-type amino acid transporter 1 expression and L-[3–18F]-α-methyl tyrosine uptake in outcome of non-small cell lung cancer. Nucl Med Biol. 2010;37:911–916. doi: 10.1016/j.nucmedbio.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7–18. doi: 10.1111/j.1440-1827.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhen HN, Zhang X, Hu PZ, Yang TT, Fei Z, Zhang JN, Fu LA, He XS, Ma FC, Wang XL. Surviving expression and its relation with proliferation, apoptosis, and angiogenesis in brain gliomas. Cancer. 2005;104:2775–2783. doi: 10.1002/cncr.21490. [DOI] [PubMed] [Google Scholar]
- 13.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe Y, Suefuji H, Hirose Y, et al. 18F-FDG uptake in primary gastric malignant lymphoma correlates with glucose transporter 1 expression and histologic malignant potential. Int J Hematol. 2013;97:43–49. doi: 10.1007/s12185-012-1225-4. [DOI] [PubMed] [Google Scholar]
- 16.Khandani AH, Dunphy CH, Meteesatien P, Dufault DL, Ivanovic M, Shea TC. Glut1 and Glut3 expression in lymphoma and their association with tumor intensity on 18F-fluorodeoxyglucose positron emission tomography. Nucl Med Commun. 2009;30:594–601. doi: 10.1097/MNM.0b013e32832cc295. [DOI] [PubMed] [Google Scholar]
- 17.Palmedo H, Urbach H, Bender H, Schlegel U, Schmidt-Wolf IG, Matthies A, Linnebank M, Joe A, Bucerius J, Biersack HJ, Pels H. FDG-PET in immunocompetent patients with primary central nervous system lymphoma: correlation with MRI and clinical follow-up. Eur J Nucl Med Mol Imaging. 2006;33:164–168. doi: 10.1007/s00259-005-1917-6. [DOI] [PubMed] [Google Scholar]
- 18.Glass J, Gluber ML, Cher L, Hochberg FH. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg. 1994;81:188–195. doi: 10.3171/jns.1994.81.2.0188. [DOI] [PubMed] [Google Scholar]
- 19.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 20.Kawai N, Zhen HN, Miyake K, Yamamaoto Y, Nishiyama Y, Tamiya T. Prognostic value of pretreatment 18F-FDG PET in patients with primary central nervous system lymphoma: SUV-based assessment. J Neurooncol. 2010;100:225–232. doi: 10.1007/s11060-010-0182-0. [DOI] [PubMed] [Google Scholar]
- 21.Kanai Y, Segawa H, Miyamoto Ki, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 22.Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neurooncol. 2010;99:217–225. doi: 10.1007/s11060-010-0117-9. [DOI] [PubMed] [Google Scholar]
- 23.Nojiri T, Nariai T, Aoyagi M, Senda M, Ishii K, Ishiwata K, Ohno K. Contributions of biological tumor parameters to the incorporation rate of L-[methyl-11C] methionine into astrocytomas and oligodendrogliomas. J Neurooncol. 2009;93:233–241. doi: 10.1007/s11060-008-9767-2. [DOI] [PubMed] [Google Scholar]
- 24.Kracht LW, Friese M, Herholz K, Schroeder R, Bauer B, Jacobs A, Heiss WD. Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging. 2003;30:868–873. doi: 10.1007/s00259-003-1148-7. [DOI] [PubMed] [Google Scholar]
- 25.Okita Y, Kinoshita M, Goto T, Kagawa N, Kishima H, Shimosegawa E, Hatazawa J, Hashimoto N, Yoshimine T. 11C-methionine uptake correlates with tumor cell density rather than with microvessel density in glioma: A stereotactic image-histology comparison. Neuroimage. 2010;49:2977–2982. doi: 10.1016/j.neuroimage.2009.11.024. [DOI] [PubMed] [Google Scholar]


