Abstract
Objective
To determine the effects of dietary-induced weight loss (D) and weight loss plus exercise (D+E) compared to exercise alone (E) on bone mineral density (BMD) in older adults with knee osteoarthritis (OA).
Design
Data come from 284 older (66.0±6.2 years), overweight/obese (BMI 33.4±3.7 kg/m2), adults with knee OA enrolled in the Intensive Diet and Exercise for Arthritis (IDEA) study. Participants were randomized to 18 months of walking and strength training (E; n=95), dietary-induced weight loss targeting 10% of baseline weight (D; n=88) or a combination of the two (D+E; n=101). Body weight and composition (DXA), regional BMD, were obtained at baseline and 18 months.
Results
E, D, and D+E groups lost 1.3±4.5 kg, 9.1±8.6 kg and 10.4±8.0 kg, respectively (p<0.01). Significant treatment effects were observed for BMD in both hip and femoral neck regions, with the D and D+E groups showing similar relative losses compared to E (both p<0.01). Despite reduced BMD, fewer overall participants had T-scores indicative of osteoporosis after intervention (9 at 18 months vs. 10 at baseline). Within the D and D+E groups, changes in hip and femoral neck, but not spine, BMD correlated positively with changes in body weight (r=0.21 and 0.54 respectively, both p=<0.01).
Conclusions
Weight loss via an intensive dietary intervention, with or without exercise, results in bone loss at the hip and femoral neck in overweight and obese, older adults with OA. Although the exercise intervention did not attenuate weight loss associated reductions in BMD, classification of osteoporosis and osteopenia remained unchanged.
Keywords: bone density, obesity, weight loss, exercise, osteoarthritis
Introduction
Osteoarthritis (OA) is a leading cause of disability in older adults1, with obesity recognized as the strongest modifiable risk factor for the condition. Even modest weight loss (i.e. 5% of baseline weight) substantially improves physical function in this population2, 3. Nonetheless, concern exists about weight loss-associated reductions in lean and bone mass4, 5, and the potential for exacerbation of age-related risk of disability6, 7 and osteoporotic fracture8, 9. Regarding the latter, although patients with obesity and OA typically present with high bone mineral density (BMD)10, paradoxically, they may be at increased risk of fracture11-13. Sustained fractures, especially at the hip, compromise quality and expectancy of life14, 15; thus, identification of adjuvants to weight loss that minimize bone loss in obese, older adults with OA is warranted16.
Numerous strategies have been proposed to reduce bone loss during weight loss, including behavioral interventions. Several studies show a positive effect of exercise training on BMD in weight stable, older adults17, 18, and it may be an effective means to prevent bone loss during weight loss. Some, but not all19-21, studies show that performing regular weight-bearing (e.g., walking) and/or muscular loading (e.g., resistance) exercise may preserve bone during weight loss22-25. Results of these studies, however, are highly variable and may not be generalizable to older adults with chronic conditions that limit exercise capacity, such as OA.
Whether exercise can prevent weight loss-associated bone loss in older, obese adults with OA is not yet known. Therefore, the primary purpose of this study is to begin to determine the effects of dietary-induced weight loss plus walking and strength training exercise (D+E) compared to dietary-induced weight loss (D) and exercise (E) alone on regional BMD and T-scores at the hip and spine in a subset of overweight and obese, older adults with OA participating in the Intensive Diet and Exercise for Arthritist (IDEA) study. Secondarily, we describe the relationships between change in BMD and change in body weight, composition, and adiposity in the treatment arms undergoing intentional weight loss (D+E and D).
Methods
Study design and participants
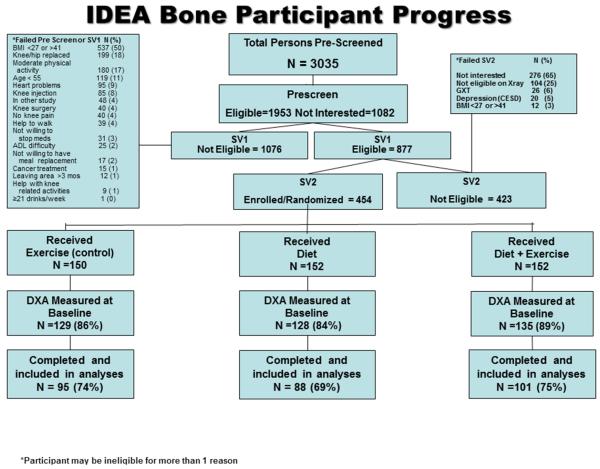
Participants included in the present analysis were enrolled in the IDEA study, a single-blinded, randomized, controlled trial (RCT) designed to determine whether significant weight loss (i.e. ≥10% reduction in body weight induced by diet, with or without exercise) improves mechanistic (knee joint loads and inflammation) and clinical (pain, function, mobility, and health-related quality of life) outcomes more than exercise alone in overweight and obese, older adults with knee OA3, 26. A total of 454 participants were originally randomized (using a computer based permuted random block design, within BMI and gender subgroups) to 18-months of E, D, or D+E, a subset of which consented to baseline, DXA-acquired, areal BMD measurement of the hip and lumbar spine (n=129, 128 and 135 for E, D, and D+E groups at baseline, respectively). All participants were offered DXA scans, but participants were allowed to refuse due to concerns about radiation exposure or schedule conflicts. Participants were lost to follow up during the trial due to study attrition (n=108), and missing data are assumed missing at random. The present analysis utilizes only the participants in whom we obtained a baseline and 18-month DXA scan (n=284, see Figure 1).
Figure 1.
IDEA Bone Consort Diagram
Individuals were eligible to participate in the IDEA study if they were ambulatory, community-dwelling, sedentary (≤ 30 minutes/week of formal exercise within the past six months), overweight or obese (27.0≤ body mass index (BMI) ≤40.1 kg/m2), older (≥55 years) adults who had grade II-III (mild to moderate) radiographic tibiofemoral OA or tibiofemoral plus patellofemoral OA of one or both knees. The Wake Forest University institutional review board approved the study, all participants signed an informed consent, and the primary outcome paper has been published3.
Interventions
Intensive dietary induced weight loss intervention
Both the D and D+E groups received the same dietary intervention, with an average weight loss goal of 10-15% of baseline weight. The dietary plan was based on partial meal replacements, including up to two meal-replacement shakes per day (General Nutrition Centers, Inc., Pittsburgh, PA.), with each shake providing 500 mg of calcium. For the third meal, participants followed a weekly menu plan and recipes that were low in fat, and high in vegetables, and provided 500-750 kcals/day. Collectively, the diet plan provided an energy-intake deficit of 800–1000 kcals/day, as predicted by resting energy expenditure (estimated resting metabolism × 1.2 activity factor), with 15–20% of calories coming from protein, < 30% from fat, and 45–60% from carbohydrate.
Body weight was monitored in weekly nutrition and behavior education sessions. Individuals trained in behavioral therapy and experienced in working with older adults ran all group and individual sessions, with one individual session and three group sessions per month for the first six months. Behavioral session topics included problem solving, goal-setting, review of a specific food topic, and tasting of several well balanced, low-fat, nutritious foods prepared with easily available ingredients. From months seven to 18, participants attended biweekly group behavioral sessions, with an individual appointment every two months.
Exercise Intervention
The D+E and E groups received the same exercise intervention, which consisted of overground walking (15 minutes), strength training (20 minutes), a second walking phase (15 minutes), and cool-down (10 minutes) three days per week for 18 months. During the first six months, participation was center-based. After the initial six-months, participants could remain in the facility program, opt for a home-based program, or combine the two. Further details of the interventions used in all treatment arms can be found in the IDEA design and rationale paper26.
Body Composition and Clinical Classification of BMD
All outcome measures were assessed by blinded study staff. Participant height and body mass were assessed at baseline and 18 months. Height was measured using a stadiometer and body mass was measured on a calibrated electronic scale. BMI was calculated as body mass in kg divided by height in meters squared. Total body fat and lean mass, and regional BMD were assessed using dual-energy X-ray absorptiometry (DXA, Hologic Delphi A 11.0 QDR, Bedford, MA) at baseline and 18 months. Specifically, bone density (BMD; g/cm2) was measured at the posterior anterior lumbar spine (L1-L4) and hip (femoral neck, trochanter, and intertrochanter space). DXA measurements were made by a certified technician blinded to treatment arm and the manufacturer’s recommendations for patient positioning, scanning, and analysis scans were followed. Osteoporosis and osteopenia were defined as location-specific T-scores ≤2.5 and between −2.5 and −1, respectively27.
Covariate Measurements
Baseline covariate assessments included self-reported demographic, medical history, and comorbidity information. Prescription bone medication usage and calcium/vitamin D supplementation were also captured via self-report at baseline.
Serum Leptin
Serum leptin concentration was measured as a biomarker of adiposity and a potential mediator of the adiposity-BMD association28. Blood draws were performed on a subsample of participants (n=116) in the fasted state at baseline and 18-months and tested using a commercial radioimmunoassay (Millipore, Billerica, MA). The subsample consisted of participants that consented to additional MRI and CT scans, who were selected as a random sample from the latter waves of study recruitment.
Statistical Analysis
Descriptive statistics were summarized by intervention group at baseline using means ± standard deviations or frequencies and percentages. The overall intervention effect on change in regional BMD was estimated using one-way ANOVA (unadjusted results) and analysis of covariance (ANCOVA, adjusted results), with results presented as means and 95% confidence intervals. The ANCOVA model testing the intervention effect included adjustment for gender, baseline BMI and baseline regional BMD value in accordance with the IDEA trial analytic plan26 to accommodate study stratification factors and to ensure that the variance is not biased. To ensure findings were not affected by bone medication and supplement use, additional ANCOVA models (as previously described) were performed, further adjusting for prescription bone medication use or calcium/vitamin D supplementation.
Associations between intentional weight (and body composition) loss and change in BMD from baseline were assessed in D and D+E arms, only. Pearson correlations were generated to measure the strength of the association between percentage changes in body weight and changes in regional BMD. Eighteen month change in regional BMD per kg change in body mass and composition or per ng/dL change in leptin was modeled using linear regression, adjusting for randomization arm, gender, baseline BMI, and baseline outcome measure. Because leptin is often considered a global adiposity biomarker, the previous association between leptin and percent BMD change was further adjusted for change in total fat mass. Lastly, to aid in clinical interpretability and detect a potential nonlinear associations between BMD and weight change, associations between change in regional BMD by overall percent weight change were modeled using ANCOVA with percent weight change tertile as the independent variable, adjusted for randomization arm, gender, baseline BMI, and baseline regional BMD.
SAS software (version 9.3, SAS Institute Inc, Cary, NC) was used for all analyses, with a Type I error rate of 0.05 for overall group comparisons and associations. Pairwise comparisons of treatment arms were deemed significant at a Bonferroni-adjusted Type I error rate of 0.0167, as prescribed in the IDEA analytic plan26.
Results
Baseline characteristics
Recruitment and retention characteristics of the present analysis are presented in Figure 1. Baseline DXA measurements were performed on 392 of the 454 IDEA trial participants, and 284 participants who had a baseline DXA scan returned for an 18-month visit scan. Race was the only significant predictor of DXA study attrition, with African-American participants more likely to withdraw (41.2%) than white participants (26.5%, p=0.02). Baseline demographic data on the IDEA study sample used in this analysis (n=284) are presented by treatment arm in Table 1. Overall, participants were 66.0 ±6.2 years of age with an average BMI of 33.4±3.7 kg/m2. Women represented 74% of the study sample and 86% self-identified as Caucasian, similar to the total IDEA sample3. Although only 9% of participants self-reported a prior diagnosis of osteoporosis, reported use of bone prescription medication was 25%, and reported use of calcium and vitamin D supplementation was 51%. Based on DXA-acquired BMD and calculated T-scores, 179 (47%) participants were classified as osteopenic and 15 (4%) participants were classified as osteoporotic at baseline.
Table 1.
Baseline descriptive characteristics according to treatment group for individuals with baseline and follow-up DXA data.
| Participant Characteristics |
Exercise (n=95) |
Diet (n=88) |
Diet + Exercise (n=101) |
|---|---|---|---|
| Age (years) | 65.8±6.3 | 66.0±6.0 | 66.1±6.4 |
| Female, n (%) | 71 (75) | 61 (70) | 77 (76) |
| White, n (%) | 80 (84) | 79 (90) | 85 (84) |
| Weight (kg) | 91.6±13.5 | 91.8±15.0 | 91.4±14.1 |
| Body mass index (kg/m2) | 33.5±3.7 | 33.2±3.6 | 33.4±3.7 |
| Total body fat mass (kg) | 36.6±7.9 | 36.5±7.1 | 36.3±8.5 |
| Total body lean mass (kg) | 55.5±11.5 | 55.1±12.4 | 54.6±10.9 |
| Leptin (ng/mL)* | 40.8±20.5 | 36.6±22.4 | 37.8±28.5 |
| Self- reported osteoporosis | 6 (6) | 9 (11) | 9 (9) |
| Self-reported bone medication use** | 24 (25) | 18 (20) | 28 (28) |
| Self-reported calcium/vitamin D use | 55 (58) | 40 (45) | 49 (49) |
| Areal bone mineral density (BMD) | |||
| Hip BMD (mg/cm2) | 968±135 | 981±162 | 962±120 |
| Femoral neck BMD (mg/cm2) | 792±109 | 805±124 | 791±109 |
| Spine BMD (mg/cm2) | 1070±176 | 1080±199 | 1070±161 |
| T-score | |||
| Hip | 0.02±0.95 | 0.05±1.11 | −0.02±0.88 |
| Femoral neck | −0.66±0.88 | −0.59±1.00 | −0.66±0.91 |
| Spine | 0.13±1.54 | 0.17±1.71 | 0.10±1.39 |
| DXA-derived osteoporosis***, n (%) | 2 (2) | 5 (6) | 3 (3) |
| DXA-derived osteopenia***, n (%) | 50 (53) | 44 (44) | 39 (44) |
Data are presented as means ± SD or n (%). Abbreviations: n = sample size; kg = kilogram; m = meter; g = gram; cm = centimeter; DXA = dual energy x-ray absorptiometry.
Leptin total subsample with BL and 18-month measures: n=116 (E: n=39; D: n=36; D+E: n=41).
Queried bone medications include Actonel, Fosamax, Boniva, and Forteo.
Baseline osteoporosis and osteopenia classifications were based on a DXA-acquired BMD T-score ≤−2.5 or −2.5<T-score<−1 at the hip, femoral neck or spine, respectively.
Intervention effect on BMD and clinical classification
Over the 18-month intervention period an average of 9.1±8.6 kg (9.7±8.5%) and 10.4±8.0 kg (11.3±8.3%) of initial body weight was lost in the D and D+E arms, respectively (both p<0.01), with a minimal but significant loss in body weight observed in the E-only group (1.3±4.5 kg, 1.4±4.6%; p<0.01). Mean diet session attendance for D (67.8±17.8%) and D+E groups (68.7±19.7%) did not significantly differ (p=0.75); likewise, mean exercise session attendance did not differ by group (E = 61.1±21.6% and D+E=63.2±20.9; p=0.49).
Unadjusted and adjusted 18-month treatment effects on BMD are presented in Table 2. Significant treatment effects were observed for both hip and femoral neck regions, with the D and D+E groups showing significant absolute and relative losses in BMD compared to E. Although 18-month hip and femoral neck BMD measurements did not significantly differ from baseline in the E-only group, it is worth noting that estimates were negative. Results were largely unchanged after adjustment for gender, baseline BMI, and baseline regional BMD value. No treatment effect was observed for spine BMD. Further analyses adjusting for baseline prescription bone medication use or calcium/vitamin D supplementation did not modify the observed treatment effects (data not shown).
Table 2.
Unadjusted and adjusted treatment effects on change in BMD by region.
| Change in BMD by Region |
Exercise (n=95) | Diet (n=88) | Diet + Exercise (n=101) | Overall p-value* |
|||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean (95% CI) | % Chg | Mean (95% CI) | % Chg | Mean (95% CI) | % Chg | ||
| Δ Total Hip BMD (mg/cm2) | |||||||
| Unadjusted | −2.1 (−8.2, 4.0) | −0.2 | −24.0 (−30.3, −17.6) | −2.5 | −19.4 (−25.3, −13.5) | −2.0 | <0.01** |
| Adjusted | −2.0 (−8.5, 4.4) | −0.2 | −23.7 (−30.3, −17.2) | −2.4 | −19.5 (−25.8, 13.2) | −2.0 | <0.01** |
| Δ Femoral Neck BMD (mg/cm2) | |||||||
| Unadjusted | −2.6 (−8.2, 2.9) | −0.3 | −15.3 (−21.0, −9.5) | −1.9 | −14.4 (−19.8, −9.1) | −1.8 | <0.01** |
| Adjusted | −0.8 (−6.6, 5.0) | −0.1 | −13.2 (−19.1, −7.3) | −1.7 | −12.5 (−18.1, −6.8) | −1.6 | <0.01** |
| Δ Spine BMD (mg/cm2) | |||||||
| Unadjusted | 5.2 (−2.1, 12.4) | 0.5 | 3.5 (−4.0, 11.0) | 0.3 | −0.9 (−7.9, 6.1) | −0.1 | 0.47 |
| Adjusted | 7.5 (−0.2, 15.1) | 0.7 | 5.4 (−2.4, 13.2) | 0.5 | 1.5 (−6.0, 9.0) | 0.1 | 0.50 |
Unadjusted estimates are based on a one-way ANOVA at 18 months. Model-adjusted estimates control for gender, baseline BMI and baseline risk factor value. Abbreviations: BMD = areal bone mineral density; n = sample size; SE = standard error.
p-value at 18 months performed based on test from a contrast statement to compare groups.
E differs significantly from D and D+E (p<0.01)
Ten participants with baseline and follow-up DXA-derived T-score values were classified as osteoporotic (1 hip, 3 femoral neck and 6 spine) and 46.8% (133) had osteopenia in at least one site at baseline. Following intervention, nine participants had T-scores indicative of osteoporosis in any region and 49.3% (140) had osteopenia. Only one participant (D group) progressed from osteopenia to osteoporosis while two participants (one E and one D) classified as osteoporotic at baseline changed to osteopenia at 18 months. Eleven participants (2 E, 3 D, 6 D+E) progressed from normal BMD to osteopenia in at least one region at 18 months.
Associations between measures of body weight, composition, and adiposity and regional BMD change
To assess the independent contribution of the magnitude of intentional weight loss on change in percent BMD, the E-only group was removed from the following analyses, and D and D+E group were combined (see Table 3). For D and D+E groups, changes in femoral neck and hip BMD correlated positively with changes in body weight (r=0.21 and 0.54, both p=<0. 01). Model-adjusted estimates controlling for randomization group, gender, baseline BMI, and baseline outcome measures revealed a 1 kg loss in body weight was associated with a 0.10± 0.03% reduction in femoral neck BMD and a 0.20±0.03% reduction in hip BMD. While changes in both fat mass and lean mass were significantly associated with percent change in hip BMD, only fat mass changes were associated with change in femoral neck BMD.
Table 3.
18-month changes in BMD from baseline per unit change in measures of body composition and leptin, D and D+E arms only.
| Change in Body Weight and Composition |
Δ Total Hip BMD (mg/cm2) |
Δ Femoral Neck BMD (mg/cm2) |
Δ Spine BMD (mg/cm2) |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| Δ Total body weight (kg) | 1.9 (1.5, 2.4) | <0.01 | 0.8 (0.3, 1.3) | <0.01 | 0.4 (−0.3, 1.01) | 0.19 |
| Δ Total body lean mass (kg) | 3.8 (2.3, 5.4) | <0.01 | 1.4 (−0.0, 2.8) | 0.06 | −0.00 (−1.8, 1.8) | 0.99 |
| Δ Total body fat mass (kg) | 2.8 (2.1, 3.4) | <0.01 | 1.2 (0.5, 1.8) | <0.01 | 0.8 (−0.0, 1.66) | 0.06 |
| Δ Leptin (ng/dL)* | 1.2 (0.7, 1.7) | <0.01 | 0.7 (0.3, 1.0) | <0.01 | 0.6 (0.0, 1.1) | 0.04 |
| Δ Leptin (ng/dL), further adjusted for fat mass change ** |
0.3 (−0.2, 0.8) | 0.26 | 0.4 (0.0, 0.8) | 0.05 | 0.5 (−0.2, 1.2) | 0.14 |
| Δ Leptin (ng/dL), further adjusted for fat mass change ** |
0.3 (−0.2, 0.8) | 0.26 | 0.4 (0.0, 0.8) | 0.05 | 0.5 (−0.2, 1.2) | 0.14 |
Model estimates adjust for randomization group, gender, baseline BMI, and baseline location BMD value.
Statistical significance (p<0.05) unchanged using log-leptin in model in place of leptin except for spine (log-leptin p=0.16).
Nonsignificance (p≥0.05) unchanged using log-leptin in place of leptin.
The relationship between change in bone density and body weight and composition was also modeled by comparing change in regional BMD by tertile of percentage weight loss for the D and D+E groups only (Table 4, no interaction was found between randomization group and tertile of weight loss). Participants experiencing the greatest weight loss (≥12.9%) showed markedly greater reductions of BMD at the femoral neck and hip, but not the spine, compared to participants in the lowest tertile of weight loss (<5.2%). Similar results were observed when losses of fat and lean mass were considered (data not shown).
Table 4.
Change in bone mineral density by tertile* of percentage weight loss, D and D+E arms only.
| Change in BMD by Region |
Tertile 1 (n=72) |
Tertile 2 (n=73) |
Tertile 3 (n=72) |
Overall p-value* |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Δ Total Hip BMD (mg/cm2) | −5.0 (−12.9, 2.9) | −18.5 (−25.8, −11.2) | −36.6 (−43.7, −29.5) | <0.01 |
| Δ Femoral Neck BMD (mg/cm2) |
−6.2 (−13.7, 1.3) | −8.0 (−14.9, −1.1) | −21.5 (−28.2, −14.8) | <0.01 |
| Δ Spine BMD (mg/cm2) | 10.1 (−4.0, 21.1) | 6.8 (−5.7, 19.3) | −1.7 (−13.6, 10.2) | 0.37 |
Where Tertile 1 experienced <5.18% weight loss, Tertile 2 experienced ≥ 5.18% but <12.86% weight loss and Tertile 3 experienced ≥12.86% weight loss.
All tertiles for total hip achieve pairwise statistical significance (p<0.0167). Additionally, tertile 3 compared to tertiles 1 and 2 for femoral neck achieves pairwise significance.
Model estimates adjusted for randomization arm, gender, baseline BMI, and baseline regional BMD.
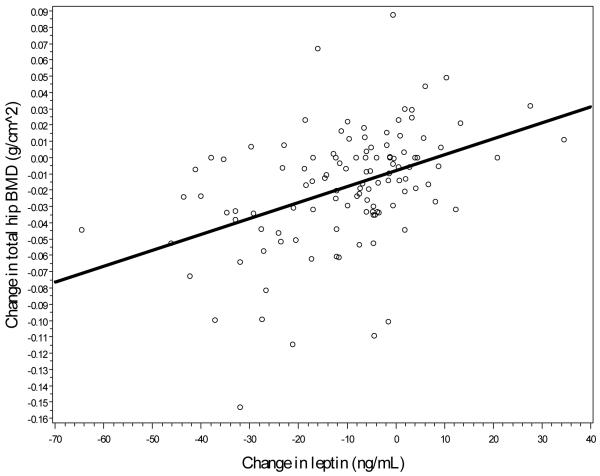
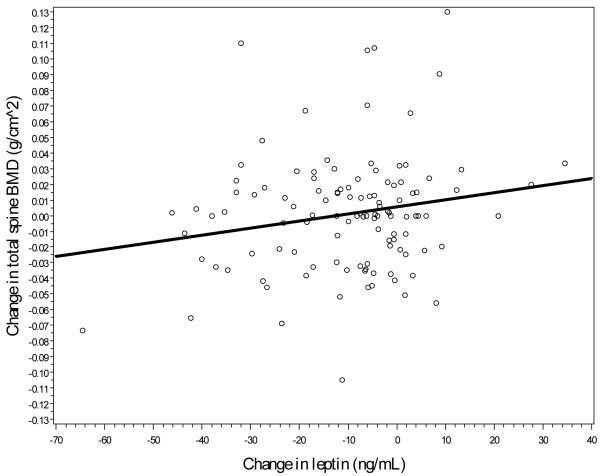
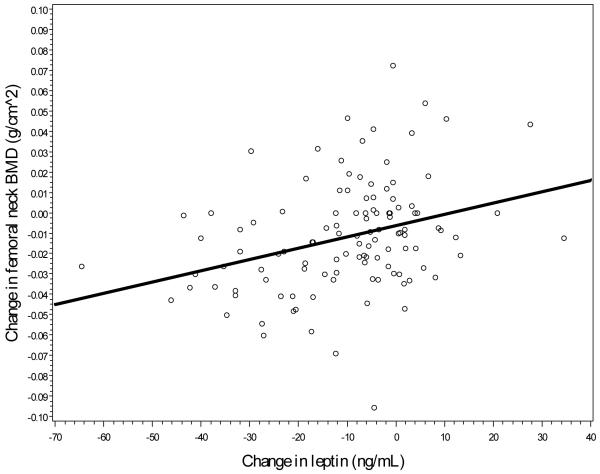
Lastly, as a potential mediator of the assosication between change in fat and bone mass, associations between change in regional bone mass and change in serum leptin were assessed. Unadjusted associations, shown in Figures 2a-2c, suggest a direct relationship, with a stronger linear association between change in leptin and change in hip BMD (r=0.43, p<0.01) and between leptin change and femoral neck change (r=0.41, p<0.01) when compared to spine BMD change and leptin change (r=0.10, p=0.09). Model-adjusted change in leptin was significantly associated with change in BMD for each location, although all associations became nonsignificant after further adjustment for total body fat mass (see Table 3).
Figure 2a.
Linear association between hip BMD changes and leptin changes
Figure 2c.
Linear association between spine BMD changes and leptin changes.
Discussion
Results from this exploratory study provide novel information about the independent and combined effects of intensive dietary-induced weight loss and exercise training on regional BMD in overweight and obese, older adults with knee OA. We report that 10% weight loss occurring over 18-months, with or without exercise, is associated with significant reductions in hip and femoral neck, but not spine, BMD. Participants who lost the most weight and fat mass also lost the most hip and femoral neck BMD, regardless of baseline BMI, BMD, or exercise status. However, despite significant BMD loss, clinical classification of osteoporosis and osteopenia remained virtually unchanged.
The addition of exercise to dietary-induced weight loss is hypothesized to attenuate the loss of BMD associated with weight loss by countering reductions in mechanical stress29. Indeed, exercise in the absence of weight loss is consistenly shown to have a positive effect on BMD in older adults17, 18. To date, however, only four RCTs (including the present study) have been designed to examine the additive effect of exercise training to dietary-induced weight loss on BMD in older adults, with conflicting results reported. The first study, conducted in 1993 by Svendsen and colleagues19, enrolled 118 overweight postmenopausal women to participate in a 3-arm (control, diet, and diet plus combined aerobic/non-aerobic exercise) 12-week RCT. Despite significant weight loss achieved in the diet groups compared to control, no consistent, major group differences in total body, spinal, or forearm BMD were observed19. These seminal findings suggest that the addition of exercise to weight loss does not prevent bone loss, although the short duration of this study intervention likely compromised the ability to observe significant changes in bone remodeling between treatment arms30. In contrast, two recent, longer RCTs suggest that exercise (high intensity resistance training23 or a combined aerobic and resistance training program25) is effective for maintaining total body23 and regional25 bone mass in overweight and obese older adults undergoing intentional weight loss.
The study design and outcomes assessed by Shah et al.25 most closely resemble the current study and, given discordant results, it is of interest to speculate which study characteristics contributed to the conflicting findings. Although total weight loss achieved in diet groups and exercise modality/frequency were similar between the studies, differences were noted in exercise duration (90 minutes/day25 versus 60 minutes/day in the present study) and adherence (83%25 versus 63% in our study, in combined diet and exercise groups). Importantly, and in contrast to our findings, the exercise intervention utilized by Shah et al.25 increased hip BMD in the exercise-only group over time, suggesting that increased compliance to exercise prescriptions of longer duration may significantly influence change in BMD. That being said, the time course of the two studies was different (12 months25 versus 18 months in our study), and a true cross-study comparison requires consideration of normal age-related bone loss (i.e. ~ 1% per year)31-33. Thus, participants in the IDEA study would have been expected to lose greater amounts of BMD simply due to the longer duration of the study, which may or may not be mitigated by exercise training. Finally, although both studies sampled overweight and obese, older adults with functional limitations (mild-to-moderate frailty25 versus OA in the present study), persons with OA have unique risks for fracture11, 12 and compromised exercise ability34, which require separate characterization. Findings from Shah et al25 and other RCTs of exercise may not be applicable to this population, as the total exercise dose realistically achievable by persons with OA may not be sufficient to prevent age- and weight loss-associated BMD loss.
Findings presented here examining the magnitude of BMD loss associated with weight loss are in line with other weight loss trials reporting a 1-2% reduction in regional BMD associated with a 10% loss in baseline weight5. Results also concur with prior literature in older adults showing that when diet and exercise groups are compared to a weight stable group, weight loss is consistently associated with reduced BMD19, 21, 25 and that changes in fat mass are directly correlated to changes in bone mass35, 36. Leptin data presented here lend support to the hypothesis that weight loss-induced reductions in leptin could belie the association between weight loss and bone loss28; however, it is worth noting that this relationship did not hold after further adjustment for fat mass change, which has been shown before37.
Clinically, weight loss-associated loss in BMD is concerning due to the well-known association between low BMD and fracture risk9, 38. Indeed, BMD is the strongest predictor of future osteoporotic fracture,39 and observational data consistently link weight loss in late life with loss of BMD8, 9; yet, whether the magnitude of BMD loss observed in this study translates into increased fracture risk remains unknown. Encouragingly, clinical classification of osteoporosis and osteopenia was unchanged in IDEA participants undergoing weight loss. Moreover, although the exercise prescription utilized in IDEA did not prevent reductions in BMD, fracture risk assessment is multifactorial and overall risk may be reduced due to parallel improvements in pain and function attributable to the study intervention3.
Strengths of the present study include the RCT design, large sample size and serial measures of BMD at clinically important sites of osteoporotic fracture. This study is not without limitations, however. First, the main IDEA trial was conceived and executed to explore the effect of E, D, and D+E on IL-6 and knee compressive forces26; thus, the present analysis is an exploratory investigation of the effect of body composition changes on BMD within a random subgroup of the IDEA trial. Because this specific analytic plan and study power were not determined a priori at the time of the trial’s inception, findings should be considered hypothesis-generating rather than confirmatory. Second, although DXA-acquired areal BMD is the primary metric by which osteoporosis is assessed, it is insufficient to quantify future fracture risk40, and presents a number of methodological limitations in the context of obesity and weight loss41;42. Future studies evaluating intervention effectiveness on skeletal health would be strengthened by the integration of measures of bone quality43, such as volumetric BMD, thickness and strength estimates, which should improve fracture risk predictive power. Third, the age and proclivity of our study population to develop osteophytes44 may have influenced BMD measures, and results may not be generalizable to older adults without OA. Additionally, the possibility that overweight and obese adults develop leptin resistance raise questions about whether our sample is appropriate for studying any leptin-bone association45. Lastly, the protective effect of calcium supplementation5 osteoporosis medications on BMD during weight loss is strong46 and, although statistical adjustment for calcium/vitamin D intake or medication use did not affect study results, the protective effect of pharmacotherapy on BMD may have compromised our ability to observe lifestyle-based differences.
In summary, 18-months of intensive dietary induced weight loss, with or without exercise training, in overweight and obese older adults with OA results in bone loss at the hip and proximal femur. Although the exercise intervention did not attenuate weight loss-associated reductions in BMD, clinical classification of osteoporosis and osteopenia in the population remained unchanged. Future intervention studies seeking to evaluate and minimize the risk/benefit ratio associated with weight loss in overweight and obese older adults need to include osteoporosis-related fractures as an endpoint. Further, additional clarification as to the duration, intensity, and type of exercise necessary to minimize bone loss in older adults undergoing intentional weight loss – including those with disease specific conditions, such as OA, that are often ignored - is needed from well-designed RCTs.
Figure 2b.
Linear association between femoral neck BMD changes and leptin changes.
Acknowledgments
This work was supported by Grant Number AR052528 to SPM from the National Institute Of Arthritis And Musculoskeletal And Skin Diseases, the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), and additional support for DPB and RFL was provided by the Arthritis and Musculoskeletal Disease Research Center at Wake Forest School of Medicine and the Wake Forest School of Medicine Translational Science Institute.
Footnotes
Author Contributions The authors’ responsibilities were as follows—SPM, BJN and RFL: designed the research; JJN, RFL, MFL, BJN, NRW and SPM: conducted the research; DPB and KMB: analyzed the data; DBP, KMB, RFL, NW, MFL, BJN, and SAS: interpreted the data and drafted the manuscript; and DPB: had primary responsibility for the final content. All authors read and approved the final manuscript.
Competing Interests The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. None of the authors had any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration Number: NCT00381290
References
- (1).Haq I, Murphy E, Dacre J. Osteoarthritis. Postgrad Med J. 2003;79(933):377–383. doi: 10.1136/pmj.79.933.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- (3).Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31(5):743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- (5).Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- (7).Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123(2 Suppl):465–468. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- (8).Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90(4):1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- (9).Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51(12):1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- (10).Stewart A, Black AJ. Bone mineral density in osteoarthritis. Curr Opin Rheumatol. 2000;12(5):464–467. doi: 10.1097/00002281-200009000-00021. [DOI] [PubMed] [Google Scholar]
- (11).Arden NK, Crozier S, Smith H, Anderson F, Edwards C, Raphael H, et al. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis Rheum. 2006;55(4):610–615. doi: 10.1002/art.22088. [DOI] [PubMed] [Google Scholar]
- (12).Prieto-Alhambra D, Nogues X, Javaid MK, Wyman A, Arden NK, Azagra R, et al. An increased rate of falling leads to a rise in fracture risk in postmenopausal women with self-reported osteoarthritis: a prospective multinational cohort study (GLOW) Ann Rheum Dis. 2013;72(6):911–917. doi: 10.1136/annrheumdis-2012-201451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. 2012;27(1):1–10. doi: 10.1002/jbmr.1486. [DOI] [PubMed] [Google Scholar]
- (14).Cockerill W, Lunt M, Silman AJ, Cooper C, Lips P, Bhalla AK, et al. Health-related quality of life and radiographic vertebral fracture. Osteoporos Int. 2004;15(2):113–119. doi: 10.1007/s00198-003-1547-4. [DOI] [PubMed] [Google Scholar]
- (15).Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- (16).Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13(11):1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- (17).Bolam KA, van Uffelen JG, Taaffe DR. The effect of physical exercise on bone density in middle-aged and older men: A systematic review. Osteoporos Int. 2013 doi: 10.1007/s00198-013-2346-1. [DOI] [PubMed] [Google Scholar]
- (18).Martyn-St JM, Carroll S. High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int. 2006;17(8):1225–1240. doi: 10.1007/s00198-006-0083-4. [DOI] [PubMed] [Google Scholar]
- (19).Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med. 1993;95(2):131–140. doi: 10.1016/0002-9343(93)90253-l. [DOI] [PubMed] [Google Scholar]
- (20).Nakata Y, Ohkawara K, Lee DJ, Okura T, Tanaka K. Effects of additional resistance training during diet-induced weight loss on bone mineral density in overweight premenopausal women. J Bone Miner Metab. 2008;26(2):172–177. doi: 10.1007/s00774-007-0805-5. [DOI] [PubMed] [Google Scholar]
- (21).Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93(6):2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ryan AS, Nicklas BJ, Dennis KE. Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol. 1998;84(4):1305–1310. doi: 10.1152/jappl.1998.84.4.1305. [DOI] [PubMed] [Google Scholar]
- (23).Daly RM, Dunstan DW, Owen N, Jolley D, Shaw JE, Zimmet PZ. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int. 2005;16(12):1703–1712. doi: 10.1007/s00198-005-1906-4. [DOI] [PubMed] [Google Scholar]
- (24).Hosny IA, Elghawabi HS, Younan WB, Sabbour AA, Gobrial MA. Beneficial impact of aerobic exercises on bone mineral density in obese premenopausal women under caloric restriction. Skeletal Radiol. 2012;41(4):423–427. doi: 10.1007/s00256-011-1196-1. [DOI] [PubMed] [Google Scholar]
- (25).Shah K, Armamento-Villareal R, Parimi N, Chode S, Sinacore DR, Hilton TN, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26(12):2851–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Messier SP, Legault C, Mihalko S, Miller GD, Loeser RF, DeVita P, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. BMC Musculoskelet Disord. 2009;10:93. doi: 10.1186/1471-2474-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organization; Geneva: 1994. [PubMed] [Google Scholar]
- (28).Motyl KJ, Rosen CJ. Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie. 2012;94(10):2089–2096. doi: 10.1016/j.biochi.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Skerry TM. The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys. 2008;473(2):117–123. doi: 10.1016/j.abb.2008.02.028. [DOI] [PubMed] [Google Scholar]
- (30).Heaney RP. The bone-remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res. 1994;9(10):1515–1523. doi: 10.1002/jbmr.5650091003. [DOI] [PubMed] [Google Scholar]
- (31).Davis JW, Grove JS, Ross PD, Vogel JM, Wasnich RD. Relationship between bone mass and rates of bone change at appendicular measurement sites. J Bone Miner Res. 1992;7(7):719–725. doi: 10.1002/jbmr.5650070702. [DOI] [PubMed] [Google Scholar]
- (32).Ahlborg HG, Johnell O, Karlsson MK. Long term effects of oestrogen therapy on bone loss in postmenopausal women: a 23 year prospective study. BJOG. 2004;111(4):335–339. doi: 10.1111/j.1471-0528.2004.00068.x. [DOI] [PubMed] [Google Scholar]
- (33).Von Thun NL, Sukumar D, Heymsfield SB, Shapses SA. Does bone loss begin after weight loss ends? Results 2 years after weight loss or regain in postmenopausal women. Menopause. 2013 doi: 10.1097/GME.0b013e3182a76fd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hunter DJ, Eckstein F. Exercise and osteoarthritis. J Anat. 2009;214(2):197–207. doi: 10.1111/j.1469-7580.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Blain H, Carriere I, Favier F, Jeandel C, Papoz L. Body weight change since menopause and percentage body fat mass are predictors of subsequent bone mineral density change of the proximal femur in women aged 75 years and older: results of a 5 year prospective study. Calcif Tissue Int. 2004;75(1):32–39. doi: 10.1007/s00223-003-0192-4. [DOI] [PubMed] [Google Scholar]
- (36).Bleicher K, Cumming RG, Naganathan V, Travison TG, Sambrook PN, Blyth FM, et al. The role of fat and lean mass in bone loss in older men: findings from the CHAMP study. Bone. 2011;49(6):1299–1305. doi: 10.1016/j.bone.2011.08.026. [DOI] [PubMed] [Google Scholar]
- (37).Goulding A, Taylor RW. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 1998;63(6):456–458. doi: 10.1007/s002239900557. [DOI] [PubMed] [Google Scholar]
- (38).Langlois JA, Visser M, Davidovic LS, Maggi S, Li G, Harris TB. Hip fracture risk in older white men is associated with change in body weight from age 50 years to old age. Arch Intern Med. 1998;158(9):990–996. doi: 10.1001/archinte.158.9.990. [DOI] [PubMed] [Google Scholar]
- (39).Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341(8837):72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- (40).Nguyen ND, Eisman JA, Center JR, Nguyen TV. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab. 2007;92(3):955–962. doi: 10.1210/jc.2006-1476. [DOI] [PubMed] [Google Scholar]
- (41).Hangartner TN, Johnston CC. Influence of fat on bone measurements with dual-energy absorptiometry. Bone Miner. 1990;9(1):71–81. doi: 10.1016/0169-6009(90)90101-k. [DOI] [PubMed] [Google Scholar]
- (42).Tothill P. Dual-energy x-ray absorptiometry measurements of total-body bone mineral during weight change. J Clin Densitom. 2005;8(1):31–38. doi: 10.1385/jcd:8:1:031. [DOI] [PubMed] [Google Scholar]
- (43).Bouxsein ML, Seeman E. Quantifying the material and structural determinants of bone strength. Best Pract Res Clin Rheumatol. 2009;23(6):741–753. doi: 10.1016/j.berh.2009.09.008. [DOI] [PubMed] [Google Scholar]
- (44).van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15(3):237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- (45).Myers MG, Jr., Heymsfield SB, Haft C, Kahn BB, Laughlin M, Leibel RL, et al. Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 2012;15(2):150–156. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Gozansky WS, Van Pelt RE, Jankowski CM, Schwartz RS, Kohrt WM. Protection of bone mass by estrogens and raloxifene during exercise-induced weight Loss. J Clin Endocrinol Metab. 2005;90(1):52–59. doi: 10.1210/jc.2004-0275. [DOI] [PubMed] [Google Scholar]