Abstract
Importance
Age-related cognitive decline among older individuals with normal cognition is a complex trait that potentially derives from processes of aging, inherited vulnerabilities, environmental factors, and common latent diseases that can progress to cause dementia, viz., Alzheimer’s disease (AD) and vascular brain injury (VBI).
Objective
Here we used CSF biomarkers to gain insight into this complex trait.
Design, Setting, Participants
Secondary analyses of an academic multicenter cross sectional (n=315) and longitudinal (n=158) study of five neuropsychological tests (Immediate Recall, Delayed Recall, Trails A, Trails B, Category Fluency) in cognitively normal individuals aged 21 to 100 years.
Main Outcome Measure(s)
to investigate the association of these test results with age, gender, level of education, inheritance of the ε4 allele of the apolipoprotein E gene (APOE), and cerebrospinal fluid (CSF) concentrations of Aβ42 and tau (biomarkers of AD) as well as F2-isoprostanes (IsoPs; measures of free radical injury).
Results
Age and education were broadly predictive of cross sectional cognitive performance: of the genetic and CSF measures, only greater CSF F2-IsoP concentration was significantly associated with poorer executive function (adjusted R2 up to 0.31). Longitudinal measures of cognitive abilities, except Category Fluency, also were associated broadly with age; of the genetic and CSF measures, only lower baseline CSF Aβ42 concentration was associated with longitudinal measures of immediate and delayed recall (marginal R2 up to 0.31).
Conclusions and Relevance
Our results suggest that age and level of education accounted for a substantial minority of variance in cross sectional or longitudinal cognitive test performance in this large group of cognitively normal adults. Latent AD and other diseases that produce free radical injury, like VBI, accounted for a small amount of variation in cognitive test performance across the adult human life span. Likely, additional genetic and environmental factors contribute substantially to age-related cognitive decline.
INTRODUCTION
Cognitive function, especially declarative memory and executive function, decreases with age in non-human primates1 and humans, even in individuals who have not crossed the clinical thresholds for Mild Cognitive Impairment (MCI) or dementia.2 This age-related cognitive decline appears to be related to several factors such as events that occurred earlier in life, which include the genetic complement inherited from parents, environment, processes of aging, and latent disease. However, the extent to which these different factors drive age-related cognitive decline remains unclear.
Observational studies using neuropathologic examination of adults who were cognitively normal proximate to death suggest that latent Alzheimer’s disease (AD) and vascular brain injury (VBI) are prevalent in those 65 years old and older.3–5 Accumulation of cerebral amyloid by molecular neuroimaging and changes in cerebrospinal fluid (CSF) concentrations of Aβ42 and tau that are characteristic of individuals diagnosed with amnestic MCI or AD dementia, also occur in a proportion of cognitively normal adults, raising the possibility that some amount of age-related cognitive decline may be the result of the earliest expression of AD.6–8 Similarly, if one assumes that white matter hyperintensities as revealed by T2-weighted MRI are at least in part a consequence of µVBI,9 then structural neuroimaging further supports a possible role for µVBI in age-related cognitive decline.10 Finally, we and others have studied CSF F2-Isoprostanes (IsoPs), biomarkers of oxidative injury to the brain, and shown that these are characteristically elevated in research subjects with MCI, AD dementia, and/or VBI; these associations have been validated mechanistically in animal models.11–14 In combination, these laboratory-based studies raise the possibility that some, or perhaps even most, age-related cognitive decline is the earliest expression of latent diseases of brain. This is an important point because, if true, it would suggest that existing therapies to control risk factors for µVBI and hopefully soon to be developed disease-modifying therapies for AD could have a high impact on age-related cognitive decline.
A focus of our AD Centers is developing CSF biomarker approaches to aid in the diagnosis and management of patients with MCI and dementia. As part of these efforts, we have obtained CSF samples from a large number of research subjects across the adult lifespan, who were carefully evaluated to establish that they are cognitively healthy controls. Here we used this valuable resource to examine the association between CSF biomarkers of AD or oxidative injury and cognitive function in relation to aging, using cross-sectional and longitudinal data.
METHODS
Recruitment of Subjects
Participants in this study were recruited between 2001 and 2009 from the University Washington ADRC and collaborating ADCs, including University of California at San Diego, Oregon Health & Science University, Indiana University, the University of Pennsylvania, and the University of California at Davis. All procedures were approved by the institutional review boards of each study site. All subjects were provided written informed consent. Subjects underwent detailed neuropsychological testing, and clinical and laboratory evaluations, and were classified as having no cognitive impairment, MCI, or dementia based on standard research criteria as previously described.15 Clinical diagnosis was made at consensus conference based on history provided by informants, neurological examination, detailed neuropsychological test results, and laboratory studies (including neuroimaging with MRI in the case of MCI or AD). Exclusion criteria were major neurological diagnosis that may affect cognitive function such as stroke, Parkinson’s disease, multiple sclerosis and history of moderate to severe head injury; major psychiatric disorder such as schizophrenia, major affective disorder and post-traumatic stress disorder; unstable major medical conditions, such as insulin-dependent diabetes; and using illegal drug or stimulants. Subjects aged 45 years and older were asked to participate in a longitudinal study with annual follow-up visits. Additionally, six younger subjects (ages 35, 38, 40, 41, and 43 years) had follow-up visits and were included in the longitudinal analyses.
CSF Biomarkers
CSF was obtained by lumbar puncture as previously described using 24-gauge sprotte atraumatic spinal needles.16 All CSF samples were analyzed in a single laboratory17 using 0.5 ml aliquots that had been stored in polypropylene cryotubes, frozen and maintained at −80° C, and never previously thawed. CSF was analyzed for Aβ42 and total tau using multiplexed Luminex reagents from InnoGenetics (Ghent, Belgium), according to manufacturer’s instructions and as previously described.18 CSF F2-IsoPs were quantified using a stable isotope dilution assay with gas chromatography/mass spectrometry and selective ion monitoring as described previously.19 APOE genotype was determined by a restriction digest method.20 Assays were performed blind to clinical diagnosis.
Statistical Analysis
We selected five neuropsychologic tests to examine multiple domains of cognition. The Wechsler Memory Scale-Revised Logical Memory Immediate and Delayed paragraph recall tasks measure verbal episodic memory;21 we used total score for immediate recall and delayed recall (each with possible range 0–25). Category Fluency is a test of semantic memory;22 we used total number of unique animals generated in one minute. Trail Making Test, Parts A and B are timed tests of ability to adapt to shifting task demands. Time taken to complete Part A (upper bound of 150 sec) is a measure of processing speed, and time taken to complete Part B (upper bound of 300 sec) is a measure of executive function.23
Inclusion criteria for the cross-sectional investigation were (i) all subjects classified as having no cognitive impairment at baseline evaluation, (ii) CSF at baseline that had assay results for all three CSF biomarkers, and (iii) a full set of neuropsychological test scores at baseline. The longitudinal investigation included subjects from the cross-sectional study who had at least one follow-up visit at approximately 12 months with results for at least one of the cognitive tests. The number of follow-up visits and time-span they encompassed varied depending on the time of recruitment to the study and the subject’s age. The longitudinal study sample was a subset of the cross-sectional study subjects, and characteristics of each study sample are shown in Table 1. At each follow-up visit, history obtained from the informants, clinical examination and neuropsychological test data were reviewed to determine whether the cognitive status of the subject remained the same or changed to MCI or dementia.
Table 1.
Demographics and Baseline Biomarkers and Cognitive Test Scores for Control Subjects in Cross-Sectional and Longitudinal Analyses
| Subjects with No Follow-Up (Cross-Sectional Only) (N = 157)a |
Subjects with Follow-Up for Longitudinal Analysis (N = 158) |
Total Subjects for Cross-Sectional Analysis (N = 315) |
|
|---|---|---|---|
| Age at Baseline (years) | 47.7 (49) ± 18.5 [21–88] | 67.1 (68) ± 11.3 [35–100] | 57.4 (61) ± 18.1 [21–100] |
| Male | 77 (49%) | 68 (43%) | 145 (46%) |
| Caucasian | 132 (84%) | 149 (94%) | 281 (89%) |
| APOE ε4 allele(s) | 60 (38%) | 48 (30%) | 108 (34%) |
| Education (years) | 16.2 (16) ± 2.5 [11–25] | 16.1 (16) ± 2.7 [10–27] | 16.1 (16) ± 2.6 [10–27] |
| MMSE | 29.2 (29) ± 1.0 [26–30] | 29.3 (30) ± 1.0 [25–30] | 29.3 (30) ± 1.0 [25–30] |
| Number of Visits | 1 (1) ± 0 [1–1] | 5.0 (5) ± 2.2 [2–10] | 3.0 (2)± 2.5 [1–10] |
| Length of Follow Up (Years) | 0 (0) ± 0 [0–0] | 4.4 (4.2) ± 2.3 [0.3–9.5] | 2.2 (0.3)± 2.8 [0–9.5] |
| CSF Aβ42 (pg/ml) | 332 (328) ± 142 [109–857] | 326 (291) ± 152 [61–820] | 329 (309) ± 147 [61–857] |
| CSF Tau (pg/ml) | 47.3 (45.6) ± 16.9 [17.5–101.1] | 50.9 (49.3) ± 14.9 [11.7–128.2] | 49.1 (48.9) ± 16.0 [11.7–128.2] |
| CSF Tau-P181 (pg/ml) | 30.1 (27.1) ± 13.3 [7.0–117.3] | 33.4 (31.5) ± 11.8 [16.7–96.5] | 31.8 (29.0) ± 12.7 [7.0–117.3] |
| CSF F2-IsoPs (pg/ml) | 28.8 (28) ± 8.6 [11–60] | 30.6 (31) ± 9.5 [11–65] | 29.7 (28) ± 9.1 [11–65] |
| Immediate Recall | 13.5 (14) ± 4.1 [4–23] | 12.9 (13) ± 3.5 [4–21] | 13.2 (13) ± 3.8 [4–23] |
| Delayed Recall | 12.6 (12) ± 4.1 [3–23] | 11.6 (12) ± 3.8 [1–23] | 12.1 (12) ± 4.0 [1–23] |
| Trail Making Test A (sec) | 25.8 (24) ± 11.1 [12–100] | 29.3 (27.5) ± 10.7 [11–81]c | 27.4 (25) ± 11.0 [11–100] |
| Trail Making Test B (sec) | 62.0 (55) ± 29.6 [24–212] | 73.8 (64) ± 32.0 [18–240]c | 67.7 (59) ± 31.2 [18–240] |
| Category Fluency (Animal) | 23.2 (23) ± 6.0 [11–37] | 22.6 (22) ± 5.6 [11–40]c | 22.9 (23) ± 5.8 [11–40] |
| Composite Scoreb | 0.13 (0.23) ± 0.74 [−1.72–1.77] | −0.14 (−0.08) ± 0.71 [−1.88–1.55]c | 0 (0.04) ± 0.74 [−1.88–1.77] |
Mean (Median) ± SD [Range] for continuous variables; n (%) for categorical variables.
Four of these subjects had one or more clinical follow-up visits but did not take the tests at these visits.
Composite Score: The average of the z-score for Immediate Recall, the z-score for Delayed Recall, −1 times the z-score for Trail Making Test A, −1 times the z score for Trail Making Test B, and the z-score for Category Fluency.
Missing Values: 6 subjects were missing longitudinal data for Trail Making Test A, Trail Making Test B, and Category Fluency (and thus missing longitudinal data for the Composite Score) in the longitudinal analysis, thus their baseline scores are excluded from the statistical summaries in this column for these cognitive tests.
Linear regression models were used for assessing cross-sectional relationships between CSF biomarker concentrations and coincident cognitive test performance. Raw scores were used for each test, except log10-transformed times for Trails A and Trails B to remove skewness. In addition, we created a composite test score constructed by computing z-scores for each of the five cognitive tests based on the baseline mean and standard deviation (z-scores for log10-transformed Tails A and Trails B were multiplied by -1) and then averaging them. Regression models consisted of cognitive test performance as the dependent variable and baseline CSF biomarker concentrations as predictors, along with the covariates baseline age, gender, education, and APOE ε4 status (no ε4 alleles versus at least one ε4 allele).
To assess association of baseline CSF biomarker concentrations with subsequent longitudinal changes of cognitive test performance, we used linear mixed-effects models,24 with cognitive test performance as the dependent variable and time since baseline and baseline CSF biomarker concentrations as predictors, along with the covariates baseline age, gender, education, and APOE ε4 status. The associations of baseline age and CSF biomarker concentrations with change in cognitive performance were tested by including time-by-baseline age and time-by-biomarker concentration interaction terms in the models. Marginal R2s for the linear mixed-effects models were computed according to Nakagawa and Schielzeth.25
We performed several kinds of sensitivity analyses. For both the cross-sectional and longitudinal analyses, we included the ratio of tau to Aβ42 as a predictor (per Kronmal,26 both tau and Aβ42 were kept in the models as main effects as well); and we also looked at models where Aβ42 was dichotomized as ≤ 192 pg/ml versus > 192 pg/ml, based on the cutoff suggested by Shaw et al.27 Because the relationship between CSF biomarkers and cognitive function may differ between older and younger people, we restricted all analyses to those aged 60 and above. To understand the relationship between cognition and CSF biomarkers that is related to normal aging, we looked at models where we excluded subjects who subsequently converted to MCI, AD, or other dementias. For the longitudinal analyses, we also used two-stage regression (least squares slope for each test in each individual over time, then weighted regression model with slope as response variable and baseline test score included as a predictor variable),28 where weights were based on subjects having different numbers of follow-up visits at different times after baseline. Finally, to understand the role of APOE genotype in cognitive decline, we examined APOE ε4 gene dose-effect in the cross-sectional analyses by coding APOE ε4 genotype as follows: ε2/ ε2 = −2, ε2/ ε3 = −1, ε2/ ε4 = 0, ε3/ ε3 = 0, ε3/ ε4 = 1, ε4/ ε4 = 2. In the longitudinal analysis, we expanded the linear mixed effects model to allow for interaction effects between APOE ε4 status and biomarkers.
Correction for multiple comparisons taking into account six separate cognitive outcomes (the five tests plus the composite test) was based on the method of Holm.29 Statistical analyses were performed using R version 3.0.1;30 linear mixed effects models were fit using the R package nlme31 or lme4.32
RESULTS
Baseline Demographics
Table 1 presents demographics, baseline CSF biomarker levels, and cognitive test scores for the 315 eligible cognitively normal subjects in our cross-sectional analysis. Of these, 157 did not have a follow-up visit (Table 1, column 2), while 158 had follow-up visits and were subjects in our longitudinal analysis (Table 1, column 3). Subjects in the longitudinal analysis had an average length of follow-up of 4.4 years and were about 10 years older on average than those in the cross-sectional analysis. There were 4 subjects who completed only one test session but had one or more clinical follow-up visits. Of the 162 subjects with clinical follow-up, 14 (9%) subjects had converted to MCI, 7 (4%) to AD, 2 (1%) to Dementia with Lewy bodies or Parkinson’s disease dementia, and 4 (2%) to another type of cognitive impairment. eTable 1 presents baseline information stratified by final clinical diagnosis, and eFigure 1 shows baseline test score versus age, stratified by final clinical diagnosis. Subjects who converted to MCI, AD or other cognitive impairments were older at baseline and had slightly longer duration of follow-up than those who remained cognitively normal. As expected, subjects who converted to MCI or AD were more likely to be APOE ε4 carriers.
Cross-Sectional Analyses
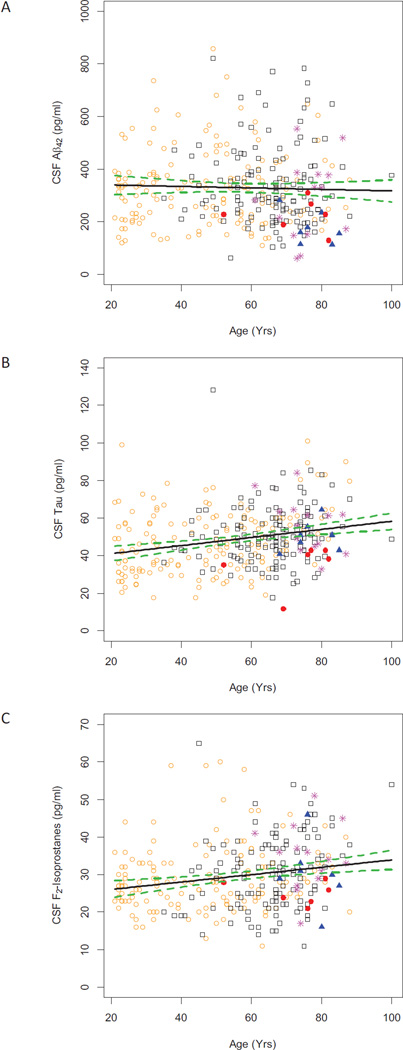
Figure 1 presents results for the three CSF biomarkers from our 315 normal subjects vs. age at baseline evaluation and stratified by final clinical diagnosis. We initially focused on associations between these baseline CSF biomarker concentrations and baseline cognitive abilities, including memory, in cognitively normal subjects. Table 2 shows the regression coefficients and p-values associated with demographic characteristics and baseline CSF biomarker levels for each cognitive test in multivariable regression models. Model 1 includes only age, gender, years of education, and presence of the APOE ε4 allele, whereas Model 2 includes the previous variables as well as concentration of CSF Aβ42, tau, and F2-IsoPs. Age was significantly (p < 0.05) associated with lower cognitive function for all cognitive tests and models, and education was associated with higher cognitive function except for Trails A. (Note that for Trails A and B, lower scores reflect better cognitive function.) CSF F2-IsoP concentration was associated with lower cognitive function for Trails A (p = 0.04), Trails B (p = 0.007), and the composite score (p = 0.02), and low Aβ42 concentrations were associated with lower cognitive function for Trails B (p = 0.048); however after adjusting for multiple comparisons based on six different cognitive measures, only the association between CSF F2-IsoPs and Trails B remained significant (Holm-corrected p = 0.042; Figure 2 shows unadjusted Trails B scores versus F2-IsoPs, along with a fitted line and 95% confidence intervals for the line). Adding biomarkers to Model 1 did not noticeably improve the adjusted R-squared for any of the cognitive tests. Phosphorylated tau was highly correlated with total tau but did not provide additional predictability in any model.
Figure 1. Cross-sectional relationships between concentration of CSF Aβ42 (Panel A), tau (Panel B), and F2-IsoPs (Panel C) versus subject age at baseline for 315 cognitively normal subjects.
Plotting symbols: orange open circle ( ) = subjects with no clinical follow-up (n=153), black open square (
) = subjects with no clinical follow-up (n=153), black open square ( ) = subjects who remained cognitively normal (n=135), magenta asterisk (
) = subjects who remained cognitively normal (n=135), magenta asterisk ( ) = converted to MCI (n=14), blue solid triangle (
) = converted to MCI (n=14), blue solid triangle ( ) = converted to AD (n=7), red solid circle (
) = converted to AD (n=7), red solid circle ( ) = converted to other dementias or cognitive impairments (n=6). Solid line is fitted least squares line unadjusted for any covariates; green dashed lines are 95% confidence bounds for the line. A) Aβ42 slope = −0.3, 95% CI = [−1.2, 0.6], r2 = 0.001, p = 0.54. B) tau slope = 0.2, 95% CI = [0.1, 0.3], r2 = 0.06, p < 0.001. C) F2-IsoPs slope = 0.1, 95% CI = [0.04, 0.15], r2 = 0.04, p < 0.001.
) = converted to other dementias or cognitive impairments (n=6). Solid line is fitted least squares line unadjusted for any covariates; green dashed lines are 95% confidence bounds for the line. A) Aβ42 slope = −0.3, 95% CI = [−1.2, 0.6], r2 = 0.001, p = 0.54. B) tau slope = 0.2, 95% CI = [0.1, 0.3], r2 = 0.06, p < 0.001. C) F2-IsoPs slope = 0.1, 95% CI = [0.04, 0.15], r2 = 0.04, p < 0.001.
Table 2. Cross-Sectional Analyses.
Relationships between baseline cognitive test scores and linear model covariates in 315 cognitively normal subjects with baseline neuropsychological testing and biomarker values.
| Cognitive Test | Adjusted R2 |
Age (Years) |
Gender (0 = Male) (1 = Female) |
Education (Years) |
APOE (0 = no ε4) (1 = any ε4) |
Aβ42 (pg/ml) |
Tau (pg/ml) |
F2-IsoPs (pg/ml) |
|---|---|---|---|---|---|---|---|---|
| Immediate Recall | ||||||||
| Model 1 | 0.07 | −0.03 (0.01); 0.004 | 0.2( 0.4); 0.61 | 0.3 (0.08); <0.001 | 0.6 (0.4); 0.17 | |||
| Model 2 | 0.07 | −0.03 (0.01); 0.01 | 0.3 (0.4); 0.55 | 0.3 (0.08); <0.001 | 0.6 (0.4); 0.15 | 9e-04 (0.002); 0.60 | −0.002 (0.02);0.90 | −0.03 (0.02); 0.21 |
| Delayed Recall | ||||||||
| Model 1 | 0.11 | −0.05 (0.01); <0.001 | 0.3 (0.4); 0.45 | 0.3 (0.08); <0.001 | 0.6 (0.4); 0.16 | |||
| Model 2 | 0.11 | −0.05 (0.01); < 0.001 | 0.4 (0.4); 0.36 | 0.3 (0.09); <0.001 | 0.7 (0.5); 0.13 | 0.001 (0.002); 0.49 | 0.002 (0.02); 0.91 | −0.04 (0.03); 0.10 |
| Log10(Trail A) | ||||||||
| Model 1 | 0.24 | 0.004 (0.0004); <0.001 | −0.03 (0.02); 0.10 | −0.005 (0.003); 0.07 | −0.004 (0.02); 0.78 | |||
| Model 2 | 0.25 | 0.004 (0.0004); <0.001 | −0.03 (0.02); 0.07 | −0.004 (0.003); 0.18 | −0.008 (0.02); 0.64 | −6e-05 ( 6e-05); 0.32 | 0.0002 (0.0006); 0.76 | 0.002 (0.0009); 0.04 |
| Log10(Trail B) | ||||||||
| Model 1 | 0.30 | 0.005 (0.0005); <0.001 | −0.03 (0.02); 0.07 | −0.02 (0.003); <0.001 | 0.03 (0.02); 0.12 | |||
| Model 2 | 0.31 | 0.004 (0.0005); <0.001 | −0.03 (0.02); 0.05 | −0.01 (0.003); <0.001 | 0.02 (0.02); 0.24 | −1e-04 (6e-05); 0.05 | 5e-04 (6e-04); 0.41 | 0.003 (0.001); 0.007 |
| Category Fluency | ||||||||
| Model 1 | 0.16 | −0.09 (0.02); <0.001 | 0.2 (0.6); 0.76 | 0.6 (0.1); <0.001 | 0.4 (0.6); 0.53 | |||
| Model 2 | 0.16 | −0.08 (0.02); <0.001 | 0.1 (0.6); 0.85 | 0.6 (0.1); <0.001 | 0.6 (0.6); 0.35 | 0.004 (0.002); 0.07 | −0.04 (0.02); 0.10 | −0.02 (0.04); 0.63 |
| Composite Score | ||||||||
| Model 1 | 0.29 | −0.02 (0.002); <0.001 | 0.1 (0.07); 0.15 | 0.08 (0.01); <0.001 | 0.05 (0.07); 0.49 | |||
| Model 2 | 0.30 | −0.02 (0.002); <0.001 | 0.1 (0.07); 0.12 | 0.07 (0.01); <0.001 | 0.08 (0.07); 0.31 | 0.0005 (0.0003); 0.09 | 0.002 (0.003); 0.42 | −0.01 (0.004); 0.02 |
Data are: Linear regression model adjusted R2; coefficient (SE); p-value. P-values less than 0.05 are colored blue and p-values less than 0.01 are colored green.
Model 1 = Age, Gender, Education, APOE ε4 (no ε4 alleles vs. at least one ε4 allele).
Model 2 = Model 1 plus baseline CSF Aβ42 (pg/ml), total tau (pg/ml), and F2-IsoPs (pg/ml).
For Immediate Recall, Delayed Recall, and Animal Category Fluency, a higher score reflects better cognitive function; for Trail Making Test Parts A and B, a lower score reflects better cognitive function.
Composite Score is the average of the z-score for Immediate Recall, the z-score for Delayed Recall, −1 times the z-score for Trail Making Test A, −1 times the z-score for Trail
Making Test B, and the z-score for Animal Category Fluency.
Figure 2. Cross-sectional relationship between Trail Making Test, Part B scores and concentration of CSF F2-IsoPs at baseline for 315 cognitively normal subjects.
Plotting symbols: orange open circle ( ) = subjects with no clinical follow-up (n=153), black open square (
) = subjects with no clinical follow-up (n=153), black open square ( ) = subjects who remained cognitively normal (n=135), magenta asterisk (
) = subjects who remained cognitively normal (n=135), magenta asterisk ( ) = converted to MCI (n=14), blue solid triangle (
) = converted to MCI (n=14), blue solid triangle ( ) = converted to AD (n=7), red solid circle (
) = converted to AD (n=7), red solid circle ( ) = converted to other dementias or cognitive impairments (n=6). Solid line is fitted least squares line for log10 score unadjusted for any covariates; green dashed lines are 95% confidence bounds for the line. Slope = 0.005, 95% CI = [0.003, 0.007], r2 = 0.06, p < 0.0001.
) = converted to other dementias or cognitive impairments (n=6). Solid line is fitted least squares line for log10 score unadjusted for any covariates; green dashed lines are 95% confidence bounds for the line. Slope = 0.005, 95% CI = [0.003, 0.007], r2 = 0.06, p < 0.0001.
Longitudinal Analyses
Our next analysis focused on association between baseline CSF biomarker concentrations and longitudinal change in cognitive performance. Table 3 shows the regression coefficients and p-values associated with baseline age and CSF biomarker levels for a 10-year change (i.e., slope coefficients are multiplied by 10) in each cognitive test based on linear mixed-effects regression models. Model 1 includes only baseline age, gender, years of education, presence of the APOE ε4 allele, time (decades since baseline), and a time by baseline age interaction term, whereas Model 2 includes the previous variables as well as concentration of CSF Aβ42, tau, and F2-IsoPs at baseline, and time by biomarker interaction terms. Baseline age was associated with declining cognitive function for all tests except Model 2 of Trails A, Model 2 of Trails B, and Category Fluency. Low baseline CSF Aβ42 concentration was associated with declining cognitive function for Immediate Recall (p = 0.004), Delayed Recall (p = 0.001), and the composite score (p = 0.007), and all of these associations remained significant after adjusting for multiple comparisons (Holm-corrected p-values: p = 0.024, p = 0.006, and p = 0.042, respectively). Also, baseline CSF tau was associated with declining cognitive function for Immediate and Delayed Recall (p = 0.03 and p = 0.04, respectively), but these associations did not remain significant after adjusting for multiple comparisons. eFigure 2 shows spaghetti plots of test score versus time, stratified by final clinical diagnosis, for all five tests. eFigure 3 shows spaghetti plots of test score versus time, stratified by baseline Aβ42 quartiles, for Immediate and Delayed Recall.
Table 3. Longitudinal Analyses Based on Linear Mixed Effects Models.
Relationships between 10-year change in cognitive test scores and linear mixed effects model covariates in 158 cognitively normal subjects with baseline neuropsychological testing and biomarker values and at least one follow-up visit.
| Cognitive Test | R2 | Time× Baseline Age |
Time× Baseline Aβ42 |
Time× Baseline Tau |
Time× Baseline F2-IsoPs |
|---|---|---|---|---|---|
| Immediate Recall | |||||
| Model 1 | 0.22 | −0.2 (0.06); <0.001 | |||
| Model 2 | 0.23 | −0.2 (0.06); 0.01 | 0.01 (0.004); 0.004 | −0.08 (0.04); 0.03 | 0.05 (0.06); 0.43 |
| Delayed Recall | |||||
| Model 1 | 0.21 | −0.2 (0.07); <0.001 | |||
| Model 2 | 0.22 | −0.2 (0.07); 0.01 | 0.02 (0.005); 0.001 | −0.08 (0.04); 0.04 | −0.03 (0.07); 0.69 |
| Log10(Trail A)* | |||||
| Model 1 | 0.26 | 0.005 (0.002); 0.04 | |||
| Model 2 | 0.31 | 0.004 (0.002); 0.10 | −0.0002 (0.0002) 0.17 | −0.001 (0.002); 0.52 | −0.0007 (0.003); 0.79 |
| Log10(Trail B)* | |||||
| Model 1 | 0.28 | 0.006 (0.003); 0.02 | |||
| Model 2 | 0.31 | 0.005 (0.003); 0.06 | −0.0001 (0.0002); 0.57 | 0.0008 (0.002); 0.65 | 0.002 (0.003); 0.50 |
| Category Fluency* | |||||
| Model 1 | 0.21 | −0.04 (0.08); 0.66 | |||
| Model 2 | 0.23 | −0.01 (0.09); 0.88 | 0.003 (0.006); 0.69 | 0.03 (0.05); 0.64 | −0.08 (0.09); 0.37 |
| Composite Score | |||||
| Model 1 | 0.37 | −0.04 (0.01); <0.001 | |||
| Model 2 | 0.40 | −0.03 (0.01); 0.004 | 0.002 (0.0008); 0.007 | −0.01 (0.007); 0.05 | −0.006 (0.01); 0.56 |
Data are: Linear mixed effects model marginal R2; coefficient (SE); p-value. P-values less than 0.05 are colored blue and p-values less than 0.01 are colored green.
Model 1 = Cognitive test score as a function of baseline age, gender, education, APOE ε4 (no ε4 alleles vs. any ε4 alleles), time (decades since baseline), and time×baseline age.
Model 2 = Model 1 plus baseline CSF Aβ42 (pg/ml), total tau (pg/ml), F2-IsoPs (pg/ml), time×Aβ42, time×tau, and time×F2-IsoPs.
6 subjects missing longitudinal data for Trail Making Test Part A, Trail Making Test Part B, and Category Fluency.
For Immediate Recall, Delayed Recall, and Category Fluency, a higher score reflects better cognitive function; for Trail Making Test Parts A and B, a lower score reflects better cognitive function.
Composite Score is the average of the z-score for Immediate Recall, the z-score for Delayed Recall, −1 times the z-score for Trail Making Test A, −1 times the z-score for Trail Making Test B, and the z-score for Animal Category Fluency.
Sensitivity Analyses
Results were similar when the tau/Aβ42 ratio was added to the cross-sectional analysis models (i.e., the ratio was not significant and did not affect the other associations), except the Trails B association with low Aβ42 became nominally non-significant (p = 0.34). When the tau/Aβ42 ratio was added to the longitudinal analysis models, it was not significant for any of the cognitive tests. When we modeled CSF Aβ42 level as a dichotomous variable (low versus high) instead of as a continuous variable (Model 2 compared to Model 3 in eTables 2 and 3), we observed in cross-section a significant association of low Aβ42 with low composite score and with poor performance in Trails A (eTable 2). For the longitudinal analyses, the association between low baseline CSF Aβ42 concentration and declining cognitive function for Immediate Recall became non-significant (eTable 3). When we restricted analyses to subjects aged 60 and older, the cross-sectional associations of CSF F2-IsoP concentration with Trails A, Trails B, and the composite score were all attenuated to non-significance (eTable 2). The relationship between baseline biomarkers and cognitive trajectories essentially remained unchanged in this restricted group of older subjects (eTable 3). When we omitted subjects who converted to MCI, AD or other dementias or cognitive impairments, our findings did not change in either the cross-sectional or longitudinal analysis (Model 2 versus Model 4 in eTables 2 and 3). Results were similar for the longitudinal analysis based on using two-stage regression with weights that account for different numbers of follow-up visits at different times after baseline compared to the results based on linear mixed effect models; however the associations between CSF tau and Immediate and Delayed Recall were no longer nominally significant.
Finally, regarding the role of APOE genotype, coding APOE genotype as a dose instead of APOE ε4 status did not change the results for the cross-sectional analyses (eTable 4). For the longitudinal analyses, when we expanded Model 2 (see Table 3) to allow for interaction effects between APOE ε4 status and biomarkers, none of these effects was significant except for Trails A, for which there was a significant interaction between APOE ε4 status and Aβ42 concentration. Low Aβ42 concentration was associated with worse 10-year change in Trails A for APOE ε4+ subjects compared to APOE ε4-subjects (difference in slopes for 10-year change in log10-transformed time (sec) for every 100 ng/ml decrease in Aβ42 = 0.1, standard deviation of difference in slopes = 0.05, p = 0.01). However, this interaction effect did not remain significant after controlling for multiple comparisons.
DISCUSSION
Cognitive decline occurs in older adults, even in those who do not cross clinical thresholds to MCI or dementia, or though stages like Clinical Dementia Rating.33 Several processes may contribute to age-related cognitive decline, including genetics, environment, and latent disease. Using the resources of the large research CSF repository built among our collaborating AD Centers, here we tested the hypothesis that age-related cognitive decline could be accounted for in part by latent AD or oxidative injury to brain as detected by CSF biomarkers. We tested our hypothesis in a cross sectional analysis of 315 adults who underwent baseline lumbar puncture and neuropsychological testing, and a longitudinal analysis of 158 of these same persons who had follow-up neuropsychological testing.
It is important to stress that our study focused on decline in cognitive abilities among cognitively normal adults, not on progression to cognitive impairment or dementia as has been investigated in previous studies using biomarkers for AD.15,34–36 Our cross sectional analysis of 315 carefully characterized individuals from across the human adult life span showed that age, gender, education, and APOE ε4 status accounted for a small percentage of the variability in cognitive performance on tests of immediate and delayed recall, executive function, and verbal fluency. Indeed, these four variables were strongest in accounting for variability in our measure of executive function (adjusted R2 = 0.31) and weakest for our measure of immediate recall (adjusted R2 = 0.08). What associations that did exist were driven largely by age and education without significant contribution by gender or APOE. Subsequent addition of the three CSF biomarkers to the model did not alter the adjusted R2 for any of the neuropsychological test results; however, after controlling for multiple endpoints, we observed a novel finding that CSF F2-IsoP concentration was significantly associated with tests of executive function in middle-aged and older adults.
We speculate that this association may derive from CSF F2-IsoPs being a more sensitive but less specific maker of age-related brain injury, and baseline executive function similarly being a more sensitive but less specific index of age-related cognitive decline. Interestingly, this association was diminished when analysis was restricted to those 60 years or older, perhaps indicating the importance of mid-life free radical injury to brain similar, and perhaps even mechanistically related, to the contribution of mid-life hypertension. The influence of age may also explain in part why no significant association was observed between CSF F2-IsoPs and longitudinal executive function since the longitudinal group was older on average.
We did not observe an association between CSF F2-IsoPs and APOE ε4 in cognitively normal adults as suggested by others.37 Neither baseline CSF Aβ42 nor tau concentration was significantly associated with any of the five cognitive test results in cognitively normal adults, similar to other smaller cross-sectional studies of cognitive function in older individuals.38,39
Our analysis of longitudinal change in neuropsychological test performance in a subset of 158 individuals using linear mixed effects models showed that only a minority of change in cognitive test performance was explained by models that used age, gender, education, APOE ε4 status, and a time by baseline age interaction term (marginal R2 ranged from 0.21 to 0.31). Addition of CSF biomarkers to these models increased the marginal R2 very little; however, after controlling for multiple endpoints, low CSF Aβ42 was significantly associated with cognitive decline in measures of both immediate and delayed recall. These findings are similar to results of another study of 165 older adults with normal cognition with or without subjective cognitive impairment.40
Overall, these results indicate that age, gender, educational level, and inheritance of APOE ε4 accounted for only a minority of variability on the five neuropsychological tests used. These results highlight the need for deeper understanding of the genetic factors that influence age-related cognitive decline and the likely important contributions of systemic disease and environmental factors, such as nutrition, drug and alcohol abuse, and traumatic brain injury, which were not captured in our study. Cognitive performance in aging may also be influenced by lifestyle factors such as cognitive stimulation, physical exercise, and socialization, which may contribute to cognitive reserve. To the extent that the variables included in our study were significantly related to cognitive performance, age and education were the dominant variables in cross section, while baseline test performance and age were the dominant variables longitudinally. One interpretation of these results is that they reinforce the cognitive reserve hypothesis, similar to recent neuroimaging studies.41
CSF biomarkers contributed little to the R2 measures of goodness-of-fit when included along with age, gender, education, and APOE ε4 status. Nevertheless, there were three significant associations that withstood correction for multiple comparisons. These were an association between CSF F2-IsoP concentration and poorer performance on executive function in cross section, and an association between low CSF Aβ42 concentration and longitudinal decline in immediate and delayed recall. The direction of these associations deserves some comment. Our measure of executive function, Trails B, is measured in seconds (up to 300 maximum) needed to complete the task; thus, a higher value indicates poorer performance. In our cross sectional analysis, higher levels of oxidative injury to brain, as measured by CSF F2-IsoPs, were associated with poorer executive function, as measured by longer time needed for Trails B (Figure 2). Interpretation of the association between low CSF Aβ42 and decline of immediate and delayed recall is more complicated. Outside of autosomal dominant forms of AD,42 current findings indicate that CSF Aβ42 concentration does not change much with aging until parenchymal Aβ42 deposition begins and ultimately progresses to clinical expression as MCI and dementia.8 Our results showed a negative correlation between decline in immediate and delayed recall and CSF Aβ42 level, suggesting that this association reflects the phase of declining CSF Aβ42 concentration and parenchymal deposition with increased risk for near-term conversion to MCI or AD dementia.15,43
Age-related cognitive decline is a complex trait that potentially derives from the confluence of multiple processes, including aging, environmental factors, inherited vulnerabilities, and latent disease. Strengths of our work are that it is relatively large for a CSF biomarker-based study, it used standardized data collection and central laboratory analysis, and subjects were relatively healthy and selected to exclude factors that obviously may have a major effect on cognition. Shortcomings of our work are that our neuropsychological test battery was relatively limited, and we may have limited variance in cognitive function because of the general healthiness of our group. Lack of statistically significant associations between CSF biomarkers and baseline test scores and decline in test scores for most of the cognitive tests could be due to issues related to statistical power, such as limited sample size especially in the longitudinal samples, relatively short duration of follow-up, and relatively large within-subject variability in the neuropsychological tests used. Future studies should consider addressing these limitations. Regardless of these concerns, the fact that even when CSF biomarkers showed statistical significance they contributed little to the R2 measures of goodness-of-fit suggests the importance of other underlying factors for cognitive health not captured by these CSF biomarkers.
With these strengths and weaknesses in mind, our analysis showed that CSF biomarkers of free radical injury and early changes of AD accounted for a small amount of variability in specific cognitive domains, and that age, education, and previous cognitive performance were the predominant predictors of cognitive function in cross section or over time. Importantly, our results also suggest that other factors not accounted for here contribute to the majority of variance in cognitive function in older cognitively normal individuals. These factors may include other genetic factors, systemic disease, environmental factors, or white matter dysfunction as suggested by some neuroimaging studies.44–46
Supplementary Material
Acknowledgements
This work was supported by NIH grants AG023185, AG031892, AG05131, AG05136, AG08017, AG033693, U01-NS082137, the Department of Veterans Affairs, the Oregon Clinical and Translational Research Institute (UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS) at the NIH, the Jane and Lee Seidman Fund, and the Nancy and Buster Alvord Endowment. We thank Dr. Kathleen Montine for editorial assistance.
None of the funding organizations or sponsors were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Montine had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures:
Dr. Leverenz serves as a consultant for Boehringer Ingelheim, Navidea Biopharmaceuticals, and Piramal Health Care.
Dr. Galasko serves as Editor of Alzheimer’s Disease Research and Treatment; serves on Data Safety Monitoring Boards for Elan, Janssen and Balance Pharmaceuticals; is a consultant for Elan Pharmaceuticals, Inc., and Genentech, Inc. He receives research support from the NIH, the Michael J Fox Foundation and the Alzheimer’s Drug Discovery Foundation.
Dr. Montine is funded by grants from the NIH and receives personal compensation in the form of honoraria from invited scientific presentations to universities and professional societies not exceeding $5,000 per year.
REFERENCES
- 1.Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997 Aug;87(1):25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- 2.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004 Sep 30;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE. Cognition and neuropathology in aging: multidimensional perspectives from the Rush Religious Orders Study and Rush Memory And Aging Project. Curr Alzheimer Res. 2011 Jun;8(4):336–340. doi: 10.2174/156720511795745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res. 2012 Jul;9(6):664–672. doi: 10.2174/156720512801322618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montine TJ, Sonnen JA, Montine KS, Crane PK, Larson EB. Adult Changes in Thought study: dementia is an individually varying convergent syndrome with prevalent clinically silent diseases that may be modified by some commonly used therapeutics. Curr Alzheimer Res. 2012 Jul;9(6):718–723. doi: 10.2174/156720512801322555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol. 2009;21(1):117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013 May 7;80(19):1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012 Aug 30;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo RY, Jagust WJ. Alzheimer's Disease Neuroimaging Initiative. Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012 Sep 25;79(13):1349–1355. doi: 10.1212/WNL.0b013e31826c1b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longstreth WT, Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009 Jul-Sep;23(3):291–294. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnen JA, Breitner JC, Lovell MA, Markesbery WR, Quinn JF, Montine TJ. Free radical-mediated damage to brain in Alzheimer's disease and its transgenic mouse models. Free Radic Biol Med. 2008 Aug 1;45(3):219–230. doi: 10.1016/j.freeradbiomed.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayer-Carter JL, Green PS, Montine TJ, et al. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011 Jun;68(6):743–752. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brys M, Pirraglia E, Rich K, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009 May;30(5):682–690. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seet RC, Lee CY, Chan BP, et al. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke. 2011 Aug;42(8):2326–2329. doi: 10.1161/STROKEAHA.111.618835. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Sokal I, Quinn JF, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007 Aug 14;69(7):631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 16.Peskind ER, Riekse R, Quinn JF, et al. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord. 2005 Oct-Dec;19(4):220–225. doi: 10.1097/01.wad.0000194014.43575.fd. [DOI] [PubMed] [Google Scholar]
- 17.Dumurgier J, Vercruysse O, Paquet C, et al. Intersite variability of CSF Alzheimer's disease biomarkers in clinical setting. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013 Jul;9(4):406–413. doi: 10.1016/j.jalz.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Mattsson N, Andreasson U, Persson S, et al. The Alzheimer's Association external quality control program for cerebrospinal fluid biomarkers. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 Jul;7(4):386–395. e386. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milatovic D, VanRollins M, Li K, Montine KS, Montine TJ. Suppression of murine cerebral F2-isoprostanes and F4-neuroprostanes from excitotoxicity and innate immune response in vivo by alpha- or gamma-tocopherol. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Nov 15;827(1):88–93. doi: 10.1016/j.jchromb.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990 Mar;31(3):545–548. [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale-Revised. New York: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 22.Gomez RG, White DA. Using verbal fluency to detect very mild dementia of the Alzheimer type. Arch Clin Neuropsychol. 2006 Dec;21(8):771–775. doi: 10.1016/j.acn.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Reitan RWD. The Halstead-Reitan neuropsychological test battery. Tuscon: Neuropsychology Press; 1985. [Google Scholar]
- 24.Pinheiro J, Bates D. Mixed-effects and models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 25.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution. 2013;4(2):133–142. [Google Scholar]
- 26.Kronmal RA. Spurious correlation and the faccaly of the ratio standard revisited. J R Statist Soc A. 1993;156(3):379–392. [Google Scholar]
- 27.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of neurology. 2009 Apr;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milliken JK, Edland SD. Mixed effect models of longitudinal Alzheimer's disease data: a cautionary note. Stat Med. 2000 Jun 15–30;19(11–12):1617–1629. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1617::aid-sim450>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Holm S. A simple sequential rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 30.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 31.Pinheiro J, DebRoy S, Sarkar D R Core Team. nlme: Linear and Nonlinear Mixed Effects Models, R package version 3.1-104. 2012 [Google Scholar]
- 32.Bates D, Maechler M, Bolker B, Walker S. Lme4: linear mixed-effects models using Eigen and S4. R package version 1.0-5. 2013 http://CRANRprojectorg/package=lme4.
- 33.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013 Oct;12(10):957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolstad S, Berg AI, Bjerke M, Johansson B, Zetterberg H, Wallin A. Cerebrospinal fluid biomarkers mirror rate of cognitive decline. J Alzheimers Dis. 2013 Jan 1;34(4):949–956. doi: 10.3233/JAD-121960. [DOI] [PubMed] [Google Scholar]
- 35.Roe CM, Fagan AM, Grant EA, et al. Cerebrospinal fluid biomarkers, education, brain volume, and future cognition. Arch Neurol. 2011 Sep;68(9):1145–1151. doi: 10.1001/archneurol.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007 Mar;64(3):343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 37.Duits FH, Kester MI, Scheffer PG, et al. Increase in Cerebrospinal Fluid F2-Isoprostanes is Related to Cognitive Decline in APOE epsilon4 Carriers. J Alzheimers Dis. 2013 Jan 1;36(3):563–570. doi: 10.3233/JAD-122227. [DOI] [PubMed] [Google Scholar]
- 38.Ewers M, Insel P, Jagust WJ, et al. CSF biomarker and PIB-PET-derived beta-amyloid signature predicts metabolic, gray matter, and cognitive changes in nondemented subjects. Cereb Cortex. 2012 Sep;22(9):1993–2004. doi: 10.1093/cercor/bhr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol. 2010 Feb;67(2):217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- 40.Rolstad S, Berg AI, Bjerke M, et al. Amyloid-beta(4)(2) is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. J Alzheimers Dis. 2011;26(1):135–142. doi: 10.3233/JAD-2011-110038. [DOI] [PubMed] [Google Scholar]
- 41.Ewers M, Insel PS, Stern Y, Weiner MW. Alzheimer's Disease Neuroimaging Initiative. Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology. 2013 Mar 26;80(13):1194–1201. doi: 10.1212/WNL.0b013e31828970c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012 Dec;11(12):1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vos SJ, van Rossum IA, Verhey F, et al. Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology. 2013 Mar 19;80(12):1124–1132. doi: 10.1212/WNL.0b013e318288690c. [DOI] [PubMed] [Google Scholar]
- 44.Ryberg C, Rostrup E, Paulson OB, et al. Corpus callosum atrophy as a predictor of age-related cognitive and motor impairment: a 3-year follow-up of the LADIS study cohort. J Neurol Sci. 2011 Aug 15;307(1–2):100–105. doi: 10.1016/j.jns.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Verdelho A, Madureira S, Moleiro C, et al. White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study. Neurology. 2010 Jul 13;75(2):160–167. doi: 10.1212/WNL.0b013e3181e7ca05. [DOI] [PubMed] [Google Scholar]
- 46.Haight TJ, Landau SM, Carmichael O, Schwarz C, Decarli C, Jagust WJ. Dissociable Effects of Alzheimer Disease and White Matter Hyperintensities on Brain Metabolism. JAMA Neurol. Jun 17;2013:1–8. doi: 10.1001/jamaneurol.2013.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.