Abstract
Objective
Despite lower socioeconomic status (SES) and higher disease burden, Hispanics in the U.S. paradoxically display equal or lower mortality on average than non-Hispanic Whites (NHW). Our objective was to determine if the “Hispanic Paradox” occurs among patients with rheumatoid arthritis (RA).
Methods
In a cohort of 706 RA patients, we compared differences in rheumatoid arthritis severity and comorbidity between Hispanic and non-Hispanic White ethnic groups at baseline. Cox proportional hazard models were used to estimate and compare mortality risk between Hispanics and NHW.
Results
We studied 706 patients with RA, of whom 434 were Hispanic, and 272 were NHW. Hispanics had significantly lower SES, greater inflammation, as well as higher tender and swollen joint counts. Patients were observed for 6,639 patient-years, during which time 229 deaths occurred by the censoring date (rate 3.4 per 100 person-years; 95% CI [3.0, 3.9]). Age- and sex-adjusted mortality was not significantly different between the two ethnic groups (HR 0.95). After adjustment for comorbidities, RA severity and level of acculturation, mortality among Hispanics was lower (HR 0.57; P=0.005).
Conclusion
Despite greater severity in most clinical manifestations and lower SES among Hispanics, their mortality, paradoxically, was not increased. Further research is needed to understand the mechanisms underlying this survival paradox.
Introduction
Rheumatoid arthritis (RA) is a chronic disease characterized by inflammation within the joints and affects approximately 1.3 million Americans.1 Although ultimate causes of death in RA patients are similar to the general population,2 mortality rates in RA patients are 1.5 – 1.6 times higher, with cardiovascular disease (CVD) being the most frequent cause of death.2,3 The disease pathology and increased mortality seen in RA is incompletely understood, though there is thought to be a genetic component to disease severity.4 Identification of a group of patients protected from RA’s higher mortality may open insights into the mechanisms of the increased mortality.
Hispanics are the fastest-growing ethnic group in the United States. According to the US Census Bureau, over half of the total population growth between 2000 and 2010 was a result of an increase in the Hispanic population.5 Yet, in spite of lower socioeconomic status (SES) and higher rates for diabetes and obesity, several studies have shown equal or lower mortality rates for Hispanics in the US when compared to non-Hispanic Whites.6–11 Given that the majority of current literature suggests lower socioeconomic status is correlated with worse health outcomes as well as greater mortality,12–15 this comparable mortality finding has been referred to as the “Hispanic Paradox”.6 There have been several proposed hypotheses to describe these findings. Most notably, the “salmon bias” hypothesis,16 cultural influences,17,18 as well as genetic variation19 are some of the key theories. The “salmon bias” hypothesis posits that foreign-born Hispanics return to their country at the end of life, and because we lack access to death records in those countries, their deaths are not recorded in the US, which makes them appear to have a lower mortality. The sociocultural characteristics of the Hispanic population, specifically Mexican-Americans, have been shown to play a role in alleviating negative effects of low SES, such as worse health outcomes.18 Furthermore, recently, genetic factors have been suggested to play a role in the Hispanic paradox in chronic obstructive pulmonary disease (COPD), a partly heritable disease.19 However, previous studies have not examined this paradox in RA.
Here, we studied mortality within a cohort of RA patients, adjusting for age, sex, socioeconomic status, and clinical features of RA as well as comorbidity. We compared mortality rates between Hispanics and Non-Hispanic Whites. Because of their lower SES and greater disease severity, we hypothesized that the Hispanic patients would have increased mortality compared to the non-Hispanic White.
Patients and Methods
Patients
The patients in this study have been described in other publications.20–22 Briefly, from 1996 to 2000, we recruited consecutive patients who met the 1987 criteria for RA23 from private and public rheumatology clinics in San Antonio, Texas. All patients participated in a comprehensive baseline evaluation of their clinical and psychosocial characteristics conducted by a physician and trained research assistants. Afterwards, we invited them for annual follow-up evaluations. We followed patients until the censoring date of May 31, 2010. We learned about deaths from public databases, physicians, relatives, neighbors and obituaries. In April and May 2010, we made further efforts to ascertain vital status by contacting patients directly. Of those we could not contact, we reviewed medical records to determine the date of last contact with health system. All deceased were confirmed by death certificate. This study received approval from the institutional review board for the current protocol.
Demographics
A trained interviewer asked patients for their date and place of birth, sex and race/ethnicity. For the latter, the interviewer asked patients to self-identify as “white,” “black,” “Asian,” “Hispanic,” or “other.” Hispanic patients were further asked if they were of Mexican, Mexican American, Puerto Rican, Cuban, Central American, or other Hispanic background. In the cohort under study, these ethnic definitions identify culturally and genetically distinct groups.22,24 For this analysis, we only included patients who selected “White” or “Hispanic”.
Social Support
The level of social support was assessed using the MOS Social Support Survey25 at baseline evaluation. The results from this survey provide four separate scales that describe how often each of the following different kinds of social support was made available to the patient: Tangible support, affectionate support, positive interaction support, and emotional/informational support. The survey asked how often the following kinds of support were available, with higher scores indicating greater amount of support (none of the time = 1, a little of the time = 2, some of the time = 3, most of the time = 4, and all of the time = 5). The tangible support scale was measured by questions such as how often there is “Someone to help you if you were confined to bed,” and “Someone to take you to the doctor if you needed it.” Affectionate support was measured by questions such as how often there is “Someone who shows you love and affection” and “Someone who hugs you.” Positive interaction support was measured by questions such as how often there is “Someone to have a good time with.” Emotional/Informational was measured by questions such as how often there is “Someone to give you good advice about a crisis” and “Someone who understands your problems.”
Acculturation
Level of acculturation was assessed using Deyo and colleagues’ four-question language based scale,26 as described previously.22 A value of 0 represents no acculturation, while 4 represents full acculturation to the prevalent US English-speaking mainstream, or Anglo, culture. Acculturation was measured in the same way for both Hispanics and NHW. The questions and scoring of possible answers are: 1) “Which was the first language you spoke as a child?” (English = 1, English and Spanish interchangeably = 0, Spanish = 0); 2) “What language do you speak at home?” (English = 1, English and Spanish interchangeably = 0, Spanish = 0); 3) “What language do you prefer to speak?” (English or English and Spanish interchangeably = 1, Spanish = 0); 4) “Do you read any English?” (Yes, some or a little = 1, no = 0).
Socioeconomic Status
We classified SES according to Nam and Powers, using years of formal education, inflation-adjusted monthly household income, and current or past occupation to calculate an SES score on an ascending scale between 0 and 100.27
Clinical Features
RA Manifestations
We defined RA duration as the interval between the date of RA diagnosis and time of study visits. At the baseline and at each of the follow-up visits, we asked patients to provide a list of their medications, and we reviewed pharmacy and medical records. We asked patients whether they were receiving glucocorticoids, and if so, we asked for the date at which these medications were first prescribed as well as the dose currently in use. We estimated cumulative oral glucocorticoid dose by multiplying the current daily dose by the number of days since glucocorticoids were initiated.28
A physician examined 48 joints for tenderness or pain on motion, swelling or deformity, and for the presence of extra-articular subcutaneous nodules as previously described.29 Joint exam Spearman-Brown reliability coefficients are 0.94 for tender/pain on motion, 0.90 for swelling and 0.98 for deformity. We quantified joint damage on a plain X-ray radiograph view of both hands and wrists according to Sharp and colleagues.30 In this study, all X-ray radiographs were scored by one rater (JFR).
For the erythrocyte sedimentation rate (ESR), we used the Westergren technique. If any ESR data were missing, we imputed the most recent adjacent follow-up visit levels into the missing data points. Approximately 57 baseline ESR values were replaced with second follow-up visits, and approximately 213 follow-up visits were replaced with adjacent ESR values. Serum rheumatoid factor (RF) was measured by the latex agglutination technique. Patients were considered seropositive if any determination during the study was positive. HLA–DRB1 genotyping was performed by Bio-synthesis, Inc. (Lewisville, TX), using polymerase chain reaction–sequence specific-primer amplification with Fastype kits (Bio-Synthesis, Inc., Lewisville, TX).31 We classified HLA–DRB1 types using the 1996 World Health Organization Nomenclature Committee for Factors of the HLA System update.32
Comorbidity
Hypertension was considered to be present if there was a physician’s diagnosis in the medical record or an antihypertensive medication was prescribed, or from the measured blood pressure throughout the study (average systolic blood pressure ≥140 mm Hg). To assess obesity, we measured height and weight to calculate the body mass index (BMI), in kg/m2. Diabetes mellitus was considered to be present if a physician had recorded the diagnosis in the medical record, if the patient had taken antidiabetic medications during the course of the study, or if the fasting blood sugar level at a study visit was ≥126 mg/dl. Hypercholesterolemia was considered to be present if a physician recorded the diagnosis in the medical record, the patient had ever been prescribed lipid-lowering medication, or the fasting plasma cholesterol level measured during a study visit was ≥200 mg/dl. Smoking was divided into three categories: current smokers, past smokers, or never smokers. Persons who had ever smoked cigarettes were classified as current smokers if they continued to smoke, or as past smokers if they had quit by the time of their most recent visit.
The comorbidity scale that we used was the Charlson Comorbidity Index.33 This index records the presence or absence of 18 health problems, each one weighted for severity according to predefined values. The following health problems receive a weight of 1 in the index: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease without hemiplegia, dementia, chronic pulmonary disease, connective tissue disease (including RA), peptic ulcer disease, mild liver disease, and diabetes mellitus without end-organ damage. The following health problems receive a weight of 2: hemiplegia, moderate or severe renal disease, diabetes with end-organ damage, malignant neoplasms, leukemia, and lymphoma. Moderate or severe liver disease receives a score of 3. Metastatic solid tumor and the acquired immunodeficiency syndrome receive a score of 6. The final score is provided by the sum of the weights of each patient’s health problems. The examining physician used a validated self-report questionnaire to interview the patients about the presence or absence of the predefined health problems.34 The physician then reviewed available medical records to verify the problems reported by the patient. If discrepancies between the medical record and the self-reported responses arose, the case was discussed among 3 or 4 physicians in order to decide on the final score. Interrater reliability was 0.94.35
Statistical Analysis
We plotted age-adjusted Kaplan-Meier survival curves for Hispanic and non-Hispanic White patients. We used Cox proportional hazards regression to compare survival, adjusting for confounders, and t-tests or chi-square, as indicated, to test differences at baseline between Hispanic and non-Hispanic White patients.36 We used generalized estimating equations (GEE) to compare clinical characteristics between Hispanics and non-Hispanic Whites over the course of the study, adjusting for confounders and accounting for repeated measures within patients.37 After comparing Hispanics to non-Hispanic Whites, we divided Hispanics into two groups, foreign-born and US-born, and compared mortality using Cox proportional hazards regression. We tested the assumption of proportional hazards for all variables in the Cox models by testing the hypothesis that the slope of their Schoenfeld residuals over time was zero,38 and by visually inspecting plots of the survival probability against time. All analyses were conducted using a desktop personal computer with the Stata statistical software package, version 9.0 (College Station, TX).
Results
We studied 706 patients who participated in a total of 4,155 observations. Time from enrollment until last follow-up, death or the censoring date was 6,639 patient-years. There were 434 Hispanic and 272 non-Hispanic White (NHW) patients.
Table 1 shows demographic and baseline clinical characteristics across ethnic groups. Hispanics were younger at the disease onset and had lower SES scores (Table 1). At baseline, Hispanics were significantly more likely to have diabetes and higher BMI, but lower cholesterol, incidence of hypertension and Charlson comorbidity scores. In regards to the Charlson comorbidity index, NHW patients showed statistically higher comorbidity scores at baseline, though this was found to be due to their older age. Furthermore, a higher percentage of NHW were found to have smoked in the past, though we found no association between smoking status at time of enrollment and comorbid conditions.
Table 1.
Demographic and clinical characteristics of 706 RA patients at baseline according to ethnic group.
| Non-Hispanic White (n=272) | Hispanic (n=434) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 60.1 (12.7) | 52.0 (12.6) | ≤ 0.001 |
| Age at onset, mean (SD) | 47.4 (14.3) | 42.4 (13.3) | ≤ 0.001 |
| Male, n (%) | 111 (41%) | 109 (25%) | ≤ 0.001 |
| Nam-Powers, mean (SD) | 50.4 (20.0) | 28.7 (17.7) | ≤ 0.001 |
| Comorbid Conditions | |||
| Diabetes, n (%) | 16 (6%) | 76 (18%) | ≤ 0.001 |
| Hypertension, n (%) | 131 (48%) | 140 (32%) | ≤ 0.001 |
| Hypercholesterolemia, n (%) | 36 (13.2%) | 17 (4%) | ≤ 0.001 |
| Past Smoker, n (%) | 138 (51%) | 154 (36%) | ≤ 0.001 |
| Current Smoker, n (%) | 53 (20%) | 80 (18%) | 0.7 |
| BMI, mean (SD) | 26.9 (5.6) | 30.0 (6.8) | ≤ 0.001 |
| Charlson Comorbidity, mean (SD) | 1.91 (1.24) | 1.62 (1.15) | 0.002 |
| RA Manifestations | |||
| Tender joints, mean (SD) | 11.2 (11.2) | 17.0 (12.5) | ≤ 0.001 |
| Swollen joints, mean (SD) | 6.4 (6.5) | 8.1 (7.1) | ≤ 0.001 |
| Deformed joints, mean (SD) | 11.6 (11.6) | 9.3 (10.8) | 0.008 |
| RA duration, mean (SD) | 13.2 (10.8) | 9.7 (10.3) | ≤ 0.001 |
| ESR, mean (SD) | 34 (26) | 44 (26) | ≤ 0.001 |
| Sharp Score*, mean (SD) | 63.2 (64.3) | 66.2 (69.5) | 0.4 |
| Nodules, n (%) | 83 (31%) | 136 (31%) | 0.8 |
| Prednisone use, n (%) | 138 (51%) | 219 (50%) | 0.9 |
| Prednisone dose, mean (SD) | 6.3 (3.4) | 6.9 (4.0) | 0.1 |
| HLA-DRB1, n (%)** | 213 (80%) | 302 (71%) | 0.006 |
| Rheumatoid factor, n (%) | 200 (74%) | 363 (84%) | ≤ 0.001 |
There were 49 patients for which no x-ray radiograph was obtained. Therefore, the values shown are for a sample of 657 patients at baseline—252 Non-Hispanic Whites and 405 Hispanics.
There were 16 patients that were not HLA-typed. The values shown are for 690 patients at baseline with corrected percentages—265 Non-Hispanic Whites and 425 Hispanics.
Regarding baseline clinical RA characteristics, the mean tender and swollen joint counts and ESR were higher in Hispanics, when compared to NHW who had greater mean deformed joint counts and disease duration. Hispanics were more likely to be positive for RF, and NHW were more likely to be positive for the HLA-DRB1 shared epitope (Table 1).
To evaluate sociocultural differences, we analyzed levels of acculturation and social support across ethnic group at baseline. Interestingly, NHWs had significantly greater positive support and emotional/informational support (Table 2).
Table 2.
Acculturation and Social Support scores of 706 RA patients at baseline according to ethnic group.
| Non-Hispanic White (n=272) | Hispanic (n=434) | P-value | |
|---|---|---|---|
| Acculturation | |||
| First language English, n (%) | 261 (96%) | 89 (20%) | ≤ 0.001 |
| Speak English most often while at home, n (%) | 269 (98%) | 167 (38%) | ≤ 0.001 |
| Prefer to speak English, n (%) | 270 (99%) | 306 (71%) | ≤ 0.001 |
| Read any English, n (%) | 272 (100%) | 379 (87%) | ≤ 0.001 |
| Total Acculturation Score, mean (SD) | 3.94 (0.28) | 2.27 (1.26) | ≤ 0.001 |
| Social Support* | |||
| Tangible support, mean (SD) | 4.05 (1.07) | 3.91 (1.03) | 0.08 |
| Affectionate support, mean (SD) | 4.39 (0.95) | 4.28 (0.99) | 0.1 |
| Positive interaction support, mean (SD) | 4.26 (1.01) | 4.02 (1.06) | 0.004 |
| Emotional/informational support, mean (SD) | 4.22 (1.01) | 3.95(1.09) | 0.001 |
There was one patient for which we were unable to obtain data on social support. The values shown are for 705 patients (NHW, n = 271; Hispanic, n = 434)
We also performed comparisons over time between Hispanics and NHW. Table 3 shows age- and sex-adjusted comparisons between the two ethnic groups. Over time, Hispanic patients had significantly more tender and deformed joints, higher ESR, and higher modified Sharp scores. There was no significant difference in the number of swollen joints, prednisone use, presence of nodules, RF positivity or Charlson comorbidity scores (Table 3).
Table 3.
Age- and sex-adjusted comparisons between Hispanics and non-Hispanic Whites in RA characteristics over time in 706 RA patients*
| Characteristic | Coefficient or OR* | P-value |
|---|---|---|
| Tender joint count (range 0 – 46) | 4.9 (3.2, 6.6)§ | ≤ 0.001 |
| Swollen joint count (range 0 – 37) | 0.49 (−0.15, 1.1)§ | 0.1 |
| Deformed joint count (range 0 – 48) | 5.0 (3.1, 6.8)§ | ≤ 0.001 |
| ESR mm/Hr (range 0 – 141) | 12.2 (8.9, 15.6)§ | ≤ 0.001 |
| Sharp score** (range 0 – 314) | 21.7 (10.6, 32.7)§ | ≤ 0.001 |
| Subcutaneous nodules (no=0, yes= 1) | 1.1 (0.8, 1.5)¶ | 0.1 |
| Prednisone use (no=0, yes=1) | 1.1 (0.9, 1.4)¶ | 0.2 |
| Rheumatoid factor (no=0, yes=1) | 1.3 (0.9, 1.8)¶ | 0.1 |
| Charlson Comorbidity Index (range 1 – 12) | −0.04 (−0.25, 0.17)§ | 0.7 |
Values represent either the regression coefficient (§) or odds ratio (OR) (¶) with 95% CI from GEE regression models in which the clinical characteristic was the dependent variable. In these models, Hispanic=1, NHW=0. Thus, a positive coefficient or an OR >1.0 signifies greater mean or prevalence in the Hispanic patients.
There were 49 patients for which no x-ray radiograph was obtained. Therefore, the values shown are for a sample of 657 patients—252 Non-Hispanic Whites and 405 Hispanics—with 2,713 observations.
By the censoring date, 229 deaths occurred, for a mortality rate of 3.4 per 100 patient-years (95% confidence interval [95% CI] = 3.0, 3.9). Among Hispanics, there were 120 deaths in 4,204 patient years for a rate of 2.8 deaths per 100 patient years (95% CI = 2.3, 3.3). Non-Hispanic Whites had 109 deaths per 2,305 patient years or 4.7 deaths per 100 patient years (95% CI = 3.9, 5.7). The unadjusted hazard ratio for mortality among Hispanics versus Non-Hispanics was 0.57 (95% CI = 0.45, 0.75) (Table 4). This difference in mortality lost significance after adjusting for age and sex with a hazard ratio of 0.96 (95% CI = 0.73, 1.26, P=0.8) for Hispanics. Figure 1 shows age-adjusted Kaplan-Meier curves illustrating probability of survival, comparing Hispanics to Non-Hispanic Whites. The lack of difference in mortality between the two ethnic groups persisted after adjusting for sex, SES, acculturation, social support and RA duration. However, when we added RA manifestations such as ESR and tender and swollen joints, as well as comorbidities to the model, the difference in mortality regained significance with the hazard ratio of 0.57 (95% CI = 0.39, 0.84, P=0.005) favoring Hispanics.
Table 4.
Mortality risk in Hispanics vs. non-Hispanic Whites in 706 RA patients
| Model* | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Unadjusted | 0.58 | 0.45, 0.75 | ≤ 0.001 |
| Age-, sex-adjusted | 0.96 | 0.73, 1.26 | 0.8 |
| Age-, sex-, SES-, acculturation level, social support-adjusted** | 0.72 | 0.49, 1.08 | 0.1 |
| Age-, sex-, SES-, acculturation level-, social support-, duration-adjusted** | 0.70 | 0.46, 1.03 | 0.08 |
| Age-, sex-, SES-, acculturation level-, social support-, duration-, tender joints-, swollen joints-, ESR-adjusted** | 0.58 | 0.39, 0.87 | 0.009 |
| Age-, sex-, SES-, acculturation level-, social support-, duration-, tender joints-, swollen joints-, ESR-, Charlson co-morbidity-adjusted** | 0.56 | 0.38, 0.84 | 0.004 |
Cox proportional hazards models
These models included 705 RA patients
Figure 1.
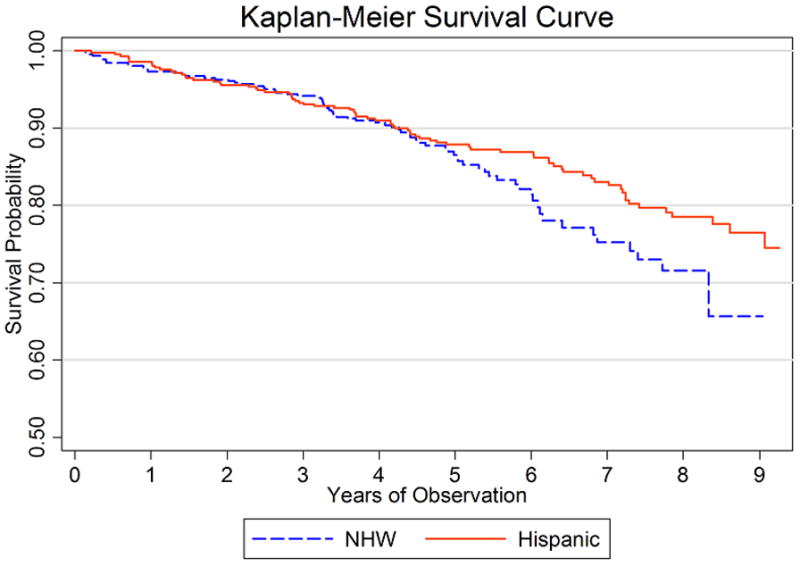
We further hypothesized that we would observe a lower mortality among foreign-born Hispanics because, as suggested by the “salmon-bias hypothesis,” foreign-born Hispanics may tend to return to die in their country of origin, where we lack access to their death records. When comparing foreign-born to US-born Hispanics, the US-born Hispanics accounted for 96 deaths in 3,157 patient years for a rate of 3.04 deaths per 100 patient years (95% CI = 2.5, 3.7). Foreign-born Hispanics had 24 deaths in 1,047 patient years for a rate of 2.3 deaths per 100 patient years (95% CI = 1.5, 3.4). Although no significant difference in mortality was observed between these two groups after age-adjustment, foreign-born Hispanics showed a hazard ratio of 0.79 (95% CI = 0.51, 1.24, P=0.310) compared to US-born Hispanics, suggesting a tendency toward a lower mortality. Furthermore, the non-Hispanic Whites still showed no significant difference in age-adjusted mortality compared to both foreign-born (P=0.316) and US-born (P=0.996) Hispanics.
Discussion
When we compared survival rates and clinical characteristics between Hispanics and Non-Hispanics Whites in a large cohort of patients with RA, we found Non-Hispanic Whites to have a greater mortality. This was true even though, on average, Hispanics in our cohort had lower SES scores and worse RA manifestations than Non-Hispanic Whites. This association remained significant (P=0.005) after adjusting for age, sex, SES scores, level of acculturation, clinical features of RA and comorbidity.
A Hispanic survival paradox was first noted in 1986, when Markides and Coreil studied the current literature on mortality within southwestern Hispanics, most of whom were Mexican American.6 These authors reviewed mortality in diseases that are leading causes of death such as cancer, diabetes, and cardiovascular diseases and found notable differences across ethnic groups, with lower mortality in Hispanics in almost all of these, with the exception of diabetes. Since then, a survival advantage among Hispanics has been documented in multiple studies,6–11,16 although some have not been able to confirm this.39–42
Our results seem to support the existence of a Hispanic mortality paradox in rheumatoid arthritis patients. Although the Hispanics in this RA sample were younger, age-adjustment eliminated the initially significant mortality difference. When other variables that indicated greater disease severity in Hispanics, such as the joint counts and ESR were added to the model, the Hispanic mortality advantage resurfaced. Each of these variables as well as the Charlson comorbidity index were significantly associated with mortality in age- and sex- adjusted Cox models in our sample, suggesting that the Hispanic patients were not affected by the negative effect of these variables in the same way as NHWs on survival.
One hypothesis given to elucidate this paradox is known as the “salmon bias”, in which foreign-born Hispanics are more likely to return to their country of origin at the end of life, resulting in an “immortal” population of Hispanics in the US.16 We were able to provide a preliminary test of this hypothesis. If the salmon-bias hypothesis were correct, we would have expected to observe a lower mortality among foreign-born Hispanics, as we did not have access to any deaths in foreign countries. Although we did not detect a significant difference between US- and foreign-born Hispanics, the direction of the effect suggests lower mortality in foreign-born Hispanics. Thus, even though our findings do not support the salmon-bias hypothesis, they did not exclude it either.
Another prevalent hypothesis suggests that culture affects mortality due to its influences on sociocultural, lifestyle and behavioral factors that act as a buffering effect.17 Several studies have suggested there are health disparities among minorities with RA,43 and some authors have attributed these to differences in treatment preferences, education, and other ethnic and cultural influences, which would contribute to poorer outcomes for minorities.44,45 Although these variables could have an effect on mortality differences,46 data suggests cultural effects cannot fully explain the paradox.39 Within our study, we adjusted for level of acculturation using a 4-item language-based scale, which has been shown to be reliable in distinguishing subsets of Mexican Americans.22 Our models revealed that after adjusting for acculturation in models already accounting for comorbidity and RA severity, the lower mortality in Hispanics became more significant. However, adding the results from all of the four social support surveys did not affect the model or mortality difference, suggesting the survival advantage in Hispanics was not explained by differences in social support.
One example of a disease reported to be less severe in Hispanics that may have a genetic basis is COPD. Hispanics in New Mexico may have lower odds of chronic pulmonary disease (COPD) and lower decline in lung function, consistent with the paradox.19 Because lung function is partly heritable,47 these authors proposed that genetic ancestry could be a factor in their findings. The severity of joint destruction in RA may also be heritable;4 therefore, it is possible that genetic differences could explain why the clinical manifestations of the disease were greater among Hispanics in the present study. There is a wide variety of genetic diversity within Hispanic subgroups in the US, with varying proportions of European, Native American and African admixture.48–50 Although the focus of this study does not include these data, pinpointing genetic protective factors in Hispanics is an area of considerable scientific interest.
In conclusion, our findings are consistent with the Hispanic Paradox, in that after adjusting for age and sex, mortality in Hispanics was not increased, despite lower SES and more severe RA. Several hypotheses have been proposed to explain this finding, such as migration effects of foreign-born Hispanics,16 sociocultural effects17,18 or genetic influences.19 Although we cannot support either the salmon-bias hypothesis or sociocultural effects, it should be of note that after adjusting for RA manifestations associated with greater mortality, the Hispanic mortality advantage increased, despite having worse outcomes on these measures. Further research is needed in order to determine the underlying factors responsible for this phenomenon, including potential genetic, environmental or sociocultural factors.
Significance and Innovations.
There is an increased mortality in RA patients compared to the general population; identifying a group less affected by this mortality may suggest strategies for interventions aimed at reducing mortality
Our findings suggest that Hispanics with RA, despite greater disease severity and lower SES, experience lower mortality than non-Hispanic Whites (NHW) with RA.
Acknowledgments
Supported in part by NIH grants R01-HL-085742, R01-HD-037151, and UL1-RR-025767
References
- 1.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis & Rheumatism. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35–61. [PubMed] [Google Scholar]
- 3.Gabriel SE, Crowson CS, O’Fallon WM. Mortality in rheumatoid arthritis: have we made an impact in 4 decades? The Journal of rheumatology. 1999;26:2529. [PubMed] [Google Scholar]
- 4.Knevel R, Gröndal G, Huizinga TW, Visser AW, Jónsson H, Víkingsson A, van der Helm-van AH. Genetic predisposition of the severity of joint destruction in rheumatoid arthritis: a population-based study. Annals of the rheumatic diseases. 2012;71:707–709. doi: 10.1136/annrheumdis-2011-200627. [DOI] [PubMed] [Google Scholar]
- 5.Ennis SR, Rios-Vargas M, Albert NG. The Hispanic Population: 2010 Census Briefs. Washington, DC: US Department of Commerce, Economics, and Statistics Administration, US Census Bureau; 2011. [Google Scholar]
- 6.Markides KS, Coreil J. The Health of Hispanics in the Southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101:253–265. [PMC free article] [PubMed] [Google Scholar]
- 7.Sorlie PD, Backlund E, Johnson NJ, Rogot E. Mortality by Hispanic status in the United States. JAMA. 1993;270:2464–2468. [PubMed] [Google Scholar]
- 8.Markides KS, Eschbach K. Aging, Migration and Mortality: Current Status of Research on the Hispanic Paradox. Journals of Gerontology, Series B: Social Sciences and Psychological Sciences. 2005;60B(Special Issue II):68–75. doi: 10.1093/geronb/60.special_issue_2.s68. [DOI] [PubMed] [Google Scholar]
- 9.Becker TM, Wiggins C, Key CR, Samet JM. Ischemic heart disease mortality in Hispanics, American Indians, and non-Hispanic whites in New Mexico, 1958–1982. Circulation. 1988;78:302–309. doi: 10.1161/01.cir.78.2.302. [DOI] [PubMed] [Google Scholar]
- 10.Goff DC, Jr, Ramsey DJ, Labarthe DR, Nichaman MZ. Acute myocardial infarction and coronary heart disease mortality among Mexican Americans and non-Hispanic whites in Texas, 1980 through 1989. Ethnicity & Disease. 1993;3:64–69. [PubMed] [Google Scholar]
- 11.Rewers M, Shetterly SM, Hoag S, Baxter J, Marshall J, Hamman RF. Is the risk of coronary heart disease lower in Hispanics than in non-Hispanic whites? The San Luis Valley Diabetes Study. Ethnicity & disease. 1993;3:44–54. [PubMed] [Google Scholar]
- 12.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: the challenge of the gradient. American Psychologist. 1994;49:15. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. American Journal of Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public health report. 2003;118:293. doi: 10.1016/S0033-3549(04)50253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosma H, van de Mheen HD, Borsboom GJ, Mackenbach JP. Neighborhood socioeconomic status and all-cause mortality. American Journal of Epidemiology. 2001;153:363–371. doi: 10.1093/aje/153.4.363. [DOI] [PubMed] [Google Scholar]
- 16.Turra CM, Elo IT. The impact of salmon bias on the Hispanic mortality advantage: New evidence from Social Security data. Population research and policy review. 2008;27:515–530. doi: 10.1007/s11113-008-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales LS, Lara M, Kington RS, Valdez RO, Escarce JJ. Socioeconomic, cultural, and behavioral factors affecting Hispanic health outcomes. Journal of health care for the poor and underserved. 2002;13:477. doi: 10.1177/104920802237532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eschbach K, Ostir GV, Patel KV, Markides KS, Goodwin JS. Neighborhood context and mortality among older Mexican Americans: is there a barrio advantage? Journal Information. 2004;94:1807–1812. doi: 10.2105/ajph.94.10.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruse S, Sood A, Petersen H, Liu Y, Leng S, Celedón JC, Tesfaigzi Y. New Mexican Hispanic smokers have lower odds of chronic obstructive pulmonary disease and less decline in lung function than non-Hispanic whites. American journal of respiratory and critical care medicine. 2011;184:1254–1260. doi: 10.1164/rccm.201103-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escalante A, del Rincón I. How much disability in rheumatoid arthritis is explained by rheumatoid arthritis? Arthritis Rheum. 1999;42:1712–21. doi: 10.1002/1529-0131(199908)42:8<1712::AID-ANR21>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Escalante A, del Rincon I. The disablement process in rheumatoid arthritis. Arthritis Rheum. 2002;47:333–342. doi: 10.1002/art.10418. [DOI] [PubMed] [Google Scholar]
- 22.Escalante A, del Rincón I, Mulrow CD. Symptoms of depression and psychological distress among Hispanics with rheumatoid arthritis. Arthritis Care Res. 2000 Jun;13(3):156–67. doi: 10.1002/1529-0131(200006)13:3<156::aid-anr5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Care Res. 2000;13:156–167. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Del Rincón I, Battafarano DF, Arroyo RA, Murphy FT, Fischbach M, Escalante A. Ethnic variation in the clinical manifestations of rheumatoid arthritis: role of HLA-DRB1 alleles. Arthritis Rheum. 2003 Apr 15;49(2):200–8. doi: 10.1002/art.11000. [DOI] [PubMed] [Google Scholar]
- 25.Sherbourne CD, Stewart AL. The MOS social support survey. Social science & medicine. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 26.Deyo RA, Diehl AK, Hazuda H, Stern MP. A simple language-based acculturation scale for Mexican Americans: validation and application to health care research. American Journal of Public Health. 1985;75:51–55. doi: 10.2105/ajph.75.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam CB, Powers MG. The Socioeconomic Approach to Status Measurement With a Guide to Occupational and Socioeconomic Status Scores. Houston, Tex: Cap & Gown Press; 1983. [Google Scholar]
- 28.del Rincón I, O’Leary DH, Haas RW, Escalante A. Effect of glucocorticoids on the arteries in rheumatoid arthritis. Arthritis & Rheumatism. 2004;50:3813–3822. doi: 10.1002/art.20661. [DOI] [PubMed] [Google Scholar]
- 29.Orces CH, del Rincon I, Abel MP, Escalante A. The number of deformed joints as a surrogate measure of damage in rheumatoid arthritis. Arthritis Care & Research. 2002;47:67–72. doi: 10.1002/art1.10160. [DOI] [PubMed] [Google Scholar]
- 30.Sharp JT, Young DY, Bluhm GB, Brook A, Brower AC, Corbett M, et al. How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis & Rheumatism. 1985;28:1326–35. doi: 10.1002/art.1780281203. [DOI] [PubMed] [Google Scholar]
- 31.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–35. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 32.Bodmer JG, Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Charron D, et al. Nomenclature for factors of the HLA system, 1996. Tissue Antigens. 1997;49:297–321. doi: 10.1111/j.1399-0039.1997.tb02759.x. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Navarro-Cano G, del Rincón I, Pogosian S, Roldán JF, Escalante A. Association of mortality with disease severity in rheumatoid arthritis, independent of comorbidity. Arthritis & Rheumatism. 2003;48:2425–2433. doi: 10.1002/art.11127. [DOI] [PubMed] [Google Scholar]
- 36.Cox DR. Regression models and life tables. J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 37.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 38.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 39.Palloni A, Arias E. Paradox lost: explaining the Hispanic adult mortality advantage. Demography. 2004;41:385–415. doi: 10.1353/dem.2004.0024. [DOI] [PubMed] [Google Scholar]
- 40.Homa DM, Mannino DM, Lara M. Asthma mortality in US Hispanics of Mexican, Puerto Rican, and Cuban heritage, 1990–1995. American journal of respiratory and critical care medicine. 2000;161:504–509. doi: 10.1164/ajrccm.161.2.9906025. [DOI] [PubMed] [Google Scholar]
- 41.Hunt KJ, Williams K, Resendez RG, et al. All cause and cardiovascular mortality among diabetic participants in the San Antonio Heart Study: Evidence Against the “Hispanic Paradox. Diabetes Care. 2002;25:1557–63. doi: 10.2337/diacare.25.9.1557. [DOI] [PubMed] [Google Scholar]
- 42.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, Hazuda HP. All-cause and cardiovascular mortality among Mexican-American and non-Hispanic White older participants in the San Antonio Heart Study—evidence against the “Hispanic paradox”. American journal of epidemiology. 2000;158:1048–1057. doi: 10.1093/aje/kwg249. [DOI] [PubMed] [Google Scholar]
- 43.Bruce B, Fries JF, Murtagh KN. Health status disparities in ethnic minority patients with rheumatoid arthritis: a cross-sectional study. The Journal of rheumatology. 2007;34:1475–1479. [PubMed] [Google Scholar]
- 44.Constantinescu F, Goucher S, Weinstein A, Fraenkel L. Racial disparities in treatment preferences for rheumatoid arthritis. Medical care. 2009;47:350–355. doi: 10.1097/MLR.0b013e31818af829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yazici Y, Kautiainen H, Sokka T. Differences in clinical status measures in different ethnic/racial groups with early rheumatoid arthritis: implications for interpretation of clinical trial data. The Journal of rheumatology. 2007;34:311–315. [PubMed] [Google Scholar]
- 46.Abraído-Lanza AF, Chao MT, Flórez KR. Do healthy behaviors decline with greater acculturation? Implications for the Latino mortality paradox. Soc Sci Med. 2005;61:1243–55. doi: 10.1016/j.socscimed.2005.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coultas DB, Hanis CL, Howard CA, Skipper BJ, Samet JM. Heritability of ventilatory function in smoking and nonsmoking New Mexico Hispanics. American Journal of Respiratory and Critical Care Medicine. 1991;144:770–775. doi: 10.1164/ajrccm/144.4.770. [DOI] [PubMed] [Google Scholar]
- 48.Salari K, Choudhry S, Tang H, Naqvi M, Lind D, Avila PC, et al. Genetic admixture and asthma-related phenotypes in Mexican American and Puerto Rican asthmatics. Genetic epidemiology. 2005;29:76–86. doi: 10.1002/gepi.20079. [DOI] [PubMed] [Google Scholar]
- 49.Hanis CL, Hewett-Emmett D, Bertin TK, Schull WJ. Origins of US Hispanics: implications for diabetes. Diabetes Care. 1991;14:618–627. doi: 10.2337/diacare.14.7.618. [DOI] [PubMed] [Google Scholar]
- 50.Collins-Scramm HE, Chima B, Morii T, Wah K, Figueroa Y, Criswell LA, et al. Mexican American ancestry-informative markers: examination of population structure and marker characteristics in European Americans, Mexican Americans, Amerindians and Asians. Hum Genet. 2004;114:263–71. doi: 10.1007/s00439-003-1058-6. [DOI] [PubMed] [Google Scholar]


