Abstract
Dendritic cells (DCs) orchestrate host defenses against microorganisms. In infectious diseases due to intracellular bacteria, the inefficiency of the immune system to eradicate microorganisms has been attributed to the hijacking of DC functions. In this study, we selected intracellular bacterial pathogens with distinct lifestyles and explored the responses of monocyte-derived DCs (moDCs). Using lipopolysaccharide as a control, we found that Orientia tsutsugamushi, the causative agent of scrub typhus that survives in the cytosol of target cells, induced moDC maturation, as assessed by decreased endocytosis activity, the ability to induce lymphocyte proliferation and the membrane expression of phenotypic markers. In contrast, Coxiella burnetii, the agent of Q fever, and Brucella abortus, the agent of brucellosis, both of which reside in vacuolar compartments, only partly induced the maturation of moDCs, as demonstrated by a phenotypic analysis. To analyze the mechanisms used by C. burnetii and B. abortus to alter moDC activation, we performed microarray and found that C. burnetii and B. abortus induced a specific signature consisting of TLR4, TLR3, STAT1 and interferon response genes. These genes were down-modulated in response to C. burnetii and B. abortus but up-modulated in moDCs activated by lipopolysaccharide and O. tsutsugamushi. This transcriptional alteration was associated with the defective interferon-β production. This study demonstrates that intracellular bacteria specifically affect moDC responses and emphasizes how C. burnetii and B. abortus interfere with moDC activation and the antimicrobial immune response. We believe that comparing infection by several bacterial species may be useful for defining new pathways and biomarkers and for developing new treatment strategies.
Introduction
Dendritic cells (DCs) are specialized antigen-processing and -presenting cells that act as sentinels at the interface between innate and adaptive immunity [1]. In peripheral tissues, DCs are considered to be immature and are characterized by a high endocytic activity, the inability to activate T-cells and the intracellular expression of major histocompatibility complex (MHC) class II molecules [2], [3]. However, encounters with microorganisms or bacterial ligands, such as lipopolysaccharide (LPS), triggers the maturation of immature DCs [4], a process that is associated in vivo with their migration to secondary lymphoid organs where they interact with T-cells to orchestrate adaptive immune responses [1], [4].
Thus, the prevention of DC maturation and/or migration may be a relevant strategy for intracellular bacteria to avoid efficient immune responses, as illustrated by some examples of bacterial infections. Salmonella enterica serovar Typhimurium interferes with the migration of intestinal DCs and hinders antigen presentation via the ubiquitination and degradation of MHC class II molecules, which prevents bacterial killing [5], [6]. Helicobacter pylori replicates within autophagosomes and retains cytosolic MHC class II molecules; H. pylori also prevents interleukin (IL)-12 production and induces IL-10 secretion, thereby inhibiting the anti-microbial Th1 response [7], [8]. Mycobacterium tuberculosis interferes with Toll-like receptor (TLR) signaling through its binding to CD209, also known as the C-type lectin dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), blocking DC maturation and IL-12 production [9], [10]. Additionally, Chlamydia trachomatis and C. psittaci release molecules that delay antigen presentation by MHC class II molecules [11].
Unfortunately, most of these reports are based on the comparison of naïve DCs and DCs stimulated by only one type of microorganism [5]–[7], [9]–[11], and such an approach cannot identify the elements common to microorganisms sharing cellular targets and the relative level of DC impairment. Therefore, we selected four intracellular bacteria responsible for infectious diseases in humans and that exhibit distinct lifestyles within target cells. These four bacteria, namely, Coxiella burnetii, Brucella abortus, Orientia tsutsugamushi and Tropheryma whipplei, are known to target myeloid cells and to be pathogenic for humans: O. tsutsugamushi, the agent of scub typhus, lives in a cytosolic [12] compartment, whereas C. burnetii, the agent of Q fever, B. abortus, the agent of brucellosis, and T. whipplei, the agent of the Whipple's disease, are found in vacuolar compartments [13]–[15]. O. tsutsugamushi induces a strong inflammatory response, including the type I interferon (IFN) response, in monocytes [16] and macrophages [17]. It has been demonstrated in vivo that O. tsutsugamushi is preferentially located in dermal DCs from the eschars of patients with scrub typhus [18]. T. whipplei is a Gram-positive bacterium; this pathogen infects macrophages, inducing apoptosis [19] and an M2 non-microbicidal profile [20]. C. burnetii, the Gram-negative bacterium infects and resides within monocytes and macrophages. This bacterium induces an M1 inflammatory profile in monocytes and an M2 immunoregulatory profile [21] and also infects monocyte-derived DCs (moDCs) in vitro, appearing to prevent their maturation [22]. B. abortus, a Gram-negative bacterium, is known to infect macrophages and evade killing via the VirB type IV secretion system [23]. B. abortus, also infects DCs [24], and it has been shown that the bacterial btp1 protein partially inhibits the maturation of mouse bone marrow-derived DCs by inhibiting the Toll-like receptor (TLR)-2 and TLR4 pathways [24].
Here, we investigate the functions of moDCs infected with four intracellular bacterial pathogens to reappraise the concept of DC activation within the context of infectious diseases. Although O. tsutsugamushi induced moDC maturation, as did LPS, C. burnetii and B. abortus were less efficient. A transcriptional analysis identified an alteration of the IFN response pathway in moDCs infected with C. burnetii and B. abortus, whereas this pathway was fully operative in O. tsutsugamushi-infected moDCs. These results demonstrate that C. burnetii and B. abortus both interfere with the activation of human moDCs at the level of the IFN response, thus positioning this pathway in the pathogenesis of Q fever and brucellosis.
Materials and Methods
Ethics Statement
The experimental protocol was approved by the institutional animal care and use committee of Aix-Marseille University (décret N° 8 87–848, October 19, 1987, Paris). Blood was obtained from the Etablissement Français du Sang. It is a commercial product and the Ethics Committee agreement is not required.
Bacteria
O. tsutsugamushi strain Kato (CSUR R163) was propagated in L929 cells, as previously described [12]. Briefly, highly infected cells were sonicated, or lysed using glass beads for O. tsutsugamushi, and centrifuged at 500× g for 10 minutes to remove the cell debris. The supernatants were collected and centrifuged at 10,000× g for 10 minutes to pellet the bacteria. The live bacteria were quantified using infected cell-counting units. C. burnetii organisms (RSA493 Nile Mile strain) were obtained by passage in BALB/c mice and culture in L929 cells. The concentration of organisms was determined by immunofluorescence using specific antibodies (Abs) and/or PCR using known concentrations of bacterial DNA. The quantification of organisms was performed as previously described [13]. Bacterial viability was assessed using the LIVE/DEAD BacLight bacterial viability kit (Invitrogen, Cergy Pontoise, France) [25]. B. abortus strain 2308 was grown on tryptic soy agar (Sigma-Aldrich, Saint-Quentin Fallavier, France) at 37°C for 4–5 days, as previously described [26]. The Twist-Marseille (CNCM I-2202) strain of Tropheryma whipplei, a bacterium known to live in macrophages, was cultured in HEL cells and purified as described elsewhere. The quantification of inocula was performed by measuring the percentage of infected cells by immunofluorescence, as previously described [19].
Isolation and stimulation of moDCs
Blood from healthy donors was obtained from the Etablissement Français du Sang. Peripheral blood mononuclear cells (PBMCs) from buffy coats were recovered from the Ficoll-Hypaque interface after centrifugation at 700× g for 20 minutes. Monocytes and T-lymphocytes were isolated from PBMCs using magnetic beads coupled to Abs specific for CD14 and CD3, respectively, as described by the manufacturer (Miltenyi Biotec, Paris, France). The purity of the monocyte and T-cell preparations was assessed by flow cytometry and was greater than 98%. The monocytes were then incubated in RPMI 1640 containing 20 mM HEPES, 2 mM glutamine, 10% fetal calf serum (FCS) (Invitrogen), 0.1 ng/mL IL-4 and 1 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Lille, France) for 7 days to obtain moDCs. The T-lymphocytes were frozen in a medium containing 10% dimethylsulfoxide. The moDCs were stimulated by 100 ng/mL Escherichia coli LPS (Sigma-Aldrich) or 20 bacterial cells per moDC. For the microarray experiments, 300 B. abortus cells per moDC were used to maximize the infection of moDCs. In some experiments, C. burnetii LPS, kindly provided by Dr. R. Toman) [13] and B. abortus LPS [27] were used at 1 µg/mL.
Functional activity of moDCs
The endocytic activity of moDCs was examined by measuring the uptake of fluorescein isothiocyanate (FITC)-coupled albumin. moDCs were cultured in 6-well plates (106 cells per well) in RPMI 1640 containing 10% FCS and stimulated with pathogens, or LPS as a control of maturation, for 24 hours at 37°C. After washing in phosphate-buffered saline (PBS), the moDCs were incubated with 20 µg/mL FITC-coupled albumin (Invitrogen) for 1 hour; the moDCs were then washed, scraped and fixed in 3% paraformaldehyde (PFA) for 15 minutes. The cell fluorescence was analyzed by flow cytometry using the FACS Canto II flow cytometer (BD Biosciences, Le Pont de Claix, France). The mean fluorescence intensity (MFI) was recorded, and the results are expressed relative to the fluorescence of unstimulated DCs.
The ability of moDCs to induce T-cell proliferation was investigated as follows. moDCs (2×105/mL) were cultured in 12-well plates and stimulated with bacteria or LPS for 24 hours. Autologous T-lymphocytes (106 cells/mL) were labeled in 500 µL of PBS containing 10% of bovine serum albumin and 10 µM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) for 15 minutes. The T-lymphocytes were co-cultured with moDCs (ratio of 5∶1) for 5 days, and the fluorescence of the T-lymphocytes was analyzed by flow cytometry. The T-lymphocyte proliferation was determined as the percentage of cells with decreased CFSE fluorescence. The results are expressed relative to the fluorescence of unstimulated moDCs.
Expression of DC maturation markers
moDCs were stimulated in 6-well plates (106 cells) in RPMI 1640 containing 10% FCS with bacterial pathogens or LPS extracted from E. coli, C. burnetii or B. abortus for 24 hours. The moDCs were scraped, centrifuged at 400× g for 5 minutes and incubated with conjugated with FITC-CD80, phycoerythrin (PE)-CD83, PE-CD86 and FITC-HLA-DR for 30 minutes. The moDCs were fixed with 3% PFA for 20 minutes, washed and incubated in 500 µL of PBS prior to the flow cytometry analysis. The fluorescence of 20,000 cells per sample was quantified using the FACS Canto II flow cytometer (BD Biosciences). The results are expressed as the fold change (FC) defined as the MFI of stimulated moDCs and the MFI of unstimulated moDCs.
Microarrays
moDCs (5×106 cells per well) were plated in 6-well plates and stimulated with bacteria (MOI of 20∶1, except for B. abortus at an MOI of 300∶1) or LPS for 6 hours, and total RNA was then extracted using the RNeasy minikit (Qiagen, Courtaboeuf, France) and DNase treatment. This study utilized the 4X44k Human Whole Genome microarrays (Agilent Technologies, Les Ulis, France), representing 44,000 probes, as recently described [25]. Reverse transcription, sample labeling and hybridization were performed according to the protocols specified by the manufacturer (One-Color Microarray-Based Gene Expression Analysis). Three samples per experimental condition were included in the analysis. The slides were scanned at a 2-µm resolution with a G2505C DNA microarray scanner (Agilent Technologies). The data were generated in compliance with the Minimum Information About a Microarray Experiment (MIAME) guidelines and were deposited in the Gene Expression Omnibus of the National Center for Biotechnology Information (accession number: GSE49016).
Image analysis and intra-array signal corrections were performed using Agilent Feature Extractor Software A.9.1.3. The microarray data analysis was performed using R (v.3.0.1) and the Bioconductor software suite, and the raw data were filtered and normalized using the Agi4x44PreProcess library. Unsupervised and supervised analyses were performed using hierarchical clustering and a principal component analysis (PCA) (made4 library [28] and Linear Models for Microarray Analysis (limma library) [29]). Genes were considered to be differentially expressed if the False Discovery Rate (FDR) [30] was below 0.1% and the absolute fold change (FC) was above 2.0. The core program of the moDCs induced by O. tsutsugamushi, C. burnetii and B. abortus was annotated using the DAVID annotation tool [31] and Kyoto Encyclopedia of Gene and Genome (KEGG) pathways [32]. The integration and rendering of the pathway-based data were performed using the library pathview [33].
Real-time RT-PCR
The genes in the IFN pathway that were identified by the microarray as being modulated were selected and validated by quantitative real-time reverse transcriptase PCR (qRT-PCR), as previously described [25]. In brief, reverse transcription of 150 ng of RNA was performed using an Moloney murine leukemia virus (MMLV) RT kit (Invitrogen). The first-strand cDNA was obtained using oligo(dT) primers and MMLV RT (Invitrogen), and the qPCR experiments were performed using SYBR Green Fast Master Mix (Roche Diagnostics, Meylan, France) and an ABI7900 Fast Real-Time PCR System (Life Technologies). The primers were designed using Primer3. The results were normalized to the housekeeping gene β-actin and are expressed as the median of fold change (FC) = 2−ΔΔCt, where ΔΔCt = (CtTarget − CtActin)stimulated − (CtTarget − CtActin)unstimulated, as previously described [34].
Phosphorylation of p38
moDCs were stimulated with 1 µg/mL E. coli LPS or pathogens for different periods. The activation of the mitogen-activated protein kinase (MAPK) p38 was determined by immunoblotting using Abs specific for phospho-p38 (1/1000 dilution, Cell Signaling). The expression of activated p38 was quantified by densitometric scanning after normalization with structural p38. The results are expressed in relative intensities.
IFN-β immunoassay
moDCs (105 cells/mL) plated in 24-well plates were stimulated with pathogens or different concentrations of E. coli LPS for 16 hours, and the supernatants were centrifuged at 1000×g for 10 minutes and frozen at −80°C. The Verikine Human IFN-β ELISA kit (R&D Systems) was used to determine IFN-β production by the moDCs; the sensitivity of the kit was 3 pg/ml, and the inter- and intra-assay specificity was ≤ 8%.
Statistical analysis
The results are expressed as the means ± SEM and were compared using Student's t-test. When the comparisons involved more than two conditions, the analysis was made with ANOVA test. P values less than 0.05 were considered significant.
Results
C. burnetii and B. abortus partly induce moDC maturation
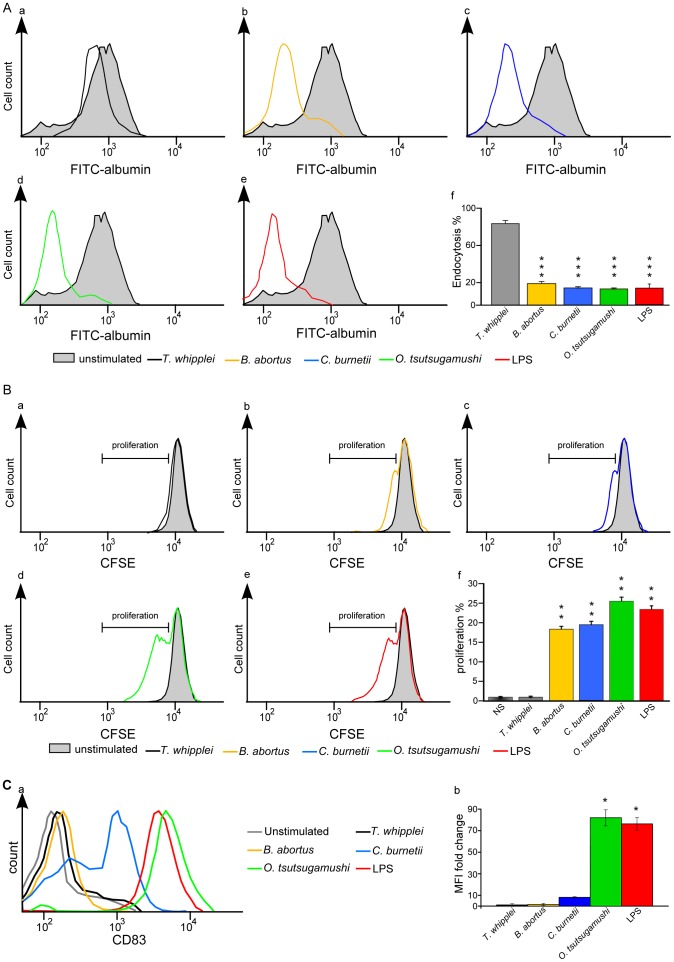
We investigated the maturation of moDCs in response to the cytoplasmic bacterial pathogen O. tsutsugamushi and three vacuolar bacteria, C. burnetii, B. abortus and T. whipplei, using three criteria: the loss of endocytosis, the ability to induce lymphocyte proliferation and the expression of CD83. First, the endocytosis activity of the moDCs stimulated with T. whipplei was not different (80% ± 8%) from that of unstimulated cells ( Figure 1A , a and f ). In contrast, the endocytosis activity of the moDCs stimulated with B. abortus C. burnetii and O. tsutsugamushi was dramatically reduced compared to the control moDCs (21% ± 3%, 18% ± 2% and 17% ± 5%, respectively, Figure 1A , b–d and f ). Note that the endocytosis activity of the moDCs stimulated with B. abortus, C. burnetii and O. tsutsugamushi was similar to that observed in moDCs stimulated with E. coli LPS (18% ± 2%, Figure 1 , e and f ). Second, the ability of moDCs to induce proliferation of autologous T-cells after moDC infection was assessed by flow cytometry. We found that T. whipplei was unable to affect T-cell proliferation ( Figure 1B , a and f ), as recorded by CFSE fluorescence. In contrast, B. abortus, C. burnetii and O. tsutsugamushi induced a significant T-cell proliferation compared to the unstimulated moDCs ( Figure 1B , b, c, d and f ): T-cell proliferation was similar to that observed in LPS-stimulated moDCs ( Figure 1B , e and f ). Third, we found that the measurement of CD83 expression was more discriminative than the two other features of moDC maturation. O. tsutsugamushi induced a strong CD83 expression (when compared to the unstimulated condition, the FC of MFI values was >80) that was similar to that induced by LPS (MFI FC>75). In contrast, CD83 was only weakly expressed in the C. burnetii-stimulated moDCs (MFI FC<10) and was absent in the moDCs stimulated by B. abortus or T. whipplei ( Figure 1C , a and b ). Taken together, these results demonstrate that O. tsutsugamushi was fully efficient to induce the maturation of moDCs whereas C. burnetii and B. abortus partly induce moDC maturation.
Figure 1. Functional study of moDC maturation.
A, moDCs were stimulated with T. whipplei (a), B. abortus (b), C. burnetii (c), O. tsutsugamushi (d) or E. coli LPS (e) for 24 hours and then incubated with FITC-albumin (1 mg/mL) for 1 hour. The fluorescence intensities of unstimulated moDCs and stimulated moDCs were determined by flow cytometry. The results are expressed as the ratio of stimulated moDC fluorescence over unstimulated moDC fluorescence (f). They represent the mean ± SD of three independent experiments. ***p<0.001 represents the comparison of stimulated moDCs vs unstimulated DCs using Student's t-test. B, moDCs stimulated with T. whipplei (a), B. abortus (b), C. burnetii (c), O. tsutsugamushi (d) or E. coli LPS (e) were co-cultured with CFSE-labeled autologous T-lymphocytes for 5 days. The gating strategy used to determine lymphocyte proliferation is shown for each stimulation (a–e). The results are expressed as the percentage of new T-cells (cells with decreased fluorescence intensity) in the total T-cell population (f). They represent the mean ± SD of three independent experiments. **p<0.02 represents the comparison of stimulated moDCs vs unstimulated DCs using Student's t-test. C, The membrane expression of CD83 by moDCs incubated with bacterial pathogens or LPS for 24 hours was determined by flow cytometry using PE-coupled anti-CD83 Abs (a). The histograms are representative of three different experiments. The FC of MFI values after stimulation was compared to unstimulated condition (b). *p<0.03 represents the comparison of stimulated moDCs vs unstimulated DCs using Student's t-test.
B. abortus and C. burnetii did not share the same ability to activate moDCs
As the expression of CD83 was differently affected by pathogens, we addressed whether these bacteria affected other features of moDC maturation, including the membrane expression of such costimulation molecules as CD80 and CD86 and that of HLA-DR, a MHC class II molecule. In agreement with its ability to induce the maturation of moDCs, we found that O. tsutsugamushi markedly increased the expression of CD80 (MFI FC of 10), CD86 (MFI FC of 10) and HLA-DR (MFI FC of 12), as did LPS. In contrast, C. burnetii only slightly affected the membrane expression of CD80 (MFI FC of 5), CD86 (MFI FC of 8) and HLA-DR (MFI FC of 8); B. abortus also poorly affected the expression of CD80 (MFI FC of 2) and CD86 (MFI FC of 4) but not that of HLA-DR. It should be noted that T. whipplei had a limited effect on the expression of CD80, CD86 and HLA-DR ( Figure 2 ). Taken together, these results emphasize the profound deficiency of B. abortus-stimulated moDCs and the relative alteration of C. burnetii-stimulated moDCs to express activation markers.
Figure 2. Phenotypic study of moDC maturation.
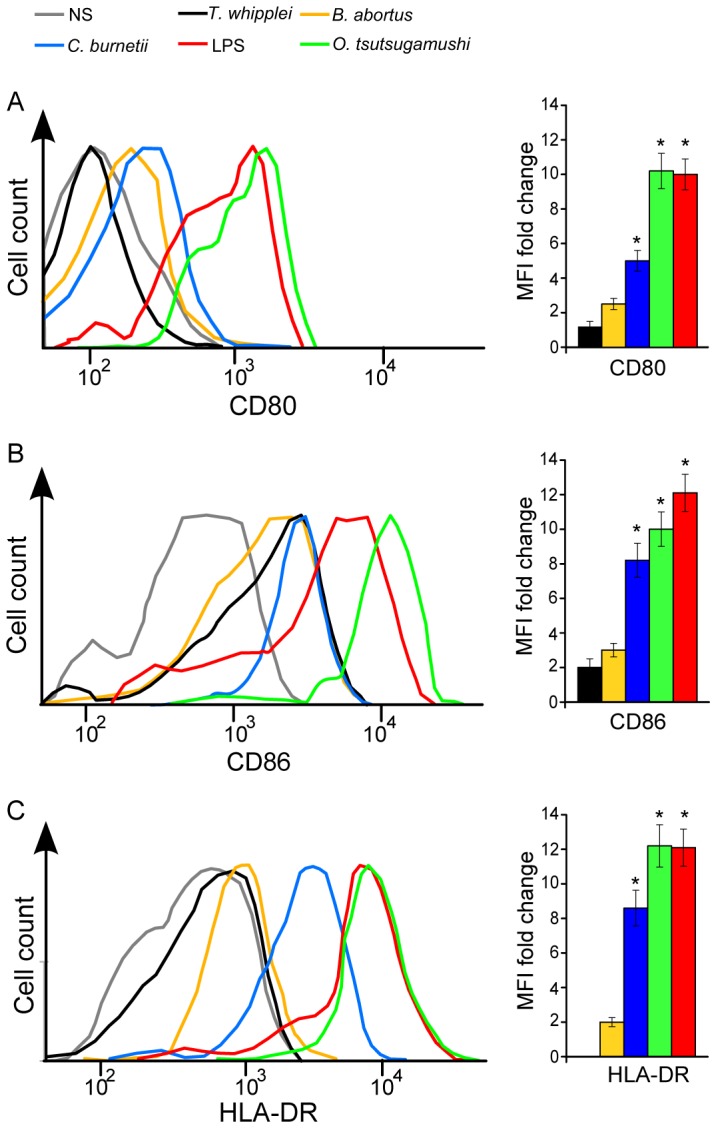
moDCs were stimulated with bacterial pathogens or E. coli LPS for 24 hours. The cells were then incubated with FITC-coupled anti-CD80 (A), PE-coupled anti-CD86 (B) and FITC-coupled anti-HLA-DR (C) Abs for 30 minutes and analyzed by flow cytometry. The histograms are representative of three different experiments. The bar charts represent the MFI FC values of stimulated moDCs compared with unstimulated moDCs. *p<h0.05.
Microarray analysis reveals specific responses to intracellular bacteria
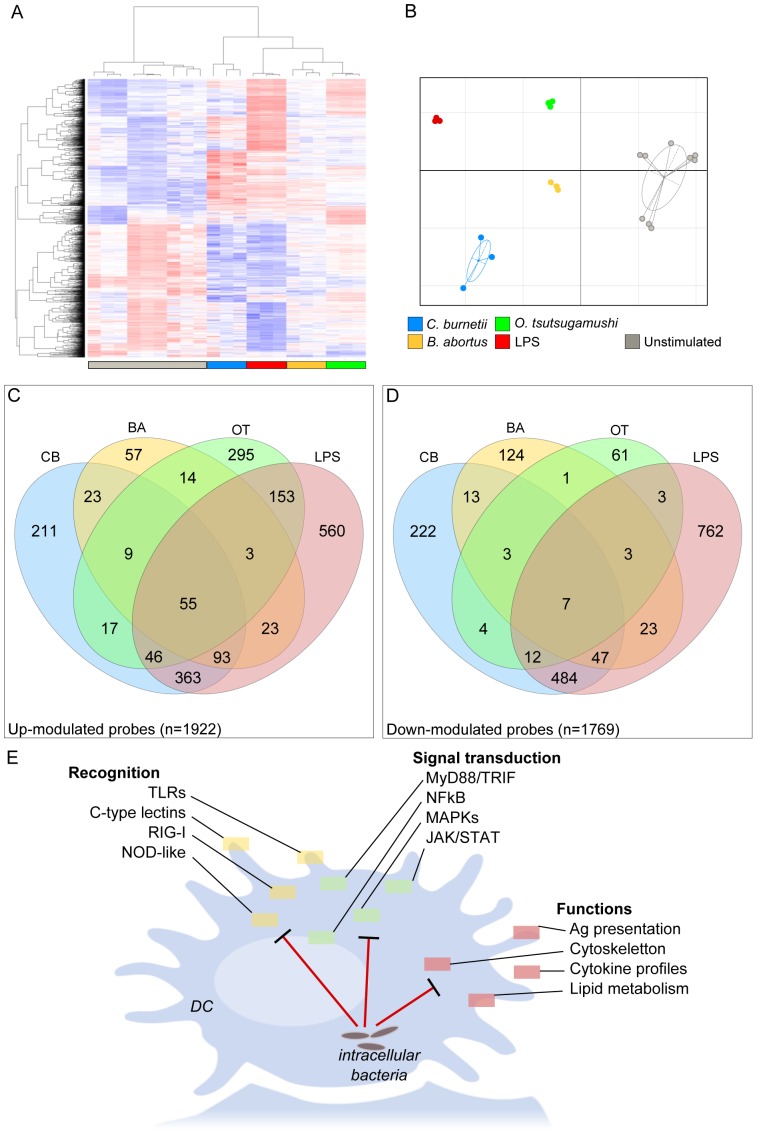
We used a systems biology approach to investigate the mechanisms leading to the different patterns of activation in response to LPS, C. burnetii, B. abortus and O. tsutsugamushi and found that the stimulated moDCs were present in a cluster that was different from that of the unstimulated moDCs ( Figure 3A ), with 3,610 modulated probes (2,609 distinct genes). We removed T. whipplei-stimulated moDCs from the analysis because we were unable to identify a transcriptional signature that was not different from background analysis. Hierarchical clustering and PCA also discriminated the global transcriptional signature of each bacterium; note that the global signatures induced by O. tsutsugamushi and LPS were largely different from those induced by C. burnetii and B. abortus ( Figure 3B ). Using Venn diagrams, we identified a core response to LPS and pathogens: this core response included 62 genes (55 up-modulated and 7 down-modulated). We also identified specific signatures for LPS and each bacterium ( Figure 3C and 3D ).
Figure 3. Transcriptional program of stimulated moDCs.
moDCs were stimulated with B. abortus, C. burnetii, O. tsutsugamushi or E. coli LPS for 6 hours. RNA was extracted, and a microarray was performed. A, A heatmap representation of the 3,610 modulated probes in stimulated moDCs compared to unstimulated moDCs (NS). The probes are shown in the rows and the samples in the columns. The expression levels are color coded from blue to red. B, Graphical representation of the samples based on the correspondence analysis of the modulated probes. The samples are colored according to the stimulus. C–D, Venn diagrams highlighting the core and specific signatures induced in moDCs stimulated with LPS and pathogens, showing up-modulated probes (C) and down-modulated probes (D). E, Summary of the pathways in moDCs modulated by intracellular bacteria. Ba: B. abortus; Cb: C. burnetii; Ot: O. tsutsugamushi.
We then analyzed the gene pathways stimulated by LPS and bacterial pathogens. Among the 2,609 genes modulated, we found that 469 genes (20%) were implicated in several canonical immune response pathways (extracted from the KEGG database, Table 1 ), such as recognition pathways (the TLR signaling pathway, NOD-like signaling pathway, RIG-I-like signaling pathway and cytosolic DNA-sensing pathway), signal processing (the Jak-STAT and MAPK signaling pathways) or functions (cytokine-cytokine receptor interaction, chemokine signaling pathway and apoptosis) ( Figure 3E ). The analysis of each pathway revealed numerous differences, which were specific for each bacterium (Results S1). Taken together, these results showed that, in addition to a core program related to the immune response, each microorganism is able to specifically modulate this response.
Table 1. KEGG pathways identified in stimulated moDCs.
| KEGG pathway name (#ID) | Number of genes | Corrected P value |
| Cytokine-cytokine receptor interaction (#04060) | 99 | 2.0×10−18 |
| Jak-STAT signaling pathway (#04630) | 51 | 2.0×10−6 |
| RIG-I-like receptor signaling pathway (#04622) | 30 | 4.5×10−6 |
| NOD-like receptor signaling pathway (#04621) | 27 | 8.5×10−6 |
| Cytosolic DNA-sensing pathway (#04623) | 25 | 8.3×10−6 |
| Chemokine signaling pathway (#04062) | 55 | 1.2×10−5 |
| Toll-like receptor signaling pathway (#04620) | 35 | 3.5×10−5 |
| Apoptosis (#04210) | 29 | 6.8×10−4 |
| P53 signaling pathway (#04115) | 22 | 9.8×10−3 |
| MAPK signaling pathway (#04010) | 58 | 3.3×10−2 |
| Focal adhesion (#04510) | 46 | 3.4×10−2 |
moDCs were stimulated with E. coli LPS or bacterial pathogens for 6 hours. RNAs were extracted, and microarrays were performed. The modulated genes were analyzed using the KEGG database. The KEGG pathways, the number of modulated genes and P values are shown.
C. burnetii and B. abortus interfere with the type I IFN response pathway
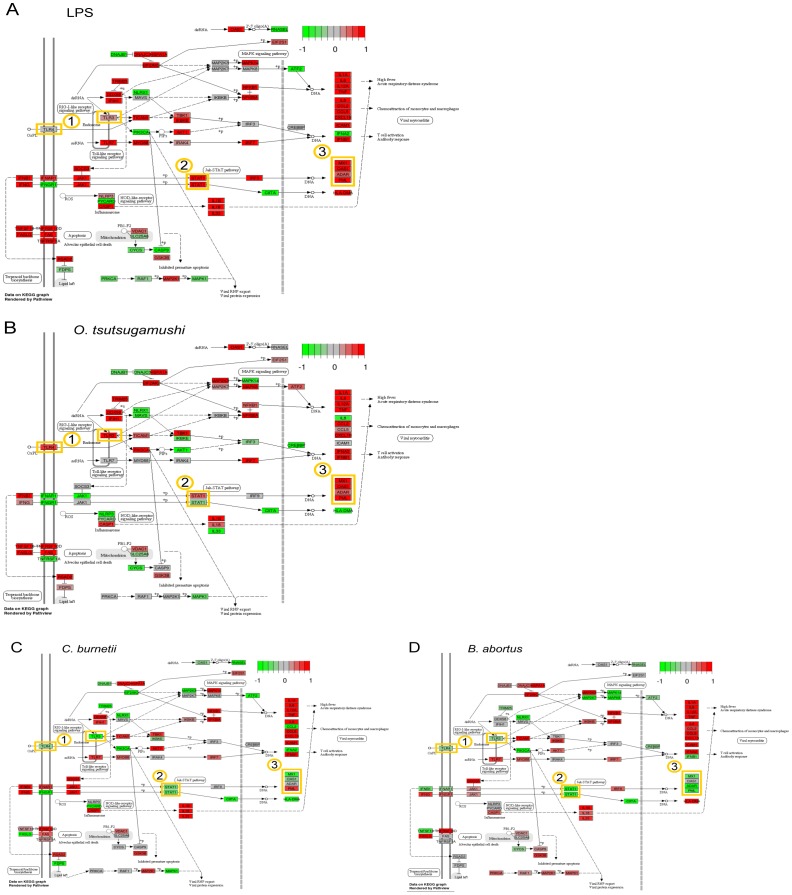
Although each microorganism elicited a specific signature in moDCs, the C. burnetii- and B. abortus-stimulated responses were clearly distinct from the O. tsutsugamushi- and LPS-stimulated responses, suggesting major differences between those agonists that induced full maturation and the others. By selecting the genes that were up-modulated by LPS and O. tsutsugamushi and down-modulated by C. burnetii and B. abortus, we identified a series of genes implicated in apoptosis regulation, innate immune response and type I IFN “antiviral” response ( Table 2 ). A set of genes involved in the IFN pathway and that were down-modulated in response to C. burnetii and B. abortus was confirmed by qRT-PCR (Table S1). In addition, a comparison of the KEGG “Influenza” pathway (which summarizes the main modules of the type I IFN response pathway) in the stimulated moDCs highlighted three nodes that were down-modulated by C. burnetii and B. abortus ( Figure 4 ), corresponding to the genes encoding TLR3/TLR4, the transcription factor Stat1 and such antiviral response genes as MX1, MX2, OAS1, IFI44 and ADAR.
Table 2. Genes differentially expressed by bacterial pathogens.
| Symbol | C. burnetii | B. abortus | O. tsutsugamushi | LPS |
| ATP10A | 0.45 | 0.85 | 1.20 | 6.00 |
| BRIP1 | 0.48 | 1.08 | 6.85 | 13.51 |
| CCL2 | 0.38 | 1.80 | 3.47 | 5.01 |
| FAM59A | 0.26 | 1.45 | 3.65 | 2.89 |
| GMPR | 0.24 | 0.78 | 2.10 | 6.24 |
| HERC5 | 0.47 | 1.30 | 14.08 | 36.91 |
| HSH2D | 0.39 | 0.91 | 0.99 | 6.02 |
| IFI44 | 0.45 | 0.97 | 3.63 | 11.52 |
| MX1 | 0.46 | 0.98 | 2.48 | 6.43 |
| MX2 | 0.49 | 0.95 | 1.63 | 13.35 |
| OR52K3P | 0.31 | 0.80 | 3.58 | 5.28 |
| P2RY6 | 0.34 | 0.91 | 0.96 | 2.06 |
| SAMD9 | 0.43 | 0.91 | 3.55 | 5.30 |
| SH2D1B | 0.49 | 1.24 | 3.32 | 4.75 |
| SIX1 | 0.40 | 1.19 | 3.73 | 3.43 |
| TRIL | 0.40 | 0.96 | 3.32 | 9.13 |
| USP18 | 0.46 | 1.10 | 4.16 | 13.52 |
| XAF1 | 0.50 | 1.00 | 1.27 | 5.69 |
moDCs were stimulated with bacterial pathogens or E. coli LPS for 6 hours. RNAs were extracted, and microarrays were performed. The genes that were up-modulated by O. tsutsugamushi and LPS and down-modulated by C. burnetii and B. abortus are listed in alphabetic order. The values of FC are presented and IFN response genes are underlined.
Figure 4. Activation of the type I IFN response pathway by bacteria.
The KEGG pathway hsa05164 of influenza response was modified to represent the type I IFN response. The genes depicted on this pathway are colored according to the modulation induced by E. coli LPS (A), O. tsutsugamushi (B), C. burnetii (C) or B. abortus (D).
The transcriptional alterations in the moDCs stimulated by C. burnetii and B. abortus were associated with defective activation of the MAPK p38. Hence, the phosphorylation of p38, 15 and 30 minutes after C. burnetii and B. abortus stimulation, was lower that observed in the moDCs stimulated with LPS or O. tsutsugamushi ( Figure 5 , A and B). The transcriptional alterations induced by C. burnetii and B. abortus were also associated with defective IFN-β production Indeed, C. burnetii was unable to induce the release of IFN-β, a type I IFN, from the moDCs. Similarly, B. abortus-stimulated moDCs released low levels of IFN-β. In contrast, O. tsutsugamushi induced the release of high levels of IFN-β from the moDCs. LPS, which induces type I IFN genes, induced the release of IFN-β in a dose-dependent manner ( Figure 5C ). These results demonstrate that the defective maturation of moDCs in response to C. burnetii and B. abortus is associated with defective gene expression within the type I IFN pathway, defective p38 activation and defective IFN-β production.
Figure 5. p38 phosphorylation and IFN-β release.
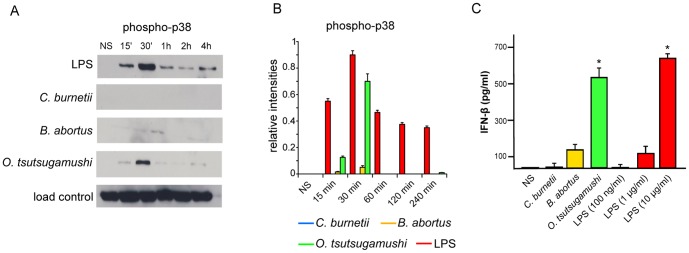
A, moDCs were stimulated with bacterial pathogens or E. coli LPS for different durations, ranging from 15 minutes to 4 hours, and immunoblotting was used to assess MAPK p38 phosphorylation. B, Densitometric scanning was used to quantify phosphorylation changes of p38 and the results are the mean ± SD of three experiments. C, moDCs were stimulated with bacterial pathogens or different concentrations of LPS for 16 hours. The release of IFN-β by moDCs was determined by ELISA. The results are expressed in pg/mL and represent the mean ± SD of three independent experiments.*p<0.03.
Discussion
This study addressed how intracellular bacteria responsible for human infectious diseases affect the response of moDCs, thereby creating an immune environment that is favorable to bacterial survival. We selected two intraphagosomal bacteria, C. burnetii and B. abortus, and one intracytosolic microorganism, O. tsutsugamushi. Although each pathogen has specificity in metabolism, virulence and replication rate, these bacteria share the ability to target myeloid cells [17], [18], [21], [22], [24]; the former two cause infectious diseases with a risk of chronic evolution, whereas the latter is responsible for acute infections. Such observations suggest that the immune mechanisms, including DC activation, induced by C. burnetii and B. abortus are different from those induced by O. tsutsugamushi. The combined use of functional tests of maturation and the measurement of activation marker membrane expression revealed that the responses of moDCs to O. tsutsugamushi were similar to those induced by LPS, corresponding to DC maturation. It has also been reported that O. tsutsugamushi activates moDCs and drives the proliferation of CD4+ T-cells [12], [18]. The combined approach in the present study showed that C. burnetii and B. abortus partly induce the maturation of moDCs, with B. abortus being less efficient than C. burnetii. These findings are consistent with previous reports showing that C. burnetii partially impairs the maturation of moDCs [22] and that B. abortus inhibits DC maturation in a murine model of infection [24]. Our results demonstrate that the use of whole functional tests, including the loss of endocytic activity and T-cell proliferation, were not sufficiently accurate to discriminate among the responses of DCs to bacteria. These assays might also lead to erroneous conclusions, as O. tsutsugamushi, C. burnetii and B. abortus share the ability to induce the loss of endocytosis and T-cell proliferation. The discrepancy between the expression of membrane antigens and functional tests of maturation has been described in Mycobacterium tuberculosis infection. Indeed, the subsets of DCs that are infected at high frequency poorly stimulate M. tuberculosis antigen-specific CD4+ T-cells, despite the expression of surface MHC class II molecules and costimulatory molecules [35]. Alternatively, the production of IL-2 and IL-15 by DCs in response to infectious stimuli may be involved in T-cell proliferation [36], [37]. We analyzed the microarray data from stimulated moDCs and found that the IL15 gene was overexpressed in response to C. burnetii, O. tsutsugamushi and LPS. Similarly, the IL2 gene was upmodulated in response to C. burnetii and LPS while the IL2RA gene was overexpressed in response to O. tsutsugamushi, C. burnetii and B. abortus (Table S2). These findings are consistent with the hypothesis that the expression of IL-2 and IL-15 pathways is sufficient to induce T-cell proliferation whereas the modulation of membrane molecules requires additional signals.
As our phenotypic assays identified defective moDC maturation in response to C. burnetii and B. abortus, we used a high-throughput transcriptional approach to characterize the underlying mechanisms. We used the E. coli LPS signature and the signature induced by O. tsutsugamushi as positive controls of maturation. The absence of LPS from the cell wall of O. tsutsugamushi [17] rules out wall LPS as stimulating O. tsutsugamushi-induced full DC maturation, which is emphasized by the failure of LPS-expressing C. burnetii and B. abortus to fully induce the maturation of DCs. Nevertheless, the LPS of these bacteria are atypical. C. burnetii LPS is poorly endotoxinic and exhibits a specific molecular composition with two unusual sugars: virenose and dihydroxystreptose [13]. The treatment of moDCs with C. burnetii LPS up-regulated the expression of CD86 and HLA-DR but not that of CD80 and CD83, suggesting that bacterial LPS contributes to the phenotype of C. burnetii-stimulated DCs (Figure S1). However, these results do not support the hypothesis of Shannon et al. that C. burnetii LPS interferes with C. burnetii-activated DCs [22]. We found that B. abortus LPS induced an moDC phenotype similar to that induced by the entire bacterium, as only CD80 and CD86 were expressed at the moDC membrane (Figure S1), suggesting that B. abortus LPS is unable to fully activate moDCs. Indeed, B. abortus LPS might act along with the btp1 protein [24] to prevent the proper DC-driven immune response. Here, we confirm that the LPSs from C. burnetii and B. abortus are not sufficient to activate moDC responses.
We focused on the moDC responses induced by C. burnetii and B. abortus. The activation of the MAPK p38 was defective in response to C. burnetii and B. abortus. The defective activation of p38 by C. burnetii has been reported in macrophages and related to the escape of bacteria to degradative compartments [13]. The modulation of p38 by Brucella sp. is debated. The LPS O chain from Brucella sp. restricts the activation of p38 [38] and the intracellular replication of Brucella melitensis is mainly dependent of p38 [39]. In contrast, B. abortus activates p38 in murine astrocytes [40]. The transcriptional signature induced by C. burnetii and B. abortus compared to that induced by O. tsutsugamushi and LPS was composed of a series of down-modulated genes. The comparison of the KEGG “Influenza” response pathway led to the identification of nodes targeted by C. burnetii and B. abortus to down-modulate TLR4/TLR3, STAT1 and type I IFN-responsive genes. We recently explored the transcriptional datasets that are deposited in public repertories, such as the Gene Expression Omnibus, and we focused the main transductional pathways that lead to the type I IFN response during bacterial infections. We found that the type I IFN pattern induced by vacuolar bacteria is completely different from the signatures induced by viruses and cytosolic bacteria [41]. The IFN response pathway has been described in transcriptomics analysis of tuberculosis in which it remains silent in latent tuberculosis and was fully active in patients with active tuberculosis [42]. As C. burnetii and B. abortus share the ability to cause a chronic evolution of disease and bacterial persistence, such a strategy may be operative for their persistence. C. burnetii was unable to stimulate the release of IFN-β, and B. abortus only induced the release of low levels of IFN-β compared to O. tsutsugamushi. It has been recently demonstrated that B. abortus induced the production of IFN-β by moDCs in mice [43]. The recognition of O. tsutsugamushi by cytosolic sensors and the activation of the type I IFN signaling pathway [44] may be related to the ability of O. tsutsugamushi to escape phagosomes and enter the cytosol [45], as found for Francisella tularensis [46] and Listeria monocytogenes [47], [48]. IFN-β was also reported to be produced by monocytes and macrophages in response to O. tsutsugamushi [16], [17], suggesting that the intracellular lifestyle of O. tsutsugamushi is critical for the production of type I IFN.
In conclusion, we showed that intracellular bacteria, such as C. burnetii and B. abortus, are able to modulate the maturation of moDCs. The classical functional tests of DC maturation are not sufficiently accurate to detect impaired DC responses. However, using a systems biology approach, we found that C. burnetii and B. abortus dampen the type I IFN response. This effect may lead to inefficient immune response and bacterial persistence within the host, conferring a critical role for the type I IFN pathway in antibacterial immunity.
Supporting Information
Phenotypic study of moDC maturation. moDCs were stimulated with C. burnetii-LPS, B. abortus-LPS or E. coli-LPS for 24 hours. The cells were then incubated with FITC-coupled anti-CD80, PE-coupled anti-CD83, PE-coupled anti-CD86 and FITC-coupled anti-HLA-DR Abs for 30 minutes and analyzed by flow cytometry. The curves are representative of three different experiments.
(TIF)
qRT-PCR validation of genes involved in the IFN pathway.
(DOC)
IL-15 and IL-12-associated genes.
(DOC)
Pathway analysis of transcriptional modulation of moDC by intracellular bacteria.
(PDF)
Acknowledgments
We are grateful to Catherine Lépolard for her technical expertise.
Funding Statement
The authors have no support or funding to report.
References
- 1. Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- 2. Shortman K, Liu Y-J (2002) Mouse and human dendritic cell subtypes. Nat Rev Immunol 2: 151–161. [DOI] [PubMed] [Google Scholar]
- 3. Palucka K, Banchereau J, Mellman I (2010) Designing vaccines based on biology of human dendritic cell subsets. Immunity 33: 464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alvarez D, Vollmann EH, von Andrian UH (2008) Mechanisms and consequences of dendritic cell migration. Immunity 29: 325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zenk SF, Jantsch J, Hensel M (2009) Role of Salmonella enterica lipopolysaccharide in activation of dendritic cell functions and bacterial containment. J Immunol 183: 2697–2707. [DOI] [PubMed] [Google Scholar]
- 6. Wei L, Wu S, Li Y, Chu Y, Huang R (2012) Salmonella enterica serovar Typhi plasmid impairs dendritic cell responses to infection. Curr Microbiol 65: 133–140. [DOI] [PubMed] [Google Scholar]
- 7. Andres S, Schmidt HMA, Mitchell H, Rhen M, Maeurer M, et al. (2011) Helicobacter pylori defines local immune response through interaction with dendritic cells. FEMS Immunol Med Microbiol 61: 168–178. [DOI] [PubMed] [Google Scholar]
- 8. Wang YH, Wu J-J, Lei HY (2009) When Helicobacter pylori invades and replicates in the cells. Autophagy 5: 540–542. [DOI] [PubMed] [Google Scholar]
- 9. Balboa L, Romero MM, Yokobori N, Schierloh P, Geffner L, et al. (2010) Mycobacterium tuberculosis impairs dendritic cell response by altering CD1b, DC-SIGN and MR profile. Immunol Cell Biol 88: 716–726. [DOI] [PubMed] [Google Scholar]
- 10. Geijtenbeek TBH, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, et al. (2003) Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 197: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ojcius DM, Bravo de Alba Y, Kanellopoulos JM, Hawkins RA, Kelly KA, et al. (1998) Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol 160: 1297–1303. [PubMed] [Google Scholar]
- 12. Choi JH, Cheong TC, Ha NY, Ko Y, Cho CH, et al. (2013) Orientia tsutsugamushi subverts dendritic cell functions by escaping from autophagy and impairing their migration. PLoS Negl Trop Dis 7: e1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barry AO, Boucherit N, Mottola G, Vadovic P, Trouplin V, et al. (2012) Impaired stimulation of p38α-MAPK/Vps41-HOPS by LPS from pathogenic Coxiella burnetii prevents trafficking to microbicidal phagolysosomes. Cell Host Microbe 12: 751–763. [DOI] [PubMed] [Google Scholar]
- 14. Celli J (2006) Surviving inside a macrophage: the many ways of Brucella . Res Microbiol 157: 93–98. [DOI] [PubMed] [Google Scholar]
- 15. Desnues B, Raoult D, Mege JL (2005) IL-16 is critical for Tropheryma whipplei replication in Whipple's disease. J Immunol 175: 4575–4582. [DOI] [PubMed] [Google Scholar]
- 16. Tantibhedhyangkul W, Prachason T, Waywa D, El Filali A, Ghigo E, et al. (2011) Orientia tsutsugamushi stimulates an original gene expression program in monocytes: relationship with gene expression in patients with scrub typhus. PLoS Negl Trop Dis 5: e1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tantibhedhyangkul W, Ben Amara A, Textoris J, Gorvel L, Ghigo E, et al. (2013) Orientia tsutsugamushi, the causative agent of scrub typhus, induces an inflammatory program in human macrophages. Microb Pathog 55: 55–63. [DOI] [PubMed] [Google Scholar]
- 18. Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, et al. (2012) Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis 6: e1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gorvel L, Al Moussawi K, Ghigo E, Capo C, Mege JL, et al. (2010) Tropheryma whipplei, the Whipple's disease bacillus, induces macrophage apoptosis through the extrinsic pathway. Cell Death Dis 1: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Desnues B, Lepidi H, Raoult D, Mege JL (2005) Whipple disease: intestinal infiltrating cells exhibit a transcriptional pattern of M2/alternatively activated macrophages. J Infect Dis 192: 1642–1646. [DOI] [PubMed] [Google Scholar]
- 21. Benoit M, Barbarat B, Bernard A, Olive D, Mege JL (2008) Coxiella burnetii, the agent of Q fever, stimulates an atypical M2 activation program in human macrophages. Eur J Immunol 38: 1065–1070. [DOI] [PubMed] [Google Scholar]
- 22. Shannon JG, Howe D, Heinzen RA (2005) Virulent Coxiella burnetii does not activate human dendritic cells: role of lipopolysaccharide as a shielding molecule. Proc Natl Acad Sci U S A 102: 8722–8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Celli J, de Chastellier C, Franchini D-M, Pizarro-Cerda J, Moreno E, et al. (2003) Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med 198: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, et al. (2008) Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog 4: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ben Amara A, Ghigo E, Le Priol Y, Lépolard C, Salcedo SP, et al. (2010) Coxiella burnetii, the agent of Q fever, replicates within trophoblasts and induces a unique transcriptional response. PloS One 5: e15315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pizarro-Cerdá J, Méresse S, Parton RG, van der Goot G, Sola-Landa A, et al. (1998) Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun 66: 5711–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martirosyan A, Ohne Y, Degos C, Gorvel L, Moriyón I, et al. (2013) Lipopolysaccharides with acylation defects potentiate TLR4 signaling and shape T cell responses. PloS One 8: e55117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Culhane AC, Thioulouse J, Perrière G, Higgins DG (2005) MADE4: an R package for multivariate analysis of gene expression data. Bioinformatics 21: 2789–2790. [DOI] [PubMed] [Google Scholar]
- 29. López-Romero P (2011) Pre-processing and differential expression analysis of Agilent microRNA arrays using the AgiMicroRna Bioconductor library. BMC Genomics 12: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hochberg Y, Benjamini Y (1990) More powerful procedures for multiple significance testing. Stat Med 9: 811–818. [DOI] [PubMed] [Google Scholar]
- 31. Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, et al. (2003) DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol 4: P3. [PubMed] [Google Scholar]
- 32. Okuda S, Yamada T, Hamajima M, Itoh M, Katayama T, et al. (2008) KEGG Atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res 36: W423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo W, Brouwer C (2013) Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformics 29: 1830–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ben Amara A, Gorvel L, Baulan K, Derain-Court J, Buffat C, et al. (2013) Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. J Immunol 191: 5501–5514. [DOI] [PubMed] [Google Scholar]
- 35. Wolf AJ, Linas B, Trevejo-Nuñez GJ, Kincaid E, Tamura T, et al. (2007) Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol 179: 2509–2519. [DOI] [PubMed] [Google Scholar]
- 36. Feau S, Facchinetti V, Granucci F, Citterio S, Jarrossay D, et al. (2005) Dendritic cell-derived IL-2 production is regulated by IL-15 in humans and in mice. Blood 105: 697–702. [DOI] [PubMed] [Google Scholar]
- 37. Granucci F, Andrews DM, Degli-Esposti MA, Ricciardi-Casragnoli P (2002) IL-2 mediates adjuvant effect of dendritic cells. Trends Immunol 23: 169–171. [DOI] [PubMed] [Google Scholar]
- 38. Jimenez de Baquies MP, Gross A, Terraza A, Dornand J (2005) Regulation of the mitogen-activated protein kinases by Brucella spp. expressing a smooth and rough phenotype: relationship to pathogen invasiveness. Infect Immun 73: 3178–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Demitrakopoulos O, Liopeta K, Dimitracopoulos G, Paliogianni F (2013) Replication of Brucella melitensis inside primary human monocytes depends on mitogen-activated protein kinase signaling. Microbes Infect 15: 450–460. [DOI] [PubMed] [Google Scholar]
- 40. Miraglia MC, Scian R, Samartino CG, Barrionuevo P, Rodriguez AM, et al. (2013) Brucella abortus induces TNF-α-dependent astroglial MMP-9 secretion through mitogen-activated protein kinases. J Neuroinflammation 10: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Textoris J, Capo C, Mege JL (2012) Type I interferons and bacterial infectious diseases: new features. Recent Res Devel Immunology 8: 49–74. [Google Scholar]
- 42. Berry MPR, Graham CM, McNab FW, Xu Z, Bloch SAA, et al. (2010) An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466: 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Almeida LA, Carvalho NB, Oliveira FS, Lacerda TLS, Vasconcelos AC, et al. (2011) MyD88 and STING signaling pathways are required for IRF3-mediated IFN-β induction in response to Brucella abortus infection. PLoS One 6: e23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boldrick JC, Alizadeh AA, Diehn M, Dudoit S, Liu CL, et al. (2002) Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc Natl Acad Sci U S A 99: 972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ge Y, Rikihisa Y (2011) Subversion of host cell signaling by Orientia tsutsugamushi . Microbes Infect 13: 638–648. [DOI] [PubMed] [Google Scholar]
- 46. Bar-Haim E, Gat O, Markel G, Cohen H, Shafferman A, et al. (2008) Interrelationship between dendritic cell trafficking and Francisella tularensis dissemination following airway infection. PLoS Pathog 4: e1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Edelson BT (2012) Dendritic cells in Listeria monocytogenes infection. Adv Immunol 113: 33–49. [DOI] [PubMed] [Google Scholar]
- 48. Tam MA, Wick MJ (2004) Dendritic cells and immunity to Listeria: TipDCs are a new recruit. Trends Immunol 25: 335–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypic study of moDC maturation. moDCs were stimulated with C. burnetii-LPS, B. abortus-LPS or E. coli-LPS for 24 hours. The cells were then incubated with FITC-coupled anti-CD80, PE-coupled anti-CD83, PE-coupled anti-CD86 and FITC-coupled anti-HLA-DR Abs for 30 minutes and analyzed by flow cytometry. The curves are representative of three different experiments.
(TIF)
qRT-PCR validation of genes involved in the IFN pathway.
(DOC)
IL-15 and IL-12-associated genes.
(DOC)
Pathway analysis of transcriptional modulation of moDC by intracellular bacteria.
(PDF)