Abstract
AIMS
To assess the efficacy of topical dorzolamide for treating cystoid macular edema in patients with retinitis pigmentosa and minimize the secondary effects of maintenance therapy in patients with retinitis pigmentosa (RP) who present with chronic microcystic macular edema.
METHODS
To replace acetazolamide systemic treatment, with a topical treatment using 2% dorzolamide in three patients. The methods performed were OCT scan with a Spectralis HRA-OCT, for the measurement of macular thickness and morphology; best corrected visual acuity was assessed using Early Treatment Diabetic Retinopathy Study (ETDRS), was assessed slit-lamp biomicroscopy, ocular tonometry, fundus biomiocrosopy, and color fundus photography. This therapeutic protocol has been applied and described in three patients.
RESULTS
In all three tested patients, following the administration of dorzolamide in eye drop, we observed a remarkable decrease in macular edema, almost comparable to that obtained with acetazolamide per os.
CONCLUSION
The study confirms the anti-edematogenic effect of topical dorzolamide in RP with recurring macular cysts, as this can have a favorable response with topical dorzolamide. In all the three examined patients, the instillation of topical dorzolamide caused a remarkable reduction in their macular edema, as highlighted on OCT.
Keywords: retinitis pigmentosa, cystoid macular edema, dorzolamide, acetazolamide
Introduction
Retinitis pigmentosa (RP) is a diffuse retinal dystrophy that mainly affects the rod cells. RP can occur as a sporadic ocular disorder without any familial history, or it can be inherited as a dominant autosomal, recessive autosomal, or X-linked disease.1
The disease affects both eyes, although asymmetrically, and it has a progressive evolution, leading to eventual blindness. The subjective symptomatology consists of hemeralopia, night blindness, and a reduction of the visual field which, in its early stages, is completely unperceived because it affects only the peripheral part. Only after several years does the presence of a central scotoma appear, which tends to extend both towards the periphery and towards the center, eventually producing the characteristic tunnel vision that indicates extreme and belated narrowing of the visual field.2
The classic ophthalmoscopic triad of RP is represented by:
Attenuation of artery caliber.
Retinal pigmentation in the form of bone spicules (the spots of black pigment that run along the course of the vessels have a characteristic shape; their morphology resembles that of osteoblasts, hence the name). These spots are more numerous at the retinal periphery and they tend to move, while thinning, towards the posterior pole, which is usually free. In fact, only in the final stages do they also invade the central region. The analysis of the anatomic–pathological alterations of these ailments has highlighted a diffuse abiotrophic process that disrupts the pigment epithelium, whose pigment migrates along the course of the vessels which, undergoing hyaline degeneration, tend to become obliterated.
Optic disc pallor (commonly known as “chamois yellow”).
The long-term prognosis is unfavorable, as there is a final loss of central vision through the direct involvement of the fovea from the pigmentosa itself and/or the maculopathy. Daily administration of vitamin A, if established prematurely, can delay the progression of the disease.1,3
Since some of the complications that are often connected with this disease, and which are able to further compromise the patient’s vision, are susceptible to treatment, it is good to subject patients to regular follow-up with the aim of identifying any complications to treat them early. The complications that manifest themselves most frequently include the posterior subcapsular cataract (common in all forms of pigmentosa; these are treatable) and the more serious maculopathy. The latter may appear in atrophic form (ie, cellophane maculopathy), or they may cause microcystic macular edema, leading to the increased permeability of the perifoveal capillary network.4 For the latter form, good results can be obtained through systemic administration of acetazolamide.9 Nevertheless its effectiveness is still controversial.5–8 Acetazolamide is a diuretic and inhibitor of carbonic anhydrase, an enzyme that plays a key role in the transport of H+ ions in various parts of the body; this leads to an increase in the renal elimination of Na/HCO3 and K+, thus causing a decrease in plasma bicarbonate and, therefore, metabolic acidosis (hence the use of these drugs in the treatment of edematous patients or in cases of metabolic alkalosis).10
Unfortunately, acetazolamide may notoriously lead to the onset of severe side effects. That is the reason why the frequent and important side effects of acetazolamide do not allow it to be administered for a prolonged period of time (3–5 days maximum). In fact, due to the distribution of carbonic anhydrase throughout the whole organism, it is possible to observe alterations involving different systems: at the renal level, it is possible to develop calculi, and both polyuria and nycturia can be present (especially at the beginning of the treatment); at the digestive level, gastric and intestinal problems often occur, sometimes accompanied by diarrhea; at the hematic level, hypokalemia may arise due to the renal excretion of K+ followed by experiences of general discomfort, acidosis and, in very rare cases, agranulocytosis; paraesthesia at limb extremities is very common.8,9
For this reason, it is necessary to find an alternative therapy that can be administered to these patients constantly to prevent their visual acuity impairment, even before retinitis has run its course. To this end, we describe three case reports of RP patients treated with topical dorzolamide, a molecule that, to date, has not shown any systemic secondary effects, three times daily to compare the efficacy and side effects induced by the different molecules used.
In accordance with other literature,19,22,23 we defined a favorable response as a significant improvement in the degree of cystic macular lesions, which was determined by measuring changes in the central foveal thickness and the foveal zone thickness on optical coherence tomography (OCT).
Materials and Methods
In the study three patients were included. Before initiating treatment, a baseline OCT scan was obtained on each patient with a Spectralis HRA-OCT produced by Heidelberg Engineering GmbH (Heidelberg, Germany) with a volumetric 512 × 49 scan for the measurement of macular thickness and morphology. The evaluation of vision was carried out pretherapy at baseline T0; best corrected visual acuity was assessed using Early Treatment Diabetic Retinopathy Study (ETDRS) tables placed at a distance of 4 m by slit-lamp biomicroscopy, ocular tonometry (using a Goldmann applanation tonometer), fundus biomiocrosopy, and color fundus photography.
None of the patients in our study had additional ocular conditions or were on other medications that could potentially affect their visual acuity and/or retinal function.
At subsequent follow-up visits, OCT images for all three patients were obtained. A central foveal thickness was calculated as an average of the measurements obtained. A change of more than 16% in foveal thickness and more than 11% in foveal zone thickness were considered significant.9 We compared all subsequent retinal thicknesses.
After the first follow-up visit, each patient was seen several times (follow-up visits ranged from 2–6 months from the previous visit) to monitor the patient’s response to treatment. At each visit, a repeat OCT measurement was obtained.
This study was approved by the local ethics committee.
Statistical analysis
The analysis was conducted calculating median and range for the quantitative variables. Moreover, Wilcoxon Signed Ranks and Friedman Test were performed in order to verify if the differences between the same patients were significant considering the three time periods (T0, T1 and T2).
The analysis was performed using SPSS for Windows, release 19.0.
The statistical significance was set at P < 0.05.
Results
Case 1
A 39-year-old man suffering from RP demonstrated microcystic macular edema on OCT, with a visual acuity of 4/10 in oculus dexter (OD) and of 3/10 in oculus sinister (OS).
In order to keep the edema under control, he was treated with an acetazolamide tablet (250 mg per os; one tablet a day for 8 days), leading to resolution of the microcystic edema and a rise in visual acuity to 8/10 in OD and 7/10 in OS.
After 12 days from the cessation of therapy, a relapse of the edema provoked a new reduction in visual acuity. Systemic treatment with acetazolamide was reinstated, but it was suspended after only 36 hours because of the patient’s intolerance to the drug (renal colic).
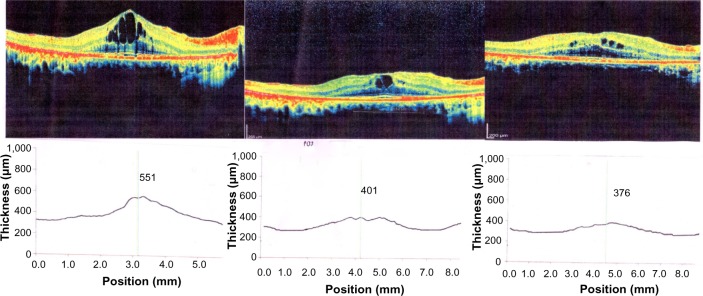
In an attempt to control the edema, treatment was started with three times daily instillation of dorzolamide in each eye. This resulted in reduction of macular edema in 7 days as documented by OCT (Fig. 1).
Figure 1.
Images of the right eye at baseline (T0) optical coherence tomography (OCT), central macular thickness (CMT) 551, 3 weeks after starting treatment CMT 401, 6 months after starting treatment (T2) CMT 376.
Case 2
A 24-year-old woman suffering from RP presented with macular edema in both eyes, as documented upon OCT, with a visual acuity of 5/10 in OD and 3/10 in OS. Oral therapy with acetazolamide was started (half a tablet 3 times a day for a week).
After 7 days, the patient showed edema resolution in OD and considerable edema reduction in OS; her visual acuity increased to 8/10 in OD and 6/10 in OS. Due to the occurrence of paraesthesias and asthenia, the woman was forced to interrupt the therapy. Within 10 days, a relapse of the edema was witnessed. Topical therapy began using dorzolamide three times daily in each eye, with considerable improvement of the edema (higher in OD than in OS), which was detectable with OCT around the 15th day of treatment (Fig. 2).
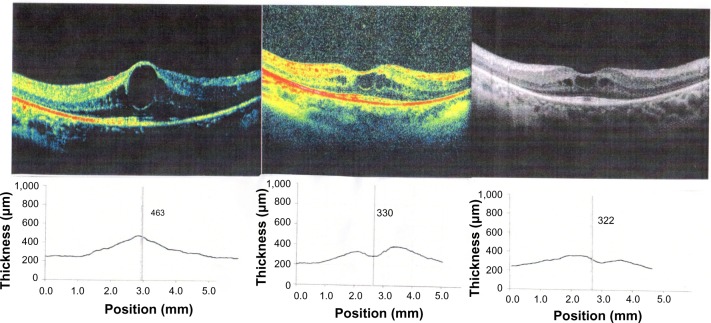
Figure 2.
Images of the left eye at baseline (T0) optical coherence tomography (OCT), central macular thickness (CMT) 463, 3 weeks after starting treatment CMT 330, 6 months after .starting treatment (T2) CMT 322.
Case 3
A 28-year-old woman suffering from RP presented with macular edema in both eyes, as documented by OCT; she also had a visual acuity of 4/10 in each eye.
During anamnesis, the patient informed us about her history of renal calculi; for this reason, we opted to directly treat her with topical therapy using dorzolamide eye drop three times daily in each eye which, in 20 days, induced a marked improvement of the edema (higher in OD than in OS) (Table 3), as seen on OCT measurements. However, the improvement in visual function in this patient proved to be only subjective.
Table 3.
Test statistics on Visual Acuity values (decimal notation and ETDRS) and CMT values from baseline (T0) to the first follow-up visit within 3 weeks of starting therapy with dorzolamide (T1) and follow-up visit at 6 months (T2).
| V.A. T0 | ETDRS T0 | V.A. T1 | ETDRS T1 | CMT_T0 | CMT_T1 | CMT_T2 | ||
|---|---|---|---|---|---|---|---|---|
| N | Valid | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Median | 4,00 | 39,50 | 4,00 | 46,00 | 459,00 | 373,00 | 348,00 | |
| Minimum | 3 | 34 | 3 | 39 | 449 | 318 | 270 | |
| Maximum | 5 | 41 | 5 | 50 | 551 | 410 | 395 |
Best visual acuity did not change between T0 and T1 (median 4/10; range: 3/10–5/10 for both time periods) (P = 1.00), as well as ETDRS values (at T0 median 39.5, range 34–41; at T1 median 46, range 39–50) (P = 0.068).
Concerning the central macular thickness (CMT), significant reductions were observed over time (at T0 median 459, range 449–551; at T1 median 373, range 318–410; at T2 median 348, range 270–395) (P = 0.002).
Discussion and Conclusions
There are no codified, uniform guidelines for the treatment of clinically significant macular edema.11 Therefore, various therapeutic options have been considered.12
Some protocols call for the administration of topical nonsteroidal anti-inflammatory drugs (NSAIDs) (indomethacin eye drop, one drop, four times a day for 2 months) in combination with systemic NSAIDs (indomethacin 50 mg/day per os for 4 weeks) with the addition, in the case of a lack of response, of acetazolamide (with a daily dosage of 500 mg/day for 10 days).10 Other, more frequently used protocols call for the combination of NSAIDs (indomethacin/diclofenac/ketorolac/flurbiprofen/suprofen), diuretics (acetazolamide), and synthetic corticosteroids.13,14 The main indications for acetazolamide administration are imbalanced trabecular blockage glaucoma and several other types of secondary glaucoma.15
Due to its considerable hypotonizing and diuretic efficacy, acetazolamide is used in cases where a quick and strong reduction of the intraocular pressure is sought. Nevertheless, due to the possibility of serious side effects arising (drowsiness, confusion, allergic reactions, paresthesias, myelosuppression, renal calculi, loss of potassium, or hyperchloremic metabolic acidosis from extended use), it is preferable that treatment not be prolonged over an extended period of time.
For this reason, a new compound has been developed that is able to exert a significant hypotonizing action following topical administration: dorzolamide in eye drop, which is free from the secondary effects that are attributable to administration via the systemic route. Moreover, this drug has such liposolubility and hydrophilia that it can cross the cornea (this is different from acetazolamide, which is not very liposoluble), and has high affinity for the enzyme. The latter is a very important characteristic that allows dorzolamide to inhibit 98% of carbonic anhydrase.1
The main indications for the topical administration of dorzolamide are imbalanced trabecular blockage glaucoma (in combination with beta-blockers), and all those cases in which beta-blocker therapy is contraindicated.16,17
Furthermore, this drug seems to have several therapeutic possibilities that would make it a suitable therapy for managing various types of edema. The instillation of eye drop, whose anti-edematogenic effect is indisputable,18 is in fact not subject to time restrictions because its prolonged use does not induce the onset of any side effects. 19 The only exception is that it can lead to a bitter taste in the mouth due to the inhibition of carbonic anhydrase in the taste buds;20 this effect is possible to neutralize by digitally compressing the lacrimal sac during eye drop instillation with a cotton wool puff.21
The reduction of CMT generally results in an improvement in visual acuity.13 Although clinical or statistically significant differences in improvements in visual acuity were not observed in our patients, it is possible to retain the integrity of the external membrane, as well as of the inner and outer segments of the photoreceptor interface.23
In all the three examined patients, the instillation of topical dorzolamide caused a remarkable reduction in their macular edema, as highlighted in the OCT.22
Despite this compound being less potent than acetazolamide per os, in the treatment of edema, it could be a valid alternative due to the lack of drug-induced secondary effects.1 It has the potential to be used continuously as maintenance therapy in the management of chronic macular microcystic edema.22 However, the therapeutic characteristics of dorzolamide will be better defined only with further clinical studies.
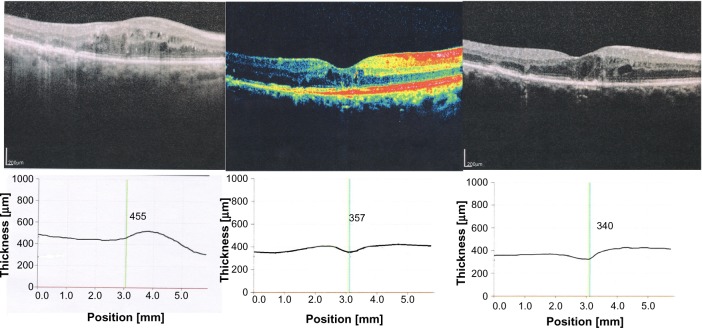
Figure 3.
Images of the right eye at at baseline (T0) optical coherence tomography (OCT), CMT 455, 3 weeks after starting treatment CMT 357, 6 months after starting treatment (T2) CMT 340.
Table 1.
Demographic characteristics of the study population and changes from baseline (TO) to the first follow-up visit within 3 weeks of starting therapy with dorzolamide (T1) in Visual Acuity (decimal notation and ETDRS).
| PATIENT NO | SEX | AGE | EYE | V.A. T0 | V.A. (NUMBER OF LETTERS ETDRS) BASELINE T0 | V.A. T1 | V.A. (NUMBER OF LETTERS ETDRS) T1 |
|---|---|---|---|---|---|---|---|
| 1 | M | 39 | O.D. | 4/10 | 40 | 8/10 | 50 |
| O.S. | 3/10 | 35 | 7/10 | 47 | |||
| 2 | F | 24 | O.D. | 5/10 | 41 | 8/10 | 49 |
| O.S. | 3/10 | 34 | 6/10 | 45 | |||
| 3 | F | 28 | O.D. | 4/10 | 39 | 4/10 | 39 |
| O.S. | 4/10 | 40 | 4/10 | 40 |
Abbreviations: F, female; M, male; OS, left eye; OD, right eye; VA, Visual Acuity; ETDRS, Early Treatment Diabetic Retinopathy Study.
Table 2.
Changes from baseline (T0) to the first follow-up visit within 3 weeks of starting therapy with dorzolamide (T1) and follow-up visit at 6 months (T2) in central macular thickness (CMT).
| PATIENT NO | EYE | CMT (μm) T0 | CMT (μm) T1 | CMT (μm) T2 |
|---|---|---|---|---|
| 1 | O.D. | 463 | 339 | 322 |
| O.S. | 470 | 389 | 356 | |
| 2 | O.D. | 450 | 318 | 270 |
| O.S. | 551 | 401 | 376 | |
| 3 | O.D. | 455 | 357 | 340 |
| O.S. | 449 | 410 | 395 |
Footnotes
ACADEMIC EDITOR: Joshua Cameron, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
Author Contributions
Conceived and designed the experiments: EP, VS, FP. Ana-lyzed the data: EP, GLT, FP. Wrote the first draft of the manuscript: VS, FP, PT. Contributed to the writing of the manuscript: EP, VS, FP, LA, MRC. Agree with manuscript results and conclusions: EP, GLT, FP, LA, VS, PT, MRC. Jointly developed the structure and arguments for the paper: EP, GLT, FP, LA, VS, PT, MRC. Made critical revisions and approved final version: EP, GLT, FP, LA, VS, PT, MRC. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Kellner U, Renner AB, Tillack H. Hereditary retinochoroidal dystrophies. Part 2: differential diagnosis. Ophthalmologe. 2004;101(4):397–412. doi: 10.1007/s00347-003-0945-5. quiz 413. German. [DOI] [PubMed] [Google Scholar]
- 2.Souied E, Soubrane G, Coscas G. Hereditary retinal diseases. Rev Prat. 1996;46(14):1730–6. French. [PubMed] [Google Scholar]
- 3.Schmidt JG, Zinser J. Long-term follow-up of deterioration of the visual field in visual acuity in retinitis pigmentosa. Fortschr Ophthalmol. 1989;86(6):614–8. German. [PubMed] [Google Scholar]
- 4.Rothova A. Medical treatment of cystoid macular edema. Ocul Immunol Inflamm. 2002;10(4):239–46. doi: 10.1076/ocii.10.4.239.15589. [DOI] [PubMed] [Google Scholar]
- 5.Fishman GA, Gilbert LD, Fiscella RG, Kimura AE, Jampol LM. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol. 1989;107(10):1445–52. doi: 10.1001/archopht.1989.01070020519031. [DOI] [PubMed] [Google Scholar]
- 6.Ismail RA, Sallam A, Zambarakji HJ. Pseudophakic macular edema and oral acetazolamide: an optical coherence tomography measurable, dose-related response. Eur J Ophthalmol. 2008;18(6):1011–3. doi: 10.1177/112067210801800626. [DOI] [PubMed] [Google Scholar]
- 7.Meyer KM, Klink T, Ugurel S, Bröcker EB. Regression of paclitaxel-induced maculopathy with oral acetazolamide. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):463–4. doi: 10.1007/s00417-011-1642-8. [DOI] [PubMed] [Google Scholar]
- 8.Apushkin MA, Fishman GA, Grover S, Janowicz MJ. Rebound of cystoid macular edema with continued use of acetazolamide in patients with retinitis pigmentosa. Retina. 2007;27(8):1112–8. doi: 10.1097/IAE.0b013e31805f6b79. [DOI] [PubMed] [Google Scholar]
- 9.Stewart WC, Halper LK, Johnson-Pratt L, Polis A, Hartenbaum D. Tolerability and efficacy of dorzolamide versus acetazolamide added to timolol. J Ocul Pharmacol Ther. 2002;18(3):211–20. doi: 10.1089/108076802760116133. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan NM. Diuretics as a basis of antihypertensive therapy. An overview. Drugs. 2000;59(Suppl 2):21–25. doi: 10.2165/00003495-200059002-00003. discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 11.Chung H, Hwang JU, Kim JG, Yoon YH. Optical coherence tomography in the diagnosis and monitoring of cystoid macular edema in patients with retinitis pigmentosa. Retina. 2006;26(8):922–7. doi: 10.1097/01.iae.0000250008.83779.23. [DOI] [PubMed] [Google Scholar]
- 12.Pacella E, La Torre G, Impallara D, et al. Efficacy and safety of the intravitreal treatment of diabetic macular edema with pegaptanib: a 12-month follow-up. Clin Ter. 2013;164(2):e121–6. doi: 10.7417/CT.2013.1543. [DOI] [PubMed] [Google Scholar]
- 13.Pacella E, Vestri AR, Muscella R, et al. Preliminary results of an intravitreal dexamethasone implant (Ozurdex®) in patients with persistent diabetic macular edema. Clin Ophthalmol. 2013;7:1423–8. doi: 10.2147/OPTH.S48364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Momont AC, Mills RP. Glaucoma screening: current perspectives and future directions. Semin Ophthalmol. 2013;28(3):185–90. doi: 10.3109/08820538.2013.771200. [DOI] [PubMed] [Google Scholar]
- 15.Kaur IP, Smitha R, Aggarwal D, Kapil M. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm. 2002;248(1–2):1–14. doi: 10.1016/s0378-5173(02)00438-6. [DOI] [PubMed] [Google Scholar]
- 16.Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999;97(3–4):387–97. doi: 10.1023/a:1002143802926. [DOI] [PubMed] [Google Scholar]
- 17.Gulkilik G, Oba E, Odabası M. Comparison of fixed combinations of dorzolamide/timolol and brimonidine/timolol in patients with primary open-angle glaucoma. Int Ophthalmol. 2011;31(6):447–51. doi: 10.1007/s10792-011-9495-z. [DOI] [PubMed] [Google Scholar]
- 18.Pacella E, Pacella F, Cavallotti C, et al. The combination latanoprost-timolol versus twice daily 0.50% timolol administration either associated or not with latanoprost: efficacy and tolerability in the primary open-angle glaucoma. Eur Rev Med Pharmacol Sci. 2010 May;14(5):477–80. [PubMed] [Google Scholar]
- 19.Genead MA, Fishman GA. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with retinitis pigmentosa and usher syndrome. Arch Ophthalmol. 2010;128(9):1146–50. doi: 10.1001/archophthalmol.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishman GA, Apushkin MA. Continued use of dorzolamide for the treatment of cystoid macular oedema in patients with retinitis pigmentosa. Br J Ophthalmol. 2007;91:743–5. doi: 10.1136/bjo.2006.107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez-Juan P, Combarros O. Gustatory nervous pathway syndromes. Neurologia. 2001;16(6):262–71. Spanish. [PubMed] [Google Scholar]
- 22.Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001. [Google Scholar]
- 23.Grover S, Fishman GA, Fiscella RG, Adelman AE. Efficacy of dorzolamide hydrochloride in the management of chronic cystoid macular edema in patients with retinitis pigmentosa. Retina. 1997;17(3):222–31. doi: 10.1097/00006982-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda Y, Yoshida N, Notomi S, et al. Therapeutic effect of prolonged treatment with topical dorzolamide for cystoid macular oedema in patients with retinitis pigmentosa. Br J Ophthalmol. 2013 Sep;97(9):1187–91. doi: 10.1136/bjophthalmol-2012-303005. [DOI] [PubMed] [Google Scholar]