Abstract
Chronic stress may impose a vulnerability to develop maladaptive fear-related behaviors after a traumatic event. Whereas previous work found that chronic stress impairs the acquisition and recall of extinguished fear, it is unknown how chronic stress impacts nonassociative fear, such as in the absence of the conditioned stimulus (CS) or in a novel context. Male rats were subjected to chronic stress (STR; wire mesh restraint 6h/d/21d) or undisturbed (CON), then tested on fear acquisition (3 tone-footshock pairings), and two extinction sessions (15 tones/session) within the same context. Then each group was tested (6 tones) in the same context (SAME) or a novel context (NOVEL), and brains were processed for functional activation using Fos immunohistochemistry. Compared to CON, STR showed facilitated fear acquisition, resistance to CS extinction on the first extinction day, and robust recovery of fear responses on the second extinction day. STR also showed robust freezing to the context alone during the first extinction day compared to CON. When tested in the same or a novel context, STR exhibited higher freezing to context than did CON, suggesting that STR-induced fear was independent of context. In support of this, STR showed increased Fos-like expression in the basolateral amygdala and CA1 region of the hippocampus in both the SAME and NOVEL contexts. Increased Fos-like expression was also observed in the central amygdala in STR-NOVEL vs. CON-NOVEL. These data demonstrate that chronic stress enhances fear learning and impairs extinction, and affects nonassociative processes as demonstrated by enhanced fear in a novel context.
Post traumatic stress disorder (PTSD) is a debilitating and increasing public health problem, especially in combat-exposed populations. The lifetime prevalence of PTSD in the United States has been reported to be ~6% (Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012). PTSD develops in a subset of those experiencing a traumatic event (Breslau, Davis, Andreski, & Peterson, 1991), which suggests individual differences in the susceptibility and resilience to the development of the disorder after trauma exposure. One biological risk factor that has been identified for PTSD is reduced hippocampal volume (Gilbertson et al., 2002). Functional imaging studies in PTSD patients corroborate the reduced hippocampal volume findings, but also reveal compromised neural integrity within the hippocampus, reduced volume and responsivity within the medial prefrontal cortex (mPFC), as well as heightened amygdala responsivity (Shin et al., 2004; Shin, Rauch, & Pitman, 2006). Although these observed regional changes provide putative neural substrates for PTSD research, whether these alterations are contributing factors to, or outcomes from the disorder is unknown.
Animal models can help approach questions raised in clinical research in prospective designs under controllable conditions. Chronic stress leads to structural and behavioral alterations in rodents that parallel the changes observed in humans with PTSD. Within the amygdala, chronic stress causes dendritic hypertrophy (Padival, Blume, & Rosenkranz, 2013; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002; Vyas, Pillai, & Chattarji, 2004) and hyperexcitability (Rosenkranz, Venheim, & Padival, 2010). These stress-induced structural and physiological changes correspond to changes in emotionally-laden behavior including increases in anxiety-like behaviors (Vyas et al., 2002), facilitated acquisition of Pavlovian fear learning (Conrad, LeDoux, Magarinos, & McEwen, 1999; Hoffman, Armstrong, Hanna, & Conrad, 2010; Sandi, Merino, Cordero, Touyarot, & Venero, 2001), and resistance to fear extinction (Izquierdo, Wellman, & Holmes, 2006). In contrast to the amygdala, chronic stress causes dendritic retraction within the hippocampus (McLaughlin, Gomez, Baran, & Conrad, 2007) and mPFC (Brown, Henning, & Wellman, 2005; Cook & Wellman, 2004), changes that correspond to impaired hippocampal-dependent spatial learning and memory (Conrad, 2010; Hoffman et al., 2011) and compromised mPFC-dependent fear extinction retention (Baran, Armstrong, Niren, Hanna, & Conrad, 2009; Miracle, Brace, Huyck, Singler, & Wellman, 2006). Therefore, manipulating chronic stress in animal models allows for the induction of neural and behavioral changes that parallel outcomes that may lead to insights into factors that predispose individuals to develop PTSD symptomatology.
Pavlovian fear conditioning is a widely used model to study the neurobiology of fear and PTSD. In this paradigm, a neutral stimulus (such as a tone) serves as the conditioned stimulus (CS) and is paired with an aversive stimulus (such as a footshock)—the unconditioned stimulus (US). The animal learns the association between CS and US, and exhibits a conditioned response (CR, such as freezing) in the presence of the CS. Analogous to exposure therapy in humans, a common PTSD treatment approach, fear extinction occurs with repeated unreinforced CS presentations that result in a new, inhibitory memory trace, or a CS-no US association. One challenge with PTSD populations is the relapse of symptoms between extinction sessions, i.e., fear responding recovers between exposure therapy sessions and outside the therapy context (discussed in Hamner, Robert, & Frueh, 2004). Previous work has shown that chronic stress facilitates the spontaneous recovery of extinguished cue-elicited fear (Baran et al., 2009; Miracle et al., 2006), which is consistent with the fear responding recovery in PTSD cases. However, it is unknown how a history of chronic stress impacts nonassociative fear, such as in the absence of the CS or in a novel context (Kamprath & Wotjak, 2004), which is pertinent to the hyperarousal symptom cluster in PTSD patients (Yehuda & LeDoux, 2007). Furthermore, how the chronically stressed brain becomes engaged during the retrieval of a fear memory has been virtually unexplored. The current study aimed to investigate (1) how a history of chronic stress impacts both cued and context extinction following cued fear conditioning, (2) how chronic stress affects fear responding in a novel context following extinction, and (3) how chronic stress impacts functional activation of limbic structures involved in fear learning and extinction during retrieval of a cued fear memory.
Method
Subjects
Twenty male Sprague-Dawley rats weighing approximately 250–275g upon arrival (approx. 2 months old; Charles River Laboratories) were pair-housed in light and sound attenuating chambers (21–22°C) on a 12:12 reverse light cycle (lights off at 6am) according to conditions specified by the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Science, National Research Council, 1996). Food and water were available ad libitum except during restraint procedures (described below). All procedures occurred during the dark phase of the light cycle.
Prior to group assignments, all animals were tested in a single open field (OF) for anxiety-like behavior and locomotion profiles. OF testing was consistent with our previously published procedures (Huynh, Krigbaum, Hanna, & Conrad, 2011) and helped to distribute similar profiles across groups (Bellani, Luecken, & Conrad, 2006). Briefly, animals were placed at pseudorandom locations in an open square arena (110cm × 110cm, 30cm height) under low light intensity (200lx) and given 10min to explore then returned to their home cage. The OF arena was cleaned after each trial with pet deodorizer. OF behavior was recorded using an overhead video camera for offline scoring. Behavior was scored using 1) grid crossings, defined as the front two paws traversing a center or peripheral grid line, and 2) center grid time, recorded from the time the front two paws crossed the center grid until the front two paws exited the center grid.
Following OF testing, animals were divided into non-stressed control (CON) or chronically stressed groups (STR), n=10/group, and further subdivided into subgroups for the same and novel context testing condition (described below). All groups had similar locomotor and anxiety-like behavior profiles in OF (data not shown).
Stress Manipulation
Rats were chronically stressed via repeated wire mesh restraint (STR) or not (CON), and were weighed weekly. During the designated restraint period, STR rats were restrained in their home cages in wire mesh restrainers for 6h/d/21d. Wire mesh restrainers were 18cm circumference x 24cm long (wire mesh from Flynn and Enslow Inc, San Francisco, CA) with wire ends sealed with grip guard sealer (ACE Hardware). CON rats were handled briefly each day, with their food and water restricted while the STR rats were restrained to keep food and water access similar across treatment conditions.
Fear Conditioning: Apparatus
Rodent fear conditioning chambers (25 cm depth × 29 cm height × 26 cm width: Coulbourn Instruments, E10-18TC) were contained in sound-attenuating cubicles (Coulbourn, E10-23, white). A PC interface card (Coulbourn, PCI-3-KIT) adapted to a PC, a universal link (Coulbourn, L91-04S), and Graphic State software (v 3.03 GS3.03) controlled the stimulus presentation. A frequency generator (Coulbourn, E12-01) produced a tone (75 dB, ~3.0 kHz) through a speaker located in the side panel of the conditioning chamber. The shock (500 ms, 0.35mA, Coulbourn Animal Shock Generator, H13-15) was administered as a current, equally distributed through a metal grid floor (Coulbourn, E10-18RF). Behavior was videotaped for off-line analysis using a camera (Coulbourn, E27-91) mounted on the ceiling and a videocassette recorder. Infrared lights were located on the side panels of the chamber to denote the onset and offset of the tone, because no audio was recorded. A house light (Coulbourn, E11-01) was mounted in the side panel to illuminate the chamber at all times.
Two distinct chamber contexts (contexts A and B) were utilized for different fear conditioning testing phases. Context A consisted of white and silver paneled walls, a wire bar shock floor with a white catch pan, and was cleaned with 70% ethanol. Context B consisted of striped paneled walls, a smooth Plexiglas® floor insert and a dark catch pan, and was cleaned with an orange scented cleaner (method® clementine all purpose natural surface cleaner, methodhome.com).
Fear Conditioning: Procedure
During the last two days of restraint stress, all testing groups were transported by cart in their home cage into the fear conditioning testing room and left on the cart for 30min to acclimate to the transport process and room. One day following the end of the stress procedure, rats were acclimated to testing chambers (context A) for 10min with the house light on. The following day they were placed into the conditioning chamber (context A) and given three-30s tones that co-terminated with a 0.35mA, 500ms shock, (ITI 120-360s) and were then transported back to their home colony. Over the following two days (Extinction 1 and 2, respectively), rats were subjected to extinction testing in context A that consisted of 15 tone-alone trials (ITI 120-360s), which were averaged into blocks of 3 trials (5 blocks/extinction session). The next day, STR and CON groups were subdivided and tested (6 tone-alone trials) in either the same context (STR-SAME, CON-SAME) or a novel chamber context (context B; STR-NOVEL, CON-NOVEL), n=5/group. An experimental timeline is illustrated in Fig. 1A.
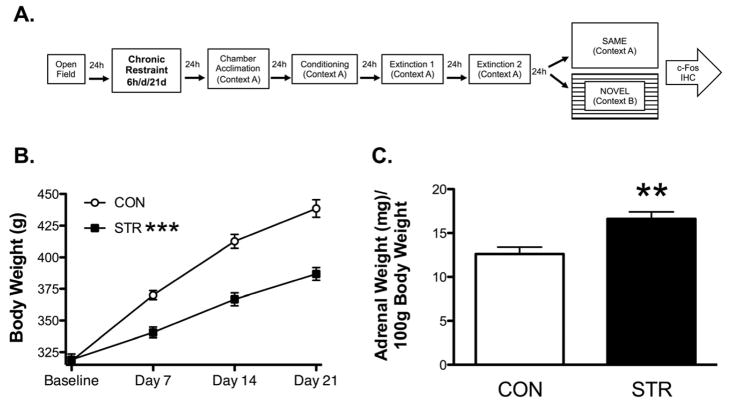
Figure 1. Timeline and Stressor Effectiveness.
(A) Experimental Timeline. All groups were tested on the open field then the following day were either subjected to chronic wire mesh restraint (6h/d/21d) or handled briefly. Following chronic restraint, rats were acclimated to the conditioning chamber (Context A), and the next day were trained with 3x tone CS-footshock pairings. The next two days (Extinction 1 and 2, respectively), both groups underwent cued fear extinction with 15 CS-alone trials in Context A. Then the next day, both groups were either tested (6x CS) in the same (Context A) or a novel context chamber (Context B) and were euthanized 90min later and brains were processed for Fos immunohistochemistry. (B). Chronic restraint stress attenuated body weight gain across 21d of wire mesh restraint. (C) Chronically stressed rats had significantly greater adrenal weight per 100g of body weight. Data are represented as mean ± SEM; n=9–10/group. ***p<0.001 vs. CON on Days 7, 14, and 21. **p<0.01 vs. CON.
Fear Conditioning: Dependent Variable
Behavior was videotaped for scoring later by observers blind to experimental conditions. For cued fear conditioning, the dependent variable measured was the number of seconds freezing during each 30s tone presentation, whereas contextual fear was defined as the number of seconds freezing during the 30s prior to tone onset. Freezing was defined as the absence of all movements except those associated with respiration (Baran, Armstrong, Niren, & Conrad, 2010; Baran et al., 2009; Blanchard & Blanchard, 1969; Conrad et al., 1999; Hoffman et al., 2010; Quirk, Russo, Barron, & Lebron, 2000). Data analyses were performed on raw data (number of seconds spent freezing during the 30s tone). However, for clarity of presentation, these same data will be represented as a percentage of freezing during the 30s tone, or respective context interval. One rat from the control group was eliminated from behavioral and Fos analyses due to almost no movement during behavioral testing, leaving n=9 for CON during extinction analyses. Another rat was excluded from the behavioral results because it was a statistical outlier, as calculated by a Grubb’s test, n=4 for CON-SAME data for freezing to CS and context.
Tissue Preparation and Fos immunohistochemistry
To capture peak Fos protein expression (Nestler, Barrot, & Self, 2001; Sonnenberg, Macgregor-Leon, Curran, & Morgan, 1989), all rats were overdosed with sodium pentobarbital (100mg/kg, i.p.) 90 minutes following placement in the testing chambers for SAME or NOVEL conditions (described above). Adrenal glands were collected and rats were then transcardially perfused with phosphate buffered saline (pH 7.4) and 4% paraformaldehyde (pH 7.4), and brains were removed and post-fixed in 4% paraformaldehyde and stored at 4°C overnight. Brains were then cryoprotected in 15% and 30% sucrose over 2d, and stored at 4°C until sectioning.
Brains were sectioned on a cryostat at 40μm within two weeks of perfusion. Multiple series of slides were taken at each level of section for separate cresyl violet and immunohistochemistry staining procedures. Sections mounted on slides were then stored at −80°C until tissue processing. One series of slides was stained with cresyl violet to identify and confirm subregions of interest for Fos analysis.
Another series of slides containing subregions of interest were processed for immunohistochemistry against Fos protein, which will be termed Fos-like immunoreactive (Fos-IR) labeling. Target sections were washed three times in 1x phosphate buffered saline (1xPBS, pH 7.4) and incubated in 5% normal goat serum/1xPBS/0.4% Triton X for 60min at room temperature. Rabbit polyclonal antibody (anti-Fos, Santa Cruz Biotechnology, sc-52) was utilized to recognize Fos in specific sections containing the dorsal hippocampus, amygdala, and medial prefrontal cortex. This antibody was used at a dilution of 1:2500 in 5% normal goat serum/1xPBS/0.4% Triton X. Following incubation (48h, 4 °C), sections were incubated with avidin-biotin-peroxidase complex (Vectastain ABC kit) for 45 min, then washed again in 1xPBS and processed using DAB with nickel-intensification (DAB peroxidase substrate kit, Vector Laboratories). Brain sections from each experimental group were processed similarly throughout all stages of the procedure. This procedure was adapted from Nikulina, et al. (2004) and used recently (Hoffman et al., 2013).
Fos Protein Analysis
Tissue sections were examined for the presence of a blue-black reaction product indicating immobilized antigen. For each group, data were obtained from 2–6 sections/rat through each brain subregion in both hemispheres, and averaged to obtain a mean value. Selected areas (30,000 μm2 for the hippocampal regions and 150,000 μm2 for the mPFC and amygdala regions) were captured and digitized using a camera (CX9000, MicroBrightField, Burlington, VT) interfaced to a microscope (Olympus BX51) with a 20x objective. A profile was considered labeled if its pixel intensity was more than 2 standard deviations darker than the background, as calculated by Stereo Investigator software (MBF Biosciences). For hippocampal analyses, targeted subregions included CA1, CA3, and the suprapyramidal (or dorsal, upper) and infrapyramidal (or ventral, lower) blades of the dentate gyrus (DGsup and DGinf, respectively). Sampling regions within each subregion were identified consistently among each hippocampal slice (Fig. 5B). Once each subregion was identified at 20x, the subregion was outlined and Stereo Investigator calculated the area (mm2). All positively labeled profiles were quantified and that value was divided by the area value to determine a density value. For the mPFC and amygdala analyses, adjacent cresyl violet stained sections were used to localize subregions with high confidence because these regions express poorly defined borders; for mPFC, analyzed subregions included anterior cingulate cortex (ACG), prelimbic cortex (PL), and infralimbic cortex (IL), for the amygdala, analyzed subregions included basolateral amygdala (BLA), central amygdala (CEA), and medial amygdala (MEA). Fos-IR labeling was quantified using a systematic random approach to achieve unbiased counts by an experimental blind to treatment conditions. Stereo Investigator software partitioned each image into 20 equal counting frames (100 × 75μm each), half of which were randomly selected and analyzed. The number of labeled Fos profiles was counted separately for each frame, excluding any overlapping labeled profiles on the left and bottom borders. Labeling density was calculated by dividing the estimated total number of labeled profiles by the total area analyzed. This procedure was adapted from Fanous et al. (2011) and reported recently (Hoffman et al., 2013).
Data Analysis
Data were analyzed by analysis of variance (ANOVA), and when significant effects were detected at p-value of 0.05 or less, post hoc analyses were performed. Additionally, planned comparisons were performed on data for which expected outcomes were anticipated based upon published findings. Proportions of freezing to CS were determined for associative memory during extinction 1 and 2 and were analyzed by one-sample t-tests with test statistic being 0.5. P-values that ranged between 0.05 and 0.1, were reported, whereas p-values greater than 0.1 were not reported. Data were analyzed by SPSS Version 19 and are represented as means ± SEM, with 8–10 animals/group for CON and STR and 4–5 animals/group for testing on the last day and Fos analyses.
Results
Stressor Effectiveness
Body weight gain and adrenal weight measures established chronic restraint as an effective stressor. A mixed factor ANOVA for restraint stress across weeks displayed a significant stress × week interaction (F(3,54)= 72.19, p< 0.001), a significant main effect across weeks (F(3,54)=872.179, p<0.001), and a significant main effect of stress (F(1,18)=24.328, p<0.001). Whereas both STR and CON exhibited similar body weights at the start of the study (baseline), STR rats gained weight more slowly than did CON over the course of the three-week stress paradigm (Fig. 1B). A one-way ANOVA on stress history for comparing adrenal weight per 100 grams of body weight showed a significant effect of restraint stress (F(1,17)= 13.029, p< 0.01). The STR group showed significantly greater adrenal weights per 100 grams of body weight than the CON groups (Fig. 1C).
Fear Conditioning: Acquisition
Chronic stress facilitated fear acquisition during the last training trial. A mixed factors ANOVA for stress history across the three training trials revealed a significant effect of trial (F(2,34)= 248.81, p< 0.001), demonstrating that both groups increased freezing across training trials. While no significant main effect for stress history or interaction between stress history and trial was found, past work has reported chronic stress to facilitate acquisition in fear conditioning at the second and third trials (Conrad et al., 1999; Hoffman et al., 2010). Consequently, planned comparisons were performed on trials 2 and 3 and revealed a significant effect of stress history on trial 3 (F(1,17)=4.25, p= 0.05), with STR freezing more than did CON (Fig. 2A).
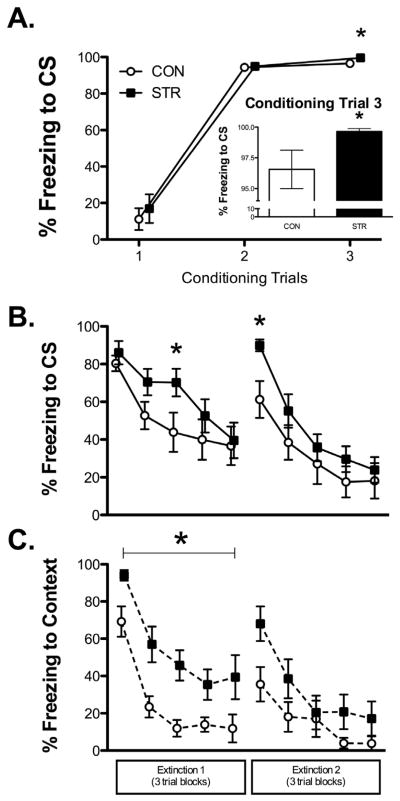
Figure 2. Conditioning and CS and Context Extinction.
(A) Freezing to CS across conditioning trials, all groups showed greater freezing across conditioning trials, whereas chronic stress enhanced fear acquisition as evidenced by increased freezing to CS during trial 3 of conditioning (inset). (B) CS Fear Extinction. Chronic stress (STR) slowed extinction acquisition, demonstrated by increased freezing to CS midway through Extinction 1. STR also showed impaired extinction retention, with robust freezing at the beginning of Extinction 2 compared to CON. (C) Contextual Fear Extinction. STR had significantly greater contextual fear during Extinction 1 compared to CON. Data are represented as mean ± SEM; n=9–10/group. *p<0.05 vs. CON.
Fear Conditioning: Tone Extinction
While both groups exhibited decreased levels of freezing to tone across extinction blocks during Extinction 1, chronic stress slowed extinction learning. A mixed factors omnibus ANOVA for stress history across extinction blocks revealed a significant effect of block (F(4,68)=18.08, p<0.001) with no other significant effects. Given the findings reported by Izquierdo and colleagues (2006) that chronic stress slows the rate of extinction to tone, we performed planned comparisons during extinction; a one-way ANOVA for extinction block 3 revealed a significant effect of stress history (F(1,17)=5.01, p<0.05) with STR freezing more during to the CS during this block, midway through the session (Fig. 2B).
A history of chronic stress also caused robust recovery of freezing to tone 24h following the last trial of the first extinction session. A mixed factors ANOVA for stress history across the last block of the first extinction session and the first block of the second extinction session revealed a significant effect across blocks (F(1,17)=26.19, p<0.001), with both groups showing spontaneous recovery of fear to the CS, and a marginal stress x block interaction (p=0.06), suggesting that this increase may differ depending on stress history. The main effect of stress was not significant, (F(1,17)=2.579, p=0.127), however a one-way ANOVA revealed a significant effect of stress for freezing to tone during the first block of Extinction 2, (F(1,17)=8.58, p<0.001), suggesting that chronic stress caused significantly greater recovery of fear responding to tone after extinction.
Both groups decreased freezing to tone during Extinction 2. A mixed factors ANOVA for stress history across blocks of extinction during Extinction 2 revealed a significant effect of block (F(4,68)=29.40, p<0.001). No other effects were significant.
Fear Conditioning: Context Extinction
For all rats, Extinction 1 and 2 occurred in the conditioning context, and freezing to the context was sampled during the 30s prior to tone onset for each trial during each extinction session. For both groups, freezing to context declined across Extinction 1, though chronic stress significantly impacted contextual fear as exhibited by greater levels of freezing prior to tone onset during Extinction 1. This was supported by a mixed factors ANOVA for stress history across context extinction blocks revealing a significant effect of block (F(4,68)=27.03, p<0.001), and a significant main effect of stress history (F(1,17)=5.76, p<0.05). There were no group differences during the first block of Extinction 2 for freezing to context, although STR had a non-significant increase in freezing to context during block 1 compared to CON (F(1,17)=3.182, p=0.092). During the rest of Extinction 2, STR froze similarly to context as CON, although both groups froze less to the context as extinction blocks progressed, which was supported by a significant effect of trial, (F(1,17)=18.85, p<0.001), with no other significant effects (Fig. 2C).
Fear Conditioning: Proportion of freezing to CS during Extinction 1 and 2
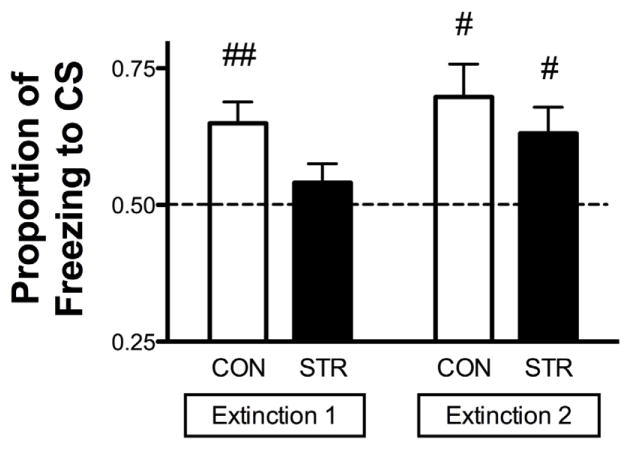
To determine the relative contribution of freezing to the tone over the context for both CON and STR groups, proportions of freezing to CS were determined for Extinction 1 and 2. For both Extinction 1 and 2, freezing to either tone or context (30s prior to tone onset) was collapsed across all trials within a session and a proportion was computed as follows: the average number of seconds freezing during tone presentations divided by the sum, which was the average number of seconds freezing during tone presentations and the average number of seconds freezing to context just prior to tone onset [mean CS/(mean CS + mean Context)]. A proportion of 1.0 indicates freezing to tone only without freezing to context (selective freezing to tone), whereas a proportion of 0.5 represents equal freezing to both tone and context (nonassociative freezing). As an index for CS selectivity, proportions of freezing to CS for each group were analyzed by a one-sample t-test compared to the test statistic of 0.5 to evaluate selective freezing to the tone CS. During Extinction 1, only the CON group showed significant selective freezing to the CS (CON t(7)=3.901, p<0.01), whereas the STR group froze similarly to the CS and context (STR, p=0.27). During Extinction 2, both CON and STR groups showed selective freezing to the CS (CON, t(7)=3.273, p<0.05; STR t(9)=2.705, p<0.05) (Fig. 3). From these data, we conclude that the STR group exhibited non-selective freezing during Extinction 1, which contrasts to the CON group that demonstrated selective freezing to CS for both Extinction 1 and 2.
Figure 3. Proportion of Freezing to CS.
During Extinction 1, CON displayed significantly increased freezing in response to the CS than the background, as indicated by a significantly higher proportion of freezing to CS compared to chance (0.5), while STR displayed equivalent freezing to the CS and context. Both groups displayed increased freezing to CS than background during Extinction 2. Data are represented as mean ± SEM; n=9–10/group. ##p<0.01 vs. chance (0.5); #p<0.05 vs. chance (0.5).
Fear Conditioning: Fear Generalization
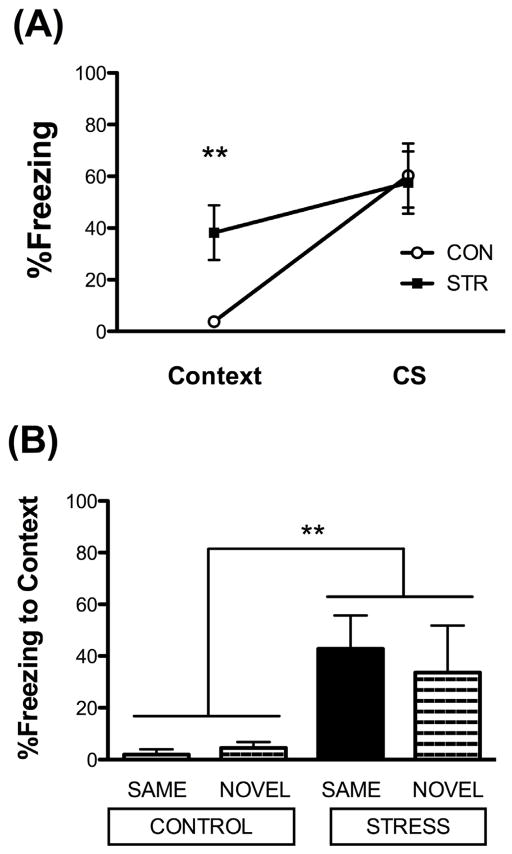
Twenty-four hours following the second extinction session, both groups were subdivided and tested in either the same (SAME) or a novel chamber context (NOVEL), to assess fear generalization. Freezing behavior was analyzed prior to and during the first tone in the session. A 2 × 2 × 2 mixed factors ANOVA was performed for two between subjects factor for stress history (CON, STR) and test context (SAME, NOVEL) and one within subject factor for stimulus (freezing prior to (context) and during the first CS trial). While there was a significant effect for stimulus (F(1,14)=35.805, p<.001), indicating that rats increased freezing during CS presentation compared to the context preceding the CS, we also found a significant stress x stimulus interaction, F(1,14)=8.595, p=0.01. Post hoc analyses revealed that chronically stressed rats showed high levels of freezing that barely changed between context (38.2 ± 10.6%) and CS (57.6 ± 12.0%), whereas CON showed significant increases in freezing from context (3.8 ± 1.6%) to CS (64.7 ± 11.8%, Fig. 4A). Specifically, chronically stressed rats froze significantly more to the context prior to CS presentation than did the CON (t(16)=2.862, p=0.01) and the chronically stressed rats freezing to context increased during freezing to the CS (t(9)=2.686, p<0.05). In contrast, CON barely froze to context, and freezing increased significantly during CS presentation (t(7)=4.916, p<0.01, Fig. 4A).
Figure 4. Chronic stress induces fear generalization to a novel context.
(A) Chronic stress enhanced freezing to context prior to the first CS presentation when tested for extinction memory, while all groups showed similar levels of freezing during the first CS presentation. There was no impact of test context (SAME and NOVEL collapsed within each stress condition; n=8–10/group). (B) Fear Generalization. STR displayed increased freezing to the context prior to trial 1, regardless of test context (SAME or NOVEL; n=4–5/group). Data are represented as mean ± SEM; **p=0.01 vs. CON.
Equally important, there was no significant contribution of test context for either stress condition (SAME, NOVEL). Consequently, STR froze more and similarly to context, regardless of whether they were tested within the same context or a novel context than did CON (further illustrated in Fig. 4B). Together, these data suggest stimulus generalization in the STR cohort. No other effects were significant from the omnibus ANOVA.
Fos IR labeling: Amygdala
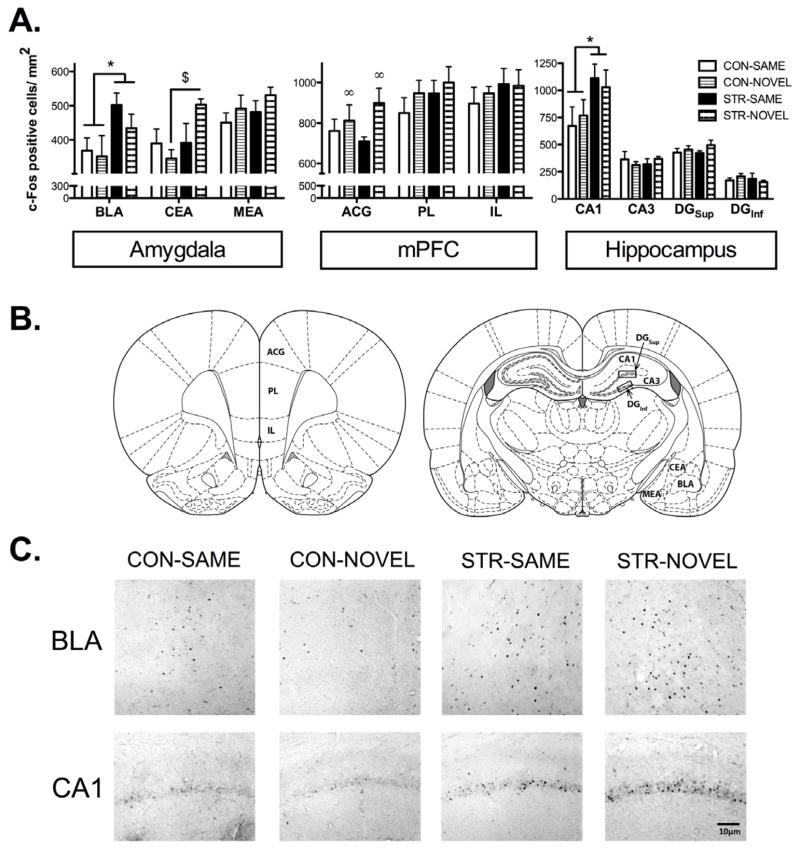
Ninety minutes following the test in either the same or a novel context, all rats were perfused and brain tissue was processed for Fos immunohistochemistry. A two-way ANOVA for stress history and test context for Fos IR labeling within the basolateral (BLA) subregion revealed a significant main effect of stress history (F(1,15)=6.31, p<0.05), showing that regardless of test context, chronic stress increased Fos labeling during fear memory retrieval (Fig. 5A). The same analysis for the central amygdala (CEA) revealed a significant stress history x test context interaction (F(1,14)=4.36, p=0.05), with post hoc analyses showing STR-NOVEL had increased Fos IR labeling in the CEA vs. CON-NOVEL (p<0.001; Fig. 5A). There were no other significant effects for these subregions or for the MEA.
Figure 5. Fos IR labeling: Amygdala, mPFC, Hippocampus.
(A) Regardless of test context, STR showed greater Fos IR compared to CON in amygdala BLA and dorsal hippocampus CA1. When tested in a novel context, STR showed increased Fos IR in amygdala CEA compared to CON. Regardless of stress history, when either group was tested in a novel context, Fos IR was in the mPFC ACG. (B) Regions of interest in coronal sections based on the rat brain atlas by Paxinos and Watson (2007). (C) Representative Fos photomicrographs observed in the BLA and CA1. Data are represented as mean ± SEM; n=4–5/group. *p<0.05 vs. CON; $p<0.001 vs. CON-NOVEL; ∞p<0.05 vs. SAME.
Fos IR labeling: mPFC
There was greater functional activation within the anterior cingulate (ACG) subregion of the mPFC when rats were tested in a novel context, regardless of stress history (Fig. 5B). This was supported by a two-way ANOVA for stress history and test context for Fos IR labeling within the anterior cingulate (ACG), revealing a significant main effect of test context (F(1,14)=4.30, p=0.05), no other effects were significant.
Fos IR labeling: Hippocampus
Within the hippocampal CA1 subregion, chronic stress enhanced functional activation during fear memory retrieval. A two-way ANOVA for stress history and test context for Fos IR labeling within the CA1 subregion revealed a significant main effect of stress history (F(1,15)=5.102, p<0.05), showing that regardless of test context, chronic stress increased Fos expression during fear memory retrieval. (Fig. 5C) There were no other significant effects.
Discussion
The current study aimed to address how a history of chronic stress affects fear extinction and nonassociative fear, and corresponding functional activation in brain regions sensitive to stress and involved with extinction. Here, we corroborate others’ findings by demonstrating that a history of chronic stress impairs extinction learning (Izquierdo et al., 2006) and memory (Baran et al., 2009; Miracle et al., 2006). Importantly, we extend these findings and are the first to show that chronic stress increases contextual fear during extinction, and fear generalization to a novel context. We also investigated patterns of functional activation using Fos IR labeling in brain areas sensitive to stress and fear extinction during an extinction recall test in either the same or a novel context. We found that regardless of test context, chronically stressed rats showed increased Fos IR labeling in both the BLA and dorsal hippocampus CA1 region, compared to nonstressed controls. We also show that when tested in a novel context, chronic stress increased Fos expression in the CEA compared to nonstressed controls. Lastly, control or stressed rats that were tested in a novel context had more Fos IR labeling in the ACG compared to those tested in the same context. These data suggest that chronic stress increases the functional activation in limbic regions associated with fear extinction (amygdala and hippocampus). Therefore, chronic stress appears to affect nonassociative fear and alter fear extinction behavior and respective functional activation within limbic regions associated with fear extinction.
Compared to non-stressed controls, chronically stressed rats exhibited slower fear extinction within the first extinction session and robust spontaneous recovery at the beginning of subsequent extinction sessions. In addition, during the first extinction session, chronic stress produced enhanced contextual fear. While chronically stressed animals froze more to the CS and context during extinction 1 and 2 (Fig. 2B) and to the context in extinction 1 (Fig. 2C), we also showed that this group had a lower proportion of freezing to the CS during extinction 1 (Fig. 3), demonstrating nearly equal freezing to the CS and context. Moreover, STR also demonstrated more freezing to the context when tested in a novel context, suggesting fear generalization (Fig. 4). These behavioral effects during CS extinction mimic the challenges faced with treating PTSD patients who are resistant to exposure-based therapy (Craske et al., 2008; Pitman et al., 1996). Additionally, the effects on nonassociative fear (sensitization, generalization) are relevant to the cluster of hyperarousal symptoms seen in PTSD patients (Yehuda & LeDoux, 2007). Taken together, the current study supports the use of chronic restraint stress and fear conditioning as a paradigm that can be implemented to investigate an animal model for PTSD, with face validity for a PTSD-like behavioral phenotype.
Fos expression during post-extinction fear memory retrieval in the same and in a novel context revealed interesting patterns within subregions of the amygdala and dorsal hippocampus. We found that regardless of test context (SAME, NOVEL), chronically stressed animals had greater Fos IR labeling in the BLA and hippocampal CA1 region. These parallel patterns of activation are noteworthy, given the reciprocal connections between the amygdala and CA1 region of the hippocampus (Pitkanen, Pikkarainen, Nurminen, & Ylinen, 2000), and supports the recent findings that amygdala-hippocampal functional connectivity is enhanced following chronic stress (Ghosh, Laxmi, & Chattarji, 2013). As test context had no impact on behavior for our chronically stressed groups, the Fos effects within BLA and CA1 tended to reflect the behavioral patterns that we observed with generalization, however within the CEA we observed greater Fos expression in the chronically stressed group compared to controls when these groups were tested in a novel context. This activation pattern may be due to greater contextual freezing in the STR-NOVEL group, and may suggest a role for the CEA in contextual fear, as this group showed generalization to a novel context, while there was virtually no freezing to context in the CON-NOVEL group. Another explanation may be that detecting changes in CEA Fos expression following a repeated presentation of a familiar stimulus, such as investigating CON and STR in the SAME context, may be difficult because Fos induction is optimal in response to a novel stimulus than it is with a repeated stimulus (Senba and Ueyama, 1997). We also found that regardless of stress history (CON and STR), rats that were tested in a novel context had greater functional activation in the anterior cingulate cortex (ACG). These results are consistent with previous work that has indicated a role for the ACG to be involved in discriminating stimuli in fear conditioning paradigms (Morgan & LeDoux, 1995; Powell, Watson, & Maxwell, 1994). In the current paradigm, we did not observe significant differences in the activation of IL or PL of the mPFC, as found in another study with a similar paradigm (Knapska & Maren, 2009); however, subtle differences between studies may contribute to the disparate findings. For example, the additional extinction session may lead to less Fos induction, as Fos is known to habituate as conditions become less novel (Papa, Pellicano, Welzl, & Sadile, 1993). More research is needed to isolate chronic stress effects on the contribution of associative and nonassociative fear to patterns of relevant brain activation.
A history of chronic stress resulted in a neurobiological and behavioral vulnerability to develop exaggerated fear responses during fear conditioning and extinction, and may be considered as an environmental risk factor for the development of PTSD following exposure to a traumatic event. As discussed earlier, chronic stress creates a structural imbalance of brain morphology in regions associated with fear processing, favoring the amygdala with deficits in regions involved in emotional regulation (mPFC), which may be mediating the persistent and generalized fear responding. In the current study, we are the first to show increased functional activation (via Fos IR labeling) within amygdala subregions (BLA and CEA) following chronic stress during recall of a fear memory. This parallels human functional imaging data that show greater amygdala activation in human populations with PTSD (Liberzon et al., 1999; Shin et al., 2006). Moreover, it has been observed that there is a reduction in hippocampal volume in PTSD patients (Bremner et al., 1995; Woon, Sood, & Hedges, 2010), but despite this, many functional imaging studies have reported greater hippocampal activation in this patient population (Osuch et al., 2001; Sachinvala, Kling, Suffin, Lake, & Cohen, 2000; Shin et al., 2006; Thomaes et al., 2009; Werner et al., 2009). These outcomes observed in humans with PTSD parallel what many have observed following chronic stress considering dendritic atrophy in the hippocampus as an indirect measure of volume (Hoffman et al., 2011; Tata & Anderson, 2010; Watanabe, Gould, & McEwen, 1992). Furthermore, here we are the first to show greater Fos expression in the hippocampus following chronic stress during retrieval of a fear memory. Thus, this is the first study to show both behavioral and functional neurobiological parallels in an animal model of PTSD. Chronic stress induced alterations in limbic regions implicated in PTSD suggest that the amygdala and hippocampal functional network is disrupted and may underlie exaggerated fear and impairments in context discrimination.
Highlights.
Chronic stress increases contextual fear during extinction
Chronic stress-enhanced fear memories generalize to a novel context
Chronic stress enhances amygdala and hippocampal Fos expression during fear memory
Chronic stress in rats induces a PTSD-like phenotype after fear conditioning
Acknowledgments
Funding was provided by NIDA R03-DA032632 (Sanabria), NIMH R03 MH094562 (Sanabria) and the College of Liberal Arts and Sciences (Conrad). The authors gratefully acknowledge the thoughtful contributions of Julia Anglin, Danya Anouti, Heather Bimonte-Nelson, Alyssa Campbell, Jeffery Hanna, Peter Kufhal, Gabriel Mazur, Pooja Paode, Sara Taylor, and Stephanie Yahn.
List of Abbreviations
- ACG
anterior cingulate gyrus
- ANOVA
analysis of variance
- BLA
basolateral amygdala
- CEA
central amygdala
- CON
nonstressed control
- CR
conditioned response
- CS
conditioned stimulus
- DG
dentate gyrus
- IL
infralimbic cortex
- ITI
inter-trial-interval
- MEA
medial amygdala
- mPFC
medial prefrontal cortex
- OF
open field
- PBS
phosphate buffered saline
- PL
prelimbic cortex
- PTSD
post-traumatic stress disorder
- SEM
standard error of the mean
- STR
chronic stress
- US
unconditioned stimulus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baran SE, Armstrong CE, Niren DC, Conrad CD. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learning and Memory. 2010;17(5):267–278. doi: 10.1101/lm.1778010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of Learning and Memory. 2009;91(3):323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67(3):370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. The American Journal of Psychiatry. 1995;152(7):973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of General Psychiatry. 1991;48(3):216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex. 2005;15(11):1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Progress in Neuropsychopharmacoology and Biological Psychiatry. 2010;34(5):742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113(5):902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behavioral Research Therapy. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Fanous S, Lacagnina MJ, Nikulina EM, Hammer RP., Jr Sensitized activation of Fos and brain-derived neurotrophic factor in the medial prefrontal cortex and ventral tegmental area accompanies behavioral sensitization to amphetamine. Neuropharmacology. 2011;61(4):558–564. doi: 10.1016/j.neuropharm.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Laxmi TR, Chattarji S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. Journal of Neuroscience. 2013;33(17):7234–7244. doi: 10.1523/JNEUROSCI.0638-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner MB, Robert S, Frueh BC. Treatment-resistant posttraumatic stress disorder: strategies for intervention. CNS Spectrums. 2004;9(10):740–752. doi: 10.1017/s1092852900022380. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Anouti DP, Lacagnina MJ, Nikulina EM, Hammer RP, Jr, Conrad CD. Experience-dependent effects of context and restraint stress on corticolimbic c-Fos expression. Stress. 2013 doi: 10.3109/10253890.2013.804505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AN, Armstrong CE, Hanna JJ, Conrad CD. Chronic stress, cyclic 17beta-estradiol, and daily handling influences on fear conditioning in the female rat. Neurobiology of Learning and Memory. 2010;94(3):422–433. doi: 10.1016/j.nlm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Krigbaum A, Ortiz JB, Mika A, Hutchinson KM, Bimonte-Nelson HA, Conrad CD. Recovery after chronic stress within spatial reference and working memory domains: correspondence with hippocampal morphology. European Journal of Neuroscience. 2011;34(6):1023–1030. doi: 10.1111/j.1460-9568.2011.07820.x. [DOI] [PubMed] [Google Scholar]
- Huynh TN, Krigbaum AM, Hanna JJ, Conrad CD. Sex differences and phase of light cycle modify chronic stress effects on anxiety and depressive-like behavior. Behavioural Brain Research. 2011;222(1):212–222. doi: 10.1016/j.bbr.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. Journal of Neuroscience. 2006;26(21):5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learning and Memory. 2004;11(6):770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learning and Memory. 2009;16(8):486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Research. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of Learning and Memory. 2006;85(3):213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109(4):681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, Post RM. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biological Psychiatry. 2001;50(4):246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa M, Pellicano MP, Welzl H, Sadile AG. Distributed changes in c-Fos and c-Jun immunoreactivity in the rat brain associated with arousal and habituation to novelty. Brain Research Bulletin. 1993;32(5):509–515. doi: 10.1016/0361-9230(93)90299-q. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Altman B, Longpre RE, Poire RE, Macklin ML, Steketee GS. Emotional processing and outcome of imaginal flooding therapy in Vietnam veterans with chronic posttraumatic stress disorder. Comparative Psychiatry. 1996;37(6):409–418. doi: 10.1016/s0010-440x(96)90024-3. [DOI] [PubMed] [Google Scholar]
- Powell DA, Watson K, Maxwell B. Involvement of subdivisions of the medial prefrontal cortex in learned cardiac adjustments in rabbits. Behavioral Neuroscience. 1994;108(2):294–307. doi: 10.1037//0735-7044.108.2.294. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biological Psychiatry. 2010;67(12):1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachinvala N, Kling A, Suffin S, Lake R, Cohen M. Increased regional cerebral perfusion by 99mTc hexamethyl propylene amine oxime single photon emission computed tomography in post-traumatic stress disorder. Military Medicine. 2000;165(6):473–479. [PubMed] [Google Scholar]
- Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102(2):329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neuroscience Research. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Sonnenberg JL, Macgregor-Leon PF, Curran T, Morgan JI. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron. 1989;3(3):359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Tata DA, Anderson BJ. The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiology and Behavior. 2010;99(2):186–193. doi: 10.1016/j.physbeh.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer NP, de Ruiter MB, Elzinga BM, van Balkom AJ, Veltman DJ. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiatry Research. 2009;171(1):44–53. doi: 10.1016/j.pscychresns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588(2):341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Werner NS, Meindl T, Engel RR, Rosner R, Riedel M, Reiser M, Fast K. Hippocampal function during associative learning in patients with posttraumatic stress disorder. Journal of Psychiatric Research. 2009;43(3):309–318. doi: 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]