Summary
Since DNA double-strand breaks (DSBs) contribute to the genomic instability that drives cancer development, DSB repair pathways serve as important mechanisms for tumor suppression. Thus, genetic lesions, such as BRCA1 and BRCA2 mutations, that disrupt DSB repair are often associated with cancer susceptibility. In addition, recent evidence suggests that DSB “mis-repair”, in which DSBs are resolved by an inappropriate repair pathway, can also promote genomic instability and presumably tumorigenesis. This notion has gained currency from recent cancer genome sequencing studies which have uncovered numerous chromosomal rearrangements harboring pathological DNA repair signatures. In this perspective, we discuss the factors that regulate DSB repair pathway choice and their consequences for genome stability and cancer.
Keywords: DNA Double strand break, repair pathway choice, microhomology-mediated end joining, DNA ends, resection, 53BP1-BRCA1
Introduction
DNA double-strand breaks (DSBs) are generated when the phospho-sugar backbones of both DNA strands are broken at the same position or in sufficient proximity to allow physical dissociation of the double helix into two separate molecules. In contrast to single-strand DNA breaks (SSBs), in which the genetic information retained on the complementary strand is still available to template repair, faithful restoration of DSBs can be problematic. In addition to loss of genetic information, DSBs can lead to fragmentation, loss or rearrangement of chromosomes. Indeed, in some circumstances, formation of a single DSB in a metazoan cell is sufficient to induce lethality ([1, 2]). Clearly, the various cellular pathways that mediate DSB repair must address the challenges of restoring both the physical integrity of the chromosome and its genetic information. In addition, the proper choice of DSB repair pathway has a critical impact on genomic integrity and cancer.
Types of DSBs
It is estimated that a cell experiences up to 105 spontaneous DNA lesions per day [2]. Among these, approximately 10 are thought to be DSBs [3]. DSBs are generated by the action of exogenous agents, such as ionizing radiation (radioactive decay, cosmic radiation or medical x-rays) or radiomimetic chemicals that mimic the action of ionizing radiation. The latter comprise several distinct classes of chemicals. These include base alkylating agents such as methyl methane sulfonate (MMS), as well as crosslinking agents that introduce covalent crosslinks between bases of the same (intrastrand) or complementary strands (interstrand or ICLs), including mitomycin C, platinum derivatives, psoralens, and nitrogen mustards. In addition, DNA topoisomerase inhibitors induce the formation of SSBs or DSBs by trapping topoisomerase-DNA intermediates (cleavable complexes) during isomerization reactions. For example, the camptothecins and their derivatives (irinotecan and topotecan), which inhibit type IB topoisomerases, generate DSBs primarily during DNA replication. In contrast, etoposide, mitoxantrone, teniposide and doxorubicin trap type II topoisomerases on DNA and thereby generate DSBs throughout the cell cycle [4]. Drugs that generate DSBs are widely used in cancer chemotherapy since tumor cells are often more sensitive to DSBs than normal cells.
DSBs are also generated during normal cell metabolism. The reactive oxygen species (ROS) produced during cellular metabolism can oxidize bases and trigger both single and double strand breaks. Other physiological sources of endogenous DSBs include DNA replication and meiotic recombination, as well as programmed rearrangements of the immunoglobulin and T cell receptor loci during lymphoid cell development. DNA replication is thought to be the major source of DSBs in proliferating cells since the DNA intermediates at replication forks are fragile and susceptible to breakage. Notably, breaks can occur following DNA polymerase stalling, which leads to the generation of persistent single-strand DNA (ssDNA) intermediates. Broken or collapsed replication forks containing ssDNA resemble DSBs at different stages of processing and are also a source of genomic instability if not properly repaired.
Finally, site-specific DSBs have been generated experimentally by heterologous expression of endonucleases such HO, which normally introduces a specific double-strand break in the mating-type locus (MAT) of Saccharomyces cerevisiae [5]. In vertebrate cells, experimental DSB induction can be achieved by expression of meganucleases such as I-SceI [6-8], chimeric zinc finger nucleases [9], transcription activator-like effector nucleases (TALENs) [10] and, more recently, bacterial RNA-guided Cas9 nucleases [11].
Significance
Inherited defects in DSB repair are implicated in a variety of human pathologies, including increased cancer susceptibility, neurological defects and/or immunodeficiencies, in disease syndromes such as Ataxia telangiectasia, Nijmegen Breakage syndrome or the severe combined immunodeficiencies (SCID) [12]. In addition, the genomic instability that arises from compromised DSB repair function is thought to be responsible for the heightened cancer susceptibility of women who carry germline lesions of the BRCA1 and BRCA2 genes, both of which encode proteins required for proper DSB repair. Indeed, the tumor cells of these BRCA1 mutation carriers display an ongoing genomic instability characterized by both, aneuploidy and extensive chromosomal rearrangements, as well as an inherent deficiency in DSB repair by homologous recombination. These observations clearly demonstrate that proper DSB repair is an effective mechanism for tumor suppressor [13]. Recent studies suggest that mis-repaired DSBs are equally problematic as they are responsible for chromosomal rearrangements such as translocations. The advances in sequencing of tumor genomes reveal that these events are much more frequent than originally thought and underscore the potential role of DSB repair not only in tumor suppression but also in oncogenic transformation.
Additionally, acute DSB formation is the central mechanism of action for many cancer treatments, including radiotherapy and chemotherapeutic agents such as topoisomerase inhibitors, anti-metabolites and DNA cross-linking agents. How these lesions are repaired greatly influences the efficacy of these treatments.
DSB repair pathways: direct end-joining vs. homology-directed repair
The major pathways of DSB repair were classically defined based on whether sequence homology is used to join the DSB ends. Non-homologous end-joining (NHEJ), which does not require sequence homology, is active throughout the cell cycle and constitute the primary pathway in vertebrate cells [3]. To initiate NHEJ, the Ku70/80 heterodimer (KU) binds to blunt or near-blunt DNA ends. DSB-bound KU then recruits and activates the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) that triggers an extensive signaling cascade that orchestrates downstream repair processes [14]. NHEJ repair, which normally involves minimal DNA processing, is facilitated by scaffold proteins XRCC4 and XLF (also called Cernunos) that bind DNA Ligase 4, the enzyme responsible for sealing the break. If DNA ends need nucleolytic processing before ligation, the Artemis endonuclease, a DNA-PKcs-interacting protein, provides this activity [15, 16].
In contrast to NHEJ, homology-directed repair (HDR) requires the use of homologous sequences to align DSB ends prior to ligation. In vertebrate cells, HDR occurs largely during the S phase of the cell cycle, when there is a replicated sister chromatid that can be used as a homologous template to copy and restore the DNA sequence missing on the damaged chromatid. While HDR is the preferred pathway of DSB repair in yeast G2 cells, recent studies in mammalian cells suggest that NHEJ is the prominent mode of repair in mammalian G2 cells [17] [18].
The search for sequence homology to template HDR repair requires the presence of single-strand DNA at the DSB end. This intermediate can be generated by the nucleolytic degradation of the 5′ strand of a DSB end in a process called DNA end resection. Resection is initiated by the MRE11/RAD50/NBS1 complex (MRN, Mre11/Rad50/Xrs2 in yeast), which can directly bind DSB ends. The MRE11 protein, which harbors separable endo- and exo-nuclease activities, generates 3′ ssDNA overhangs through a combination of endonucleolytic cleavage followed by 3′-5′ exonucleolytic processing [19]. In addition, the NBS1 subunit of MRN recruits CtIP (Sae2 in yeast), a distinct endonuclease that is essential, directly or indirectly, for resection by Mre11. Once MRN/CtIP initiates resection, the EXO1 and DNA2 nucleases perform the bulk of end-resection required for HDR. In this process, DNA2 acts in complex with the RecQ helicases BLM and/or WRN [20, 21]. The checkpoint kinases ATM and ATR are also required for proper DSB resection and modulate the activities of several resection factors including MRE11, RAD50, CtIP, DNA2 and EXO1 [22, 23].
The ssDNA overhangs generated by DNA resection are initially coated by the RPA complex to form an RPA-ssDNA nucleoprotein filament. This process is required to allow extensive resection by the EXO and DNA2 nuclease and to prevent degradation or self-annealing of the nascent ssDNA [24]. Subsequently, various mediator proteins, including RAD52, BRCA2 and PALB2, promote nucleation of Rad51 binding, displacement of RPA, and assembly of the Rad51-ssDNA nucleoprotein filament to form the recombinase that promotes homology search and strand invasion. Vertebrates have five RAD51 paralogs (RAD51B, RAD51C, RAD51D, XRCC2 and XRCC3), which are all essential for cell viability and cooperate in the repair of DSBs by HDR. Since HDR uses an undamaged DNA template to restore chromosome integrity, it has the potential to repair DSBs more faithfully than NHEJ.
DSB repair of the third kind: annealing-dependent mechanisms
While NHEJ and HDR appear to be the major pathways of DSB repair in eukaryotic cells, other modes of DSB repair, such as microhomology mediated end-joining (MMEJ) and single strand annealing (SSA), are also observed in both normal and pathological cellular settings. For example, since the Ku70/80 heterodimer preferentially binds flush or near-flush DNA ends, resection not only renders a DSB end suitable for HDR, but also reduces its potential to serve as a substrate for NHEJ repair [3, 25]. If, however, HDR function is compromised, as in BRCA1/2-deficient cells, resected DSB ends may instead be resolved by annealing-dependent mechanisms that entail base-pairing between tracks of exposed homologous sequences on separate ssDNA overhangs, followed by further processing to fill in the resultant gaps and ligate the nicks. Annealing-dependent repair can be initiated by homologous regions of various lengths. While very short homology tracks of only a few nucleotides are sufficient to promote MMEJ [26, 27], larger tracks ranging from 10 bp to several kilobases are generally used during SSA. Notably, these pathways are independent of the core NHEJ factors, DNA-PKcs, KU complex and Lig4.
Since annealing requires some resection to expose microhomologies, the MMEJ and SSA pathways are dependent on the MRN-CtIP complex. For example, MMEJ is suppressed upon MRE11 down-regulation by either depletion with siRNAs or inhibition with the small molecule mirin [28]. Likewise, inactivation of Nbs1 or CtIP decreases MMEJ [29, 30]. SSA also requires MRN- and CtIP-dependent resection, but is independent of Rad51.
Increasing evidence suggests that inappropriate use of annealing-mediated DSB repair can elicit the formation of pathological chromosomal translocations. For example, cancer genomes frequently harbor of microhomologies indicative of MMEJ at the breakpoints junctions of chromosomal rearrangements (see below).
DSB repair pathway choice: impact of the nature of DNA ends
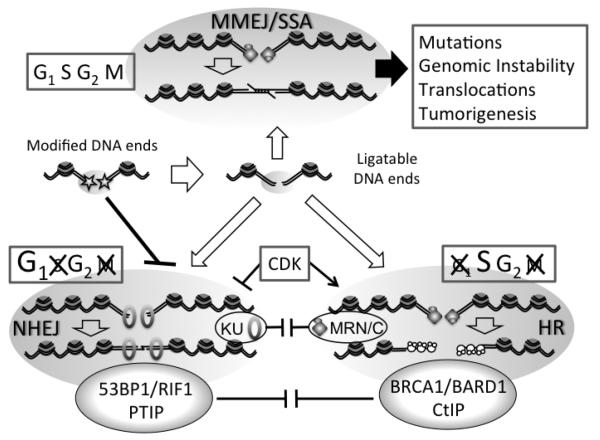
The chemical nature of DNA ends also influences the modality of repair. In principle, DNA ends that possess 3′ hydroxyl and 5′ phosphate groups can be directly ligated or extended by DNA polymerases. Indeed, many experimental systems in use to study DSB repair are based on nucleases that generate “clean” DSBs (e.g., HO or I-SceI), which serve as suitable substrates for either NHEJ or HDR (Figure 1). In vivo, however, most DSBs are unlikely to harbor such readily ligatable DNA ends. In contrast, DSBs generated by irradiation or radiomimetic drugs exhibit a variety of chemical modifications, including 5′ hydroxyls, 3′ phosphates, abasic sites, covalently-bound adenylate groups and protein-DNA adducts. These modifications must be removed from DNA ends before repair since they can potentially block any of several DSB repair steps, such as KU binding, resection initiation and DNA ligation. Given the wide variety of chemical lesions encountered in vivo, cells employ multiple mechanisms to render modified DSB ends suitable for ligation.
Figure 1. DSB repair pathway choices and their regulation.
The three major modalities of DSB repair (NHEJ, HDR and MMEJ/SSA) are depicted. NHEJ can process ligatable DNA ends but is inhibited by modified/damaged DNA ends. In contrast, MMEH or HDR, which both initiate by resection, can process both types of ends. Competition between NHEJ and HDR takes place directly on DNA ends between KU and MRN, as well as in the vicinity of the break between 53BP1 and BRCA1. HDR is not operating in G1 due to the absence of a sister chromatin as homologous template. NHEJ in S-phase is toxic. See text for details.
In some cases, these ends can be restored to a ligatable state through the actions of specialized enzymes. For example, PNKP (polynucleotide kinase 3′ phosphatase) adds a phosphate group to a 5′-OH end and removes 3′ phosphates [31]. Ku itself can remove abasic sites near DSBs [32] while Aprataxin catalyzes the removal of adenylate groups [33]. The phosphodiesterases TDP1 and TDP2 remove tyrosyl phosphate bonds to eliminate the DNA-topoisomerase adducts arising from treatment with topoisomerase poisons [34, 35]. All these processing mechanisms yield ends suitable for NHEJ. Alternatively, DNA-end lesions can be removed by nucleases that catalyze cleavage upstream of the lesion. These include Artemis, which harbors 5′ endonuclease and 5′-3′ exonuclease activities, the Aprataxin-and-PNK-like factor (APLF), an endonuclease and 3′ to 5′ exonuclease [36], and WRN, a 3′-5′ helicase with exonuclease activity. Additionally, nucleolytic processing by the MRN/CtIP complex can release covalently-attached protein adducts from DSB ends to allow subsequent initiation of repair [37, 38].
These nucleases generally act in the NHEJ pathway, producing short ssDNA overhangs that largely retain the capacity to bind the Ku70/80 heterodimer. Nonetheless, it is conceivable that Artemis, APLF or WRN could generate some ssDNA overhangs too long to be processed efficiently by NHEJ, thus favoring MMEJ. Of note, the chemical modifications generated by irradiation or radiomimetic drugs can also be removed by the endo- and exo-nuclease activities of MRN/CtIP complex. In this case however, the ability of MRN/CtIP to initiate resection and recruit the highly-processive EXO and DNA2 resection nucleases would obviate subsequent repair through the NHEJ pathway.
Since DSB repair has been studies in many experimental settings ranging from budding yeast to human cells, it is important to note that significant differences exist between these model systems. Budding yeast cells, in which the bulk of genetics experiments have been performed, are highly proficient for HDR and appear to favor this mode of DSB repair over NHEJ, while in mammalian cells NHEJ is the preferred DSB repair pathway. In addition, processing of modified ends appears to be restricted to the action of the MRX/Sae2 complex in yeast, whereas NHEJ components such as KU and Artemis (see above) also facilitate end joining in higher eukaryotes [16, 32]. Finally, although yeast cells can tolerate the loss of certain DSB repair proteins, such as the MRX complex, the corresponding MRN complex is essential for the viability of metazoans. These differences could reflect the increased size and complexity of metazoan genomes.
Regulation of DSB repair pathway choice
The competition between NHEJ and HDR, which stems from the molecular interplay between KU and MRN/CtIP at DSB ends, has primarily been studied in yeast. Nonetheless, the central aspects of this process are well conserved in higher eukaryotes, as might be expected given the high degree of conservation between the DSB repair factors of yeast and mammals (see Table 1). In addition, however, mammalian cells also employ several ancillary factors to regulate DSB pathway choice, such as the Fanconi anemia and BRCA1 – 53BP1 proteins.
Table 1.
Repair factors implicated in DSB repair pathway choice
| Human | S. cerevisiae | Function in DSB repair | Impact on repair pathway of choice | |
|---|---|---|---|---|
|
| ||||
| NHEJ | 53BP1 | Rad9 | Mediator/adaptor protein, chromatin reader | Inhibition of BRCA1 mediated resection |
| MDC1 | Rad9 | Mediator/adaptor protein | ||
| Rif1 | - | Inhibitor of DNA end resection | Inhibition of BRCA1 mediated resection | |
| PTIP | - | Recruitment of 53BP1 | Inhibition of BRCA1 mediated resection | |
| H2AX | H2AX | DDR signaling | Prevents resection in VDJ recombination | |
| Ku70 | YKu70 | dsDNA end binding, resection inhibition | End protection, prevents resection | |
| Ku80 | YKu80 | dsDNA end binding, resection inhibition | End protection, prevents resection | |
| LIG4 | Dnl4/Lig4 | DNA ligase | ||
| XRCC4 | Lif1 | Scaffold protein | ||
| XLF/Cernunnos | Nej1 | Scaffold protein | ||
| DNA-PKcs | - | DSB-responsive PIKK family protein kinase | ||
| Artemis | Pso2/Snm1 | Nuclease, end processing | ||
| LIG1 | Cdc9 | DNA ligase (alternative end joining) | ||
| LIG3 | - | DNA ligase (alternative end joining) | ||
| Aprataxin | APTX | End processing | ||
| APLF | - | End processing | ||
| PNKP | Tpp1 | End processing | ||
| DNA pol λ | Pol4 | End processing, gap filling | ||
| DNA pol μ | Pol4 | End processing, gap filling | ||
| Tdp1 | Tdp1 | 3′ tyrosyl-DNA phosphodiesterase | ||
| TTRAP/TDP2 | Tdp2 | 3′ and 5′ tyrosyl-DNA phosphodiesterase | ||
| HR | BRCA1 | - | Mediator/adaptor, ubiquitin ligase | |
| BARD1 | - | Constitutive RING-mediated heterodimerization with BRCA1 |
||
| BACH1/BRIP1/FANCJ | - | 3′ to 5′ helicase | ||
| TopBP1 | Dpb11 | S-phase checkpoint | ||
| RAP80 | - | BRCA1 recruitment to Chromatin | ||
| Abraxas | - | BRCA1 recruitment to Chromatin | ||
| Mre11 | Mre11 | Endonuclease, 3′-5′ exonuclease, MRN/X complex | Resection initiation, KU release | |
| Rad50 | Rad50 | ATPase/Scaffold, MRN/X complex | Resection initiation, KU release | |
| Nbs1/Nibrin | Xrs2 | Adaptor/checkpoint roles, MRN/X complex | Resection initiation, KU release | |
| CtIP/RBBP8 | Sae2, Com1 | Endonuclease | Initiation of DNA end resection | |
| EXO1 | Exo1 | 5′-3′ exonuclease | Initiation and long-range resection | |
| DNA2 | DNA2 | Helicase, 5′-3′ exonuclease | Long-range resection | |
| BLM | Sgs1 | Helicase | Long-range resection | |
| WRN | Sgs1 | Helicase, 3′-5′ exonuclease (WRN) | Long-range resection | |
| BRCA2 (FANCD1) | - | Recombination mediator, Rad51 loading | Facilitates resection | |
| RPA | Rfa | Trimeric ssDNA binding protein | Facilitates resection in yeast | |
| RAD51 paralogs (RAD51C = FANCO) |
Rad51 | Recombinase, Homology pairing | ||
| CDKs | Cdc28 | Cell cycle kinases | Stimulates resection (p-CtIP, p-DNA2) | |
| FA | 16 FANC genes | Mph1 (FANCM) | Collectively inhibit NHEJ in S-phase | |
Impact of KU
As noted above, since the KU complex binds 3′-overhangs poorly, the initiation of DNA resection inhibits NHEJ and commits DSB repair to the HDR, MMEJ, or SSA pathways. [39, 40]. Resection is normally restricted to the S and G2 phases of cell cycle progression, when sister chromatids are available to serve as templates for repair (Figure 1). Not surprisingly, the induction of DNA resection at the onset of S-phase is dependent of cyclin-dependent kinases (CDKs) [41-44]. For example, CDK phosphorylation of a conversed site in the C-terminus of Sae2/CtIP is required to promote resection in budding yeast (Sae2-S267), Xenopus extracts (CtIP-T806) and human cells (CtIP-T847) [45-47]. CDK also phosphorylates Dna2 to stimulates its recruitment to DSBs and the extent of long-range resection in yeast [48]. In addition, Exo1 is phosphorylated by CDKs, although the functional consequence of this modification is not known [44].
In yeast cells lacking KU, CDK activity is no longer required for resection initiation by MRX-Sae2, suggesting that CDK might also negatively regulate KU [49]. Nonetheless, CDK activity is still needed for long-range resection by Exo1 or Sgs1-Dna2 in the absence of KU [50].
In yeast, KU binding to DSBs ends antagonizes end resection [50, 51]. For instance, in the absence of MRX, Exo1-dependent resection is inhibited by KU [51]. This suggests a direct competition between KU and MRX at DSBs [52, 53]. HDR is more efficient in the absence of KU, suggesting that cells attempt to repair DSBs by NHEJ before resorting to DNA resection [54, 55]. Together, these observations suggest that cells favor DSB repair by NHEJ if the ends are suitable for joining, while DNA resection is activated if joining fails, particularly when DNA ends have not been made suitable for NHEJ. Moreover, a recent study suggests that the endonuclease activity associated with MRE11 is responsible for removing KU from DSBs in mammalian cells. A small molecule analog of mirin [56], which specifically inhibits MRE11-associated endonuclease, promotes NHEJ [19].
Fanconi anemia
Additional evidence points to an inhibitory relationship between NHEJ and HDR specifically during S-phase. Fanconi anemia is a complex genetic disease characterized by congenital abnormalities, infertility, cancer susceptibility and bone-marrow failure. This phenotype can result from mutations in any of at least different 16 genes, which encode the FANCA, FANCB, FANCC, FANCD1 (BRCA2), FANCD2, FANCE, FANCF, FANCG (XRCC9), FANCI, FANCJ (BRIP1), FANCL (PHF9), FANCM, FANCN (PALB2), FANCO (RAD51C), FANCP (SLX4) and FANCQ (XPF) proteins [57]. The Fanconi anemia (FA) pathway participates in crosslink repair and is also involved in DNA replication fork stability [58, 59] [60]. Accordingly, defects in the FA pathway result in replication-dependent genomic instability [61] [62]. Notably, these cellular defects can be rescued by inhibiting NHEJ in Caenorhabditis elegans, DT40 chicken cells, and mammalian cells [63] [64]. This suggests that the FA pathway facilitates DSB repair by HDR during S-phase while suppressing NHEJ. NHEJ inhibition could be due in part to the WRN helicase [65]. In the absence of the FA pathway, DSBs generated during DNA replication are repaired by NHEJ resulting in genome rearrangements. FA could facilitate DSB processing specifically in the context of DNA replication. While there is no direct evidence for DNA processing by FA proteins, FA cells show reduced repair of DSBs by MMEJ consistent with the idea that the FA could facilitate resection [66].
BRCA1 – 53BP1
Another layer of regulation of DSB pathway choice has recently emerged from studies of the functional interactions between BRCA1 and 53BP1 [67]. Women who carry germline mutations of the BRCA1 tumor suppressor gene face a dramatically increased risk of breast and ovarian cancer [68]. The protein product of BRCA1 has been implicated in various cellular processes, including multiple aspects of the cellular response to DNA damage [69], Most notably, BRCA1 is required for efficient repair of DSBs through the HDR pathway [13]. Although the biochemical mechanisms by which BRCA1 mediates HDR remain unclear, the HDR functions of BRCA1 correlate particularly well with its tumor suppression activity in animal models of hereditary breast cancer [70, 71]. Interestingly, BRCA1 is required for both HDR and cell viability in a broad range of cell types, despite the fact that the tumor susceptibility of BRCA1 mutation carriers is largely restricted to breast and ovarian cancers. While the tissue specificity of tumor risk in these patients is not understood, a longstanding, but as yet unproven hypothesis, posits that breast and ovarian tissues may be especially dependent on BRCA1 for HDR repair of DNA adducts generated by estrogens during cell proliferation [72] [73]. In any case, the breast tumors that arise in BRCA1 mutation carriers belong to a particular subtype of breast cancer that features a “basal-like” histopathology and a “triple-negative” (ER−, PR−, HER2−) phenotype [74]. Notably, basal-like breast tumors display the characteristic signs of HDR-deficient cells, including aneuploidy, extensive chromosomal rearrangements, and a heightened sensitivity to genotoxins that induce DSBs and interstrand DNA crosslinks.
Early work established that the 53BP1 protein promotes NHEJ under certain settings, such as long-range V(D)J recombination and immunoglobulin class switching during lymphoid development [67]. However, recent studies demonstrate that 53BP1 also serve as a linchpin in the pathway choice of DSB repair. This new insight emerged from the remarkable observation that the repair defects and cellular lethality associated with BRCA1 loss are rescued upon simultaneous inactivation of 53BP1 [75-77]. For example, 53BP1 depletion ameliorates most of the phenotypic abnormalities characteristic of Brca1-mutant mouse embryonic fibroblast and murine embryonic stem cells, including proliferative senescence, spontaneous chromosomal instability, heightened genotoxin sensitivity, and compromised HDR function. Indeed, similar effects of 53BP1 loss are also apparent at the organismal level, in that cellular senescence and apoptosis, as well as overall embryonic lethality, is greatly diminished in double-mutant Brca1Δ11/Δ1153BP1−/− mice relative to single-mutant Brca1Δ11/Δ11 animals. Moreover, the fact that mammary carcinogenesis is rare in adult Brca1Δ11/Δ1153BP1−/− mice, as compared to adult Brca1-mutant mice rescued by p53 deficiency (e.g., Brca1Δ11/Δ11p53+/–), suggests that 53BP1 loss also counters the tumor susceptibility associated with Brca1 inactivation, perhaps in part by restoring HDR function and chromosomal stability [76].
To account for these observations, Bunting et al. [76] proposed that 53BP1 promotes NHEJ by blocking CtIP-dependent DNA resection and thereby allowing DSBs to retain the flush DNA ends suitable for recognition by DNA ligase 4, the enzyme that mediates DNA sealing during NHEJ repair. Furthermore, they showed that the formation of complex chromosomal rearrangements in Brca1-mutant cells is dependent on both 53BP1 and DNA ligase 4. These findings imply that, in the absence of BRCA1 function, DSBs that would normally be repaired by HDR are instead shunted to the NHEJ pathway, which aberrantly joins them to yield the chromosomal abnormalities characteristic of BRCA1-mutant cells. However, the DSB ends of BRCA1-mutant cells would, upon 53BP1 depletion, be rendered susceptible to DNA resection, allowing assembly of recombinogenic Rad51/ssDNA filaments and restitution of HDR function. This scenario implies that BRCA1 normally functions to promote DNA resection, and indeed several, though not all, studies have observed a modest diminution of resection upon BRCA1 depletion [78-81]. Given that BRCA1 interacts directly with CtIP, it was appealing to propose that BRCA1 regulates resection through its association with MRN/CtIP, and one study reported that resection-dependent modes of DSB repair, such as HDR and SSA, are defective in chicken DT40 cells expressing a CtIP mutant that fails to bind BRCA1 [82]. However, subsequent work has established that the BRCA1/CtIP interaction is not required for DNA resection, HDR, and/or SSA in multiple settings, including chicken DT40 cells [83], Xenopus cell-free extracts [47] and mammalian cells [84]. Thus, if BRCA1 promotes DNA resection, it is likely to do so through a mechanism that is independent of its interaction with CtIP.
The ability of 53BP1 to inhibit DNA resection is dependent on its associated factor RIF1 [79, 85-88]. Interestingly, Escribano-Diaz et al. [79] have shown that the antagonistic relationship between BRCA1 and 53BP1 that regulates repair pathway choice is based upon reciprocal exclusion of RIF1 and BRCA1 polypeptides at DSB sites during cell cycle progression. Thus, during the G1 phase, when NHEJ repair is preferred, RIF1 blocks the recruitment of BRCA1 to DSB ends and acts downstream of 53BP1 to suppress DNA resection and thus HDR. Conversely, in S and G2 cells, which possess suitable templates for homology-directed repair, BRCA1 and the CtIP resection protein both antagonized the recruitment of RIF1 to DSB ends, allowing for DNA resection and HDR. Accordingly, this regulatory circuit ensures proper coordination of repair pathway choice with respect to the cell cycle.
Microhomologies and cancer
Recent obsevations suggest that the MMEJ pathway may promote the formation of abberant chromosomal rearrangements, including the oncogenic chromosome translocations associated human cancer. Recurrent chromosome translocations with oncogenic potential are the hallmarks of most hematological malignancies, such as Burkitt’s lymphoma and chronic myelogenous leukemia. Traditional cytogenetic methods have also identified some recurrent translocations in epithelial tumors. However, truly recurrent translocations may be masked in carcinoma cells due to the excessive levels of chromosome rearrangements typically associated with solid tumors. Nonetheless, recurrent translocations have been described in papillary thyroid carcinomas, prostate cancers, and, more recently, non-small cell lung cancers (reviewed in [89, 90]). Deep sequencing studies have confirmed that carcinomas, including breast tumors, harbor chromosomal rearrangements of exceptional degree and complexity [91, 92].
The extent of DNA resection may be a critical factor in generating these pathological rearrangements. Interesting, microhomologies are frequently found at the junctions of these rearrangements, raising the prospect that aberrant repair of DSBs through the MMEJ pathway can mediate the formation of oncogenic chromosome translocations [91, 93]. Moreover, since mouse fibroblasts that lack key NHEJ factors, such as KU or DNA ligase 4, spontaneously accumulate high levels of chromosomal rearangements, NHEJ activity appears to suppress chromosomal instability in normal cells [94, 95]. Furthermore, using an experimental model of interchromosomal translocation, Zhang et al. (2011) showed that downregulation of CtIP dramatically reduces the translocation frequency and microhomology usage in mouse embryonic stem (ES) cells [96], suggesting that most translocations in these cells are generated through MMEJ.
Conclusion
Maintaining the proper balance of DNA resection is critical to prevent pathologic chromosome rearrangements and subsequent tumor development. Aberrant DSB repair can arise from multiple sources. On one hand, relieving NHEJ inhibition during S phase promotes genomic instability and cancer as seen in Fanconi anemia patients. On the other hand, inhibition of NHEJ during G1 favors untimely resection in a context that is not suitable for HDR, thereby leading to annealing-dependent joining and possibly chromosomal rearrangement. Finally, inhibition of long-range resection and/or HDR while MRN/CtIP is still active will yield persistent ssDNA intermediates that can serve as substrates for MMEJ-mediated chromosomal translocation. Because MMEJ is active throughout the cell cycle, it may be responsible for the bulk of these toxic, and potentially oncogenic, rearrangements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature. 2000;407:777–783. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- [2].Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- [3].Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rudin N, Haber JE. Efficient repair of HO-induced chromosomal breaks in Saccharomyces cerevisiae by recombination between flanking homologous sequences. Mol Cell Biol. 1988;8:3918–3928. doi: 10.1128/mcb.8.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colleaux L, D’Auriol L, Galibert F, Dujon B. Recognition and cleavage site of the intron-encoded omega transposase. Proc Natl Acad Sci U S A. 1988;85:6022–6026. doi: 10.1073/pnas.85.16.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jasin M. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 1996;12:224–228. doi: 10.1016/0168-9525(96)10019-6. [DOI] [PubMed] [Google Scholar]
- [8].Kass EM, Helgadottir HR, Chen CC, Barbera M, Wang R, Westermark UK, Ludwig T, Moynahan ME, Jasin M. Double-strand break repair by homologous recombination in primary mouse somatic cells requires BRCA1 but not the ATM kinase. Proc Natl Acad Sci U S A. 2013;110:5564–5569. doi: 10.1073/pnas.1216824110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- [10].Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, Dulay GP, Hua KL, Ankoudinova I, Cost GJ, Urnov FD, Zhang HS, Holmes MC, Zhang L, Gregory PD, Rebar EJ. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- [11].Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McKinnon PJ. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu Rev Pathol. 2012;7:303–321. doi: 10.1146/annurev-pathol-011811-132509. [DOI] [PubMed] [Google Scholar]
- [13].Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis, Nature reviews. Molecular cell biology. 2010;11:196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dynan WS, Yoo S. Interaction of Ku protein and DNA-dependent protein kinase catalytic subunit with nucleic acids. Nucleic Acids Res. 1998;26:1551–1559. doi: 10.1093/nar/26.7.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mohapatra S, Yannone SM, Lee SH, Hromas RA, Akopiants K, Menon V, Ramsden DA, Povirk LF. Trimming of damaged 3′ overhangs of DNA double-strand breaks by the Metnase and Artemis endonucleases. DNA repair. 2013;12:422–432. doi: 10.1016/j.dnarep.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ma Y, Pannicke U, Schwarz K, Lieber MR. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108:781–794. doi: 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- [17].Shahar OD, Raghu Ram EV, Shimshoni E, Hareli S, Meshorer E, Goldberg M. Live imaging of induced and controlled DNA double-strand break formation reveals extremely low repair by homologous recombination in human cells. Oncogene. 2012;31:3495–3504. doi: 10.1038/onc.2011.516. [DOI] [PubMed] [Google Scholar]
- [18].Shibata A, Conrad S, Birraux J, Geuting V, Barton O, Ismail A, Kakarougkas A, Meek K, Taucher-Scholz G, Lobrich M, Jeggo PA. Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J. 2011;30:1079–1092. doi: 10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shibata A, Moiani D, Arvai AS, Perry J, Harding SM, Genois MM, Maity R, van Rossum-Fikkert S, Kertokalio A, Romoli F, Ismail A, Ismalaj E, Petricci E, Neale MJ, Bristow RG, Masson JY, Wyman C, Jeggo PA, Tainer JA. DNA Double-Strand Break Repair Pathway Choice Is Directed by Distinct MRE11 Nuclease Activities. Molecular cell. 2014;53:7–18. doi: 10.1016/j.molcel.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nimonkar AV, Genschel J, Kinoshita E, Polaczek P, Campbell JL, Wyman C, Modrich P, Kowalczykowski SC. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011;25:350–362. doi: 10.1101/gad.2003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liao S, Guay C, Toczylowski T, Yan H. Analysis of MRE11’s function in the 5′-->3′ processing of DNA double-strand breaks. Nucleic Acids Res. 2012;40:4496–4506. doi: 10.1093/nar/gks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jasin M, Rothstein R. Repair of strand breaks by homologous recombination. Cold Spring Harb Perspect Biol. 2013;5:a012740. doi: 10.1101/cshperspect.a012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- [24].Chen H, Lisby M, Symington LS. RPA coordinates DNA end resection and prevents formation of DNA hairpins. Molecular cell. 2013;50:589–600. doi: 10.1016/j.molcel.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu D, Topper LM, Wilson TE. Recruitment and dissociation of nonhomologous end joining proteins at a DNA double-strand break in Saccharomyces cerevisiae. Genetics. 2008;178:1237–1249. doi: 10.1534/genetics.107.083535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Decottignies A. Alternative end-joining mechanisms: a historical perspective. Front Genet. 2013;4:48. doi: 10.3389/fgene.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deriano L, Roth DB. Modernizing the Nonhomologous End-Joining Repertoire: Alternative and Classical NHEJ Share the Stage. Annu Rev Genet. 2013;47:433–455. doi: 10.1146/annurev-genet-110711-155540. [DOI] [PubMed] [Google Scholar]
- [28].Rass E, Grabarz A, Plo I, Gautier J, Bertrand P, Lopez BS. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nature structural & molecular biology. 2009;16:819–824. doi: 10.1038/nsmb.1641. [DOI] [PubMed] [Google Scholar]
- [29].Bennardo N, Gunn A, Cheng A, Hasty P, Stark JM. Limiting the persistence of a chromosome break diminishes its mutagenic potential. PLoS Genet. 2009;5:e1000683. doi: 10.1371/journal.pgen.1000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bennardo N, Cheng A, Huang N, Stark JM. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 2008;4:e1000110. doi: 10.1371/journal.pgen.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jilani A, Ramotar D, Slack C, Ong C, Yang XM, Scherer SW, Lasko DD. Molecular cloning of the human gene, PNKP, encoding a polynucleotide kinase 3′-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. The Journal of biological chemistry. 1999;274:24176–24186. doi: 10.1074/jbc.274.34.24176. [DOI] [PubMed] [Google Scholar]
- [32].Roberts SA, Strande N, Burkhalter MD, Strom C, Havener JM, Hasty P, Ramsden DA. Ku is a 5′-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature. 2006;443:713–716. doi: 10.1038/nature05164. [DOI] [PubMed] [Google Scholar]
- [34].Pouliot JJ, Yao KC, Robertson CA, Nash HA. Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science (New York, N Y ) 1999;286:552–555. doi: 10.1126/science.286.5439.552. [DOI] [PubMed] [Google Scholar]
- [35].Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- [36].Kanno S, Kuzuoka H, Sasao S, Hong Z, Lan L, Nakajima S, Yasui A. A novel human AP endonuclease with conserved zinc-finger-like motifs involved in DNA strand break responses. EMBO J. 2007;26:2094–2103. doi: 10.1038/sj.emboj.7601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hartsuiker E, Neale MJ, Carr AM. Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Molecular cell. 2009;33:117–123. doi: 10.1016/j.molcel.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436:1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mimori T, Hardin JA. Mechanism of interaction between Ku protein and DNA. The Journal of biological chemistry. 1986;261:10375–10379. [PubMed] [Google Scholar]
- [40].Foster SS, Balestrini A, Petrini JH. Functional interplay of the Mre11 nuclease and Ku in the response to replication-associated DNA damage. Mol Cell Biol. 2011;31:4379–4389. doi: 10.1128/MCB.05854-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Barlow JH, Lisby M, Rothstein R. Differential regulation of the cellular response to DNA double-strand breaks in G1. Molecular cell. 2008;30:73–85. doi: 10.1016/j.molcel.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, Haber JE, Foiani M. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ferretti LP, Lafranchi L, Sartori AA. Controlling DNA-end resection: a new task for CDKs. Front Genet. 2013;4:99. doi: 10.3389/fgene.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huertas P, Jackson SP. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. The Journal of biological chemistry. 2009;284:9558–9565. doi: 10.1074/jbc.M808906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huertas P, Cortes-Ledesma F, Sartori AA, Aguilera A, Jackson SP. CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature. 2008;455:689–692. doi: 10.1038/nature07215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Peterson SE, Li Y, Chait BT, Gottesman ME, Baer R, Gautier J. Cdk1 uncouples CtIP-dependent resection and Rad51 filament formation during M-phase double-strand break repair. J Cell Biol. 2011;194:705–720. doi: 10.1083/jcb.201103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen X, Niu H, Chung WH, Zhu Z, Papusha A, Shim EY, Lee SE, Sung P, Ira G. Cell cycle regulation of DNA double-strand break end resection by Cdk1-dependent Dna2 phosphorylation. Nature structural & molecular biology. 2011;18:1015–1019. doi: 10.1038/nsmb.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang Y, Shim EY, Davis M, Lee SE. Regulation of repair choice: Cdk1 suppresses recruitment of end joining factors at DNA breaks. DNA repair. 2009;8:1235–1241. doi: 10.1016/j.dnarep.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Clerici M, Mantiero D, Guerini I, Lucchini G, Longhese MP. The Yku70-Yku80 complex contributes to regulate double-strand break processing and checkpoint activation during the cell cycle. EMBO Rep. 2008;9:810–818. doi: 10.1038/embor.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mimitou EP, Symington LS. Ku prevents Exo1 and Sgs1-dependent resection of DNA ends in the absence of a functional MRX complex or Sae2. EMBO J. 2010;29:3358–3369. doi: 10.1038/emboj.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Eid W, Steger M, El-Shemerly M, Ferretti LP, Pena-Diaz J, Konig C, Valtorta E, Sartori AA, Ferrari S. DNA end resection by CtIP and exonuclease 1 prevents genomic instability. EMBO Rep. 2010;11:962–968. doi: 10.1038/embor.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tomimatsu N, Mukherjee B, Deland K, Kurimasa A, Bolderson E, Khanna KK, Burma S. Exo1 plays a major role in DNA end resection in humans and influences double-strand break repair and damage signaling decisions. DNA repair. 2012;11:441–448. doi: 10.1016/j.dnarep.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pierce AJ, Hu P, Han M, Ellis N, Jasin M. Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev. 2001;15:3237–3242. doi: 10.1101/gad.946401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Frank-Vaillant M, Marcand S. Transient stability of DNA ends allows nonhomologous end joining to precede homologous recombination. Molecular cell. 2002;10:1189–1199. doi: 10.1016/s1097-2765(02)00705-0. [DOI] [PubMed] [Google Scholar]
- [56].Dupre A, Boyer-Chatenet L, Sattler RM, Modi AP, Lee JH, Nicolette ML, Kopelovich L, Jasin M, Baer R, Paull TT, Gautier J. A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nature chemical biology. 2008;4:119–125. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bogliolo M, Schuster B, Stoepker C, Derkunt B, Su Y, Raams A, Trujillo JP, Minguillon J, Ramirez MJ, Pujol R, Casado JA, Banos R, Rio P, Knies K, Zuniga S, Benitez J, Bueren JA, Jaspers NG, Scharer OD, de Winter JP, Schindler D, Surralles J. Mutations in ERCC4, encoding the DNA-repair endonuclease XPF, cause Fanconi anemia. Am J Hum Genet. 2013;92:800–806. doi: 10.1016/j.ajhg.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hinz JM, Nham PB, Salazar EP, Thompson LH. The Fanconi anemia pathway limits the severity of mutagenesis. DNA repair. 2006;5:875–884. doi: 10.1016/j.dnarep.2006.05.039. [DOI] [PubMed] [Google Scholar]
- [59].Wang LC, Stone S, Hoatlin ME, Gautier J. Fanconi anemia proteins stabilize replication forks. DNA repair. 2008;7:1973–1981. doi: 10.1016/j.dnarep.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Knipscheer P, Raschle M, Smogorzewska A, Enoiu M, Ho TV, Scharer OD, Elledge SJ, Walter JC. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science. 2009;326:1698–1701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493:356–363. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Adamo A, Collis SJ, Adelman CA, Silva N, Horejsi Z, Ward JD, Martinez-Perez E, Boulton SJ, La Volpe A. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Molecular cell. 2010;39:25–35. doi: 10.1016/j.molcel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- [64].Pace P, Mosedale G, Hodskinson MR, Rosado IV, Sivasubramaniam M, Patel KJ. Ku70 corrupts DNA repair in the absence of the Fanconi anemia pathway. Science. 2010;329:219–223. doi: 10.1126/science.1192277. [DOI] [PubMed] [Google Scholar]
- [65].Aggarwal M, Banerjee T, Sommers JA, Iannascoli C, Pichierri P, Shoemaker RH, Brosh RM., Jr. Werner syndrome helicase has a critical role in DNA damage responses in the absence of a functional fanconi anemia pathway. Cancer Res. 2013;73:5497–5507. doi: 10.1158/0008-5472.CAN-12-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lundberg R, Mavinakere M, Campbell C. Deficient DNA end joining activity in extracts from fanconi anemia fibroblasts. The Journal of biological chemistry. 2001;276:9543–9549. doi: 10.1074/jbc.M008634200. [DOI] [PubMed] [Google Scholar]
- [67].Zimmermann M, de Lange T. 53BP1: pro choice in DNA repair. Trends in cell biology. 2013 doi: 10.1016/j.tcb.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wooster R, Weber BL. Breast and ovarian cancer. The New England journal of medicine. 2003;348:2339–2347. doi: 10.1056/NEJMra012284. [DOI] [PubMed] [Google Scholar]
- [69].Li ML, Greenberg RA. Links between genome integrity and BRCA1 tumor suppression. Trends in biochemical sciences. 2012;37:418–424. doi: 10.1016/j.tibs.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bouwman P, Jonkers J. Mouse models for BRCA1 associated tumorigenesis: from fundamental insights to preclinical utility. Cell Cycle. 2008;7:2647–2653. doi: 10.4161/cc.7.17.6266. [DOI] [PubMed] [Google Scholar]
- [71].Shakya R, Reid LJ, Reczek CR, Cole F, Egli D, Lin CS, deRooij DG, Hirsch S, Ravi K, Hicks JB, Szabolcs M, Jasin M, Baer R, Ludwig T. BRCA1 tumor suppression depends on BRCT phosphoprotein binding, but not its E3 ligase activity. Science. 2011;334:525–528. doi: 10.1126/science.1209909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gaikwad NW. Metabolomic profiling unravels DNA adducts in human breast that are formed from peroxidase mediated activation of estrogens to quinone methides. PloS one. 2013;8:e65826. doi: 10.1371/journal.pone.0065826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hickey M. Untangling BRCA mutations, sex hormones, and cancer risk. The lancet oncology. 2013;14:1151–1152. doi: 10.1016/S1470-2045(13)70481-9. [DOI] [PubMed] [Google Scholar]
- [74].Tischkowitz MD, Foulkes WD. The basal phenotype of BRCA1-related breast cancer: past, present and future. Cell Cycle. 2006;5:963–967. doi: 10.4161/cc.5.9.2713. [DOI] [PubMed] [Google Scholar]
- [75].Bouwman P, Aly A, Escandell JM, Pieterse M, Bartkova J, van der Gulden H, Hiddingh S, Thanasoula M, Kulkarni A, Yang Q, Haffty BG, Tommiska J, Blomqvist C, Drapkin R, Adams DJ, Nevanlinna H, Bartek J, Tarsounas M, Ganesan S, Jonkers J. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nature structural & molecular biology. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, Xu X, Deng CX, Finkel T, Nussenzweig M, Stark JM. A. Nussenzweig, 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cao L, Xu X, Bunting SF, Liu J, Wang RH, Cao LL, Wu JJ, Peng TN, Chen J, Nussenzweig A, Deng CX, Finkel T. A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency. Molecular cell. 2009;35:534–541. doi: 10.1016/j.molcel.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen L, Nievera CJ, Lee AY, Wu X. Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair. The Journal of biological chemistry. 2008;283:7713–7720. doi: 10.1074/jbc.M710245200. [DOI] [PubMed] [Google Scholar]
- [79].Escribano-Diaz C, Orthwein A, Fradet-Turcotte A, Xing M, Young JT, Tkac J, Cook MA, Rosebrock AP, Munro M, Canny MD, Xu D, Durocher D. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Molecular cell. 2013;49:872–883. doi: 10.1016/j.molcel.2013.01.001. [DOI] [PubMed] [Google Scholar]
- [80].Schlegel BP, Jodelka FM, Nunez R. BRCA1 promotes induction of ssDNA by ionizing radiation. Cancer Res. 2006;66:5181–5189. doi: 10.1158/0008-5472.CAN-05-3209. [DOI] [PubMed] [Google Scholar]
- [81].Zhao GY, Sonoda E, Barber LJ, Oka H, Murakawa Y, Yamada K, Ikura T, Wang X, Kobayashi M, Yamamoto K, Boulton SJ, Takeda S. A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Molecular cell. 2007;25:663–675. doi: 10.1016/j.molcel.2007.01.029. [DOI] [PubMed] [Google Scholar]
- [82].Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nakamura K, Kogame T, Oshiumi H, Shinohara A, Sumitomo Y, Agama K, Pommier Y, Tsutsui KM, Tsutsui K, Hartsuiker E, Ogi T, Takeda S, Taniguchi Y. Collaborative action of Brca1 and CtIP in elimination of covalent modifications from double-strand breaks to facilitate subsequent break repair. PLoS Genet. 2010;6:e1000828. doi: 10.1371/journal.pgen.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Reczek CR, Szabolcs M, Stark JM, Ludwig T, Baer R. The interaction between CtIP and BRCA1 is not essential for resection-mediated DNA repair or tumor suppression. J Cell Biol. 2013;201:693–707. doi: 10.1083/jcb.201302145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chapman JR, Barral P, Vannier JB, Borel V, Steger M, Tomas-Loba A, Sartori AA, Adams IR, Batista FD, Boulton SJ. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Molecular cell. 2013;49:858–871. doi: 10.1016/j.molcel.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Di Virgilio M, Callen E, Yamane A, Zhang W, Jankovic M, Gitlin AD, Feldhahn N, Resch W, Oliveira TY, Chait BT, Nussenzweig A, Casellas R, Robbiani DF, Nussenzweig MC. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. Science. 2013;339:711–715. doi: 10.1126/science.1230624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Feng L, Fong KW, Wang J, Wang W, Chen J. RIF1 counteracts BRCA1-mediated end resection during DNA repair. The Journal of biological chemistry. 2013;288:11135–11143. doi: 10.1074/jbc.M113.457440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zimmermann M, Lottersberger F, Buonomo SB, Sfeir A, de Lange T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science. 2013;339:700–704. doi: 10.1126/science.1231573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhang Y, Gostissa M, Hildebrand DG, Becker MS, Boboila C, Chiarle R, Lewis S, Alt FW. The role of mechanistic factors in promoting chromosomal translocations found in lymphoid and other cancers. Adv Immunol. 2010;106:93–133. doi: 10.1016/S0065-2776(10)06004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lieber MR, Yu K, Raghavan SC. Roles of nonhomologous DNA end joining, V(D)J recombination, and class switch recombination in chromosomal translocations. DNA repair. 2006;5:1234–1245. doi: 10.1016/j.dnarep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- [91].Stephens PJ, McBride DJ, Lin ML, Varela I, Pleasance ED, Simpson JT, Stebbings LA, Leroy C, Edkins S, Mudie LJ, Greenman CD, Jia M, Latimer C, Teague JW, Lau KW, Burton J, Quail MA, Swerdlow H, Churcher C, Natrajan R, Sieuwerts AM, Martens JW, Silver DP, Langerod A, Russnes HE, Foekens JA, Reis-Filho JS, van ’t Veer L, Richardson AL, Borresen-Dale AL, Campbell PJ, Futreal PA, Stratton MR. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462:1005–1010. doi: 10.1038/nature08645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal S, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Quail MA, Burton J, Swerdlow H, Carter NP, Morsberger LA, Iacobuzio-Donahue C, Follows GA, Green AR, Flanagan AM, Stratton MR, Futreal PA, Campbell PJ. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Weinstock DM, Jasin M. Alternative pathways for the repair of RAG-induced DNA breaks. Mol Cell Biol. 2006;26:131–139. doi: 10.1128/MCB.26.1.131-139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ferguson DO, Sekiguchi JM, Chang S, Frank KM, Gao Y, DePinho RA, Alt FW. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6630–6633. doi: 10.1073/pnas.110152897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Boboila C, Jankovic M, Yan CT, Wang JH, Wesemann DR, Zhang T, Fazeli A, Feldman L, Nussenzweig A, Nussenzweig M, Alt FW. Alternative end-joining catalyzes robust IgH locus deletions and translocations in the combined absence of ligase 4 and Ku70. Proc Natl Acad Sci U S A. 2010;107:3034–3039. doi: 10.1073/pnas.0915067107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhang Y, Jasin M. An essential role for CtIP in chromosomal translocation formation through an alternative end-joining pathway. Nature structural & molecular biology. 2011;18:80–84. doi: 10.1038/nsmb.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]