Abstract
Background
Myocardial infarction results in the formation of scar tissue populated by myofibroblasts, a phenotype characterized by increased contractility and matrix deposition. Mesenchymal stem cells (MSC) delivered to the myocardium can attenuate scar growth and restore cardiac function, though the mechanism is unclear.
Methods
This study describes a simple yet robust 3D in vitro co-culture model to examine the paracrine effects of implanted MSC on resident myofibroblasts in a controlled biochemical and mechanical environment. The fibrosis model consisted of fibroblasts embedded in a 3D collagen gel cultured under defined oxygen tensions and exposed to either cyclic strain or interstitial fluid flow. MSC were injected into this model, and the effect on fibroblast phenotype was evaluated 48 hours after cell injection.
Results
Analysis of gene and protein expression of the fibroblasts indicated that injection of MSC attenuated the myofibroblast transition in response to reduced oxygen and mechanical stress. Assessment of VEGF and IGF-1 levels demonstrated that their release by fibroblasts was markedly upregulated in hypoxic conditions, but attenuated by strain or fluid flow. In fibroblast-MSC co-cultures, VEGF levels were increased by hypoxia but not affected by mechanical stimuli, while IGF-1 levels were generally low and not affected by experimental conditions.
Discussion
This study demonstrates how a 3D in vitro model of the cardiac scar can be used to examine paracrine effects of MSC on the phenotype of resident fibroblasts, and therefore illuminates the role of injected progenitor cells on the progression of cardiac fibrosis.
Keywords: Cardiac Fibrosis, Cell Therapy, Hypoxia, Co-culture, Mesenchymal Stem Cells
Introduction
The benefits of injecting adult stem cells into the myocardium post-infarction have been widely examined, but there is little consensus on the degree, duration, and mechanism of cardiac improvement that can be consistently achieved using such treatment [1,2]. Moreover, the success of such cell therapy can vary depending on the timing and mode of cell delivery [3,4]. Variability in the cell source may be influenced by hypertension, diabetes, and other pathologies that have comorbidity with heart disease. Additionally, a myriad of biochemical and mechanical factors within the microenvironment of the post-infarcted myocardium can affect the response of the transplanted cells. The scar environment is characterized by dense extracellular matrix, decreased capillary density, and the presence of contractile myofibroblasts [5,6,7]. Mechanically, the scar is stiffer than surrounding tissue, and the degree of cyclic deformation from systole and diastole is also reduced. It is therefore important to characterize and understand the response of injected stem cells to these exogenous stimuli, in order to optimize cell therapy for the treatment of heart damage.
It is likely that transplanted adult stem cells benefit the myocardium through a paracrine mechanism [8]. Originally, adult stem cells transplanted into the myocardium were thought to differentiate into cardiomyocytes [9] or fuse with damaged cells to restore function [10]. However, bulk regeneration of damaged heart muscle in this manner is unlikely, as it would require the transplanted cells to differentiate into a fully functional cardiomyocyte phenotype, as well as to self-assemble in the correct mechanical and electrical micro-architecture. Improperly integrated heart muscle could give rise to systolic dysfunction or entry points for arrhythmias. More recently, there is a growing consensus that implanted stem cells release paracrine factors that have cardioprotective and angiogenic effects on the damaged myocardium [11]. Identification of these factors would allow their therapeutic delivery in the absence of cells. However, it is likely that stem cell-mediated paracrine release has both a spatial- and time-dependence, which complicates delivery of a growth factor cocktail. Model systems are therefore required to characterize this paracrine action of the implanted stem cells and their interaction with resident cardiac cell types.
A variety of methods have been used to examine the paracrine effects of transplanted stem cells on cardiac cells. A common approach involves culturing cardiomyocytes or cardiac fibroblasts in conditioned media from adult stem cells [12,13], and conditioned media from human mesenchymal stem cells (MSC) has also been injected into animal models to analyze its effect on infarcted heart function [14]. Although useful for studying the potential for stem cell-mediated benefit, these experiments cannot capture the full spectrum of molecular communication between injected stem cells and cardiac cell types. To address this issue, cell co-culture systems have been created [15]. Such systems yield insight into the effect of stem cells on cardiomyocytes, and also illuminate how the presence of myocytes affects stem cell response. However, these simple systems typically do not include the potentially very important effects of mechanical and biochemical stimuli present in microenvironment of the native myocardium.
Animal models have also been used to study the paracrine effects of injected stem cells. These experiments have the advantage that they include the relevant myocardial architecture, presence of cardiomyocytes, fibroblasts, and vascular cell types, as well as mechanical stresses during systole and diastole. A recent study showed that MSC injected into the myocardium improved wall thickness and left ventricular ejection fraction by stimulating angiogenesis [16], and improvement in left ventricular function has also been observed in human studies [17], as well as following intracoronary cell delivery [18]. MSC delivered by intramyocardial injection were shown to increase the presence of myofibroblasts in the scar region [19], while intracoronary delivery of MSC embedded in alginate attenuated the presence of myofibroblasts [20]. Genetic modification to induce overexpression of key paracrine factors such as VEGF [21], Akt [22], CXCR4 [23] and Wnt [24] has yielded some insight into the mechanisms of therapeutic action. However, isolating the effects of specific cell-to-cell interactions and discerning the responses to particular exogenous stimuli remains a challenge when using animal models.
The goal of the present study was to create a co-culture system that mimics key features of the myocardial scar environment, but still allows resolution of the stem cell response to specific mechanical and biochemical stimuli. Our focus in this study was on the paracrine effects of MSC on resident cardiac fibroblasts, since this interplay has direct relevance to the etiology and treatment of cardiac fibrosis. Fluorescently labeled mesenchymal stem cells (MSC) were injected into a cardiac fibroblast-seeded 3D collagen gel to create a co-culture system, and these tissue constructs were then exposed to defined levels of fluid shear, solid stress, and hypoxia. The effect of MSC injection on the phenotype of the resident fibroblasts was evaluated using immunohistochemistry and analysis of gene expression at a distance of 250 μm from the injection site. Secretion of vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF) by the both the injected MSC and resident fibroblasts was also examined by immunoassay. These studies provide insight into how stem cells injected into the myocardium interact with resident cardiac fibroblasts, under mechanical and biochemical conditions that are found in the heart.
Materials and Methods
Cell culture and collagen gel fabrication
Cardiac fibroblasts were isolated from the ventricles of 2–3 day old Sprague-Dawley rats using procedures approved by the Institutional Animal Use and Care Committee at the University of Michigan. Briefly, isolated ventricles were minced and digested in 0.125% trypsin and 0.15% pancreatin (Sigma-Aldrich, St. Louis, MO) at 37 °C in consecutive 15-minute steps. Fibroblasts were separated from myocytes in a pre-plating step and were culture expanded. Fibroblasts to be used in experiments were cultured in M199 containing 5% fetal bovine serum and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO) for one week.
Three-dimensional (3D) collagen hydrogels were fabricated using acid solubilized bovine collagen type I (MP Biomedicals, Solon, OH) using techniques similar to previous studies [25]. Briefly, isolated cardiac fibroblasts were suspended at a concentration of 5.0 × 105 cells/ml in a gel precursor solution composed of collagen Type I (2.0 mg/ml), 10 % FBS, DMEM, and 0.1 M NaOH at physiological pH. Gelation was then initiated by incubation at 37 °C in a humidified 5% CO2 incubator for 45 minutes. The resulting collagen hydrogels had an open fibrillar structure with cells embedded directly inside the matrix, as characterized in previous studies [26,27]. In this study, fibroblast-seeded gels were prepared in custom-made polydimethylsiloxane (PDMS, Dow Corning, Midland, MI) wells, which prevented compaction of the gel matrix by the resident cells. This culture system has been developed and characterized previously and allows controlled application of 5% cyclic strain and/or perfusion with 10 μL/min cross flow [28]. These values of strain are relevant for cells in the heart wall in both rodent and human models, and are comparable values have been used in previous studies [29,30].
Rat mesenchymal stem cells were purchased from Invitrogen and cultured to passage 4. MSC for use in experiments were plated in T-75 tissue culture flasks at a density of 3.3 × 103 cells/cm2. To achieve transduction with eGFP, lentiviral-mediated gene transfer was initiated by adding viral particles to DMEM media containing 5 μg/mL Polybrene (Santa Cruz Biotech, Santa Cruz, CA) for 14 hours. Viral particles were added at a density of 4.4 × 103 particles/cm2, resulting in an MOI of 1.33. After the viral incubation, the medium was replaced with fresh DMEM and the cells were allowed to proliferate for three days before being frozen in liquid nitrogen prior to injection at passage 5.
Mesenchymal stem cell delivery
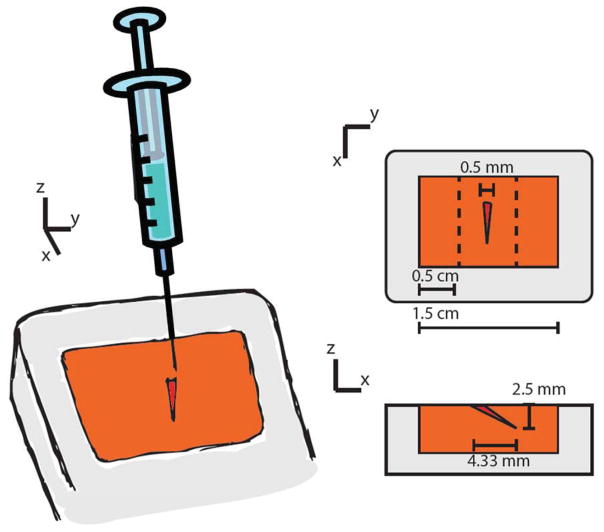
A micromanipulator was used to assure that MSC injections were accurate and consistent in quantity and location in the 3D collagen constructs. MSC injection was performed 12 hours after fabrication of the collagen hydrogel. A 1 mL syringe equipped with a 1-inch 25 gauge needle was mounted on the manipulator at an angle of 30 degrees from the gel surface. The 3 axis manipulator allowed for axial displacement control, so the needle tip penetrated 5 mm into the gel. A schematic of the dimensions of the injection is shown in Figure 1. Once the needle reached its desired position, 40 μL of 6.25 × 103 cells/mL MSC solution was injected into each gel, resulting in the addition of 250,000 MSC. Gels were then incubated for one hour at 37 °C before being returned to culture medium.
Figure 1.
Schematic of injection placement within the 3D fibroblast-seeded model of the cardiac scar. A micromanipulator was used to consistently inject MSC at a 30° angle from the gel surface. The dotted lines show where the gels were sectioned to analyze the fibroblast response.
Mechanical stimulation and hypoxic culture
The apparatus used to apply cyclic mechanical strain and interstitial fluid flow to cell-seeded 3D collagen constructs has been described and characterized previously [28,31]. Briefly, constructs were cultured at 37 °C with 100% humidity and 5% CO2 for 24 hours after gel polymerization (and 12 hours following MSC injection). Constructs analyzed at this 24 hour point served as initial controls, while the remaining constructs were transferred to the mechanical strain/flow apparatus or to an oxygen-controlled incubator set at 2.5% O2 (hypoxia) for an additional 48 hours. Parallel constructs transferred to an incubation bath but not exposed to loading served as the control condition. Mechanically stimulated constructs were exposed to either 5% strain at a frequency of 1 Hz or 10 μL/min of interstitial fluid flow.
Immunofluorescence
Constructs were fixed, permeabilized, and stained using previously described protocols [5]. Briefly, gels were fixed in 3.7% paraformaldehyde (Sigma-Aldrich) for 10 min at room temp, washed with PBS, and permeabilized in 0.2% Triton X-100 (Sigma-Aldrich) for 10 min. Immunostains included fluorescent DAPI (1:50 dilution) and phalloidin conjugated to Texas Red (1:50). Cells were visualized using confocal microscopy, with a focus on a plane 250 μm from the MSC injection site. Projection image Z-stacks of approximately 50 μm were collected using a confocal microscope. To quantify cell migration, ImageJ was used to quantify the distribution of GFP-fluorescing cells, and the migration line was drawn when fluorescence had diminished to 2% of the value within the cone.
Quantitative reverse transcription PCR
A guanidium thiocyanate-phenol-chloroform extraction protocol (TRIzol, Invitrogen Inc.) was used to isolate mRNA from the cells within 3D constructs. Briefly, gels were homogenized and dissolved in TRIzol. mRNA was further purified with chloroform, precipitated and washed with isopropanol and then ethanol, and then dissolved in RNAse-free water. Reverse transcription of mRNA was carried out with a high-capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) in combination with a C-1000 Thermocycler (Bio-Rad, Hercules, CA).
Quantitative PCR was then performed using TaqMan gene expression assay kits (Applied Biosystems, Foster City CA), and the housekeeping gene GAPDH using a 7500 Fast Sequence Detection system (Applied Biosystems, Foster City CA). Collagen type I, collagen type III, TGF-β, and α-SMA were examined as markers of the cardiac fibroblast phenotype [23]. Data are expressed in fold change relative to the initial control samples, calculated based on the ΔΔCT method. Duplicates of each sample were analyzed.
Enzyme-Linked Immunosorbent Assays (ELISA)
Quantikine ELISA assays purchased commercially (R&D Systems, Minneapolis MN) were used to quantify the levels of VEGF and IGF-1 in the supernatants from the co-culture hydrogels. After removal of the constructs from the PDMS wells, the remaining 100–150 μL of supernatant culture medium were used for cytokine analysis. This solution was collected and stored at −20 °C until ELISA was performed.
Statistical analysis
Results show sample sizes of three to eight separate experiments, with data expressed as mean +/− standard deviation. An unpaired Student’s t test was used to determine statistical significance for comparisons of two groups, with p<0.05 considered significantly different.
Results
The effect of MSC injection on fibroblast activation
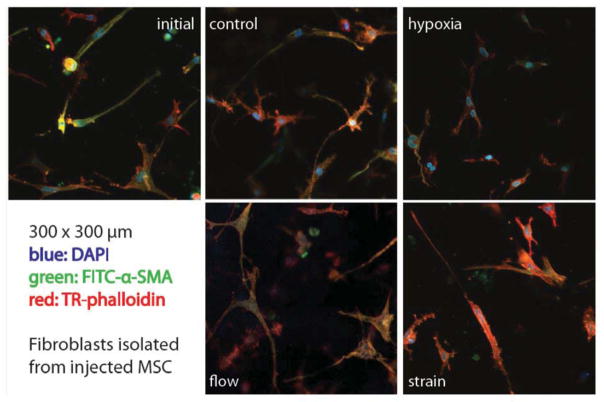
This study was designed to examine the paracrine interactions between injected MSC and resident cardiac fibroblasts in a mechanically stimulated 3D model of cardiac tissue. The primary indicator of these interactions examined was the level of activation of the resident fibroblasts. In our previous work, we showed that fibroblasts seeded into 3D collagen hydrogels exhibit a baseline level of activation, as indicated by increased expression of collagens I and III, TGFβ, and αSMA, and that this level of activation is augmented by fluid flow and hypoxia [32]. We examined fibroblasts in a plane 250 μm from the injection site by immunostaining for αSMA, a marker of the activated myofibroblast phenotype, as shown in Figure 2. The morphology of the cardiac fibroblasts was similar for all time points and stimulation conditions, though the cells exposed to hypoxia were less spread with fewer cell processes extending into the extracellular matrix. Staining for αSMA was somewhat stronger at the initial time point and in the flow condition, compared to the other samples. However, none of the conditions exhibited prominent stress fibers and high levels of αSMA staining, suggesting that the fibroblasts were not strongly activated.
Figure 2.
Immunofluoresence of cardiac fibroblasts at a position approximately 250 μm from the MSC injection site. Each image is 300 × 300 μm. DAPI (blue) indicates cell nuclei; phalloidin (red) indicates the actin cytoskeleton; anti-α-SMA antibody (green) indicates smooth muscle actin expression. Images best viewed in color.
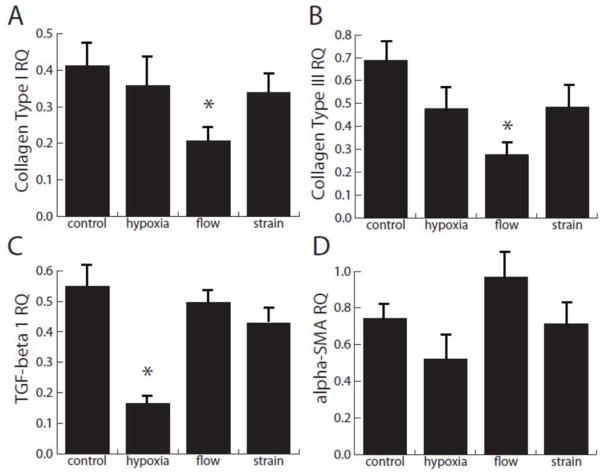
In order to further quantify the fibroblast response to injected MSC, portions of the hydrogel approximately 250 μm to either side of the injection site were homogenized to examine gene expression, as shown in Figure 3. All markers of fibroblast activation were significantly reduced relative to initial control levels under all treatment conditions, except the level of αSMA message in constructs under flow, which remained unchanged. Interestingly, the presence of flow caused a significant decrease in collagen I and III message, compared to control. Hypoxia strongly attenuated TGFβ levels in these co-culture constructs. The gene expression levels observed in this study contrast significantly with the levels produced by gels seeded with fibroblasts alone and exposed to the identical experimental treatments [32]. Overall, these results suggest that not only does the addition of MSC attenuate fibroblast activation, but it may reverse the fibroblast to myofibroblast transition.
Figure 3.
Quantification of message levels of cardiac fibroblasts at a position approximately 250 μm from the MSC injection site. Four markers of the fibroblast to myofibroblast transition were measured and compared to initial control levels. * denotes significance from the control test condition; all levels are significant from initial levels except αSMA in response to flow.
MSC migration in response to mechanical and biochemical stimulation
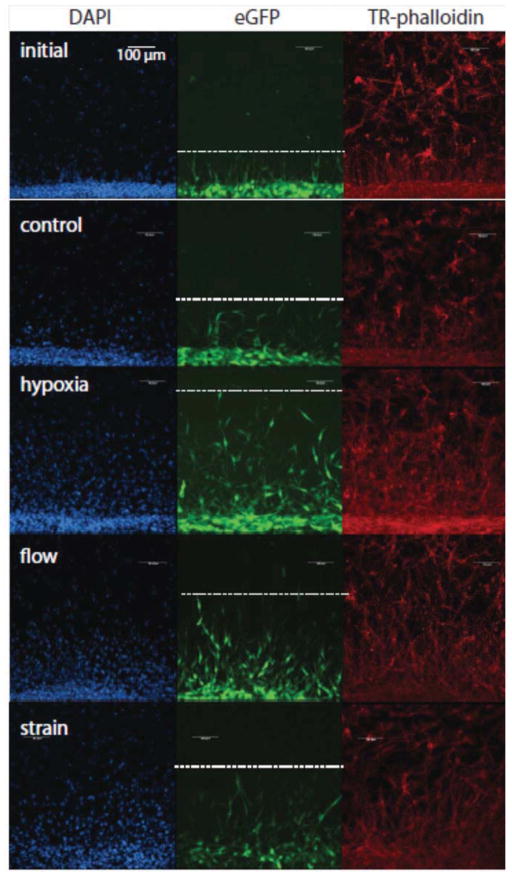
In this study, MSC were injected as a bolus of 250,000 cells. Once implanted, the MSC resided in the injection cone created by needle insertion, and subsequently began to attach to either the surrounding collagen gel and/or neighboring cells. Over time, MSC migrated out of the injection cone and into the surrounding collagen matrix. Images of fluorescent staining of the interface between the injection cone and the collagen gel at the initial time point (12 h after MSC injection) and at the 48 h time point under specific treatment conditions are shown in Figure 4. This figure shows the nuclei of all cells in the constructs (DAPI, first column), the labeled MSC (eGFP, second column), and the actin cytoskeleton of all cells in the construct (phalloidin, third column). The dashed line in the second column indicates the migration front of the injected MSC. The migration distance of MSC for these conditions was quantified and the results are shown in Figure 5. As seen in both the immunofluorescence images and quantified data, hypoxic conditions resulted in the greatest migration distance. Interestingly, mechanical stimulation by both interstitial fluid flow and cyclic strain produced statistically the same levels of migration. All treatment stimuli resulted in significantly higher migration distances than both control and initial conditions. The highest migration distance approached 500 μm, which translates to an average cell velocity of about 10 μm/h. Interestingly, this is approximately the same average velocity as the initial time point, which migrated about 100 μm in a time of 12 hours. This result suggests that initial migration velocities decrease under most conditions, but are maintained under hypoxic conditions.
Figure 4.
Immunofluorescence imaging of a region near the edge of the MSC injection cone (bottom of each image) under different experimental conditions. DAPI (blue) indicates cell nuclei; eGFP (green) indicates the cytoplasm of transfected MSC; phalloidin (red) indicates the actin cytoskeleton. Dotted lines represent the calculated penetration distance of MSC into the surrounding matrix. Images best viewed in color.
Figure 5.
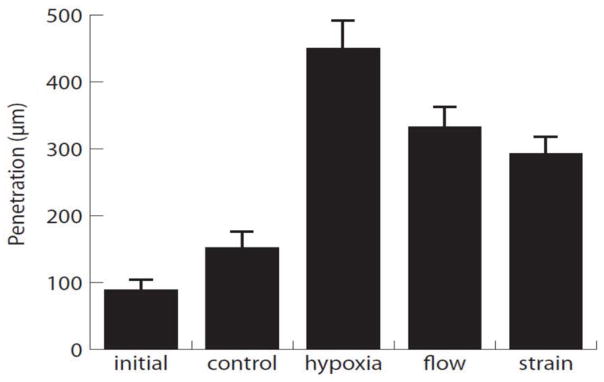
Quantification of the penetration distance of MSC from the wall of the injection cone into the surrounding collagen matrix at 48 hours after the initial time point.
Release of VEGF and IGF-1 in stimulated co-cultures
Having demonstrated that the injected MSC affect fibroblast activation, studies were conducted to identify the paracrine factors responsible for this effect. Analysis of the supernatant from cultured constructs was used to examine the levels of two growth factors commonly associated with the paracrine signaling by MSC: VEGF and IGF-1. Both fibroblast-MSC co-cultures and fibroblast-only constructs were examined, in an effort to determine the relative influence of both cell types. The data are presented as levels of expression by co-cultures with the levels from fibroblast-only gels subtracted (as an estimation of the contribution of the injected MSC), and the fibroblast-only levels are provided for reference (Figures 6 and 7). This normalization does not directly yield the amount of growth factor produced by the MSC, because the presence of MSC may affect fibroblast release. However it provides an overall measure of the effect of MSC on total paracrine release by these two cell types.
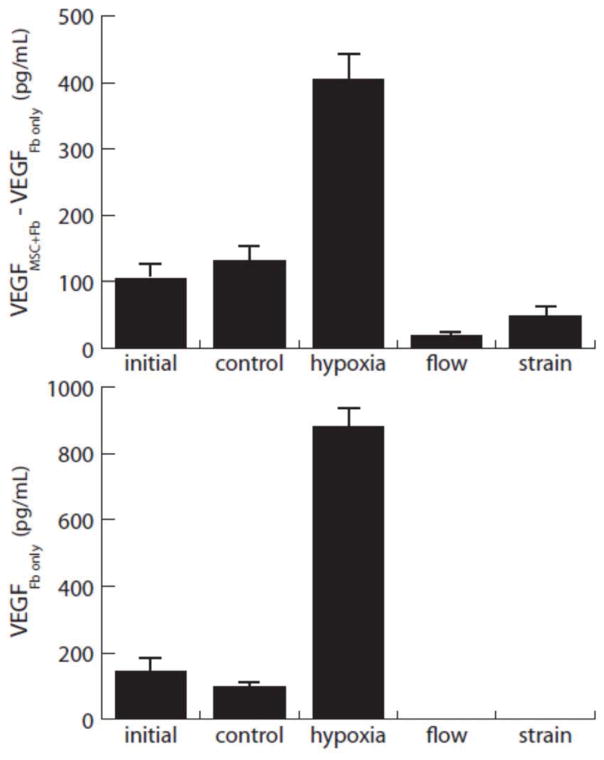
Figure 6.
VEGF release by MSC injected into the collagen gel model. Upper plot shows contribution to VEGF levels by injected MSC, estimated by subtracting the levels in fibroblast-only gels (lower plot) from the combined fibroblast-MSC cultures.
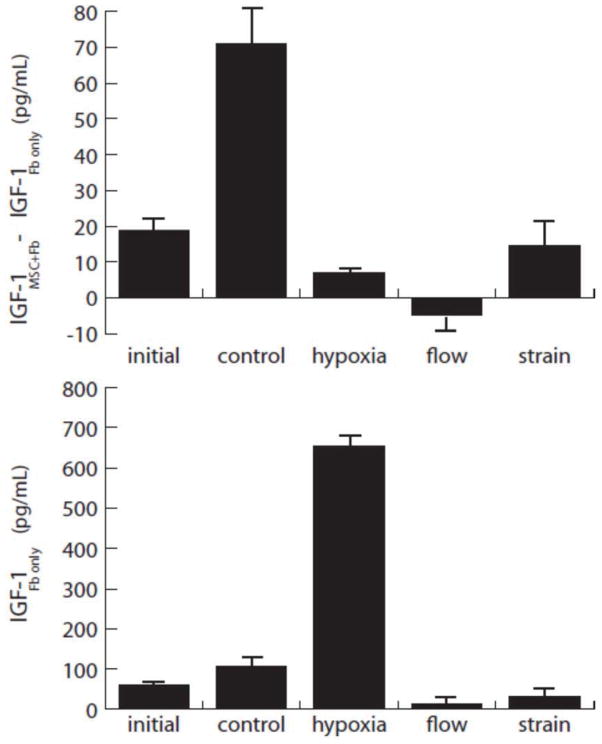
Figure 7.
IGF-1 release by MSC injected into the collagen gel model. Upper plot shows contribution to IGF levels by injected MSC, estimated by subtracting the levels in fibroblast-only gels (lower plot) from the combined fibroblast-MSC cultures.
Expression of VEGF is shown in Figure 6. VEGF release increased with time in the MSC, as evidenced by the increase in control expression levels over the initial levels. Hypoxia stimulated markedly increased VEGF release from fibroblasts and from implanted MSC, relative to controls. Interestingly, mechanical stimulation in the form of both interstitial fluid flow and cyclic mechanical strain resulted in decreased VEGF levels, relative to controls. These stimuli completely abrogated the release from fibroblast-only constructs, and the levels from combined constructs were also low. The results suggest that increasing VEGF release is an important mediator in response to hypoxia, but not mechanical stimulation.
Figure 7 shows IGF-1 levels in both combined fibroblast-MSC and fibroblast-only constructs. The production of IGF-1 by fibroblasts alone mirrored the release profile of VEGF stimulated by the treatment conditions. However, absolute levels of IGF-1 from fibroblast-MSC cultures were considerably lower than VEGF levels, and no treatment condition produced more than 100 pg/mL. Fibroblasts alone secreted higher levels, and in response to hypoxia produced very high (> 600 pg/mL) levels of this growth factor. Under control conditions (no strain, no hypoxia), IGF-1 levels in the combined constructs increased significantly over initial conditions. However, this increase was not evident in the other experimental treatments. These results suggest that IGF-1 production is generally downregulated in fibroblast-MSC co-cultures, and is not stimulated by either hypoxia or mechanical stimulation.
Discussion
The present study describes a 3D co-culture system in which isolated MSC can be injected into a rudimentary model of fibrotic myocardial tissue. Our focus in the present work is on the first 48 h following cell injection, which is a critical phase in the function of MSC transplants. Our model allows for examination of the response of injected MSC to specific biochemical and mechanical stimuli, and also allows characterization of the effect of MSC implantation on cardiac fibroblast phenotype. The results of this study indicate that the presence of MSC significantly inhibits the fibroblast to myofibroblast transition that is a characteristic of the fibrotic response in the heart. This effect was observed under all experimental treatments, and was supported by a decrease in message levels of collagens I and III, TGFβ, and αSMA, relative to uninjected controls. Interestingly, the behavior of the injected MSC was affected by the applied experimental conditions. Hypoxia resulted in increased cell migration, which supports recent findings that HIF-1alpha improves MSC-mediated repair [33]. Levels of VEGF and IGF-1 were essentially unaffected by mechanical stress, though hypoxia had a substantial effect on VEGF production in fibroblast-MSC co-cultures. Gels seeded only with fibroblasts also produced significant VEGF and IGF-1 levels under hypoxic conditions, indicating that fibroblasts themselves contribute to the paracrine environment in the ischemic myocardium.
The co-culture model presented in this study provides a relative simple yet robust platform to study the interaction of implanted stem cells with the fibrotic environment in the heart. Injected stem cells are surrounded in three dimensions by cardiac fibroblasts, which allows for crosstalk between the cell types. The model therefore adds key components representative of the cellular microenvironment in tissues, but it does not match the full complexity of the in vivo environment. Our model focuses on the resident fibroblasts in cardiac scar tissue, because of their central role in mediating the fibrotic response. However fibrotic tissue in the heart also includes other cues, including potential signals from smooth muscle cells and endothelial cells in the local vasculature, and/or immune cells that are recruited to the injury site. These cells and signals can influence the local environment and modulate stem cell function and response. While this additional complexity is not captured by the present model, it may be possible to create more physiologically relevant models by incorporating additional cell types or extracellular matrix components in a 3D protein hydrogel system.
One of the main challenges in developing consistent and effective stem cell-based therapies is the inherent variability in stem cell function caused by the age and disease state of the donor, among other factors. A consistent 3D, multicellular in vitro model that incorporates physiological stimuli may therefore be useful as a screening tool to identify populations of cells that are most likely to produce a beneficial effect upon transplantation. However, the elements of MSC function that are important to their therapeutic potential remain unclear. Our results show that MSC migration rate can be influenced by hypoxia and mechanical stimuli, but cell migration may also lead to alterations in the complex microstructure of the heart tissue that can lead to undesired sequelae such as arrhythmias. The utility of 3D culture models will increase as the relationships between the component cell types and the applied stimuli are more fully understood.
The results of this study also support the concept that the paracrine action of implanted MSC and the effect on resident cells is a time-dependent and cell-orchestrated process. The need for such dynamic and responsive delivery of growth factors in healing heart tissue provides support for cell-based therapies, since the transplanted cells can sense and respond to the local tissue environment. The system used for this work allows these dynamics to be characterized and studied. Migration and change in the differentiated state of injected MSC took place over time after implantation, and in this study were evaluated at the 48 hour time point. Levels of VEGF and IGF-1 also changed over time, as would be expected in a remodeling and healing environment, and this system could also be used to study other paracrine factors known or hypothesized to be involved in phenotype transitions in the heart. The ability to observe both the spatial location of the cells and the temporal profile of growth factor release provides insight into the interplay between cell types that leads to tissue remodeling and regeneration.
Taken together, these studies demonstrate that a 3D in vitro model of the cardiac scar can be used to examine the paracrine effects of implanted MSC under relevant physiological conditions. Our findings show that environmental stimuli affect both the resident fibroblasts and the injected MSC, as well as the interactions between these cell types. Such studies have the potential to illuminate the cellular interactions that are involved in repair of cardiac tissue, and may have relevance to the cell-based treatment of cardiac fibrosis.
Acknowledgments
This work was partially supported by a Microfluidics in Biomedical Sciences Training Program, sponsored by the National Institute of Biomedical Imaging and Bioengineering.
Footnotes
Disclosure of Interest
No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elnakish MT, Kuppusamy P, Khan M. Stem cell transplantation as a therapy for cardiac fibrosis. J Pathol. 2013;229(2):347–54. doi: 10.1002/path.4111. [DOI] [PubMed] [Google Scholar]
- 2.Duran JM, Taghavi S, George JC. The need for standardized protocols for future clinical trials of cell therapy. Transl Res. 2012;160(6):399–410. doi: 10.1016/j.trsl.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF) Am Heart J. 2011;161(6):1078–87. doi: 10.1016/j.ahj.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Jaquet K, Krause KT, Denschel J, Faessler P, Nauerz M, Geidel S, et al. Reduction of myocardial scar size after implantation of mesenchymal stem cells in rats: what is the mechanism? Stem Cells Dev. 2005;14(3):299–309. doi: 10.1089/scd.2005.14.299. [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Chen L, Fan L, Wu L, Chen X, Li W, et al. Enhanced therapeutic effects of mesenchymal stem cells on myocardial infarction by ischemic postconditioning through paracrine mechanisms in rats. J Mol Cell Cardiol. 2011;5:839–47. doi: 10.1016/j.yjmcc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: the Rubik’s cube of cell therapy for heart disease. Dis Model Mech. 2009;2(7–8):344–58. doi: 10.1242/dmm.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galie PA, Stegemann JP. Reduced serum content and increased matrix stiffness promote the cardiac myofibroblast transition in 3D collagen matrices. Cardiovascular Pathology. 2011;20(6):325–33. doi: 10.1016/j.carpath.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivedi P, Tray N, Nguyen T, Nigam N, Gallicano GI. Mesenchymal stem cell therapy for treatment of cardiovascular disease: helping people sooner or later. Stem Cells Dev. 2010;19(7):1109–20. doi: 10.1089/scd.2009.0465. [DOI] [PubMed] [Google Scholar]
- 9.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, et al. Mesenchymal Stem Cells from Adult Human Bone Marrow Differentiate into a Cardiomyocyte Phenotype In Vitro. Exp Biol Med. 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 10.Nygren J, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, et al. Bone-marrow derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nature Medicine. 2004;10(5):494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 11.Siegel G, Krause P, Wöhrle S, Nowak P, Ayturan M, Kluba T, et al. Bone marrow-derived human mesenchymal stem cells express cardiomyogenic proteins but do not exhibit functional cardiomyogenic differentiation potential. Stem Cells Dev. 2012;21(13):2457–70. doi: 10.1089/scd.2011.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27(11):2734–43. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Letters. 2007;581(21):3961–6. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Research. 2011;6(3):206–14. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Koninckx R, Hensen K, Daniëls A, Moreels M, Lambrichts I, Jongen H, et al. Human bone marrow stem cells co-cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy. 2009;11(6):778–92. doi: 10.3109/14653240902988818. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Wang S, Yu Z, Hoyt RF, Sachdev V, Vincent P, et al. Direct injection of autologous mesenchymal stromal cells improves myocardial function. Biochem Biophys Res Comm. 2009;390(3):902–7. doi: 10.1016/j.bbrc.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beeres SL, Bax JJ, Dibbets P, Stokkel MP, Zeppenfeld K, Fibbe WE, et al. Effect of intramyocardial injection of autologous bone marrow-derived mononuclear cells on perfusion, function, and viability in patients with drug-refractory chronic ischemia. Journal of Nucl Med. 2006;47(4):574–80. [PubMed] [Google Scholar]
- 18.Poynter JA, Herrmann JL, Manukyan MC, Wang Y, Abarbanell AM, Weil BR, Brewster BD, Meldrum DR. Intracoronary Mesenchymal Stem Cells Promote Postischemic Myocardial Functional Recovery, Decrease Inflammation, and Reduce Apoptosis via a Signal Transducer and Activator of Transcription 3 Mechanism. J American College Surgeons. 2011;213(2):253–60. doi: 10.1016/j.jamcollsurg.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Du YY, Yao R, Pu S, Zhao XY, Liu GH, Zhao LS, et al. Mesenchymal stem cells implantation increases the myofibroblasts congregating in infarct region in a rat model of myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2012;40(12):1045–50. [PubMed] [Google Scholar]
- 20.Wright EJ, Farrell KA, Malik N, Kassem M, Lewis AL, Wallrapp C, et al. Encapsulated glucagon-like peptide-1-producing mesenchymal stem cells have a beneficial effect on failing pig hearts. Stem Cells Transl Med. 2012;1(10):759–69. doi: 10.5966/sctm.2012-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan BS, Lou JY. Enhancement of angiogenic effect of co-transfection human NGF and VEGF genes in rat bone marrow mesenchymal stem cells. Gene. 2011;485(2):167–71. doi: 10.1016/j.gene.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, et al. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27(4):971–9. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, Wang T, Zhang D, Zhao T, Dai B, Ashraf A, et al. Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev. 2012;21(5):778–89. doi: 10.1089/scd.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo S, Jones WK, Li H, He Z, Pasha Z, Yang Y, et al. Paracrine effect of Wnt11-overexpressing mesenchymal stem cells on ischemic injury. Stem Cells Dev. 2012;21(4):598–608. doi: 10.1089/scd.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25(17):3699–706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 26.Rowe SL, Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7(11):2942–8. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe SL, Stegemann JP. Microstructure and mechanics of collagen-fibrin matrices polymerized using ancrod snake venom enzyme. J Biomech Eng. 2009;131(6):061012. doi: 10.1115/1.3128673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galie PA, Stegemann JP. Simultaneous application of interstitial flow and cyclic mechanical strain to a three-dimensional cell-seeded hydrogel. Tissue Eng Part C Methods. 2011;17(5):527–36. doi: 10.1089/ten.tec.2010.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285(5):H1871–81. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 30.MacKenna DA, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101(2):301–10. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galie PA, Spilker RL, Stegemann JP. A linear, biphasic model incorporating a brinkman term to describe the mechanics of cell-seeded collagen hydrogels. Ann Biomed Eng. 2011;39(11):2767–79. doi: 10.1007/s10439-011-0371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galie PA, Russell MW, Westfall MV, Stegemann JP. Interstitial fluid flow and cyclic strain differentially regulate cardiac fibroblast activation via AT1R and TGF-β1. Experimental Cell Res. 2012;318(1):75–84. doi: 10.1016/j.yexcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerrada I, Ruiz-Saurí A, Carrero R, Trigueros C, Dorronsoro A, Sanchez-Puelles JM, et al. Hypoxia-inducible factor 1 alpha contributes to cardiac healing in mesenchymal stem cells-mediated cardiac repair. Stem Cells Dev. 2013;22(3):501–11. doi: 10.1089/scd.2012.0340. [DOI] [PubMed] [Google Scholar]