Abstract
The human metabolome is a measurable outcome of interactions among an individual's inherited genome, microbiome, and dietary intake. We explored the relationship between dietary intake and serum untargeted metabolomic profiles in a subsample of 1,977 African Americans from the Atherosclerosis Risk in Communities (ARIC) Study in 1987–1989. For each metabolite, we conducted linear regression to estimate its relationships with each food group and food category. Potential confounding factors included age, sex, body mass index (weight (kg)/height (m)2), energy intake, kidney function, and food groups. We used a modified Bonferroni correction to determine statistical significance. In total, 48 pairs of diet-metabolite associations were identified, including multiple novel associations. The food group “sugar-rich foods and beverages” was inversely associated with 5 metabolites in the 2-hydroxybutyrate–related subpathway and positively associated with 5 γ-glutamyl dipeptides. The hypothesized mechanism of these associations may be through oxidative stress. “Sugar-rich foods and beverages” were also inversely associated with 7 unsaturated long-chain fatty acids. These findings suggest that the contribution of a sugar-rich dietary pattern to increased cardiovascular disease risk may be partially attributed to oxidative stress and disordered lipid profiles. Metabolomics may reveal novel metabolic biomarkers of dietary intake and provide insight into biochemical pathways underlying nutritional effects on disease development.
Keywords: African Americans, dietary habits, metabolomics
An unhealthy diet is considered 1 of the factors that has contributed to a rapid increase in the incidence of cardiovascular diseases. The framework in contemporary nutrition science has been largely reductionist in nature (e.g., focusing on deviations from the norm, 1 nutrient at a time) (1). However, emerging technologies, including metabolomics, permit investigation of the complex relationships among diet, lifestyle, and genes, and these relationships are reflected in metabolic intermediates of multiple physiological and cellular processes (2). Furthermore, metabolomics offers the potential to facilitate broad assessment of nutritional status (3) because it measures a range of small molecules that characterize the overall biological state of human health (4). Therefore, metabolomics could play an important role in the identification of novel biomarkers of dietary intake, as well as in the investigation of the mechanism of dietary effects on health and disease.
In a well-characterized, population-based sample of African Americans from the Atherosclerosis Risk in Communities (ARIC) Study, we explored cross-sectional associations of multiple named metabolites quantified by an untargeted high-throughput chromatography/mass spectrometry–based protocol with usual dietary intake measured by a semiquantitative food frequency questionnaire (FFQ). We aimed to identify metabolites pointing to pathways of nutrient metabolism.
STUDY DESIGN AND POPULATION
The ARIC Study is a prospective cohort study designed to investigate the causes and outcomes of cardiovascular disease in 15,792 individuals from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland). Detailed descriptions of its design, objectives, and procedures have been published elsewhere (5). ARIC participants underwent interviews, fasting venipuncture, and measurement of anthropometric characteristics at the baseline examination. Trained interviewers ascertained basic demographic data, medical history, and information about personal dietary habits.
At baseline, dietary intake was assessed by a 66-item interviewer-administered semiquantitative FFQ, which was a modified version (6) of the 61-item instrument developed by Willett et al. (7). Participants indicated the frequency with which they consumed specific foods and beverages according to 9 predefined frequency categories, ranging from never or less than 1 time per month to 6 or more times per day (standard portion sizes were given as a reference for intake estimation; pictures and food models were shown to the participants by the interviewers). Brand names of breakfast cereals most commonly consumed and use of salt in cooking and at the table were also ascertained. The reliability and validity of this FFQ has been assessed (6, 8). Food and beverages from the FFQ were categorized into 29 categories (9), and 20 categories were further collapsed into the following 5 food groups: meat (red and processed meat, pork, and chicken); dairy (milk, yogurt, cheese, and ice cream); fruits and vegetables; grains (whole grain and refined grain products, including biscuits, corn bread, white bread, snack chips, rice, pasta, and ready-to-eat cereal but not desserts); and sugar-rich foods and beverages (SRFB) (sugar-sweetened beverages, chocolate candy, candy without chocolate, cake, cookies, pie, donuts, Danish pastries, and brownies) (9). The other 9 single-food categories were tea, coffee, diet beverages, fruit juice, fried foods, fish and seafood, nuts and peanut butter, fat (butter and margarine), and eggs. Nutrient intakes were derived from the FFQ responses by using the Harvard Nutrient Database (10).
Metabolomic profiles were measured in a subsample of African American ARIC participants at the baseline examination, including individuals randomly selected from the Jackson, Mississippi, field center. The participants consented to genetic research, provided high-quality dietary intake data, and fasted for 8 hours or more before the baseline examination (n = 1,977). Among these participants with available metabolomic data, 1,500 randomly selected participants were included in our discovery set, and the remaining 477 participants made up the replication set. Metabolite profiling was completed in June 2010 using fasting serum samples that had been stored at −80°C since their collection at baseline in 1986–1987. An untargeted, gas chromatography/mass spectrometry and liquid chromatography/mass spectrometry–based metabolomic quantification protocol was used by Metabolon, Inc. (Durham, North Carolina) to detect and analyze serum samples (11, 12). A summary of the assay procedures has been presented in a previous publication (13). This untargeted approach identified and quantified named compounds the chemical identities of which were known, as well as additional unnamed compounds that did not currently have chemical standards. Only the named compounds were included in the current study. Local institutional review boards approved the ARIC protocol, and all subjects gave written informed consent.
Body mass index (weight (kg)/height (m)2) was measured by field center staff. Race/ethnicity was self-reported. Estimated glomerular filtration rate (in mL/min/1.73 m2) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (14). Alcohol consumption was ascertained in a dietary interview conducted by trained interviewers. Subjects were asked whether they currently drank alcoholic beverages and, if not, whether they ever had. Cigarette smoking status was self-reported and categorized as current or noncurrent smoker. Prevalent diabetes was defined as having a fasting glucose level of 126 mg/dL or higher (15) or currently taking diabetes medication. Prevalent hypertension was defined as having systolic blood pressure of 140 mm Hg or higher, having diastolic blood pressure of 90 mm Hg or higher, or currently taking antihypertensive medication.
STATISTICAL ANALYSIS
On the basis of both practical and theoretical considerations, we divided the 356 named metabolites into 3 groups according to their percent of values that were missing or below the detection limit (BDL) for that metabolite among all 1,977 observations in the combined sample. Group 1 contains 308 metabolites with less than 50% of observations having values that were missing or BDL. The levels of group 1 metabolites were presented by a continuous variable during data analysis with missing/BDL values replaced by the lowest detected values. Group 2 contains 29 metabolites that have a moderate amount of missing/BDL values (50%–80% of observations). For group 2 metabolites, we considered the missing/BDL values as category 1, the measured (i.e., nonmissing/non-BDL) values below the median as category 2, and the measured values above the median as category 3. Group 3 contains 19 metabolites that have more than 80% of observations with missing/BDL values, and we considered the missing/BDL values as category 1 and the measured values as category 2. During data analysis, the levels of group 2 and group 3 metabolites were presented by an ordinal variable using the above-mentioned categories in each group.
Data were presented as means and standard errors for the continuous variables and frequencies (%) for the categorical variables. Baseline characteristics were compared using the χ2 test for categorical variables and a 2-sample t test for continuous variables. For each metabolite, linear regression was conducted to estimate its relationship with each of the food groups/categories in both the discovery sample and the replication sample. Adjustments were made for age, sex, body mass index, energy intake per day, kidney function measured by estimated glomerular filtration rate, and food groups. The food groups were entered in the regression together, whereas the single-food categories were entered separately, as shown in the following formula. For food groups in quintiles:
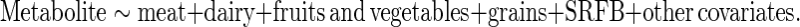 |
For each single-food category in quintiles:
 |
The β coefficients in the linear models represent the change in standard deviation of each metabolite in group 1, or the change in the categorical unit of each metabolite in groups 2 and 3 for an increase of 1 quintile of each food group/category. The corresponding P values for trend across quintiles of each food group/category were also calculated. Linearity in the associations of food groups/categories with metabolite levels was examined and not generally rejected by introducing a quadratic term of the respective food group/category variable in the above linear regressions (data not shown).
A modified Bonferroni procedure was used in the discovery set to consider the correlations among metabolites and to correct for multiple comparisons (16, 17). This adjustment takes into account the full correlation matrix of metabolites and uses the mean correlation among the metabolites in the formula, where the new α level for the kth hypothesis for k = 1, 2, …, K is readjusted for each individual metabolite according to  , where
, where 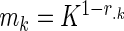 ,
, 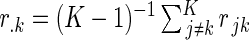 , and
, and  is the correlation coefficient between the jth and kth metabolites. When the average of the correlation coefficients is 0, this adjustment is according to the Bonferroni procedure, and when it is 1, the adjusted and the unadjusted P values are the same. In the discovery set, for the test of association of a metabolite and food groups, the significance level was 1.9 × 10−4; and for the test of association of a metabolite and each single-food category, the significance level was 2.1 × 10−5, acknowledging the multiple single-food categories (n = 9) with pure Bonferroni correction.
is the correlation coefficient between the jth and kth metabolites. When the average of the correlation coefficients is 0, this adjustment is according to the Bonferroni procedure, and when it is 1, the adjusted and the unadjusted P values are the same. In the discovery set, for the test of association of a metabolite and food groups, the significance level was 1.9 × 10−4; and for the test of association of a metabolite and each single-food category, the significance level was 2.1 × 10−5, acknowledging the multiple single-food categories (n = 9) with pure Bonferroni correction.
In a secondary analysis including all 1,977 participants, SRFB-related metabolites were further analyzed for their associations with sugar nutrient intake (i.e., fructose, lactose, sucrose, and percent of total energy intake from carbohydrate) by general linear regression. In addition to the nutrient variable, the multivariable model included age, sex, body mass index, energy intake per day, and kidney function as adjustment covariates. The significance level was set at 4.8 × 10−5, acknowledging the 4 groups of nutrient intake measurement (i.e., fructose, lactose, sucrose, and percent of total energy intake from carbohydrate). All statistical analyses were performed in SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Summary demographic and dietary information of the study population in both the discovery and replication samples is presented in Table 1. Subjects in the study sample were generally middle-aged and overweight. As expected, participants from the discovery sample and replication sample were similar. The study participants consumed more daily servings of food groups than of single-food categories, consistent with the definition of food groups. They had more servings of fruits and vegetables and grains per day than they had of the other food groups/categories.
Table 1.
Participant Baseline Characteristics in the Discovery and Replication Samples of African Americans From the Jackson, Mississippi, Field Center of the Atherosclerosis Risk in Communities Study, 1987–1989a
| Characteristic | Discovery Set (n = 1,500) |
Replication Set (n = 477) |
||||
|---|---|---|---|---|---|---|
| No. | % | Mean (SE) | No. | % | Mean (SE) | |
| Age, years | 52.92 (5.8) | 52.74 (5.7) | ||||
| Male sex | 524 | 34.9 | 178 | 37.3 | ||
| Body mass indexb | 29.68 (6.2) | 29.58 (5.5) | ||||
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 104.30 (18.3) | 104.50 (17.9) | ||||
| Total energy intake per day, calories | 1,574.00 (639.4) | 1,579.50 (568.4) | ||||
| Current alcohol drinker | 455 | 30.3 | 126 | 26.4 | ||
| Current cigarette smoker | 438 | 29.2 | 131 | 27.5 | ||
| Prevalent diabetes | 240 | 16.0 | 79 | 16.6 | ||
| Prevalent hypertension | 789 | 52.6 | 261 | 54.7 | ||
| Food groups, servings/dayc | ||||||
| Dairy | 1.16 (1.2) | 1.14 (1.1) | ||||
| Fruits and vegetables | 3.27 (2.1) | 3.36 (2.2) | ||||
| Grains | 2.80 (1.6) | 2.84 (1.5) | ||||
| Meat | 1.51 (0.9) | 1.55 (0.8) | ||||
| Sugar-rich foods and beverages | 1.65 (1.5) | 1.61 (1.3) | ||||
| Single-food categories, servings/day | ||||||
| Coffee | 1.02 (1.3) | 0.94 (1.3) | ||||
| Diet soft drinks | 0.29 (0.6) | 0.32 (0.7) | ||||
| Eggs | 0.36 (0.5) | 0.34 (0.5) | ||||
| Fat (butter or margarine) | 0.81 (0.8) | 0.81 (0.8) | ||||
| Fish and seafood | 0.42 (0.4) | 0.41 (0.3) | ||||
| Fried foods | 0.59 (0.6) | 0.60 (0.6) | ||||
| Fruit juice | 0.50 (0.6) | 0.53 (0.7) | ||||
| Nuts and peanut butter | 0.25 (0.4) | 0.24 (0.4) | ||||
| Tea | 0.38 (0.7) | 0.44 (0.8) | ||||
Abbreviation: SE, standard error.
a No statistical significant difference was detected between the discovery set and the replication set (P < 0.05).
b Weight (kg)/height (m)2.
c “Meat” consisted of processed meat, red meat, pork and poultry; “dairy” consisted of milk, yogurt, cheese, and ice cream; “fruits and vegetables” consisted of fruits (not including juice), cruciferous vegetables, carotene vegetables, dark-leaf vegetables, legumes, potatoes, tomatoes, and other vegetables; “grains” consisted of whole grain and refined grain products (not including grain desserts); and “sugar-rich foods and beverages” consisted of candy, desserts, and sugared drinks (soda and fruit-flavored drinks).
Among the 356 named metabolites, associations of 48 metabolite-diet pairs were identified and replicated to be statistically significant (Tables 2 and 3). The SRFB group was related to more metabolites than the other food groups. There were 23 metabolites significantly related to SRFB intake, including 7 unsaturated long-chain fatty acids, five 2-hydroxybutyrate–related metabolites, 2 sex steroids, 5 γ-glutamyl dipeptides, and 4 metabolites in other pathways (Table 2). Among the SRFB-associated metabolites, we observed significant associations with sucrose intake and percent of total energy intake from carbohydrate (Table 4). These data suggest that sucrose may be the central agent underlying the observed SRFB-metabolite association.
Table 2.
Metabolites Significantly Associated With Intakes of Food Groups, “Sugar-Rich Foods and Beverages” and “Fruits and Vegetables,” Among African Americans From the Jackson, Mississippi, Field Center of the Atherosclerosis Risk in Communities Study, 1987–1989
| Metabolitea, by Food Group | Biological Characteristics | Discovery Set (n = 1,500) |
Replication Set (n = 477) |
||
|---|---|---|---|---|---|
| β | P Value | β | P Value | ||
| Sugar-rich foods and beverages | |||||
| Docosapentaenoate (n3 DPA; 22:5n3) | Unsaturated long chain | −0.10 | 1.19 × 10−4 | −0.08 | 3.56 × 10−2 |
| 10-Nonadecenoate (19:1n9) | Fatty acids | −0.11 | 1.15 × 10−5 | −0.12 | 1.01 × 10−3 |
| Adrenate (22:4n6) | −0.09 | 1.35 × 10−4 | −0.18 | 9.26 × 10−6 | |
| Dihomo-linoleate (20:2n6) | −0.14 | 1.19 × 10−8 | −0.12 | 7.51 × 10−4 | |
| Eicosenoate (20:1n9 or 11) | −0.13 | 1.12 × 10−7 | −0.12 | 1.00 × 10−3 | |
| Oleate (18:1n9) | −0.11 | 8.69 × 10−6 | −0.12 | 8.16 × 10−4 | |
| Palmitoleate (16:1n7) | −0.10 | 7.11 × 10−5 | −0.12 | 2.10 × 10−3 | |
| 2-Aminobutyrate | 2-Hydroxybutyrate–related metabolites | −0.14 | 5.45 × 10−9 | −0.12 | 3.53 × 10−3 |
| 2-Hydroxybutyrate | −0.16 | 7.45 × 10−10 | −0.14 | 6.48 × 10−5 | |
| 2-Hydroxyisobutyrate | −0.10 | 6.11 × 10−5 | −0.10 | 8.86 × 10−3 | |
| 3-Hydroxyisobutyrate | −0.12 | 1.15 × 10−6 | −0.17 | 1.20 × 10−5 | |
| α-Hydroxyisovalerate | −0.09 | 1.59 × 10−4 | −0.10 | 3.23 × 10−2 | |
| 4-Androsten-3β,17β-diol disulfate 1 | Sex steroids | −0.12 | 8.53 × 10−8 | −0.21 | 9.96 × 10−7 |
| 5α-Androstan-3β,17β-diol disulfate | −0.10 | 3.70 × 10−6 | −0.13 | 2.32 × 10−4 | |
| Glycerol | Lipid | −0.10 | 3.78 × 10−5 | −0.07 | 3.22 × 10−2 |
| γ-Glutamylglutamate | γ-Glutamyl dipeptides | 0.11 | 1.06 × 10−5 | 0.10 | 1.56 × 10−2 |
| γ-Glutamylisoleucine | 0.11 | 5.79 × 10−6 | 0.10 | 1.01 × 10−2 | |
| γ-Glutamylleucine | 0.10 | 8.78 × 10−5 | 0.11 | 1.03 × 10−2 | |
| γ-Glutamylthreonine | 0.13 | 9.05 × 10−8 | 0.14 | 1.45 × 10−3 | |
| γ-Glutamyltyrosine | 0.14 | 2.74 × 10−8 | 0.10 | 1.39 × 10−2 | |
| Creatine | In amino acid pathway | −0.10 | 9.62 × 10−6 | −0.10 | 6.02 × 10−3 |
| Malate | In Krebs cycle pathway | −0.10 | 1.25 × 10−4 | −0.11 | 2.41 × 10−3 |
| Theobromine | In xanthine metabolism | 0.10 | 2.66 × 10−5 | 0.10 | 2.57 × 10−2 |
| Fruits and vegetables | |||||
| Glycerate | In carbohydrate pathway | 0.11 | 1.21 × 10−6 | 0.13 | 1.30 × 10−4 |
a All metabolites listed were group 1 metabolites.
Table 3.
Metabolites Significantly Associated With Intakes of Single-Food Categories Among African Americans From the Jackson, Mississippi, Field Center of the Atherosclerosis Risk in Communities Study, 1987–1989
| Metabolite, by Single-Food Category | Biological Characteristics | Discovery Set (n = 1,500) |
Replication Set (n = 477) |
||
|---|---|---|---|---|---|
| β | P Value | β | P Value | ||
| Coffee | |||||
| Quinatea | Quinate is an antioxidant (51) and a phenolic product of chlorogenic acid, which is a major polyphenol in coffee (52); the other metabolites are metabolites of caffeine and exhibit both antioxidant and prooxidant properties (53) | 0.11 | 2.33 × 10−12 | 0.12 | 1.52 × 10−7 |
| Paraxanthine | 0.17 | <10−20 | 0.18 | 6.46 × 10−10 | |
| 5-Acetylamino-6-amino-3-methyluracil | 0.13 | 8.23 × 10−16 | 0.12 | 3.67 × 10−7 | |
| 1,7-Dimethylurate | 0.14 | 1.70 × 10−18 | 0.12 | 2.85 × 10−6 | |
| 1-Methylurate | 0.12 | 4.60 × 10−13 | 0.08 | 3.01 × 10−5 | |
| 1-Methylxanthinea | 0.08 | 2.33 × 10−12 | 0.07 | 4.20 × 10−4 | |
| Caffeine | 0.08 | 4.59 × 10−8 | 0.12 | 3.58 × 10−4 | |
| 1,3,7-Trimethyluratea | 0.10 | 3.14 × 10−15 | 0.07 | 2.34 × 10−3 | |
| 7-Methylxanthinea | 0.10 | 1.21 × 10−16 | 0.09 | 1.98 × 10−5 | |
| Eggs | |||||
| Docosapentaenoate (n6 DPA; 22:5n6) | An essential fatty acid | 0.15 | 2.84 × 10−14 | 0.15 | 1.31 × 10−6 |
| Fish and seafood | |||||
| Eicosapentaenoate (EPA; 20:5n3) | Essential fatty acid, reflecting habitual fish and seafood intake (54) | 0.09 | 8.39 × 10−6 | 0.06 | 4.96 × 10−2 |
| Docosahexaenoate (DHA; 22:6n3) | 0.14 | 1.14 × 10−11 | 0.13 | 3.53 × 10−5 | |
| CMPF | An endogenous metabolite in humans of furan fatty acid; found in significant amounts in fish | 0.12 | 2.66 × 10−10 | 0.07 | 1.53 × 10−2 |
| Fruit juice | |||||
| Glycerate | Carbohydrate pathway | 0.15 | 6.65 × 10−18 | 0.11 | 2.18 × 10−5 |
| Stachydrine | Both are present in citrus juices at significant levels (36) | 0.19 | <10−20 | 0.15 | 4.64 × 10−9 |
| N-methyl proline | 0.17 | 1.00 × 10−20 | 0.15 | 1.60 × 10−6 | |
| Threonate | An active metabolite of ascorbic acid | 0.11 | 1.22 × 10−9 | 0.10 | 2.56 × 10−5 |
| Scyllo-inositol | Present in grapes and citrus juices (38); a potential therapeutic agent for Alzheimer's disease (55) | 0.08 | 4.57 × 10−6 | 0.07 | 1.62 × 10−3 |
| Homostachydrine | A potential derivative of pipecolic acid; occurs in citrus plants (37) | −0.09 | 8.18 × 10−7 | −0.11 | 2.93 × 10−4 |
| Nuts and peanut butter | |||||
| Tryptophan betaine | Accumulates in the seeds of most Erythrina species (56) | 0.25 | <10−20 | 0.26 | 3.54 × 10−14 |
| 2-Methylbutyroylcarnitine | In amino acid pathway | −0.09 | 1.59 × 10−7 | −0.11 | 2.08 × 10−4 |
| 4-Vinylphenol sulfate | Responsible for the characteristic flavor of cooked soybeans (57) | 0.08 | 9.50 × 10−7 | 0.16 | 2.97 × 10−4 |
| 5α-Androstan-3β,17β-diol disulfate | Sex steroid | −0.08 | 4.18 × 10−6 | −0.09 | 9.54 × 10−4 |
| 4-Androsten-3β,17β-diol disulfate 1 | −0.08 | 9.70 × 10−7 | −0.09 | 5.62 × 10−3 | |
Abbreviations: BDL, below the detection limit; CMPF, 3-carboxy-4-methyl-5-propyl-2-furanpropanoate.
a Group 2 metabolites with 50%–80% of values that were missing/BDL. We considered these values as category 1. For the measured values (nonmissing/non-BDL), we considered values below the median as category 2 and values above the median as category 3. These categories formed an ordinal variable, which was used during data analysis. All other metabolites (without footnote designator “a” were group 1 metabolites.
Table 4.
Selected Metabolitesa Significantly Associated With Dietary Sucrose and Carbohydrate Intakes Among African Americans From the Jackson, Mississippi, Field Center of the Atherosclerosis Risk in Communities Study, 1987–1989b
| Metabolitec | Dietary Sucrose (in g) |
Carbohydrate (in % kcal) |
||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| 10-Nonadecenoate (19:1n9) | −0.004 | 6.08 × 10−7 | Not significant | |
| 2-Aminobutyrate | −0.005 | 1.59 × 10−9 | −0.016 | 4.43 × 10−11 |
| 2-Hydroxybutyrate | −0.005 | 2.05 × 10−10 | −0.014 | 1.46 × 10−9 |
| 3-Hydroxyisobutyrate | −0.004 | 1.20 × 10−6 | −0.010 | 1.66 × 10−5 |
| 4-Androsten-3β,17β-diol disulfate 1 | −0.005 | 1.87 × 10−8 | −0.015 | 2.01 × 10−10 |
| 5α-Androstan-3β,17β-diol disulfate | −0.003 | 1.50 × 10−5 | −0.010 | 2.02 × 10−6 |
| Adrenate (22:4n6) | Not significant | −0.010 | 3.34 × 10−5 | |
| α-Hydroxyisovalerate | −0.003 | 3.99 × 10−5 | −0.012 | 1.11 × 10−6 |
| Dihomo-linoleate (20:2n6) | −0.005 | 6.69 × 10−8 | Not significant | |
| Eicosenoate (20:1n9 or 11) | −0.005 | 6.61 × 10−9 | Not significant | |
| γ-Glutamylglutamate | 0.004 | 1.90 × 10−6 | 0.014 | 1.09 × 10−8 |
| γ-Glutamylisoleucine | 0.005 | 3.77 × 10−8 | 0.011 | 1.05 × 10−5 |
| γ-Glutamylleucine | 0.004 | 1.01 × 10−6 | 0.011 | 4.21 × 10−6 |
| γ-Glutamylthreonine | 0.004 | 6.92 × 10−7 | 0.010 | 2.53 × 10−5 |
| γ-Glutamyltyrosine | 0.005 | 1.74 × 10−9 | 0.010 | 2.13 × 10−5 |
| Malate | −0.005 | 7.74 × 10−8 | Not significant | |
| Oleate (18:1n9) | −0.004 | 8.25 × 10−6 | Not significant | |
| Theobromine | 0.004 | 2.02 × 10−5 | Not significant | |
a Only the significant associations between the sugar-rich food and beverage–related metabolites and sugar nutrient intake are presented in this table.
b In addition to intake of sucrose or carbohydrate, the multivariable models included age, sex, body mass index (weight (kg)/height (m)2), energy intake per day, and kidney function as adjustment covariates. The significance level was set at 4.8 × 10−5, acknowledging the 4 groups of nutrient intake measured (i.e., fructose, lactose, sucrose, and percent of total energy intake from carbohydrate). No significant association of metabolites with fructose or lactose was identified.
c All metabolites listed were group 1 metabolites.
DISCUSSION
We used untargeted metabolomics and FFQ-based dietary information to identify and replicate metabolomic biomarkers of habitual dietary intake in a well-defined sample of African Americans from the ARIC Study. In the following paragraphs, we discuss our findings of diet-metabolite associations and their potential implications in diet-disease research. Special emphasis is given to discussion of those observations which, to our knowledge, are novel.
SRFB-related metabolites
The SRFB group, which is the sum of daily servings of candy, desserts, and sugar-sweetened beverages, was inversely associated with 5 metabolites in the 2-hydroxybutyrate–related subpathway and positively associated with 5 γ-glutamyl dipeptides (Table 2). We speculate that the associations between SRFB and these metabolites may be through oxidative stress. High-sugar diets cause chronic hyperglycemia-induced oxidative stress (18), resulting in biosynthesis of γ-glutamyl dipeptides, which are then transported across cell membranes (19). 2-hydroxybutyrate is a byproduct of the methionine-to-glutathione pathway, and its production is directly related to the rate of hepatic glutathione synthesis as oxidative stress increases (20). These 2 pathways were also identified to be associated with alcohol consumption in a previous metabolomic study (21). Additionally, the metabolite creatine, which is also a potential antioxidant, was inversely associated with SRFB intake. The above information supports the hypothesis that the unfavorable effects of SRFB on health and diseases, such as hypertension (22, 23) and diabetes (24), may be at least partially through oxidative stress.
The SRFB group was inversely associated with 7 unsaturated long-chain fatty acids (Table 2). The precise mechanisms of these associations need further clarification. Sugar-sweetened beverages have been consistently reported to be inversely associated with high-density lipoprotein cholesterol levels (25, 26) and positively associated with low-density lipoproteins and triglycerides (26). Our novel finding of an inverse association between SRFB and unsaturated long-chain fatty acids suggests that the potential contribution of foods rich in added sugar to cardiovascular disease risk (27) may be partially attributable to abnormal lipid profiles.
Two sex steroids, 4-androsten-3β,17β-diol disulfate 1 (a testosterone precursor in vivo (28)) and 5α-androstan-3β,17β-diol disulfate (a metabolite of the androgen 5α-dihydrotestosterone (29)), were inversely associated with intakes of both SRFB and nuts and peanut butter (Tables 2 and 3), which is, to our knowledge, a novel observation. Sex-stratified results are presented in Figures 1 and 2, respectively, for 4-androsten-3β,17β-diol disulfate 1 and 5α-androstan-3β,17β-diol disulfate, with exogenous sex hormone takers excluded in the combined sample (n = 1,783), showing consistent associations across men and women. This finding is consistent with recent feeding studies showing that glucose ingestion induced a reduction in testosterone levels in men (30), and serum testosterone levels were higher in the fasting state (31). The influence of blood glucose on sex steroid levels may be via several mechanisms, including gene regulation (32) and modulation of hormone secretion from peripheral endocrine glands (30). Peanuts contain phytoestrogens, such as isoflavonoids and lignans, which exert estrogen-like effects (33) and, thus, may inhibit the levels of male hormones. A recent study indicates that sugar-rich beverages may be a risk factor for endometrial cancer (34), and the association between endogenous sex hormone levels and the risk of breast cancer in postmenopausal women has been well established. Therefore, our findings that dietary SRFB and nuts and peanut butter are associated with sex steroids may be of interest for future study. Our findings of the associations between SRFB and their related metabolites provide further evidence to support the 2010 Dietary Guidelines for Americans, which recommend reduced intakes of foods and beverages with added sugars (35).
Figure 1.
Least squares mean levels of 4-androsten-3β,17β-diol disulfate 1 by A) quintile of sugar-rich food and beverage consumption among women; B) quintile of sugar-rich food and beverage consumption among men; C) quintile of nut and peanut butter consumption among women; and D) quintile of nuts and peanut butter consumption among men. Sample includes 1,783 African American Atherosclerosis Risk in Communities (ARIC) Study participants not taking exogenous sex hormones, from the Jackson, Mississippi, field center of the ARIC Study, 1987–1989. The P values for trend of sex steroid levels across quintiles of sugar-rich food and beverage consumption among A) women and B) men are 8.2 × 10−3 and 7.7 × 10−9, respectively. The P values for trend across quintiles of nut and peanut butter consumption among C) women and D) men are 1.7 × 10−6 and 1.8 × 10−3, respectively.
Figure 2.
Least squares mean levels of 5α-androstan-3β,17β-diol disulfate by A) quintile of sugar-rich food and beverage consumption among women; B) quintile of sugar-rich food and beverage consumption among men; C) quintile of nut and peanut butter consumption among women; and D) quintile of nut and peanut butter consumption among men. Sample includes 1,783 African American Atherosclerosis Risk in Communities (ARIC) Study participants not taking exogenous sex hormones, from the Jackson, Mississippi, field center of the ARIC Study, 1987–1989. The P values for trend of sex steroid levels across quintiles of sugar-rich food and beverage consumption among A) women and B) men are 1.9 × 10−2 and 2.8 × 10−6, respectively. The P values for trend across quintiles of nut and peanut butter consumption among C) women and D) men are 1.0 × 10−5 and 6.9 × 10−4, respectively.
Other food-related metabolites
A few established food-metabolite associations emerged as positive controls (Table 3). First, the metabolites significantly related to coffee were established metabolites of coffee intake. Second, fish and seafood are predominant sources of intake for eicosapentaenoate and docosahexaenoate in humans, and these metabolites were significantly associated with fish and seafood intake in our study. Third, most of the identified and replicated fruit juice–related metabolites, such as stachydrine and threonate, were previously reported to be found in fruit juice (36–38).
There have been several previous studies investigating the relationship between metabolomic biomarkers and dietary intake, but most have considered only 1 or a few food groups (39–42) or used targeted measurements to focus on a few metabolic pathways (43–45). To our knowledge, this study is the first population-based nutrition study using untargeted metabolomics to investigate the association of the human metabolome with a wide spectrum of dietary habits. Nevertheless, measurement variation in dietary intake and the metabolome, unaccounted differences in the gut microbiome and other covariates, differences in sample collection (e.g., serum vs. urine), and chance variation may need to be accounted for as the field considers observed inconsistencies.
In the ARIC Study, the metabolomic data are available in a subset of African American participants, but these data represent the largest sample size of metabolomic measurements in African Americans reported thus far. The ARIC blood samples were stored for 2 decades before measurement of serum metabolome. Because the metabolomic profiling techniques have recently emerged, there is no documentation regarding the stability of measuring metabolomic markers in samples stored for such a long period of time. Multiple freeze-thaw studies (46–50) have suggested that small metabolites are more stable than other larger biomarkers. In the ARIC Study, the correlations between measurements of the common blood analytes at baseline in 1987 and those in 2010 were high (e.g., for glucose in mg/dL, r = 0.9; for cholesterol in mmol/L, r = 0.7; and for blood urea nitrogen in mg/dL, r = 0.9). Furthermore, we expect that the long-term stability would not affect our overall conclusions about the relationship of food intake with the serum metabolome.
Based on the current incomplete knowledge of the composition of foods and their metabolism, we used an untargeted “bottom-up” metabolomic approach to study the relationship between regular dietary intake and the many small molecules making up the human metabolome. In total, we identified and replicated 48 pairs of associations between a metabolite and food group/category in a large community-based sample of African Americans. Established, as well as novel, metabolomic biomarkers of dietary intake emerged. The SRFB food group was associated with more metabolites than the other food groups/categories, and some of these associations may be through oxidative stress mechanisms. These associations provide novel functional insights for diet-disease associations that have been reported in previous studies. Application of metabolomics within human nutrition science will promote research aiming to evaluate diet-disease relationships.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Human Genetics, and Environmental Sciences, University of Texas Health Science Center at Houston, Houston, Texas (Yan Zheng, Bing Yu, Eric Boerwinkle); Metabolon, Inc., Durham, North Carolina (Danny Alexander); Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (Lyn M. Steffen); and Human Genome Sequencing Center, Baylor College of Medicine, Houston, Texas (Eric Boerwinkle).
The Atherosclerosis Risk in Communities Study is a collaborative study supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN2682011-00008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The metabolomics research was sponsored by the National Human Genome Research Institute (grant 3U01HG004402-02S1). Y.Z. and B.Y. are supported in part by a training fellowship from the Burroughs Wellcome Fund (grant 1008200).
Conflict of interest: none declared.
REFERENCES
- 1.Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu Rev Nutr. 2012;32:183–202. doi: 10.1146/annurev-nutr-072610-145159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercuro G, Bassareo PP, Deidda M, et al. Metabolomics: A new era in cardiology? J Cardiovasc Med (Hagerstown) 2011;12(11):800–805. doi: 10.2459/JCM.0b013e32834a658f. [DOI] [PubMed] [Google Scholar]
- 3.Zivkovic AM, German JB. Metabolomics for assessment of nutritional status. Curr Opin Clin Nutr Metab Care. 2009;12(5):501–507. doi: 10.1097/MCO.0b013e32832f1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins SM, German JB. Toward the implementation of metabolomic assessments of human health and nutrition. Curr Opin Biotechnol. 2002;13(5):512–516. doi: 10.1016/s0958-1669(02)00363-4. [DOI] [PubMed] [Google Scholar]
- 5.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 6.Stevens J, Metcalf PA, Dennis BH, et al. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutr Res. 1996;16(5):735–745. [Google Scholar]
- 7.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 8.Bidulescu A, Chambless LE, Siega-Riz AM, et al. Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr J. 2009;8:14. doi: 10.1186/1475-2891-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities Study. Circulation. 2008;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 10.Nettleton JA, Volcik KA, Hoogeveen RC, et al. Carbohydrate intake modifies associations between ANGPTL4[E40K] genotype and HDL-cholesterol concentrations in white men from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2009;203(1):214–220. doi: 10.1016/j.atherosclerosis.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans AM, DeHaven CD, Barrett T, et al. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81(16):6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 12.Ohta T, Masutomi N, Tsutsui N, et al. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol. 2009;37(4):521–535. doi: 10.1177/0192623309336152. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Yu B, Alexander D, et al. Associations between metabolomic compounds and incident heart failure among African Americans: the ARIC Study. Am J Epidemiol. 2013;178(4):534–542. doi: 10.1093/aje/kwt004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Duynhoven J, Vaughan EE, Jacobs DM, et al. Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci U S A. 2011;108(suppl 1):4531–4538. doi: 10.1073/pnas.1000098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16(22):2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Blakesley RE, Mazumdar S, Dew MA, et al. Comparisons of methods for multiple hypothesis testing in neuropsychological research. Neuropsychology. 2009;23(2):255–264. doi: 10.1037/a0012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279(41):42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 19.Soga T, Sugimoto M, Honma M, et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol. 2011;55(4):896–905. doi: 10.1016/j.jhep.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Lord RS, Bralley JA. Clinical applications of urinary organic acids. Part I: detoxification markers. Altern Med Rev. 2008;13(3):205–215. [PubMed] [Google Scholar]
- 21.Zheng Y, Yu B, Alexander D, et al. Metabolomic patterns and alcohol consumption among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. doi: 10.3945/ajcn.113.074070. [published online ahead of print April 23, 2014] doi:10.3945/ajcn.113.074070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preuss HG, Knapka JJ, MacArthy P, et al. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. Am J Hypertens. 1992;5(9):585–591. doi: 10.1093/ajh/5.9.585. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Caballero B, Mitchell DC, et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation. 2010;121(22):2398–2406. doi: 10.1161/CIRCULATIONAHA.109.911164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu S, Yoffe P, Hills N, et al. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS One. 2013;8(2):e57873. doi: 10.1371/journal.pone.0057873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosova EC, Auinger P, Bremer AA. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J Acad Nutr Diet. 2013;113(2):219–227. doi: 10.1016/j.jand.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh JA, Sharma A, Cunningham SA, et al. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123(3):249–257. doi: 10.1161/CIRCULATIONAHA.110.972166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard BV, Wylie-Rosett J. Sugar and cardiovascular disease: a statement for healthcare professionals from the Committee on Nutrition of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2002;106(4):523–527. doi: 10.1161/01.cir.0000019552.77778.04. [DOI] [PubMed] [Google Scholar]
- 28.Chen F, Knecht K, Leu C, et al. Partial agonist/antagonist properties of androstenedione and 4-androsten-3beta,17beta-diol. J Steroid Biochem Mol Biol. 2004;91(4-5):247–257. doi: 10.1016/j.jsbmb.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caronia LM, Dwyer AA, Hayden D, et al. Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin Endocrinol (Oxf) 2013;78(2):291–296. doi: 10.1111/j.1365-2265.2012.04486.x. [DOI] [PubMed] [Google Scholar]
- 31.Sartorius G, Spasevska S, Idan A, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the Healthy Man Study. Clin Endocrinol (Oxf) 2012;77(5):755–763. doi: 10.1111/j.1365-2265.2012.04432.x. [DOI] [PubMed] [Google Scholar]
- 32.Selva DM, Hogeveen KN, Innis SM, et al. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117(12):3979–3987. doi: 10.1172/JCI32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgs J. The beneficial role of peanuts in the diet—Part 2. Nutr Food Sci. 2003;33(2):56–64. [Google Scholar]
- 34.Inoue-Choi M, Robien K, Mariani A, et al. Sugar-sweetened beverage intake and the risk of type I and type II endometrial cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2384–2394. doi: 10.1158/1055-9965.EPI-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv Nutr. 2011;2(3):293–294. doi: 10.3945/an.111.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Servillo L, Giovane A, Balestrieri ML, et al. Proline derivatives in fruits of bergamot (Citrus bergamia Risso et Poit): presence of N-methyl-L-proline and 4-hydroxy-L-prolinebetaine. J Agric Food Chem. 2011;59(1):274–281. doi: 10.1021/jf102833v. [DOI] [PubMed] [Google Scholar]
- 37.Servillo L, Giovane A, Balestrieri ML, et al. Occurrence of pipecolic acid and pipecolic acid betaine (homostachydrine) in Citrus genus plants. J Agric Food Chem. 2012;60(1):315–321. doi: 10.1021/jf204286r. [DOI] [PubMed] [Google Scholar]
- 38.Sanz ML, Villamiel M, Martínez-Castro I. Inositols and carbohydrates in different fresh fruit juices. Food Chem. 2004;87(3):325–328. [Google Scholar]
- 39.Vrhovsek U, Masuero D, Gasperotti M, et al. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J Agric Food Chem. 2012;60(36):8831–8840. doi: 10.1021/jf2051569. [DOI] [PubMed] [Google Scholar]
- 40.Wong M, Lodge JK. A metabolomic investigation of the effects of vitamin E supplementation in humans. Nutr Metab. 2012;9(1):110. doi: 10.1186/1743-7075-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pujos-Guillot E, Hubert J, Martin JF, et al. Mass spectrometry–based metabolomics for the discovery of biomarkers of fruit and vegetable intake: citrus fruit as a case study. J Proteome Res. 2013;12(4):1645–1659. doi: 10.1021/pr300997c. [DOI] [PubMed] [Google Scholar]
- 42.Redeuil K, Smarrito-Menozzi C, Guy P, et al. Identification of novel circulating coffee metabolites in human plasma by liquid chromatography–mass spectrometry. J Chromatogr A. 2011;1218(29):4678–4688. doi: 10.1016/j.chroma.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 43.Menni C, Zhai G, Macgregor A, et al. Targeted metabolomics profiles are strongly correlated with nutritional patterns in women. Metabolomics. 2013;9(2):506–514. doi: 10.1007/s11306-012-0469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mensack MM, McGinley JN, Thompson HJ. Metabolomic analysis of the effects of edible dry beans (Phaseolus vulgaris L.) on tissue lipid metabolism and carcinogenesis in rats. Br J Nutr. 2012;108(suppl 1):S155–S165. doi: 10.1017/S0007114512000827. [DOI] [PubMed] [Google Scholar]
- 45.Oberbach A, Blüher M, Wirth H, et al. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J Proteome Res. 2011;10(10):4769–4788. doi: 10.1021/pr2005555. [DOI] [PubMed] [Google Scholar]
- 46.Brinc D, Chan MK, Venner AA, et al. Long-term stability of biochemical markers in pediatric serum specimens stored at −80°C: a CALIPER Substudy. Clin Biochem. 2012;45(10-11):816–826. doi: 10.1016/j.clinbiochem.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerman LJ, Li M, Yarbrough WG, et al. Global stability of plasma proteomes for mass spectrometry–based analyses. Mol Cell Proteomics. 2012;11(6):M111.014340. doi: 10.1074/mcp.M111.014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao YC, Yuan ZB, Yang YD, et al. Effect of freeze-thaw cycles on serum measurements of AFP, CEA, CA125 and CA19-9. Scand J Clin Lab Invest. 2007;67(7):741–747. doi: 10.1080/00365510701297480. [DOI] [PubMed] [Google Scholar]
- 49.Cavalleri A, Colombo C, Venturelli E, et al. Evaluation of reactive oxygen metabolites in frozen serum samples. Effect of storage and repeated thawing. Int J Biol Markers. 2004;19(3):250–253. doi: 10.1177/172460080401900312. [DOI] [PubMed] [Google Scholar]
- 50.O'Connor KA, Brindle E, Holman DJ, et al. Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research. Clin Chem. 2003;49(7):1139–1148. doi: 10.1373/49.7.1139. [DOI] [PubMed] [Google Scholar]
- 51.Pero RW, Lund H, Leanderson T. Antioxidant metabolism induced by quinic acid. Increased urinary excretion of tryptophan and nicotinamide. Phytother Res. 2009;23(3):335–346. doi: 10.1002/ptr.2628. [DOI] [PubMed] [Google Scholar]
- 52.Gonthier MP, Verny MA, Besson C, et al. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J Nutr. 2003;133(6):1853–1859. doi: 10.1093/jn/133.6.1853. [DOI] [PubMed] [Google Scholar]
- 53.Azam S, Hadi N, Khan NU, et al. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med Sci Monit. 2003;9(9):BR325–BR330. [PubMed] [Google Scholar]
- 54.Amiano P, Dorronsoro M, de Renobales M, et al. Very-long-chain omega-3 fatty acids as markers for habitual fish intake in a population consuming mainly lean fish: the EPIC cohort of Gipuzkoa. European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr. 2001;55(10):827–832. doi: 10.1038/sj.ejcn.1601242. [DOI] [PubMed] [Google Scholar]
- 55.Salloway S, Sperling R, Keren R, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77(13):1253–1262. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preedy VR, Watson RR, Patel VB, editors. Nuts and Seeds in Health and Disease Prevention. London, United Kingdom: Academic Press; 2011. [Google Scholar]
- 57.Maarse H, editor. Volatile Compounds in Foods and Beverages. New York, NY: Marcel Dekker; 1991. [Google Scholar]




