Abstract
Huntington’s disease (HD) is a genetic neurodegenerative disorder. The most common symptom of HD is abnormal involuntary writhing movements, called chorea. Antipsychotics and tetrabenazine are used to alleviate the signs and symptoms of HD. Stem cells have been investigated for use in neurodegenerative disorders to develop cell therapy strategies. Recent evidence indicates that the beneficial effects of stem cell therapies are actually mediated by secretory molecules, as well as cell replacement. Although stem cell studies show that cell transplantation provides cellular improvement around lesions in in vivo models, further work is required to elucidate some issues before the clinical application of stem cells. These issues include the precise mechanism of action, delivery method, toxicity and safety. With a focus on HD, this review summarizes cell therapy strategies and the paracrine effect of stem cells.
Keywords: Huntington’s disease, Chorea, Cell therapy, Stem cell, Paracrine effect
INTRODUCTION
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder. It is caused by an abnormal number of coronary angiography (CAG) trinucleotide repeats in the Huntingtin gene (HTT), which encodes a 350 kDa ubiquitously expressed protein, Huntingtin (Htt).1 HD is characterized by movement disorder, cognitive impairment, dementia, and affective disturbances.2 HD patients have more than 40 CAG repeats and show abnormal involuntary writhing movements. Juvenile HD patients have more than 60 CAG repeats.1,3 The age of onset of HD is typically between 35 and 44 years old.
A HTT comprising more than 40 CAG repeats is translated into mutant Huntingtin (mHtt) protein, which causes the death of medium spiny neurons in the striatum. Normal Htt is ubiquitously expressed and is essential for embryonic development.4 The mechanism of neuronal cell death by mHtt has not been clearly established although previous studies report that it has been linked with mitochondrial dysfunction, transcriptional dysregulation, altered protein-protein interactions, abnormal protein aggregations, and excitotoxicity.5–7
TREATMENT OF HUNTINGTON’S DISEASE
Many pharmacological drugs are used in the treatment of HD (Table 1). The treatment paradigm for HD patients depends on 3 main clinical domains: movement, psychiatric, and cognitive abnormalities. Tetrabenazine is the most commonly used drug for chorea. Antipsychotic agents, including Haloperidol, Pimozide, and Clozapine, are used to treat patients with psychiatric/behavioral comorbidities. Rivastigmine and Donepezil are the preferred treatments for improving cognitive function. These treatments are the result of limited evidence presented in the literature. Furthermore, comparison of the available treatment studies is problematic due to differences in study populations, variable outcomes, the use of different instruments, and the confounding effects of drugs.
Table 1.
Pharmacological drugs on symptoms of HD
| Treatment of chorea | |
| Antidopaminergic agents8,9 | Tetrabenazine |
| Antipsychotic agents10–17 | Haloperidol, Pimozide, Clozapine, Olanzapine, Ziprasidone, Aripiprazole, Risperidone, Quetiapine |
| N-methyl-D-aspartic acid receptor antagonists18,19 | Amantadine, Memantine |
| Omega-3 fatty acids20 | Ethyl-eicosapentaenoic acid |
| Treatment of cognitive dysfunction associated with HD | |
| Cognition21,22 | Rivastigmine, Donepezil |
| Treatment of behavioral disturbances associated with HD | |
| Depression23–26 | Fluoxetine, Venlafaxine, Mirtazapine, Clozapine |
| Psychosis27 | Risperidone |
| Irritability, agitation28–33 | Olanzapine, Quetiapine, Sertraline, Buspirone, Valproate, Propranolol |
HD: Huntington’s disease.
CELL THERAPY FOR HUNTINGTON’S DISEASE
The pharmacological treatment of HD can alleviate symptoms, but it cannot cure the disease. Cell therapy strategies have been actively studied as a potential cure for HD. The ultimate goal of cell therapy is the replacement or neuroprotection of dead or dying cells. Cell therapy strategies can be classified into two broad categories based on the use of either fetal tissues/cells or stem cells. Studies using fetal brain tissue were performed using animal models of HD prior to 1990. Several clinical trials on HD patients have been performed with fetal tissues or cells. However, effective recovery has not been reported in any clinical trials, although some studies showed that fetal tissue transplantation provided cellular improvement around lesions.34,35 Moreover, fetal tissue transplantation led to localized effects only and did not persist long-term.34,36
Stem cells are being studied in various disease models, in preference to fetal tissue or cells due to the limited availability of the latter. Stem cell research focuses primarily on neurodegenerative disorders. Several types of stem cells, such as embryonic stem cells (ESCs), bone marrow mesenchymal stem cells (BM-MSCs), neural stem cells (NSCs), adipose stem cells (ASCs), and induced pluripotent stem cells (iPSCs), are used to develop cell therapy strategies.
Embryonic stem cells are pluripotent, and mouse ESCs can differentiate into neurons, astrocytes, and oligodendrocytes.37 It has been reported that human ESCs (hESCs) can differentiate into neurons in the lesions of HD animal models, attenuating progressive symptoms.38 Despite these benefits of hESCs, complications arising from their use include immune rejection, ethical concerns, and tumor formation.38 On the other hand, somatic stem cells such as BM-MSCs, NSCs, ASCs, and iPSs are ideal sources for clinical trials because these stem cells do not present the above mentioned immune rejection and ethical problems.
Murine and human NSCs (hNSCs) have been studied in vivo as cell therapy sources for HD. A study involving an hNSC treated HD animal group investigated the migration of transplanted hNSCs around a lesion site. Following tail vein or ventricle injection, a significantly greater volume of striatum was observed in the treatment group compared to the control group. Other studies reported that transplanted NSCs differentiated into neurons, oligodendrocytes, and predominantly, astrocytes, in in vivo HD models, resulting in partial functional recovery.38–42
Bone marrow mesenchymal stem cells and ASCs are easily obtained multipotent somatic stem cells that can be differentiated into neuronal cells. Moreover, these stem cells have the ability to secrete neuroprotective factors, such as growth factors, chemokines, and cytokines. Recent studies have shown that intrastriatal transplantation of BM-MSCs reduced striatal atrophy, although transplanted cells only survived for up to 7 days in transgenic HD mice. BM-MSCs can be genetically modified to provide sustained and long-term delivery of neuroprotective factors, which increase neurogenesis and protect against cell death.43–45 Genetically modified MSCs are currently under consideration for use in the treatment of neurodegenerative disorders, including HD.46
Adipose stem cells are a feasible source for cellular therapy due to ease of isolation, manipulation, and a strong safety profile in the clinic. The intrastriatal transplantation of normal human ASCs reduced lesion volumes in an HD rat model.47 To examine the long-term effect of ASC transplantation and investigate the possibility of autologous ASC transplantation in HD patients, HD patient-derived ASCs have been investigated over a period of 4 months in the YAC128 model.48 The results showed similar expression levels of growth factors, such as brain derived neurotrophic factor (BDNF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and leukemia inhibitory factor (LIF), in HD ASCs compared with normal human ASCs. However, no long-term effects of transplantation with either HD or normal ASCs were observed in YAC128.
Embryonic stem cells have two limitations regarding their clinic application: the ethical issues surrounding their use and allogenic immune rejection. iPSCs provide a potential solution because they have the ability to differentiate into various cell types and can be induced from the fibroblasts of an HD patient.49,50 iPSCs from an HD patient with 72 CAG repeats have been efficiently induced to form gamma- Aminobutyric acid neurons and were functional following transplantation into a rat model of HD.51
STEM CELL PARACRINE EFFECT BASED THERAPY IN HUNTINGTON’S DISEASE
Although stem cells have the ability to differentiate into any type of cell, recent studies indicate that the beneficial effects of stem cell therapies actually occur via secretory molecules in addition to cell replacement, the so-called paracrine effect.47,52 Stem cells secrete a variety of growth factors, cytokines, and chemokines that regulate their biology in an autocrine/ paracrine manner, and they interact with the surrounding microenvironment.53,54 VEGF, HGF, insulin- like growth factor-1 and -2 (IGF-1, -2) and stromal- derived factor-1 secreted from stem cells are important to neuronal survival, neurogenesis, and mitochondrial activation via a bystander-like mechanism. 47,55,56 These positive effects on recipient neural cells result in protection and repair, leading to the inhibition of HD progression (Figure 1).
Figure 1.
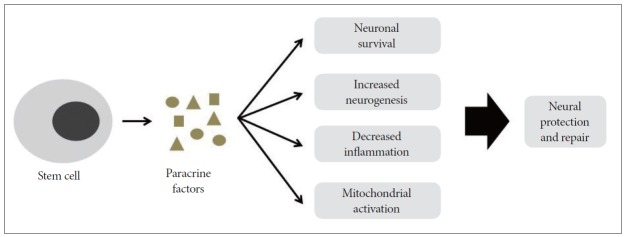
Possible mechanism of neural protection and repair by paracrine effects of stem cells. Stem cells release secretory molecules, including anti-inflammatory cytokines, various growth factors and extracellular vesicles. These factors could positively influence cell survival, neurogenesis, inflammation and mitochondrial function, leading to neural protection and repair.
Adipose stem cells are multipotent somatic stem cells. They secrete multiple antiapoptotic growth factors, including VEGF, HGF, BDNF, basic fibroblast growth factor, and IGF-1.57–59 One solution to the problem of stem cell availability may be the paracrine effect of ASCs.
The paracrine effects of human ASCs on HD pathology were investigated in cell culture experiments and HD R6/2 mouse models.47 Transplantation of ASCs resulted in reduced lesion volume and fewer apoptotic striatal cells in the HD rat model compared with control animals. The ASC transplanted group showed significant improvement in apomorphine- induced rotation tests via the paracrine effect. ASCs have been injected into the R6/2 HD mouse model, and treated mice exhibited a significantly longer survival time than control mice.
The paracrine effect of ASCs in the R6/2 HD mouse model was also investigated.60 ASC extracts were isolated and used to treat R6/2 mice via intraperitoneal injection. The results were similar to those obtained from stem cell transplantation, suggesting that the injection of these stem cell extracts could also slow HD progression.60
Taken together, the use of growth factors in HD could be an ideal stem cell strategy to protect against neuronal death, given that stem cells from an HD patient have the genetic components for autologous transplantation therapies. To implement this therapy, further works are required to elucidate the precise mechanism of the paracrine effects of ASC extracts. Prior to clinical application, thorough in vivo studies examining the delivery method, toxicity, and pharmacokinetics of therapeutic candidates are required.
CONCLUSION
Pharmacological drugs to cure HD are in development. Most of these drugs do not demonstrate significant effects, although several drugs are currently undergoing clinical trials. Stem cell therapy is an effective strategy for curing HD, and many preclinical trials show encouraging results. Although the precise mechanism of the stem cell paracrine effect has not been completely elucidated, this strategy has potential for clinical application.
Acknowledgments
This work was supported by grants from the Korea Health 21 R&D Project, Ministry of Health & Welfare (A092058) and National Research Foundation of Korea (NRF) (2011-0012728).
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
REFERENCES
- 1.Kremer B, Goldberg P, Andrew SE, Theilmann J, Telenius H, Zeisler J, et al. A worldwide study of the Huntington’s disease mutation. The sensitivity and specificity of measuring CAG repeats. N Engl J Med. 1994;330:1401–1406. doi: 10.1056/NEJM199405193302001. [DOI] [PubMed] [Google Scholar]
- 2.Huntington G. On chorea. Med Surg Rep. 1872;26:317–321. [Google Scholar]
- 3.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 4.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 5.DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington’s disease. Trends Neurosci. 1990;13:286–289. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- 6.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, et al. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg YP, Nicholson DW, Rasper DM, Kalchman MA, Koide HB, Graham RK, et al. Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat Genet. 1996;13:442–449. doi: 10.1038/ng0896-442. [DOI] [PubMed] [Google Scholar]
- 8.Jankovic J, Beach J. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology. 1997;48:358–362. doi: 10.1212/wnl.48.2.358. [DOI] [PubMed] [Google Scholar]
- 9.Ondo WG, Tintner R, Thomas M, Jankovic J. Tetrabenazine treatment for Huntington’s disease-associated chorea. Clin Neuropharmacol. 2002;25:300–302. doi: 10.1097/00002826-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Koller WC, Trimble J. The gait abnormality of Huntington’s disease. Neurology. 1985;35:1450–1454. doi: 10.1212/wnl.35.10.1450. [DOI] [PubMed] [Google Scholar]
- 11.Girotti F, Carella F, Scigliano G, Grassi MP, Soliveri P, Giovannini P, et al. Effect of neuroleptic treatment on involuntary movements and motor performances in Huntington’s disease. J Neurol Neurosurg Psychiatry. 1984;47:848–852. doi: 10.1136/jnnp.47.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Vugt JP, Siesling S, Vergeer M, van der Velde EA, Roos RA. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63:35–39. doi: 10.1136/jnnp.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogelman G, Hirschmann S, Modai I. Olanzapine and Huntington’s disease. J Clin Psychopharmacol. 2001;21:245–246. doi: 10.1097/00004714-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Bonelli RM, Mayr BM, Niederwieser G, Reisecker F, Kapfhammer HP. Ziprasidone in Huntington’s disease: the first case reports. J Psychopharmacol. 2003;17:459–460. doi: 10.1177/0269881103174009. [DOI] [PubMed] [Google Scholar]
- 15.Brusa L, Orlacchio A, Moschella V, Iani C, Bernardi G, Mercuri NB. Treatment of the symptoms of Huntington’s disease: preliminary results comparing aripiprazole and tetrabenazine. Mov Disord. 2009;24:126–129. doi: 10.1002/mds.22376. [DOI] [PubMed] [Google Scholar]
- 16.Parsa MA, Szigethy E, Voci JM, Meltzer HY. Risperidone in treatment of choreoathetosis of Huntington’s disease. J Clin Psychopharmacol. 1997;17:134–135. doi: 10.1097/00004714-199704000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249:1114–1115. doi: 10.1007/s00415-002-0741-1. [DOI] [PubMed] [Google Scholar]
- 18.Verhagen Metman L, Morris MJ, Farmer C, Gillespie M, Mosby K, Wuu J, et al. Huntington’s disease: a randomized, controlled trial using the NMDA-antagonist amantadine. Neurology. 2002;59:694–699. doi: 10.1212/wnl.59.5.694. [DOI] [PubMed] [Google Scholar]
- 19.Ondo WG, Mejia NI, Hunter CB. A pilot study of the clinical efficacy and safety of memantine for Huntington’s disease. Parkinsonism Relat Disord. 2007;13:453–454. doi: 10.1016/j.parkreldis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Puri BK, Leavitt BR, Hayden MR, Ross CA, Rosenblatt A, Greenamyre JT, et al. Ethyl-EPA in Huntington disease: a double-blind, randomized, placebo-controlled trial. Neurology. 2005;65:286–292. doi: 10.1212/01.wnl.0000169025.09670.6d. [DOI] [PubMed] [Google Scholar]
- 21.de Tommaso M, Difruscolo O, Sciruicchio V, Specchio N, Livrea P. Two years’ follow-up of rivastigmine treatment in Huntington disease. Clin Neuropharmacol. 2007;30:43–46. doi: 10.1097/01.wnf.0000240945.44370.f0. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez HH, Friedman JH, Grace J, Beason-Hazen S. Donepezil for Huntington’s disease. Mov Disord. 2000;15:173–176. doi: 10.1002/1531-8257(200001)15:1<173::aid-mds1032>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Como PG, Rubin AJ, O’Brien CF, Lawler K, Hickey C, Rubin AE, et al. A controlled trial of fluoxetine in nondepressed patients with Huntington’s disease. Mov Disord. 1997;12:397–401. doi: 10.1002/mds.870120319. [DOI] [PubMed] [Google Scholar]
- 24.Holl AK, Wilkinson L, Painold A, Holl EM, Bonelli RM. Combating depression in Huntington’s disease: effective antidepressive treatment with venlafaxine XR. Int Clin Psychopharmacol. 2010;25:46–50. doi: 10.1097/YIC.0b013e3283348018. [DOI] [PubMed] [Google Scholar]
- 25.Bonelli RM. Mirtazapine in suicidal Huntington’s disease. Ann Pharmacother. 2003;37:452. doi: 10.1345/aph.1C352. [DOI] [PubMed] [Google Scholar]
- 26.Sajatovic M, Verbanac P, Ramirez LF, Meltzer HY. Clozapine treatment of psychiatric symptoms resistant to neuroleptic treatment in patients with Huntington’s chorea. Neurology. 1991;41:156. doi: 10.1212/wnl.41.1.156. [DOI] [PubMed] [Google Scholar]
- 27.Erdemoglu AK, Boratav C. Risperidone in chorea and psychosis of Huntington’s disease. Eur J Neurol. 2002;9:182–183. doi: 10.1046/j.1468-1331.2002.0354e.x. [DOI] [PubMed] [Google Scholar]
- 28.Squitieri F, Cannella M, Piorcellini A, Brusa L, Simonelli M, Ruggieri S. Short-term effects of olanzapine in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:69–72. [PubMed] [Google Scholar]
- 29.Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47:70–72. doi: 10.1176/appi.psy.47.1.70. [DOI] [PubMed] [Google Scholar]
- 30.Ranen NG, Lipsey JR, Treisman G, Ross CA. Sertraline in the treatment of severe aggressiveness in Huntington’s disease. J Neuropsychiatry Clin Neurosci. 1996;8:338–340. doi: 10.1176/jnp.8.3.338. [DOI] [PubMed] [Google Scholar]
- 31.Bhandary AN, Masand PS. Buspirone in the management of disruptive behaviors due to Huntington’s disease and other neurological disorders. Psychosomatics. 1997;38:389–391. doi: 10.1016/S0033-3182(97)71447-6. [DOI] [PubMed] [Google Scholar]
- 32.Grove VE, Jr, Quintanilla J, DeVaney GT. Improvement of Huntington’s disease with olanzapine and valproate. N Engl J Med. 2000;343:973–974. doi: 10.1056/NEJM200009283431316. [DOI] [PubMed] [Google Scholar]
- 33.Stewart JT. Paradoxical aggressive effect of propranolol in a patient with Huntington’s disease. J Clin Psychiatry. 1987;48:385–386. [PubMed] [Google Scholar]
- 34.Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- 35.Nakao N, Grasbon-Frodl EM, Widner H, Brundin P. DARPP-32-rich zones in grafts of lateral ganglionic eminence govern the extent of functional recovery in skilled paw reaching in an animal model of Huntington’s disease. Neuroscience. 1996;74:959–970. doi: 10.1016/0306-4522(96)00238-2. [DOI] [PubMed] [Google Scholar]
- 36.Cicchetti F, Saporta S, Hauser RA, Parent M, Saint-Pierre M, Sanberg PR, et al. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc Natl Acad Sci U S A. 2009;106:12483–12488. doi: 10.1073/pnas.0904239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Pevny L, Lovell-Badge R, Smith A. Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol. 1998;8:971–974. doi: 10.1016/s0960-9822(98)70399-9. [DOI] [PubMed] [Google Scholar]
- 38.Song J, Lee ST, Kang W, Park JE, Chu K, Lee SE, et al. Human embryonic stem cell-derived neural precursor transplants attenuate apomorphine-induced rotational behavior in rats with unilateral quinolinic acid lesions. Neurosci Lett. 2007;423:58–61. doi: 10.1016/j.neulet.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 39.Dziewczapolski G, Lie DC, Ray J, Gage FH, Shults CW. Survival and differentiation of adult rat-derived neural progenitor cells transplanted to the striatum of hemiparkinsonian rats. Exp Neurol. 2003;183:653–664. doi: 10.1016/s0014-4886(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 40.Johann V, Schiefer J, Sass C, Mey J, Brook G, Krüttgen A, et al. Time of transplantation and cell preparation determine neural stem cell survival in a mouse model of Huntington’s disease. Exp Brain Res. 2007;177:458–470. doi: 10.1007/s00221-006-0689-y. [DOI] [PubMed] [Google Scholar]
- 41.McBride JL, Behrstock SP, Chen EY, Jakel RJ, Siegel I, Svendsen CN, et al. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. J Comp Neurol. 2004;475:211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- 42.Visnyei K, Tatsukawa KJ, Erickson RI, Simonian S, Oknaian N, Carmichael ST, et al. Neural progenitor implantation restores metabolic deficits in the brain following striatal quinolinic acid lesion. Exp Neurol. 2006;197:465–474. doi: 10.1016/j.expneurol.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 43.Choong PF, Mok PL, Cheong SK, Leong CF, Then KY. Generating neuron-like cells from BM-derived mesenchymal stromal cells in vitro. Cytotherapy. 2007;9:170–183. doi: 10.1080/14653240701196829. [DOI] [PubMed] [Google Scholar]
- 44.Erba P, Terenghi G, Kingham PJ. Neural differentiation and therapeutic potential of adipose tissue derived stem cells. Curr Stem Cell Res Ther. 2010;5:153–160. doi: 10.2174/157488810791268645. [DOI] [PubMed] [Google Scholar]
- 45.Zavan B, Vindigni V, Gardin C, D’Avella D, Della Puppa A, Abatangelo G, et al. Neural potential of adipose stem cells. Discov Med. 2010;10:37–43. [PubMed] [Google Scholar]
- 46.Annett G, Bauer G, Nolta JA. Mesenchymal stem cells for trinucleotide repeat disorders. Methods Mol Biol. 2013;1010:79–91. doi: 10.1007/978-1-62703-411-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee ST, Chu K, Jung KH, Im WS, Park JE, Lim HC, et al. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. 2009;66:671–681. doi: 10.1002/ana.21788. [DOI] [PubMed] [Google Scholar]
- 48.Im W, Lee ST, Park JE, Oh HJ, Shim J, Lim J, et al. Transplantation of patient-derived adipose stem cells in YAC128 Huntington’s disease transgenic mice. PLoS Curr. 2010 doi: 10.1371/currents.RRN1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Jeon I, Lee N, Li JY, Park IH, Park KS, Moon J, et al. Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. Stem Cells. 2012;30:2054–2062. doi: 10.1002/stem.1135. [DOI] [PubMed] [Google Scholar]
- 52.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 53.Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/ paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 54.Janowska-Wieczorek A, Majka M, Ratajczak J, Ratajczak MZ. Autocrine/paracrine mechanisms in human hematopoiesis. Stem Cells. 2001;19:99–107. doi: 10.1634/stemcells.19-2-99. [DOI] [PubMed] [Google Scholar]
- 55.Pluchino S, Cossetti C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia. 2013;61:1379–1401. doi: 10.1002/glia.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 58.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291:R880–R884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 59.Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 60.Im W, Ban J, Lim J, Lee M, Lee ST, Chu K, et al. Extracts of adipose derived stem cells slows progression in the R6/2 model of Huntington’s disease. PLoS One. 2013;8:e59438. doi: 10.1371/journal.pone.0059438. [DOI] [PMC free article] [PubMed] [Google Scholar]


